Abstract
Objective
To evaluate the effects of pressure threshold respiratory training (RT) on heart rate variability and baroreflex sensitivity in persons with chronic spinal cord injury (SCI).
Design
Before-after intervention case-controlled clinical study.
Setting
SCI research center and outpatient rehabilitation unit.
Participants
Persons with chronic SCI ranging from C2 to T11 that participated in RT (n=24) and untrained chronic SCI controls ranging from C2 to T9 (n=20).
Intervention
A total of 21 ± 2 of RT sessions performed 5 days a week during a four-week period using a combination of pressure threshold inspiratory and expiratory devices.
Main Outcome Measures
Forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and beat-to-beat arterial blood pressure (BP) and heart rate (HR) changes during 5-second long maximum expiratory pressure maneuver (5s MEP) and sit-up orthostatic stress test acquired before and after the RT program.
Results
In contrast to the untrained controls, individuals in RT group experienced significantly increased FVC and FEV1 (both p < .01) in association with improved quality of sleep, cough, and speech. Sympathetically (phase II) and parasympathetically (phase IV) mediated baroreflex sensitivity both significantly (p < .05) increased during 5s MEP. During orthostatic stress test, improved autonomic control over HR was associated with significantly increased sympathetic and parasympathetic modulation (low- and high-frequency change, p < .01 and p < .05, respectively).
Conclusion
The results indicate that inspiratory-expiratory pressure threshold RT is a promising technique to positively impact both respiratory and cardiovascular dysregulation observed in persons with chronic SCI.
Keywords: Autonomic nervous system, Blood pressure, Breathing exercises, Hypotension, Rehabilitation, Respiration, Spinal cord injuries
INTRODUCTION
It is estimated that the number of people living with spinal cord injury (SCI) in the U.S ranges from 243,000 to 347,0001 and while life expectancy for individuals with SCI is lower than general population, survival of patients with SCI has improved. However, medical complication rates greatly increase with the advancing age of persons with SCI2. Due to injury to the motor and autonomic nervous systems and accelerated aging mechanisms associated with immobility, persons with SCI are more likely to die from pneumonia, heart attacks, stroke, and other cardiopulmonary complications compared with their age-matched, non-injured counterparts3–8. Vascular and respiratory adaptations can occur as soon as 6 weeks after injury9. Chronic SCI can result in maladaptive blood pressure regulation and respiratory deficits leading to hypotension, bradycardia, dyspnea, and impaired cough that together may predict the development of cardiovascular disease7, 10–14. Higher SCI levels with more complete neurological lesions demonstrate greater cardiovascular and pulmonary functional deficits; however, even incomplete SCI lesions as low as L4 should be considered as risk factors for cardiopulmonary disability as they still exhibit deficits from functional impairment due to accelerated aging, lifestyle factors, and decreased mobility7, 15–17.
As stated above, co-presentation of cardiovascular and pulmonary diseases in SCI is common and could be, in part, due to the interdependence of the cardiovascular and pulmonary systems. Physiologically, these systems are closely linked: arterial blood pressure (BP) oscillates with breathing and engages the baroreceptors18–20 while chemoreceptors and pulmonary mechanoreceptors participate in heart rate (HR) and vasomotor tone regulation in response to arterial blood gas content and inspiratory volume changes18, 21. The chemoreflexes exert profound influences not only on breathing, but also on cardiovascular function. Moreover, baroreflex-chemoreflex interactions may have relevance to disease states in which baroreflex function is impaired, such as SCI22.
There is clinical evidence that this relationship still exists post-SCI: it is demonstrated that morbidity and mortality rates are significantly decreased when cardiovascular and pulmonary deficits are addressed together during the acute treatment of SCI23, 24. In the chronic phase, individuals with greater pulmonary function outcomes demonstrate higher resting BP values25. Despite this evidence, cardiovascular and pulmonary dysfunction testing and treatment in SCI are targeted independently: interventions that demonstrate increased pulmonary function outcomes are not tested for resulting changes in cardiovascular regulation26–32. Recently, we reported that orthostatic hypotension and respiratory dysfunction can be ameliorated in persons with chronic SCI after respiratory motor training33. However, much of the cardiovascular therapy currently available to persons with SCI is limited to management of unstable BP with little evidence of long-term changes in BP regulation34–36. There is therefore a gap in therapies available to persons with SCI to ameliorate cardiovascular dysregulation and subsequently decrease the risk of future cardiovascular events. Respiratory Training (RT) is commonly used in persons with SCI to increase cough capacity and respiratory capacity and improve performance27, 30–32, 37, 38. It is thus a logical intervention to test the theory of cardiovascular and pulmonary system interdependence effect in the treatment of chronic SCI subjects to prevent long term complications secondary to cardiovascular events. Therefore, we hypothesized that RT improves cardiovascular response to respiratory and cardiovascular challenges due to improved reflex activity.
METHODS
This study was approved by the Institutional Review Board for Human Research. Forty-four persons with SCI were recruited from Frazier Rehabilitation and Neuroscience institute in Louisville, KY, according to the following inclusion criteria: at least 18 years of age; chronic (a minimum of 6 months since SCI), non-progressive SCI; no ventilatory dependence; respiratory functional deficit defined as a decrease in predicted FVC and FEV1 values at least 10%; no tobacco or drug use; and no cardiovascular or respiratory diseases unrelated to SCI.
Clinical assessment
Twenty-four individuals participated in the RT protocol, while 20 physically (age, sex, height, and weight) matched participants were in the control group. The International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) were used to determine the neurological level and clinical severity of the spinal cord lesion according to the American Spinal Injury Association Impairment Scale (AIS)39. Twenty-seven participants were classified as having motor-complete (AIS-A or AIS-B) SCI and 17 participants were diagnosed with motor-incomplete (AIS-C or AIS-D) SCI, with a neurological range from C2 to T11 (Table 1).
Table 1.
Demographic Summary of Participants
| ID | Age | Sex | Height (in) | Weight (lbs) | LOI | AIS | Months Since Injury | |
|---|---|---|---|---|---|---|---|---|
| RMT Group | A58 | 40 | M | 70 | 230 | C3 | A | 22 |
| A38 + | 37 | F | 69 | 130 | C4 | A | 248 | |
| A65 * | 29 | M | 68 | 180 | C4 | A | 10 | |
| B18 * | 56 | M | 72 | 155 | C3 | B | 29 | |
| B06 + | 42 | F | 67 | 123 | C4 | B | 70 | |
| B11 | 25 | M | 70 | 185 | C4 | B | 98 | |
| B16 + | 60 | M | 71 | 220 | C4 | B | 31 | |
| B19 | 40 | M | 74 | 177 | C6 | B | 6 | |
| C33 * | 59 | M | 69 | 145 | C2 | C | 532 | |
| C27 | 58 | M | 70 | 190 | C4 | C | 47 | |
| C30 + | 19 | F | 67 | 94 | C4 | C | 12 | |
| C34 | 20 | M | 76 | 140 | C4 | C | 53 | |
| C26 | 33 | M | 72 | 165 | C6 | C | 4 | |
| C38 | 42 | M | 70 | 245 | C2 | D | 156 | |
| B17 | 59 | M | 75 | 217 | C4 | D | 15 | |
| C18 + | 31 | M | 71 | 214 | C4 | D | 36 | |
| A46 + | 47 | F | 62 | 192 | T6 | A | 43 | |
| A66 | 48 | M | 72 | 170 | T6 | A | 65 | |
| A75 + | 49 | M | 73 | 188 | T9 | A | 84 | |
| A55 | 35 | M | 68 | 165 | T11 | A | 50 | |
| C16 + | 35 | M | 72 | 185 | T1 | C | 70 | |
| C28 | 29 | M | 70 | 160 | T5 | C | 56 | |
| C24 | 40 | F | 68 | 125 | T11 | C | 110 | |
| C39 | 45 | F | 68 | 135 | T11 | C | 21 | |
| Mean ± SD | 40 (12) | NA | 70 (3) | 173 (37) | NA | NA | 76 (109) | |
| Control Group | A33 + | 58 | M | 74 | 232 | C3 | A | 20 |
| A38 + | 39 | F | 69 | 113 | C4 | A | 237 | |
| B21 * | 30 | M | 73 | 165 | C4 | B | 64 | |
| B22 | 51 | M | 70 | 180 | C4 | B | 37 | |
| B28 + | 39 | M | 68 | 163 | C6 | B | 113 | |
| C14 + | 47 | M | 76 | 130 | C3 | C | 10 | |
| D35 + | 60 | M | 71 | 220 | C2 | D | 35 | |
| A35 + | 55 | M | 73 | 200 | T3 | A | 377 | |
| A36 + | 56 | M | 68 | 210 | T3 | A | 341 | |
| A57 | 27 | F | 73 | 165 | T4 | A | 45 | |
| A69 | 26 | M | 73 | 140 | T4 | A | 27 | |
| A39 + | 36 | M | 70 | 175 | T5 | A | 185 | |
| A53 | 30 | M | 70 | 141 | T5 | A | 23 | |
| A73 + | 28 | M | 67 | 180 | T6 | A | 58 | |
| A74 + | 56 | M | 69 | 240 | T9 | A | 418 | |
| B20 + | 28 | M | 65 | 128 | T2 | B | 53 | |
| C44 | 40 | M | 71 | 173 | T4 | B | 24 | |
| C37 | 20 | F | 60 | 190 | T2 | C | 77 | |
| C36 | 23 | M | 74 | 157 | T6 | C | 9 | |
| C45 | 22 | M | 70 | 260 | T2 | D | 6 | |
| Mean ± SD | 39 (12) | NA | 70 (4) | 177 (36) | NA | NA | 128 (146) | |
Participants that could not perform the 5s MEP are marked with (*) if spasms prohibited analysis, or (+) if participants entered study prior to addition of the 5s MEP to the research protocol.
Study Protocol
Data collection occurred between 2011 and 2014. FVC and FEV1, baroreflex sensitivity (BS), and HR variability (HRV) values were assessed at baseline (called the “Pre-Test” time point) in all participants. Individuals in the RT group then participated in 20, 45-minute sessions of training using a threshold positive expiratory pressure device and inspiratory muscle trainer (Respironics Inc., Cedar Grove, NJ) assembled together using a three-way valve system (Airlife 001504, Allegiance Healthcare Corp., McGaw Park, IL) with flanged mouthpiece29, 38. Participants were trained on-site with a team member tracking progression and monitoring the training load. Each participant received 20 training sessions. No participants dropped out during the study. Training load was increased regularly so participants were training at 60% of their maximum inspiratory and expiratory airway pressure generation by the last week. Besides experimental procedures, all participants maintained their normal routine as active participants in the community fitness gym. After four weeks of training for the RT group, and four to six weeks for the control group, participants were assessed again at the “Post-Test” time point.
Data acquisition
A Finometer MIDI (Finapres Medical System B.A., Netherlands) and finger plethysmograph synchronized with FMS 3-lead ECG device recorded continuous arterial BP waveform and HR. Brachial BP measurements were acquired to calibrate BP values obtained from the finger cuff (GE’s Dinamap Patient Monitor, Boston, MA)40. Data were converted from analog to digital signals using a Powerlab 16/35 system, recorded by LabChart 7 (AD Instruments, Denver, CO). Hemodynamic variables were acquired at 1000 Hz and calculated in Labchart; BS outcomes (see below) were analyzed in R41, while HRV was analyzed using Matlab software (The MathWorks, Natick, MA)42.
Baroreflex Sensitivity
Participants were assessed in a seated position, without the aid of compression garments or abdominal binders43, 44. To assess BS, participants blew with maximal effort into a mouthpiece for the duration of an audible, 5s tone (5s MEP)45. Each event was attempted three times, with at least 30s rest in between events to allow BP values to return to baseline. The increased intrathoracic pressure decreases venous return during the maneuver, eliciting a reflex tachycardia (similar to early phase II of the Valsalva maneuver), while the systolic blood pressure (SBP) overshoot following the maneuver elicits a reflex bradycardia (similar to phase IV of the Valsalva). For phases II and IV of the 5s MEP, SBP values were plotted against the following R-R interval (RRI) using a linear regression analysis with a Pearson correlation coefficient. The slope of each phase is reported in ms/mmHg, which quantifies the change in RRI for every 1 mmHg increase or decrease of SBP46–48. Acceptable attempts were those between 5 and 7s in duration49, 50 with a Pearson correlation coefficient > .8047, 51, 52; BS outcomes were excluded if the maneuver did not meet 27 cmH2O45, or if they triggered spasms, autonomic dysreflexia, or coughs. The phases analyzed began with three consecutively increasing or decreasing RRIs47, 52; the mean of all acceptable attempts was used for statistical analysis.
Orthostatic Stress Test
The orthostatic stress test was performed in the morning, between 9:00 and 10:30AM, in a dimly lit, temperature controlled room (22°C). Participants were asked to eat a light breakfast (i.e., little to no sugar or fat), to refrain from caffeine for 12 hours prior to the experiment, and to empty their bladder prior to arrival. Participants were recumbent upon a Hausted Manual Gurney Chair (GF Health Products, Atlanta, GA) for 15 minutes and then passively moved to a sitting position for an additional 15 minutes. The participant was instructed to refrain from non-essential movement or speech for the duration of the study. The test was terminated if the participant experienced autonomic dysreflexia, uncontrolled orthostatic hypotension that would lead to syncope, or if the participant felt too uncomfortable to continue.
Spectral power of RR interval was calculated during the last 10 minutes of the passively seated position. Each interval was linearly detrended, and spectral power was estimated using Welch’s averaged periodogram method (500-point windows with 50% overlapping segments). Mean spectral power was calculated for low-frequency (LF, 0.04 - 0.15 Hz) and high-frequency (HF, 0.15 - 0.4 Hz) regions using trapezoidal integration over the specified frequency range53–55. The first 5 minutes of the passively seated position were excluded because the rapid position change often triggered spasms that altered BP and heart rate. During orthostatic stress, like in a passively seated position, the LF RRI oscillation primarily reflects sympathetic control over the heart, while HF oscillation principally reflects vagally-mediated oscillations coupled to respiration55–58. Respiration frequency obtained from respiratory belts was checked to make sure it fell within the HF range.
Statistical Analysis
Data were analyzed in R (R Foundation for Statistical Computing, Vienna, Austria) and represented as mean ± standard deviation (SD); significance was set to α = .05. The presence of multiple covariates in the data and normally distributed residuals made the linear regression an appropriate test for significance59. To compare pre- to post-test outcomes, change scores were calculated for each participant by subtracting their post-test score from their pre-test score. This new measure was used as the dependent variable in all regression models; change score of the control group was the reference to which RT was compared. To control some of the variability between participants, level of injury (cervical or thoracic) and/or AIS impairment (A, B or C/D) were covariates in models (Table 2).
Table 2.
Summary of Pulmonary Function Testing (PFT) values obtained from the Pre-Test and Post-Test timepoints
| RMT Group | FVC (% predicted) | FEV1 (% predicted) | ||
|---|---|---|---|---|
| Pre-Test | Post-Test | Pre-Test | Post-Test | |
| C33 (C2C) | 78 | 88 | 70 | 80 |
|
| ||||
| D38 (C2D) | 85 | 99 | 87 | 92 |
|
| ||||
| A58 (C3A) | 65 | 60 | 53 | 50 |
|
| ||||
| B18 (C3B) | 65 | 67 | 39 | 56 |
|
| ||||
| A38 (C4A) | 63 | 53 | 66 | 49 |
|
| ||||
| A65 (C4A) | 63 | 58 | 30 | 32 |
|
| ||||
| B06 (C4B) | 46 | 57 | 38 | 40 |
|
| ||||
| B11 (C4B) | 57 | 66 | 41 | 47 |
|
| ||||
| B16 (C4B) | 86 | 85 | 75 | 75 |
|
| ||||
| B17 (C4B) | 70 | 74 | 58 | 68 |
|
| ||||
| C27 (C4C) | 64 | 57 | 41 | 40 |
|
| ||||
| C30 (C4C) | 41 | 69 | 45 | 46 |
|
| ||||
| C34 (C4C) | 37 | 40 | 29 | 28 |
|
| ||||
| C18 (C4D) | 77 | 67 | 74 | 66 |
|
| ||||
| B19 (C6B) | 70 | 81 | 66 | 77 |
|
| ||||
| C26 (C6C) | 60 | 76 | 67 | 76 |
|
| ||||
| C16 (T1C) | 59 | 73 | 52 | 64 |
|
| ||||
| C28 (T5C) | 82 | 85 | 74 | 78 |
|
| ||||
| A46 (T6A) | 96 | 93 | 73 | 77 |
|
| ||||
| A66 (T6A) | 78 | 80 | 70 | 76 |
|
| ||||
| A75 (T9A) | 82 | 80 | 75 | 74 |
|
| ||||
| C24 (T11C) | 100 | 95 | 81 | 83 |
|
| ||||
| C25 (T11C) | 94 | 94 | 90 | 92 |
|
| ||||
| C39 (T11C) | 98 | 99 | 87 | 82 |
|
| ||||
| Mean ± SD | 76 (13) | 82 (13) ** | 68 (15) | 76 (15) ** |
| Control Group | FVC (% predicted) | FEV1 (% predicted) | ||
| Pre-Test | Post-Test | Pre-Test | Post-Test | |
|
| ||||
| Mean ± SD | 77 (27) | 73 (25) | 67 (24) | 68 (24) |
Results are reported as Mean (SD);
p < .05,
p < .01
RESULTS
There were no significant between-group differences in any measured variable prior to onset of RT. Pulmonary function outcomes increased significantly in the RT group compared to controls: FVC increased from 76 ± 13% to 82 ± 13% (p < .01), and FEV1 increased from 68 ± 15% to 76 ± 15% (p < .01).
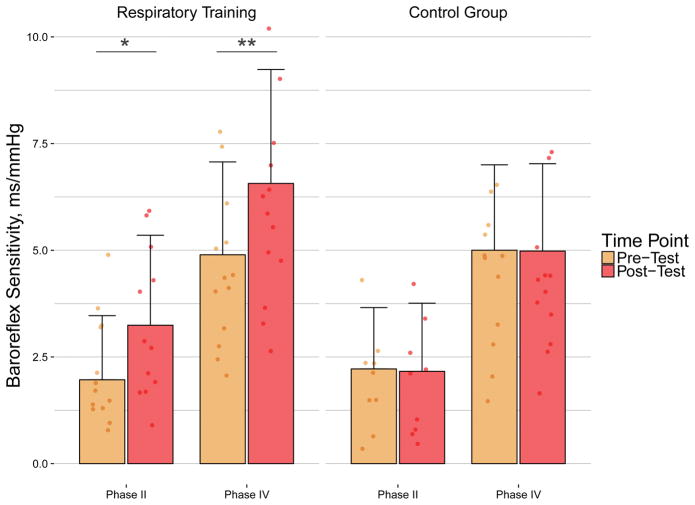
We found significant BS increases in the trained group in response to maximal, acute expiratory effort that were not seen in the control group. After training, heart rate changes in response to oscillations in systolic BP improved (Figure 1). Slope of the 5s MEP (Figure 2; Table 2) during phase II increased from 2 ± 2 to 3 ± 2 ms/mmHg (p = .01), while phase IV slope increased from 5 ± 2 to 7 ± 3 ms/mmHg (p < .01), demonstrating increased sympathetic (phase II) and parasympathetic (phase IV) control over the heart. These responses occurred without any significant differences to SBP during the maneuver (lowest SBP pre to post RT: 68 ± 18 mmHg to 71 ± 13 mmHg; peak SBP pre to post RT: 132 ± 39 mmHg to 126 ± 31 mmHg). There was also no correlation between phase II and phase IV gains in the RT group. There was a significant relationship between level of injury and phase IV in one of the models (Table 2), which could reflect the greater gains demonstrated by the cervical group compared to thoracic. There was no significant relationship between severity of injury and gains after RT (Table 2), demonstrating AIS impairment did not detectably affect gains post training.
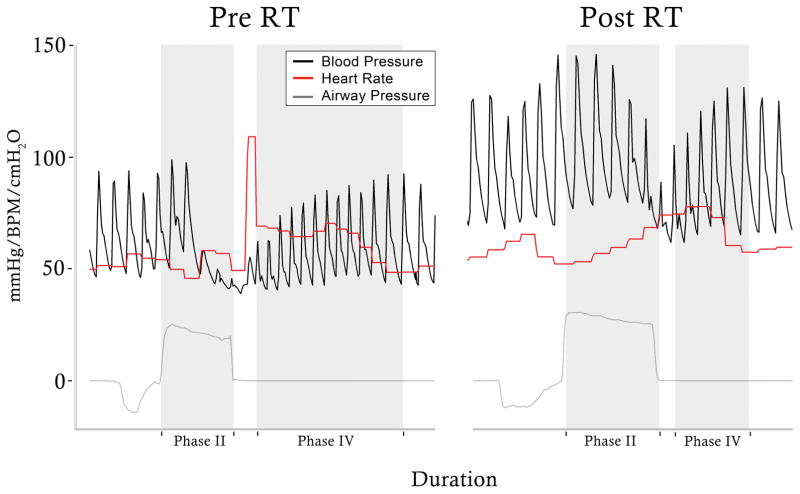
Figure 1.
Recording of the maximum expiratory pressure (5s MEP) pre- and post- respiratory training (RT) program. Sample recording of arterial blood pressure (black), heart rate (red) and airway pressure (gray) during 5s MEP from male with C6, AIS B spinal cord injury. The relationship between heart rate and systolic blood pressure oscillations improved after respiratory training, during (Phase II: 1.4 ms/mmHg to 3.4 ms/mmHg) and after (Phase IV: 6.8 ms/mmHg to 9.6 ms/mmHg) the maneuver.
Figure 2.
Baroreflex sensitivity (BS) during phases II and IV of the maximum expiratory pressure (5s MEP) maneuver. Note that there were significant increases in BS values during phase II and phase IV of 5s MEP in the respiratory training (RT) group (n=14; *p < .05; **p < .01) compared to controls (n=11), demonstrating an increased relationship between heart rate and systolic blood pressure oscillations after RT.
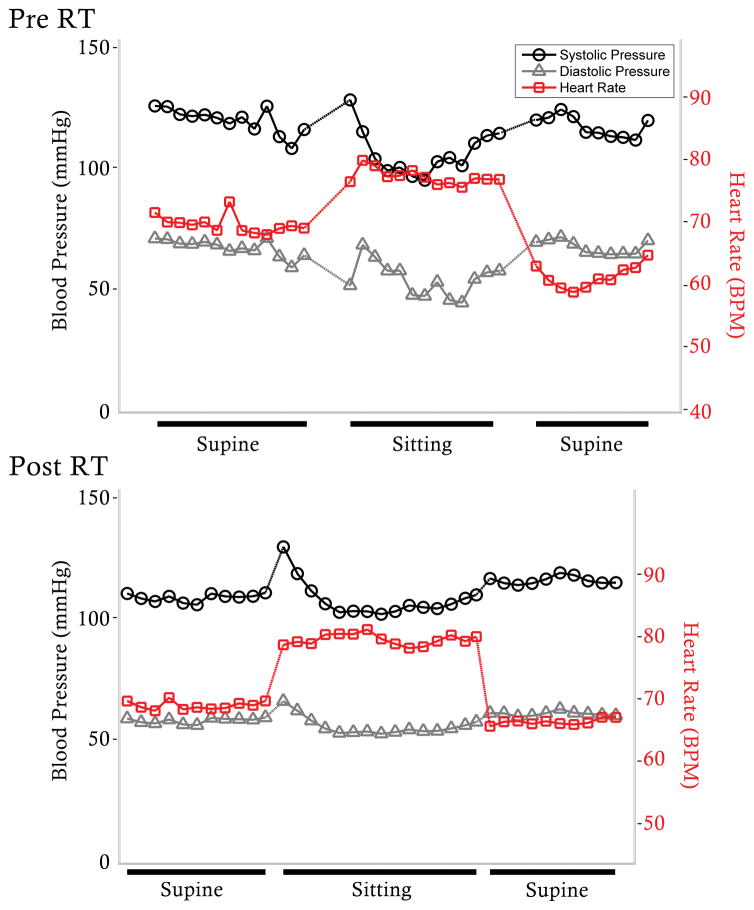
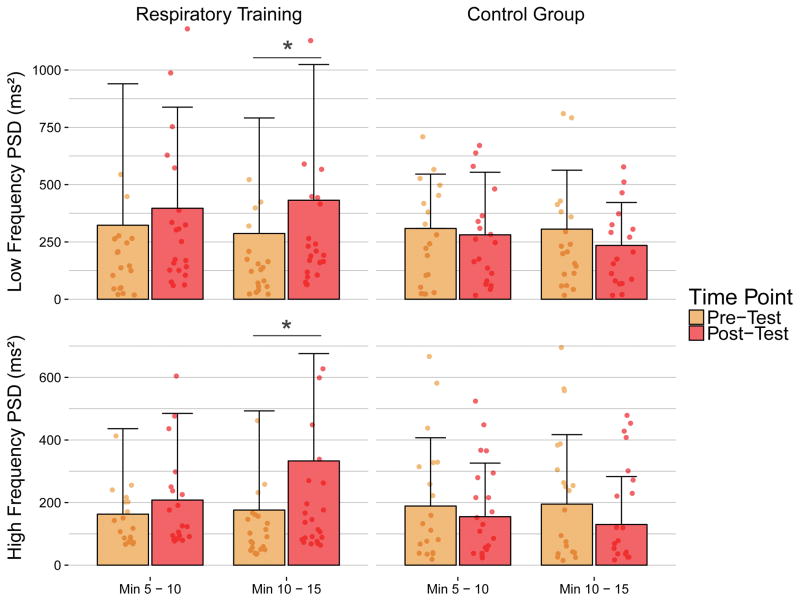
After training, the drop in sitting DBP was ameliorated (Δ pre: −14/−5 ± 26/16 mmHg; Δ post: −4/0 ± 14/9 mmHg) and there was an improved cardiac and hemodynamic response during the orthostatic stress test (Figure 3) for a number of participants, but the changes were not significant (p = .061). HRV increased in the RT group in both the LF and HF bands in the last 5 minutes of the sitting position (Figure 4): LF power increased from 284 ± 490 to 432 ± 592, (p < .01), and HF power increased from 169 ± 311 ms2 to 333 ± 343 ms2 (p < .05). There were neither significant changes in primary contributing frequency of the LF (pre and post: 0.08 ± 0.02 Hz) and HF (pre and post: 0.25 ± 0.05 Hz) bands when comparing the RT group to the control, nor were there changes to respiration frequency, demonstrating the increased power results from greater oscillation within the same frequency. There were no significant relationships between severity of injury and HRV (Table 2), reflecting gains exhibited by all participants in the training group.
Figure 3.
Blood pressure (BP) and heart rate (HR) during orthostatic stress test before (Pre) and after (Post) respiratory training (RT) program. Example from female with C4, AIS A SCI of one-minute means of systolic blood pressure (black), diastolic blood pressure (gray), and heart rate (red) during orthostatic stress test. Before (top) respiratory training, the participant demonstrated a poorly regulated response to orthostatic stress, while after (bottom) respiratory training, the hemodynamic and cardiac responses improved.
Figure 4.
Heart rate (HR) variability during orthostatic stress test. Sitting power spectral density (ms2) from the Respiratory Training (RT) (n=24) and Control (n=20) groups during minutes 5–10 and 10–15 in the low and high frequency (LF and HF, respectively) bands. There was a significant increase in LF and HF power in the last 5 minutes of the sitting position in the RT group.
DISCUSSION
Our study found that a four-week RT protocol utilizing inspiratory and expiratory training can lead to significant increases in pulmonary function outcomes, BS, and HRV as well as better cardiovascular response during orthostatic stress test indicating RT has the ability to influence and improve both respiratory and cardiac autonomic function which ameliorates cardiovascular stress response in persons with chronic SCI.
Percent-predicted values of FVC and FEV1 increased significantly after RT (Table 2). Reduction of these values are attributed to neuromuscular weakness60–62 which can lead to pulmonary infections63, 64 and increase mortality risk by three percent with each percentage decrease in function4. It is therefore possible that RT can reduce the risk of developing SCI-induced pulmonary disease by improving the ability to overcome airway obstruction and increasing respiratory endurance. Indeed, participants reported improved cough capacity, respiration, and speech, and overall improved quality of life after RT. Our data also suggest improvements in pulmonary function outcomes are not limited to cervical injuries. Participants with thoracic lesions also demonstrated improvements after RT with no significant relationship between level and severity of injury and functional gains. Previously, other researchers have found factors like smoking62, duration of injury17, weight and health status7 contribute significantly to pulmonary dysfunction, exacerbating functional impairment from SCI. This could help explain why there was no significant relationship between functional improvement and level or severity of injury: there is impairment in addition to what can be attributed to SCI that can be targeted with RT. We are not the first to find improvements to pulmonary function in persons with SCI following respiratory exercises26, 28–32, but we are the first to investigate the effects of RT on lower thoracic injuries which could lead to significant clinical benefits including improvement in endurance, lung capacity, ability to tolerate activities during transfers, exercising, and improvement in quality of life. Thus, our data suggest RT has the potential to improve pulmonary function even in persons with incomplete or low-level SCI.
We found significant increases to BS in the RT group, in both phase II and phase IV of the 5s MEP maneuver (Figure 2). The 5s MEP engages both sides of the baroreceptor reflex arc: the drop in BP during phase II increases HR via available sympathetic engagement and vagal withdrawal, while the BP overshoot during phase IV increases vagal activity (Figure 3)47, 51, 65, 66. We now report significant increases to BS post-RT without changes to the absolute peak and trough SBP values between timepoints; as such, the changes in BS are not attributable to a change in the phase relationship between SBP and RRI and instead demonstrate a more effective baroreceptor response for the same change in SBP during the maneuver. This could potentially be a reversal of the vessel stiffening common in SCI, as changes in intrathoracic pressure during training affect BP, or it could result from more effective use of available sympathetic and parasympathetic efferents. The clinical implications of this findings lead us to believe that deconditioning of the baroreflex induced by SCI can be reversed by RT, which may serve as a protective measure to maintain respiratory-cardiovascular health.
There were also significant increases in HF power during the last 5 minutes of orthostatic stress in our RT group (Figure 4). Increased power spectral density of HRV without changes to the primary contributing frequency results from increased oscillation of RRI, demonstrating an increased ability of vagal pulmonary reflexes to match RRI to inspiration67, 68. While it is true that respiration increases during orthostatic stress69, 70, we did not find any increases in respiratory rate from pre- to post-RT. We therefore speculate the increased HF power is the result of increased cardiovagal reflex activity which lead to increased oscillation of RRI from a better match of HR to inspiration.
Oscillation of RR interval in the LF band is attributed to sympathetic nervous system activity, possibly modulation of HR via the activity of the baroreceptor reflex loop71–74. We detected significant increases in sympathetic control of HR during the last 5 minutes of the seated position (Figure 4), which happened to be the time period containing the lowest BP values overall. Indeed, the last 5 minutes of the seated position was the period when orthostatic hypotension alleviated in our previously published study33. The theory that LF frequencies result only from baroreceptor activity would also align with our findings, as sympathetic recruitment by the baroreceptors increases sigmoidally as SBP drops75, which could explain why significant increases in LF power occurred during the last 5 minutes of the seated position: the lowest BP values would cause the greatest firing by the baroreceptors. This could also be subsequent to changes in respiration, as greater pulmonary function would lead to greater oscillations in BP which would increasingly activate the baroreceptors. Thus, we speculate increased sympathetic tone post-RT is related to increased baroreceptor activity, but further analysis is required. Irrespective of the mechanism it is evident that, following RT, sympathetic control of the heart via reflex-mediated oscillation increases in response to orthostatic stress. Impaired HRV has been associated with increased mortality and a greater risk of cardiac events in several populations76, 77. Improved autonomically mediated cardiovascular responses during cardiovascular stress reported herein indicate RT in patients with chronic SCI may help ameliorate long-term cardiovascular risks.
Finally, we did not see significant changes to SBP or DBP values during the sitting position in this group, perhaps because few participants demonstrated orthostatic hypotension prior to training, but several participants demonstrated an improved cardiovascular response to orthostatic stress after RT (Figure 3) which could positively impact cardiovascular fitness in chronic SCI as well as improvement in quality of life; this combined with a p-value of .061 leads us to speculate changes to absolute blood pressure values would be significant with a larger sample size.
We have thus demonstrated a therapy that targets and improves pulmonary function outcomes can ultimately lead to improved cardiac-autonomic responses to rapid changes in SBP. Moreover, these changes can be seen irrespective of injury level and completeness: except for phase IV of the 5s MEP (Table 2, Cervical vs. Thoracic), there was no outcome in which a particular group experienced greater gains than another. This is a novel and exciting finding, particularly in a population with significant increase in mortality from cardiovascular and pulmonary diseases when compared with their non-injured counterparts3–7. In addition to the accelerated aging of these organ systems, impaired BS on its own is a risk factor for development of cardiovascular disease because of poorly regulated BP78–80. Thus, RT not only has the potential to improve the pulmonary function and cardiovascular regulation in persons with SCI, it could potentially impact long-term outcomes in SCI by potentially reducing the risk of cardiovascular disease in patients with chronic SCI.
In addition to being a potentially effective therapy with benefits to cardiopulmonary function, this training has the added advantage of being easily administered: participants require an inexpensive training device and a nose clip. Training sessions can take place in their own chairs and might be at home instead of at a facility that would require a commute and clinician to administer the therapy. We also found significant changes with only 20, 45-minute sessions of RT, which means participants could see improved regulation as little as a month.
Limitations
The study was limited by group heterogeneity and available sample size due to the challenge to form demographically and clinically homogeneous randomly formed groups from a highly diverse but limited SCI population, particularly with respect to the factors related to level/severity and duration of injury. In addition, true quality of life changes were not investigated statistically.
CONCLUSION
Chronic SCI patients have a very high risk of acute admissions and increased mortality due to complications from cardiovascular and respiratory diseases. The lack of effective therapies to manage cardiovascular and respiratory deficits negatively affects the life span of persons with chronic SCI. Our findings indicate that inspiratory-expiratory pressure threshold RT is a promising technique to positively impact both respiratory and cardiovascular dysregulation observed in persons with chronic SCI.
Table 3.
Multivariate Linear Regression Models of RT Outcomes Compared to Control.
| RT vs Control+ | Cervical vs Thoracic+ | AIS A vs AIS CD+ | AIS B vs AIS CD+ | Adjusted R2 | ||
|---|---|---|---|---|---|---|
| FVC (%) | 10 (3) ** | 0 (7) | 3 (1) | 0 (4) | 0.49 | |
| FEV1 (%) | 8 (2) ** | 0 (6) | 2 (1) | 0 (3) | 0.54 | |
| BS (ms/mmHg) During 5s MEP | ||||||
| Phase II | 1.24 (0.4) * | 0.33 (0.4) | 1.09 (0.5) | 0.13 (0.6) | 0.45 | |
| Phase IV | 1.47 (0.5) ** | 1.47 (0.5) ** | −0.17 (0.4) | 0.55 (0.7) | 0.57 | |
| Power Spectral Density | ||||||
| Low Frequency R-R Interval | ||||||
| Minutes 5–10 | 122 (113) | −173 (116) | 20 (128) | 157 (155) | 0.17 | |
| Minutes 10–15 | 206 (73) ** | −56 (76) | 62 (82) | 389 (102) | 0.46 | |
| High Frequency R-R Interval | ||||||
| Minutes 5–10 | 80 (57) | 27 (51) | −21 (68) | 62 (79) | 0.08 | |
| Minutes 10–15 | 114 (50) * | −17 (51) | −14 (55) | 106 (70) | 0.23 | |
Reference factor for independent variables are marked with (+). Results are reported as beta coefficient (standard error);
p < .05,
p < .01. RT: respiratory training; FVC: forced vital capacity; FEV 1: forced expiratory volume in one second;
BS: baroreflex sensitivity; MEP: maximum expiratory pressure generation.
Acknowledgments
RESEARCH SUPPORT FUNDING: This work was funded by the Leona M. and Harry B. Helmsley Charitable Trust; Kentucky Spinal Cord and Head Injury Research Trust 9-10A, Christopher and Dana Reeve Foundation OA2-0802; Craig H. Neilsen Foundation 1000056824, U.S. Department of Education and Department of Health and Human Services H133N110007, and National Institutes of Health R01HL103750 and P30GM103507 grants.
We express our deep appreciation to the research participants; to Steven R. Williams, MD, Carie Z. Tolfo, PT, DPT, and Jamie Ochsner, PT for the clinical support of this study; to Eddie Brown, Manpreet Chopra, Sarah Ostermeier, Jesse Fischer, and Goutam Singh for data collection and processing; and to Paul Ditterline for statistical assistance.
CLINICAL TRIAL REGISTRATION NUMBER: NCT02396823
List of abbreviations
- 5s
MEP maximum expiratory pressure generation maneuver, sustained for 5s
- AIS
american spinal injury assessment impairment scale
- BP
blood pressure
- BS
baroreflex sensitivity
- FEV1
Forced Expiratory Volume in 1 second
- FVC
Forced Vital Capacity
- HF
high-frequency
- HR
heart rate
- HRV
heart rate variability
- LF
low-frequency
- RRI
R-R interval
- RT
respiratory training
- SBP
systolic blood pressure
- SCI
spinal cord injury
Footnotes
Authors’ contribution: Bonnie Legg Ditterline, Sevda Aslan, and Alexander Ovechkin: study design, data acquisition/analysis/interpretation, and manuscript writing; David Randall, Susan Harkema, and Camilo Castillo: study design, manuscript drafting and critical revision.
References
- 1.Center NSCIS. Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. J Spinal Cord Med. 2016;39(4):493–4. doi: 10.1080/10790268.2016.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50(5):365–72. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 3.Middleton JW, Dayton A, Walsh J, Rutkowski SB, Leong G, Duong S. Life expectancy after spinal cord injury: a 50-year study. Spinal Cord. 2012 doi: 10.1038/sc.2012.55. [DOI] [PubMed] [Google Scholar]
- 4.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–16. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hitzig SL, Eng JJ, Miller WC, Sakakibara BM, Team SR. An evidence-based review of aging of the body systems following spinal cord injury. Spinal cord. 2011;49(6):684–701. doi: 10.1038/sc.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen MP, Truitt AR, Schomer KG, Yorkston KM, Baylor C, Molton IR. Frequency and age effects of secondary health conditions in individuals with spinal cord injury: a scoping review. Spinal cord. 2013;51(12):882–92. doi: 10.1038/sc.2013.112. [DOI] [PubMed] [Google Scholar]
- 7.Stolzmann KL, Gagnon DR, Brown R, Tun CG, Garshick E. Longitudinal change in FEV1 and FVC in chronic spinal cord injury. American journal of respiratory and critical care medicine. 2008;177(7):781–6. doi: 10.1164/rccm.200709-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capoor J, Stein AB. Aging with spinal cord injury. Physical medicine and rehabilitation clinics of North America. 2005;16(1):129–61. doi: 10.1016/j.pmr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 9.de Groot PC, Bleeker MW, van Kuppevelt DH, van der Woude LH, Hopman MT. Rapid and extensive arterial adaptations after spinal cord injury. Arch Phys Med Rehabil. 2006;87(5):688–96. doi: 10.1016/j.apmr.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Stolzmann KL, Gagnon DR, Brown R, Tun CG, Garshick E. Risk factors for chest illness in chronic spinal cord injury: a prospective study. Am J Phys Med Rehabil. 2010;89(7):576–83. doi: 10.1097/PHM.0b013e3181ddca8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waddimba AC, Jain NB, Stolzmann K, Gagnon DR, Burgess JF, Jr, Kazis LE, et al. Predictors of cardiopulmonary hospitalization in chronic spinal cord injury. Arch Phys Med Rehabil. 2009;90(2):193–200. doi: 10.1016/j.apmr.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–9. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 13.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–52. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 14.Koseoglu BF, Safer VB, Oken O, Akselim S. Cardiovascular disease risk in people with spinal cord injury: is there a possible association between reduced lung function and increased risk of diabetes and hypertension? Spinal Cord. 2017;55(1):87–93. doi: 10.1038/sc.2016.101. [DOI] [PubMed] [Google Scholar]
- 15.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46(7):466–76. doi: 10.1038/sj.sc.3102161. [DOI] [PubMed] [Google Scholar]
- 16.Bauman WA, Kahn NN, Grimm DR, Spungen AM. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal cord. 1999;37(9):601–16. doi: 10.1038/sj.sc.3100911. [DOI] [PubMed] [Google Scholar]
- 17.Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81(6):757–63. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- 18.Burgh Daly Md. Peripheral arterial chemoreceptors and respiratory-cardiovascular integration. New York: Clarendon Press; 1997. [Google Scholar]
- 19.Scher A, O’Leary D, Sheriff D. Arterial Baroreceptor Regulation of Peripheral Resistance and of Cardiac Performance. In: Persson PB, Kirchheim HR, editors. Baroreceptor Reflexes. Springer; Berlin Heidelberg: 1991. pp. 75–125. [Google Scholar]
- 20.Sala-Mercado JA, Moslehpour M, Hammond RL, Ichinose M, Chen X, Evan S, et al. Stimulation of the cardiopulmonary baroreflex enhances ventricular contractility in awake dogs: a mathematical analysis study. Am J Physiol Regul Integr Comp Physiol. 2014;307(4):R455–64. doi: 10.1152/ajpregu.00510.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LY, Yu J. Sensory nerves in lung and airways. Comprehensive Physiology. 2014;4(1):287–324. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 22.Shamsuzzaman AS, Somers VK. Cardiorespiratory interactions in neural circulatory control in humans. Ann N Y Acad Sci. 2001;940:488–99. doi: 10.1111/j.1749-6632.2001.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 23.Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(Suppl 1):82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]
- 24.Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma. 2011;28(8):1479–95. doi: 10.1089/neu.2009.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisbie JH. Breathing and the support of blood pressure after spinal cord injury. Spinal cord. 2005;43(7):406–7. doi: 10.1038/sj.sc.3101732. [DOI] [PubMed] [Google Scholar]
- 26.Derrickson J, Ciesla N, Simpson N, Imle PC. A comparison of two breathing exercise programs for patients with quadriplegia. Phys Ther. 1992;72(11):763–9. doi: 10.1093/ptj/72.11.763. [DOI] [PubMed] [Google Scholar]
- 27.Tamplin J, Berlowitz DJ. A systematic review and meta-analysis of the effects of respiratory muscle training on pulmonary function in tetraplegia. Spinal Cord. 2014;52(3):175–80. doi: 10.1038/sc.2013.162. [DOI] [PubMed] [Google Scholar]
- 28.Van Houtte S, Vanlandewijck Y, Kiekens C, Spengler CM, Gosselink R. Patients with acute spinal cord injury benefit from normocapnic hyperpnoea training. J Rehabil Med. 2008;40(2):119–25. doi: 10.2340/16501977-0140. [DOI] [PubMed] [Google Scholar]
- 29.Zupan A, Savrin R, Erjavec T, Kralj A, Karcnik T, Skorjanc T, et al. Effects of respiratory muscle training and electrical stimulation of abdominal muscles on respiratory capabilities in tetraplegic patients. Spinal cord. 1997;35(8):540–5. doi: 10.1038/sj.sc.3100433. [DOI] [PubMed] [Google Scholar]
- 30.Gounden P. Progressive resistive loading on accessory expiratory muscles in tetraplegia. S Afr J Physiother. 1990;42:4–12. [Google Scholar]
- 31.Litchke LG, Russian CJ, Lloyd LK, Schmidt EA, Price L, Walker JL. Effects of respiratory resistance training with a concurrent flow device on wheelchair athletes. The journal of spinal cord medicine. 2008;31(1):65–71. doi: 10.1080/10790268.2008.11753983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth EJ, Stenson KW, Powley S, Oken J, Primack S, Nussbaum SB, et al. Expiratory muscle training in spinal cord injury: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91(6):857–61. doi: 10.1016/j.apmr.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Aslan SC, Randall DC, Krassioukov AV, Phillips A, Ovechkin AV. Archives of physical medicine and rehabilitation. 2016. Respiratory Training Improves Blood Pressure Regulation in Individuals With Chronic Spinal Cord Injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillis DJ, Wouda M, Hjeltnes N. Non-pharmacological management of orthostatic hypotension after spinal cord injury: a critical review of the literature. Spinal Cord. 2008;46(10):652–9. doi: 10.1038/sc.2008.48. [DOI] [PubMed] [Google Scholar]
- 35.Mills PB, Fung CK, Travlos A, Krassioukov A. Nonpharmacologic Management of Orthostatic Hypotension: A Systematic Review. Archives of Physical Medicine and Rehabilitation. 2015;96(2):366–75. e6. doi: 10.1016/j.apmr.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Phillips AA, Krassioukov AV. Contemporary Cardiovascular Concerns after Spinal Cord Injury: Mechanisms, Maladaptations & Management. J Neurotrauma. 2015 doi: 10.1089/neu.2015.3903. [DOI] [PubMed] [Google Scholar]
- 37.Berlowitz DJ, Tamplin J. Respiratory muscle training for cervical spinal cord injury. Cochrane Database Syst Rev. 2013;7:CD008507. doi: 10.1002/14651858.CD008507.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Houtte S, Vanlandewijck Y, Gosselink R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respiratory medicine. 2006;100(11):1886–95. doi: 10.1016/j.rmed.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bos WJW, van Goudoever J, van Montfrans GA, van den Meiracker AH, Wesseling KH. Reconstruction of Brachial Artery Pressure From Noninvasive Finger Pressure Measurements. Circulation. 1996;94(8):1870–5. doi: 10.1161/01.cir.94.8.1870. [DOI] [PubMed] [Google Scholar]
- 41.R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 42.Aslan SC, Randall DC, Donohue KD, Knapp CF, Patwardhan AR, McDowell SM, et al. Blood pressure regulation in neurally intact human vs. acutely injured paraplegic and tetraplegic patients during passive tilt. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1146–57. doi: 10.1152/ajpregu.00225.2006. [DOI] [PubMed] [Google Scholar]
- 43.Ovechkin A, Vitaz T, de Paleville DT, Aslan S, McKay W. Evaluation of respiratory muscle activation in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2010;173(2):171–8. doi: 10.1016/j.resp.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Aslan SC, Chopra MK, McKay WB, Folz RJ, Ovechkin AV. Evaluation of respiratory muscle activation using respiratory motor control assessment (RMCA) in individuals with chronic spinal cord injury. Journal of visualized experiments: JoVE. 2013;(77) doi: 10.3791/50178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ditterline BEL, Aslan SC, Randall DC, Harkema SJ, Ovechkin AV. Baroreceptor reflex during forced expiratory maneuvers in individuals with chronic spinal cord injury. Respiratory physiology & neurobiology. 2016;229:65–70. doi: 10.1016/j.resp.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porth CJ, Bamrah VS, Tristani FE, Smith JJ. The Valsalva maneuver: mechanisms and clinical implications. Heart Lung. 1984;13(5):507–18. [PubMed] [Google Scholar]
- 47.Smith SA, Stallard TJ, Salih MM, Littler WA. Can sinoaortic baroreceptor heart rate reflex sensitivity be determined from phase IV of the Valsalva manoeuvre? Cardiovasc Res. 1987;21(6):422–7. doi: 10.1093/cvr/21.6.422. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation. 1982;66(2):432–9. doi: 10.1161/01.cir.66.2.432. [DOI] [PubMed] [Google Scholar]
- 49.Misra U, Kalita J. Clinical Neurophysiology. 2. New Delhi, India: Elsevier; 2006. [Google Scholar]
- 50.Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology. 2011;76(23):2010–6. doi: 10.1212/WNL.0b013e31821e5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimm DR, Almenoff PL, Bauman WA, De Meersman RE. Baroreceptor sensitivity response to phase IV of the Valsalva maneuver in spinal cord injury. Clin Auton Res. 1998;8(2):111–8. doi: 10.1007/BF02267821. [DOI] [PubMed] [Google Scholar]
- 52.Palmero HA, Caeiro TF, Iosa DJ, Bas J. Baroreceptor reflex sensitivity index derived from Phase 4 of the Valsalva maneuver. Hypertension. 1981;3(6 Pt 2):II-134–7. doi: 10.1161/01.hyp.3.6_pt_2.ii-134. [DOI] [PubMed] [Google Scholar]
- 53.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 54.Di Rienzo M, Parati G, Radaelli A, Castiglioni P. Baroreflex contribution to blood pressure and heart rate oscillations: time scales, time-variant characteristics and nonlinearities. Philosophical transactions Series A, Mathematical, physical, and engineering sciences. 2009;367(1892):1301–18. doi: 10.1098/rsta.2008.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inoue K, Miyake S, Kumashiro M, Ogata H, Yoshimura O. Power spectral analysis of heart rate variability in traumatic quadriplegic humans. Am J Physiol. 1990;258(6 Pt 2):H1722–6. doi: 10.1152/ajpheart.1990.258.6.H1722. [DOI] [PubMed] [Google Scholar]
- 56.Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol. 1999;517( Pt 2):617–28. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trang H, Girard A, Laude D, Elghozi JL. Short-term blood pressure and heart rate variability in congenital central hypoventilation syndrome (Ondine’s curse) Clin Sci (Lond) 2005;108(3):225–30. doi: 10.1042/CS20040282. [DOI] [PubMed] [Google Scholar]
- 58.Wecht JM, de Meersman RE, Weir JP, Spungen AM, Bauman WA. Cardiac autonomic responses to progressive head-up tilt in individuals with paraplegia. Clin Auton Res. 2003;13(6):433–8. doi: 10.1007/s10286-003-0115-5. [DOI] [PubMed] [Google Scholar]
- 59.Vittinghoff E. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. 2. New York: Springer; 2012. [Google Scholar]
- 60.Roth EJ, Nussbaum SB, Berkowitz M, Primack S, Oken J, Powley S, et al. Pulmonary function testing in spinal cord injury: correlation with vital capacity. Paraplegia. 1995;33(8):454–7. doi: 10.1038/sc.1995.99. [DOI] [PubMed] [Google Scholar]
- 61.Jain NB, Brown R, Tun CG, Gagnon D, Garshick E. Determinants of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC in chronic spinal cord injury. Archives of physical medicine and rehabilitation. 2006;87(10):1327–33. doi: 10.1016/j.apmr.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Almenoff PL, Spungen AM, Lesser M, Bauman WA. Pulmonary function survey in spinal cord injury: influences of smoking and level and completeness of injury. Lung. 1995;173(5):297–306. doi: 10.1007/BF00176893. [DOI] [PubMed] [Google Scholar]
- 63.Fishburn MJ, Marino RJ, Ditunno JF., Jr Atelectasis and pneumonia in acute spinal cord injury. Archives of physical medicine and rehabilitation. 1990;71(3):197–200. [PubMed] [Google Scholar]
- 64.Slonimski M, Aguilera EJ. Atelectasis and mucus plugging in spinal cord injury: case report and therapeutic approaches. J Spinal Cord Med. 2001;24(4):284–8. doi: 10.1080/10790268.2001.11753586. [DOI] [PubMed] [Google Scholar]
- 65.Kihara M, Mitsui M, Nishikawa S, Nishimoto K, Takahashi M. Comparison of electrophysiologic and autonomic tests in sensory diabetic neuropathy. Clinical autonomic research: official journal of the Clinical Autonomic Research Society. 1998;8(4):213–20. doi: 10.1007/BF02267784. [DOI] [PubMed] [Google Scholar]
- 66.Aminoff MJ, Daroff RB. Encyclopedia of the neurological sciences. 2. Waltham, MA: Academic Press/Elsevier; 2014. [Google Scholar]
- 67.Ori Z, Monir G, Weiss J, Sayhouni X, Singer DH. Heart rate variability. Frequency domain analysis Cardiology clinics. 1992;10(3):499–537. [PubMed] [Google Scholar]
- 68.Elghozi JL, Laude D, Girard A. Effects of respiration on blood pressure and heart rate variability in humans. Clin Exp Pharmacol Physiol. 1991;18(11):735–42. doi: 10.1111/j.1440-1681.1991.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 69.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29(9):1876–81. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 70.Ogoh S, Nakahara H, Okazaki K, Bailey DM, Miyamoto T. Cerebral hypoperfusion modifies the respiratory chemoreflex during orthostatic stress. Clin Sci (Lond) 2013;125(1):37–44. doi: 10.1042/CS20120335. [DOI] [PubMed] [Google Scholar]
- 71.Iellamo F. Baroreflex control of heart rate during exercise: a topic of perennial conflict. J Appl Physiol. 2001;90(3):1184–5. doi: 10.1152/jappl.2001.90.3.1184. [DOI] [PubMed] [Google Scholar]
- 72.Jan YK, Anderson M, Soltani J, Burns S, Foreman RD. Comparison of changes in heart rate variability and sacral skin perfusion in response to postural changes in people with spinal cord injury. Journal of rehabilitation research and development. 2013;50(2):203–14. doi: 10.1682/jrrd.2011.08.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue K, Ogata H, Hayano J, Miyake S, Kamada T, Kuno M, et al. Assessment of autonomic function in traumatic quadriplegic and paraplegic patients by spectral analysis of heart rate variability. J Auton Nerv Syst. 1995;54(3):225–34. doi: 10.1016/0165-1838(95)00012-m. [DOI] [PubMed] [Google Scholar]
- 74.Burgess DE, Hundley JC, Li SG, Randall DC, Brown DR. First-order differential-delay equation for the baroreflex predicts the 0. 4-Hz blood pressure rhythm in rats. The American journal of physiology. 1997;273(6 Pt 2):R1878–84. doi: 10.1152/ajpregu.1997.273.6.R1878. [DOI] [PubMed] [Google Scholar]
- 75.Studinger P, Goldstein R, Taylor JA. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J Physiol. 2007;583(Pt 3):1041–8. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stein PK, Barzilay JI. Relationship of abnormal heart rate turbulence and elevated CRP to cardiac mortality in low, intermediate, and high-risk older adults. J Cardiovasc Electrophysiol. 2011;22(2):122–7. doi: 10.1111/j.1540-8167.2010.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study Circulation. 1996;94(11):2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 78.Koutelou M, Katsikis A, Flevari P, Theodorakis G, Livanis E, Georgiadis M, et al. Predictive value of cardiac autonomic indexes and MIBG washout in ICD recipients with mild to moderate heart failure. Annals of Nuclear Medicine. 2009;23(7):677–84. doi: 10.1007/s12149-009-0289-6. [DOI] [PubMed] [Google Scholar]
- 79.La Rovere MT, Pinna GD, Maestri R, Sleight P. Clinical value of baroreflex sensitivity. Netherlands heart journal: monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2013;21(2):61–3. doi: 10.1007/s12471-012-0349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]