Abstract
BACKGROUND
Quantitative Susceptibility Mapping (QSM) MRI allows accurate assessment of iron content in cerebral cavernous malformations (CCM), and a threshold increase by ≥6% in QSM has been shown to reflect new symptomatic hemorrhage (SH) in previously stable lesions.
PURPOSE/HYPOTHESIS
It is unclear how lesional QSM evolves in CCMs after recent SH, and whether this could serve as a monitoring biomarker in clinical trials aimed at preventing rebleeding in these lesions.
STUDY TYPE
This is a prospective observational cohort study.
POPULATION
16 CCM patients who experienced a SH within the past year, whose lesion was not resected or irradiated.
FIELD STRENGTH/SEQUENCE
The data acquisition was performed using QSM sequence implemented on a 3T MRI system.
ASSESSMENT
The lesional QSM assessments at baseline and yearly during 22 patient-years of follow-up were performed by a trained research staff including imaging scientists.
STATISTICAL TESTS
Biomarker changes were assessed in relation to clinical events. Clinical trial modeling was performed using two-tailed tests of time-averaged difference (assuming within-patient correlation of 0.8, power = 0.9 and alpha = 0.1) to detect 20%, 30% or 50% effects of intervention on clinical and biomarkers event rates during two years of follow-up.
RESULTS
The change in mean lesional QSM of index hemorrhagic lesions was +7.93% per patient-year in the whole cohort. There were 5 cases (31%) of recurrent SH or lesional growth, and twice as many instances (62%) with a threshold (≥6%) increase in QSM. There were no instances of SH hemorrhage or lesional growth without an associated threshold increase in QSM during the same epoch.
DATA CONCLUSION
We report novel biomarker changes which are sensitive to and twice as common as recurrent SH or lesional growth during follow-up of CCMs after recent bleed. The frequency of threshold QSM increase, or mean lesional QSM change per epoch may serve as monitoring biomarkers of CCM hemorrhage, reducing sample size requirements for proof of effect of novel therapies and enhancing the efficiency of clinical trials.
Keywords: Cerebral Cavernous Malformation, Imaging Biomarker, Clinical trials, QSM
INTRODUCTION
Cerebral cavernous malformations (CCM) is a common neurovascular disorder, affecting 0.2–0.4% of the population (1). The lesions consist of clustered dilated capillaries (caverns) with defective endothelial cell-cell junctions. Hemorrhage is a cardinal mechanism mediating clinical sequelae in CCM, and is reflected by chronic deposition of non-heme iron in lesions (2). Iron content in CCM may be quantified using advanced magnetic resonance imaging (MRI) technique of quantitative susceptibility mapping (QSM) (3,4). The QSM technique utilizes morphology enabled dipole inversion from a single-angle MR acquisition (3). It quantifies magnetic field changes caused by local susceptibility sources (such as iron), by resolving the captured signal phase-encoding data (3). In vitro phantoms with varying concentrations of iron in ferric, ferrous and ferumoxytol molecular states have been precisely correlated with QSM measurements (3).
According to FDA/NIH definitions, biomarkers refer to biological characteristics that can be objectively measured and assessed as indicators of normal or pathological processes (5). In clinical practice, biomarkers include tools and technologies that help in understanding etiology, establishing diagnosis, follow progression or assessing the responses to therapeutics. QSM has been proposed as a biomarker in a wide range of neurologic disorders where iron deposition is a cardinal feature. These include cerebral microbleeds (6), multiple sclerosis (7), brain tumors (8), differentiating hemorrhage from intracranial calcifications (9), and neurodegenerative diseases (10,11).
Despite the heterogeneity of lesional blood products at different stages of evolution, iron content in surgically excised CCM lesions precisely reflected the measured iron concentration in the same specimens by mass spectrometry (3). There was excellent inter-observer agreement of QSM assessments, and precise reproducibility using different MRI instruments (3). Consistent with the conservation of mass hypothesis, in vivo measurements of mean lesional QSM were shown to be higher in human CCMs with greater vascular permeability assessed by dynamic contrast enhanced quantitative perfusion (12), in older patients, and in cases with prior overt hemorrhages (3). More importantly, an increase in mean lesional QSM was recently associated with a new symptomatic hemorrhage (SH) or lesional growth during prospective longitudinal follow-up of previously stable CCM lesions, while a slight but significant decrease in QSM was documented in stable lesions (13).
A clinically overt SH has been defined as the prime relevant outcome parameter in the natural course of a CCM lesion (1). This outcome has been reported in a number of natural history studies (1) including a large meta-analysis using individual patient data from multiple centers (14). It is now well established that cavernomas with SH are at significantly higher risk of re-bleeding in subsequent years than lesions that had never bled, making this subset of patients the prime target of novel therapeutic investigations in upcoming clinical trials (1). However, it remains unclear how QSM might change in CCMs that recently bled, and how this might correlate with recurrent symptomatic events. This would reflect on the potential application of QSM as a monitoring biomarker.
We hypothesized that a threshold QSM increase will be registered with each recurrent symptomatic event (validation of sensitivity), and potentially in additional cases reflecting asymptomatic hemorrhage during longitudinal follow-up of CCM lesions with recent SH. We further assessed the frequency of QSM threshold events and mean change in lesional QSM per year as potential therapeutic targets in clinical trial modeling.
MATERIALS AND METHODS
Subject Enrollment
We conducted a prospective observational cohort study without therapeutic intervention. The study included patients with CCM lesion and an adjudicated SH within the prior year, where the index hemorrhagic lesion was neither resected nor irradiated. Cases were followed for at least one year or until recurrent SH or demonstrable lesional growth. Enrolled subjects underwent QSM assessments in conjunction with MRI at baseline and at annual follow-up. We excluded cases harboring additional confounding brain pathology, including prior brain irradiation. The subjects underwent informed consent per Institutional Review Board approved protocol (Table 1).
Table 1.
Demographics of enrolled CCM subjects with recent symptomatic hemorrhage, and follow-up characteristics
| Patient | Sex | Genotype | Race | Epoch | Clinical event | Biomarker event |
|---|---|---|---|---|---|---|
| 1 | F | CCM1 | White/Caucasian | 1 | Stable | No |
| 2 | F | CCM1 | White/Caucasian | 1 | Stable | Yes |
| 2 | Stable | No | ||||
| 3 | F | CCM1 | Hispanic | 1 | Growth | Yes |
| 2 | Stable | No | ||||
| 4 | M | Sporadic | White/Caucasian | 1 | Stable | No |
| 5 | F | CCM3 | White/Caucasian | 1 | Stable | Yes |
| 6 | F | CCM3 | White/Caucasian | 1 | Stable | No |
| 2 | Stable | Yes | ||||
| 7 | M | CCM3 | White/Caucasian | 1 | Hemorrhage | Yes |
| 2 | Stable | Yes | ||||
| 8 | F | CCM3 | Hispanic | 1 | Hemorrhage | Yes |
| 9 | F | CCM1 | White/Caucasian | 1 | Stable | No |
| 2 | Stable | No | ||||
| 10 | F | CCM1 | White/Caucasian | 1 | Stable | No |
| 11 | M | MFUG | White/Caucasian | 1 | Stable | No |
| 2 | Stable | Yes | ||||
| 12 | M | Sporadic | White/Caucasian | 1 | Stable | No |
| 13 | F | Sporadic | African American | 1 | Hemorrhage | Yes |
| 14 | F | Sporadic | White/Caucasian | 1 | Stable | No |
| 15 | F | Sporadic | White/Caucasian | 1 | Hemorrhage | Yes |
| 16 | F | Sporadic | White/Caucasian | 1 | Stable | No |
M/F: Male/Female; MFUG: Multifocal Unknown Genotype
Defining QSM And Clinical Events
The detailed protocol of QSM has been published (3,13). Thresholds with optimal sensitivity and specificity had been generated to segregate lesions which remained stable from those which manifested rebleed or lesion growth during prospective follow-up. Receiver operating curves (ROC) estimated a threshold change of +5.81% in mean lesional QSM with optimal sensitivity and specificity of 82.3 and 88.9%, respectively (13). We thus defined a threshold “biomarker event” as a ≥6% change in mean QSM of the hemorrhagic CCM lesion during any year epoch of follow-up (Fig. 1).
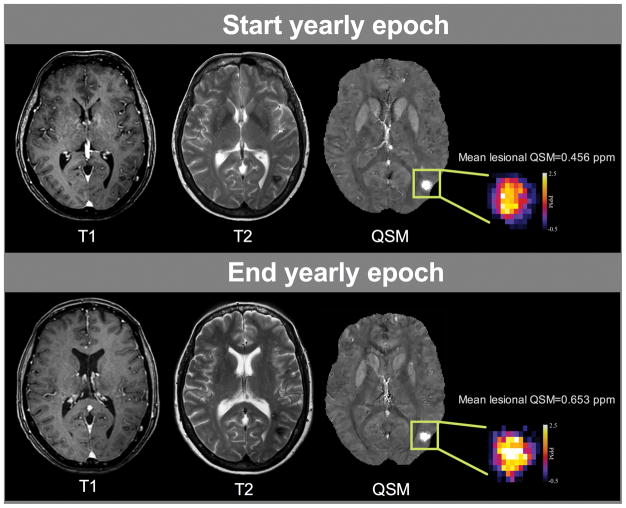
Fig. 1. Illustration of a biomarker event in a CCM familial/multifocal case, without clinical symptoms or change on conventional imaging.
The CCM lesion observed in hyposignal on T1 and T2-weighted images and in hypersignal on the QSM post-processed images showed an increased mean lesional QSM measurements of 43% between the beginning (up) and the end (down) of a yearly epoch. This increased lesional iron deposition was not correlated with relevant clinical symptom, and may be associated with occult lesional bleed, that could be monitored for testing proof of concept effect of putative therapies.
A clinical event was defined as either a SH based on adjudicated definition (13) or a demonstrable growth of a lesion by ≥2mm on comparable T1 or T2-weighted images at 3T field strength (3), clinically meaningful outcome parameters in natural history studies and potential clinical trials.
Lesional Iron Deposition Data Acquisition And Post-Processing
Image Acquisition And Post-Processing
All scans were performed using a 3T magnetic resonance imaging (MRI) system (Achieva, Philips, Best, Netherlands) with an eight-channel phased-array head coil. The QSM protocol utilized were previously published (3,13). Lesional iron deposition was quantified using a single 3D, multi-echo, gradient recalled echo (GRE) T2*-weighted, spoiled gradient echo acquisition sequence. The QSM images were then post-processed with a customized software using a morphology-enabled dipole inversion algorithm (3) generating the local susceptibility distribution by inverting the estimated tissue field map with prior information from the magnitude images. The tissue field map was obtained by removing the background field induced by large susceptibility sources (i.e. air/tissue interface) from the field map derived from the GRE phase images (3).
The post-processing routines were implemented using MATLAB platform (MathWorks, Natick, USA). The QSM datasets were acquired and post-processed by 3 experienced imaging scientists and two research clinical fellows. The operators were blinded to the clinical status of the patients throughout the image analysis.
Region Of Interest (ROI) Selections In CCM Patients
The ROI segmentation was performed using Image J software (National Institute of Health, Bethesda, MD), with previously demonstrated intra- and inter-observer consistency (3,13). On QSM post-processed maps, CCM lesions showed up as hyperintense and were matched using SWI and T2-weighted images, the latter including the CCM “hemosiderin ring”. 2D ROIs were created across multiple slices by segmenting the entire lesion and aggregating to define a 3D lesional volume (3,12). The mean lesional susceptibility was computed within the 3D ROI in parts per million (ppm).
The index hemorrhagic lesion (correlated with a qualifying SH within the year prior to enrollment) was considered at baseline and follow-up assessments (15). The index lesion was matched on T1 and T2-weighted images on follow-up scans, for the purpose of stability assessment. Changes in the index CCM lesion were used for the primary analyses in this study. We did not encounter any cases with a recurrent SH or growth in a lesion other than the index lesion that had caused the original SH. QSM assessment in lesions other than the index hemorrhagic CCM (in familial cases with multiple lesions) was performed as an additional control during follow-up.
Sample Size Calculations For Trial Modeling
We assumed that each patient would contribute two outcome measurements (at year 1 and year 2) based on intention-to-treat during two years of follow-up. For modeling an effect on the frequency of clinical or biomarker events, we hypothesized a time-averaged difference between two arms using a repeated measures analysis implemented as an unadjusted linear mixed model (16,17). For modeling the impact of intervention on the mean change in QSM change score, the same methodology was used but as a continuous measure. Effect sizes modeled were based on likely minimum clinically significant changes (for event rates), and on percent changes in lesional iron content documented in preclinical studies of novel therapies (for QSM change) (18,19).
Statistical Analysis
Analyses were done using 1-year patient epochs, with QSM assessments at the beginning and end of each epoch. Hence a patient followed for two years (total of three MRI visits) would contribute to two QSM change measurements based on three QSM assessments. We assessed the frequency of threshold biomarker events and the frequency of clinical instability (SH or lesion growth) per patient during follow-up. We also assessed the mean change in lesional QSM during follow-up.
Modeling included sample size calculations to power trials based on a hypothesized impact of intervention on the frequency of clinical outcome events or biomarker events per patient, or an effect on mean percent change in lesional QSM per year (called the QSM change score), during 2 years of follow-up of CCM with recent SH. Two-tailed tests were used, assuming within-patient correlation of 0.8, power=0.9 and alpha=0.1.
The software PASS V11 was used to perform the analysis (NCSS, Kaysville, Utah, USA, www.ncss.com). Projected needed sample sizes are summarized in Tables 2 and 3. For each model, these would need to be inflated to account for potential attrition depending on the therapy being tested.
Table 2.
Trial modeling: Sample size calculation based on frequency of clinical or biomarker events
| Endpoint | Frequency of clinical events | Frequency of biomarker events | ||
|---|---|---|---|---|
|
|
|
|||
| Difference | Group 1 size | Group 2 size | Group 1 size | Group 2 size |
|
|
||||
| 20% | 247 | 247 | 62 | 62 |
|
| ||||
| 30% | 110 | 110 | 28 | 28 |
|
| ||||
| 50% | 40 | 40 | 10 | 10 |
Two-tailed tests were used, assuming within-patient correlation of 0.8, power = 0.9 and alpha = 0.1. Each patient would contribute two outcome measurements (at year 1 and year 2). Calculation was based on observed frequency of 5 clinical events and 10 biomarker events in a cohort of 16 patients.
Table 3.
Trial modeling: Sample size calculation based on mean percent change in lesional QSM per year
| Endpoint
|
Mean percent QSM change
|
|
|---|---|---|
| Difference | Group 1 size | Group 2 size |
| 20% | 25 | 25 |
|
| ||
| 25% | 16 | 16 |
|
| ||
| 30% | 11 | 11 |
Two-tailed tests were used, assuming within-patient correlation of 0.8, power = 0.9 and alpha = 0.1. Each patient would contribute two outcome measurements (at year 1 and year 2).
Calculation was based on observed mean percent QSM change of 7.9%.
RESULTS
Demographic And CCM-related Symptoms Characteristics
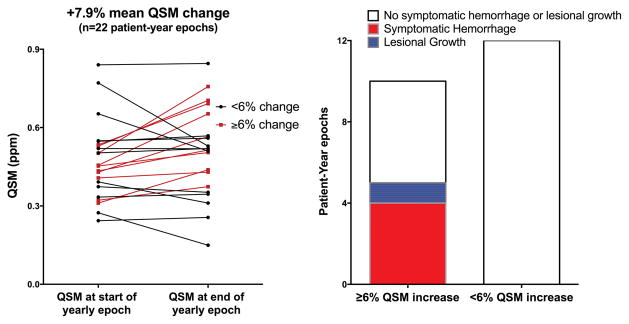
From June 2012-January 2017, 298 CCM patients were enrolled in the MRI biomarkers study. Eighty-one had a SH in the preceding year. Sixteen were followed for one or two years, with satisfactory QSM assessments at enrollment, and at each annual follow-up (Table 1). During 22 patient-years of follow-up, 5 clinical events (4 cases of recurrent SH, one case of lesional growth) and 10 QSM biomarker events occurred (Fig. 2). All clinical events were associated with threshold biomarker events as defined. Threshold biomarker events were twice as common as clinical events.
Fig. 2.
(Left) QSM measurements at the beginning and end of 22 patient-year epochs of CCM lesion that had bled within 1 year, and followed for 1–2 years. Cases underwent annual QSM, allowing paired measurements at the beginning and end of each patient-year epoch. Only QSM measurements in the lesion with initial hemorrhage were considered. Paired measurements in red identify epochs with a recorded threshold QSM increase by ≥6%. (Right) Number of patient-year epochs with and without threshold QSM change, and clinical events during these epochs. All clinical events occurred in the setting of ≥6% increase in QSM.
The mean lesional QSM change in the index hemorrhagic lesions was +7.93% per patient-year in the whole cohort. Cases with biomarker events had a mean change of lesional QSM by +28.15% per patient-year epoch, while cases without biomarker events demonstrated a mean change of −8.96% per patient-year epoch. The QSM values of non-index lesions in multifocal cases (24 lesions, followed up for 30 epochs, none of which manifested clinical or imaging change) showed a mean QSM change of −0.5%, similar to previously reported slight decrease in mean lesional QSM of clinically stable lesions (13).
Clinical Trial Modeling An Effect On Mean Percent Change In Lesional QSM Per Year (QSM change score) Requires Fewest Subjects
Trial modeling (Tables 2 and 3) revealed that 494, 220, and 80 subjects (half randomized assigned to placebo or treatment) would be needed to detect 20%, 30% or 50% effects of intervention, respectively, on clinical event rates during two years of follow-up, at the postulated power and type 1 error. However, only 124, 56, and 20 subjects respectively would be needed to detect the same respective effect on the frequency of biomarker events. To detect 20, 25 and 30% absolute differences between the mean QSM change score in each arm, based on two annual change scores measured per patient (at year 1 and 2), sample sizes of 50, 32 or 22 subjects would be required, respectively.
DISCUSSION
Our analysis focused on CCM lesions that had bled within the past year, as these are at significantly higher risk of re-bleed (1,14), and will be the most likely target of clinical trials. Our results showed that a pre-articulated threshold QSM increase (“biomarker event”) occurred twice as often as SH or lesional growth.
There were no instances of clinical event without an associated threshold biomarker event during the same epoch. Cases with a new biomarker event manifested a large increase in QSM, likely representing lesional bleeds, even in the absence of symptoms or lesional growth. Conversely, cases without a new threshold biomarker event were never associated with a clinical event. These manifested a net decrease in QSM during follow-up, consistent with clearance of iron from the lesions.
Preclinical studies have demonstrated a favorable effect of experimental therapies on CCM lesional hemorrhage in murine models (18,19), using contemporaneous randomized treatment assignment versus placebo, and blinded outcome assessment per NINDS guidelines (20). Our results suggest that lesional QSM may be used as a monitoring biomarker (5) of lesional hemorrhage during follow-up of CCMs that recently bled. The enhanced sensitivity to detect lesional hemorrhage offers a significant efficiency in trial sample size needed to test a potential effect of therapies. Two-tailed trial designs would postulate a decrease in QSM as a measure of therapeutic proof of concept effectiveness, while an increase in QSM would raise a concern about safety, signaling increased lesional hemorrhage. This would best be applied in probing proof of concept effect in Phase I or IIA trials of putative therapies, and potentially as certifiable outcome parameters in Phase IIB or III trials of orphan therapies (5). The approach is particularly relevant in go/no-go decisions when comparing multiple therapies and doses during early stages of therapeutic development.
The study generates hypotheses for further research. A clinical readiness project is underway to verify QSM measurement accuracy, reproducibility and precision at multiple sites, and to validate clinical event rates and biomarker changes during follow-up of CCMs with recent hemorrhage. This may provide more reliable pilot data for trial modeling and correct the potential biases of referral and follow-up inherent to a single research site. The study was further limited by a small number of subjects with clinical events, and results will need confirmation in larger cohort.
In conclusion, we have shown that QSM can serve as a qualitative (frequency of threshold events) or quantitative (mean percent change) monitoring biomarker of lesional hemorrhage. The use of this technique improves clinical trials efficiency by reducing sample size requirements.
Acknowledgments
FUNDING
This work was partially supported by NIH grant (R21NS087328) to IAA and the William and Judith Davis Fund in Neurovascular Surgery Research. Funding sources played no role in the formulation of research questions nor the interpretation of results.
We would like to acknowledge the assistance of Carol B Thompson, MS, MBA of the Bloomberg School of Public Health at Johns Hopkins University for assistance with part of the statistical design, and support for this from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
References
- 1.Akers A, Al-Shahi Salman R, Awad I, et al. Synopsis of Guidelines for the Clinical Management of Cerebral Cavernous Malformations: Consensus Recommendations Based on Systematic Literature Review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery. 2017;80:665–680. doi: 10.1093/neuros/nyx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiger HJ, Markwalder TM, Reulen HJ. Clinicopathological relations of cerebral cavernous angiomas: observations in eleven cases. Neurosurgery. 1987;21:879–884. doi: 10.1227/00006123-198712000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Tan H, Zhang L, Mikati AG, et al. Quantitative Susceptibility Mapping in cerebral cavernous malformations: Clinical correlations. AJNR Am J Neuroradiol. 2016;37:1209–1215. doi: 10.3174/ajnr.A4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan H, Liu T, Wu Y, et al. Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Invest Radiol. 2014;49:498–504. doi: 10.1097/RLI.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker Qualification: Toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98:34–46. doi: 10.1002/cpt.136. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Surapaneni K, Lou M, Cheng L, Spincemaille P, Wang Y. Cerebral microbleeds: burden assessment by using quantitative susceptibility mapping. Radiology. 2012;262:269–278. doi: 10.1148/radiol.11110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551–559. doi: 10.1148/radiol.12120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deistung A, Schweser F, Wiestler B, et al. Quantitative susceptibility mapping differentiates between blood depositions and calcifications in patients with glioblastoma. PLoS One. 2013;8:e57924. doi: 10.1371/journal.pone.0057924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Zhu W, Kovanlikaya I, et al. Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping. Radiology. 2014;270:496–505. doi: 10.1148/radiol.13122640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotfipour AK, Wharton S, Schwarz ST, et al. High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson’s disease. J Magn Reson Imaging. 2012;35:48–55. doi: 10.1002/jmri.22752. [DOI] [PubMed] [Google Scholar]
- 11.Fritzsch D, Reiss-Zimmermann M, Trampel R, Turner R, Hoffmann KT, Schafer A. Seven-tesla magnetic resonance imaging in Wilson disease using quantitative susceptibility mapping for measurement of copper accumulation. Invest Radiol. 2014;49:299–306. doi: 10.1097/RLI.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 12.Mikati AG, Tan H, Shenkar R, et al. Dynamic permeability and quantitative susceptibility: related imaging biomarkers in cerebral cavernous malformations. Stroke. 2014;45:598–601. doi: 10.1161/STROKEAHA.113.003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girard R, Fam MD, Zeineddine HA, et al. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg. 2017;127:102–110. doi: 10.3171/2016.5.JNS16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horne MA, Flemming KD, Su IC, et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol. 2016;15:166–173. doi: 10.1016/S1474-4422(15)00303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell PG, Jabbour P, Yadla S, Awad IA. Emerging clinical imaging techniques for cerebral cavernous malformations: a systematic review. Neurosurg Focus. 2010;29:E6. doi: 10.3171/2010.5.FOCUS10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown H, Prescott R. Applied mixed models in medicine. John Wiley & Sons; 2014. [Google Scholar]
- 17.Liu H, Wu T. Sample size calculation and power analysis of time-averaged difference. J Mod Appl Stat Methods. 2005;4:9. [Google Scholar]
- 18.Shi C, Shenkar R, Zeineddine HA, et al. B-Cell depletion reduces the maturation of cerebral cavernous malformations in murine models. J Neuroimmune Pharmacol. 2016;11:369–377. doi: 10.1007/s11481-016-9670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenkar R, Shi C, Austin C, et al. RhoA Kinase inhibition with Fasudil versus Simvastatin in murine models of cerebral cavernous malformations. Stroke. 2017;48:187–194. doi: 10.1161/STROKEAHA.116.015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis SC, Amara SG, Asadullah K, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]