Abstract
The central extended amygdala (EAc)—including the bed nucleus of the stria terminalis (BST) and central nucleus of the amygdala (Ce)—plays a critical role in triggering fear and anxiety and is implicated in the development of a range of debilitating neuropsychiatric disorders. Although it is widely believed that these disorders reflect the coordinated activity of distributed neural circuits, the functional architecture of the EAc network and the degree to which the BST and the Ce show distinct patterns of functional connectivity is unclear. Here, we used a novel combination of imaging approaches to trace the connectivity of the BST and the Ce in 130 healthy, racially diverse, community‐dwelling adults. Multiband imaging, high‐precision registration techniques, and spatially unsmoothed data maximized anatomical specificity. Using newly developed seed regions, whole‐brain regression analyses revealed robust functional connectivity between the BST and Ce via the sublenticular extended amygdala, the ribbon of subcortical gray matter encompassing the ventral amygdalofugal pathway. Both regions displayed coupling with the ventromedial prefrontal cortex (vmPFC), midcingulate cortex (MCC), insula, and anterior hippocampus. The BST showed stronger connectivity with the thalamus, striatum, periaqueductal gray, and several prefrontal territories. The only regions showing stronger functional connectivity with the Ce were neighboring regions of the dorsal amygdala, amygdalohippocampal area, and anterior hippocampus. These observations provide a baseline against which to compare a range of special populations, inform our understanding of the role of the EAc in normal and pathological fear and anxiety, and showcase image registration techniques that are likely to be useful for researchers working with “deidentified” neuroimaging data.
Keywords: affective neuroscience, amygdala, anxiety, bed nucleus of the stria terminalis (BST/BNST), central extended amygdala
1. INTRODUCTION
When extreme, fear and anxiety can become debilitating (Grupe & Nitschke, 2013; Salomon et al., 2015). Anxiety disorders are common and challenging to treat, imposing a staggering burden on public health, and underscoring the need to develop a more complete understanding of the distributed neural circuits governing the expression of fear and anxiety in humans (Bystritsky, 2006; Craske et al., 2017; DiLuca & Olesen, 2014; Global Burden of Disease Collaborators, 2016; Griebel & Holmes, 2013).
Converging lines of anatomical, mechanistic, and physiological evidence make it clear that the central extended amygdala (EAc) is a key hub in this circuitry (Figure 1a,b) (Avery, Clauss, & Blackford, 2016; Davis, Walker, Miles, & Grillon, 2010; Fox & Shackman, in press; Goode & Maren, 2017; Gungor & Paré, 2016; Shackman & Fox, 2016; Tovote, Fadok, & Luthi, 2015). The EAc encompasses a collection of subcortical regions with similar cellular compositions, neurochemistry, gene expression, and structural connectivity and it encompasses the bed nucleus of the stria terminalis (BST), the central nucleus of the amygdala (Ce), the sublenticular extended amygdala (SLEA), and portions of the accumbens shell (Alheid & Heimer, 1988; Fox, Oler, Tromp, Fudge, & Kalin, 2015a; Oler et al., 2017; Yilmazer‐Hanke, 2012). It has long been recognized that the amygdala is connected to the BST via two major fiber bundles—the ventral amygdalofugal pathway (VA) and the stria terminalis (ST) (Avery et al., 2014; Kamali et al., 2016, 2015; Nauta, 1961) (Figure 1c)—and more recent tracing studies have identified a third, indirect pathway centered on the SLEA (Ce ↔ SLEA ↔ BSTL) (deCampo & Fudge, 2013; Fudge et al., 2017; Oler et al., 2017). Anatomically, the Ce and the BST are both poised to trigger or orchestrate key signs of fear and anxiety—including alterations in arousal, behavioral inhibition, and neuroendocrine activity—via dense mono‐ and polysynaptic projections to brainstem and subcortical effector regions (Fox et al., 2015a; Freese & Amaral, 2009; Fudge et al., 2017).
Figure 1.
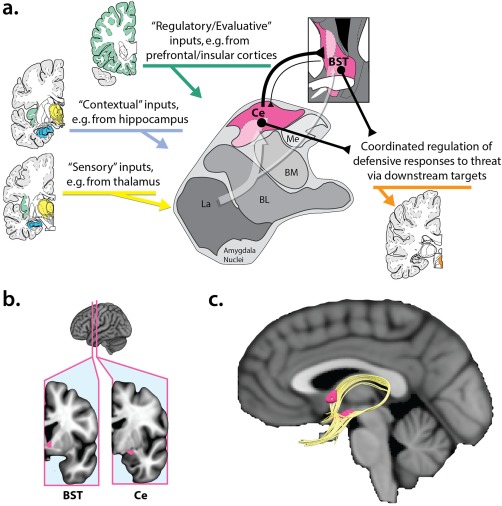
The EAc. (a) Simplified schematic of key EAc inputs and outputs in humans and other primates. The EAc (magenta) encompasses the BST, which encircles the anterior commissure, and the Ce. As shown by the translucent white arrow at the center of the figure, much of the sensory (yellow), contextual (blue), and regulatory (green) inputs to the EAc are indirect (i.e., polysynaptic), and first pass through adjacent amygdala nuclei before arriving at the Ce or the BST. Both regions are poised to orchestrate momentary states of fear and anxiety via dense projections to downstream effector regions (orange). Portions of this figure were adapted from the atlas of (Mai, Paxinos, & Voss, 2007; see also Yilmazer‐Hanke, 2012). (b) BST and Ce seeds. Figure depicts the location of the BST and Ce seeds used in the present study. See Supporting Information, Figure S5 for bilateral views and a more detailed description of seed derivation. (c) Structural connections of the EAc. In humans and other primates, the BST (dorsorostral magenta region) and the Ce (ventrocaudal magenta region) are structurally connected via two major fiber bundles (gold), the ventral amygdalofugal pathway and the stria terminalis (Johnston, 1923; Nauta, 1961; Yilmazer‐Hanke, 2012). From the Ce, the ventral amygdalofugal pathway courses forward and medially, passing through the SLEA, a bridge of neurons harbored within the substantia innominata. The stria terminalis, which arches dorsally over the thalamus, provides a second, less direct connection between the two major divisions of the central extended amygdala. Figure depicts deterministic tractography (gold) of these two fiber bundles. Image kindly provided by Do Tromp. Abbreviations: BL = basolateral nucleus of the amygdala; BM = basomedial nucleus of the amygdala; BST = bed nucleus of the stria terminalis; Ce = central nucleus of the amygdala; EAc = central division of the extended amygdala; La = lateral nucleus of the amygdala; Me = medial nucleus of the amygdala; SLEA = sublenticular extended amygdala [Color figure can be viewed at http://wileyonlinelibrary.com]
Consistent with this neuroanatomy, mechanistic studies in rodents indicate that microcircuits within and between the BST and the Ce play a critical role in organizing defensive responses to a range of potentially threat‐relevant cues and contexts (Calhoon & Tye, 2015; Davis et al., 2010; Fox & Shackman, in press; Goode & Maren, 2017; Gungor & Paré, 2016; Lange et al., 2017; Tovote et al., 2015) (Figure 1c). Although the BST and the Ce are often viewed as passive output relays for amygdala‐mediated emotional learning (e.g., La → Ce/BST → effector regions; LeDoux, 2000, 2007; Pare & Duvarci, 2012), more recent work in rodents has expanded this role to include relaying information about pain and aversive reinforcers (Yu et al., 2017), guiding attention to motivationally salient stimuli (Davis & Whalen, 2001; Roesch, Esber, Li, Daw, & Schoenbaum, 2012; Shackman et al., 2016a), learning aversive associations (Ciocchi et al., 2010; Han, Soleiman, Soden, Zweifel, & Palmiter, 2015; Li et al., 2013; Penzo, Robert, & Li, 2014; Penzo et al., 2015; Sato et al., 2015; Yu et al., 2017), and actively gating and regulating defensive responses (Ehrlich et al., 2009; Fadok et al., 2017; Gungor & Paré, 2016; Pare & Duvarci, 2012).
Although the causal contribution of the BST has yet to be explored in primates, the Ce has been shown to control defensive responses to potential threat in monkeys (Kalin, 2017; Kalin et al., 2016; Kalin, Shelton, & Davidson, 2004). Similarly, rodents, monkeys, and humans with amygdala damage exhibit a profound lack of fear and anxiety in response to a broad spectrum of learned and innate dangers (Antoniadis, Winslow, Davis, & Amaral, 2007; Bechara et al., 1995; Choi & Kim, 2010; Davis & Whalen, 2001; Feinstein, Adolphs, Damasio, & Tranel, 2011; Feinstein, Adolphs, & Tranel, 2016; Izquierdo, Suda, & Murray, 2005; Kalin et al., 2004; Korn et al., 2017; Mason, Capitanio, Machado, Mendoza, & Amaral, 2006; Oler, Fox, Shackman, & Kalin, 2016).
Neuroimaging research indicates that heightened activity in the EAc is associated with elevated signs of fear and anxiety in both monkeys and humans (Alvarez et al., 2015; Banihashemi, Sheu, Midei, & Gianaros, 2015; Cheng, Knight, Smith, & Helmstetter, 2006; Cheng, Richards, & Helmstetter, 2007; Fox et al., 2015b; Fox, Shelton, Oakes, Davidson, & Kalin, 2008; Kalin, Shelton, Fox, Oakes, & Davidson, 2005; Knight, Nguyen, & Bandettini, 2005; Kragel & LaBar, 2015; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Shackman et al., 2013; Somerville et al., 2013; van Well, Visser, Scholte, & Kindt, 2012; Wood, Ver Hoef, & Knight, 2014). Among humans, the amygdala responds to a variety of threat‐related cues (Costafreda, Brammer, David, & Fu, 2008; Fusar‐Poli et al., 2009; Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Sabatinelli et al., 2011; Sergerie, Chochol, & Armony, 2008) and work using high‐resolution fMRI indicates that the dorsal amygdala in the region of the Ce is particularly sensitive to aversive visual stimuli (Hrybouski et al., 2016).
Although less intensively studied than the Ce, the BST is sensitive to emotional faces (Sladky et al., 2017), aversive images (Brinkmann et al., 2018), and a variety of threat‐related cues (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011; Brinkmann et al., 2017b; Choi, Padmala, & Pessoa, 2012; Grupe, Oathes, & Nitschke, 2013; Herrmann et al., 2016; Klumpers et al., 2015; McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014; Mobbs et al., 2010; Pedersen et al., 2017; Somerville, Whalen, & Kelley, 2010; Somerville et al., 2013). While imaging research hints at potential functional differences between the two regions (Alvarez et al., 2011; Fox et al., 2015b; Meyer, Padmala, & Pessoa, 2017; Shackman et al., 2017; Somerville et al., 2013), methodological limitations preclude decisive inferences (Fox & Shackman, in press; Shackman & Fox, 2016). Importantly, other work suggests that alterations in EAc function likely plays a key role in the development, maintenance, and recurrence of anxiety disorders, depression, and substance abuse (Avery et al., 2016; Brinkmann et al., 2017a, 2017b, 2018; Buff et al., 2017; Fox & Kalin, 2014; Kaczkurkin et al., 2016; Münsterkötter et al., 2015; Shackman et al., 2016a, 2016b; Stevens et al., 2017; Williams et al., 2015; Wise & Koob, 2014).
Although this vast literature leaves little doubt that the EAc plays a crucial role in evaluating and responding to a variety of potential threats, it does not act in isolation. Fear and anxiety reflect functional circuits that extend well beyond the borders of the EAc (Chang, Gianaros, Manuck, Krishnan, & Wager, 2015; Fox & Shackman, in press; Kragel, Knodt, Hariri, & LaBar, 2016; Nummenmaa & Saarimaki, in press; Pessoa, 2017; Shackman & Fox, 2018; Shackman, Fox, & Seminowicz, 2015; Wager et al., 2015). Anatomically, the BST and the Ce are embedded within a complex web of mono‐ and polysynaptically connected brain regions (Figure 1a) (Carrive & Morgan, 2012; Fox et al., 2015a; Freese & Amaral, 2009; Fudge et al., 2017; Oler et al., 2017; Ongur & Price, 2000). This structural backbone includes subcortical regions, such as the periaqueductal gray (PAG), that are responsible for triggering specific signs of fear and anxiety (Amano et al., 1982; Assareh, Sarrami, Carrive, & McNally, 2016; Bandler, Price, & Keay, 2000; Chen et al., 2015; Fadok et al., 2017; Faull & Pattinson, 2017; Motta, Carobrez, & Canteras, 2017; Nashold, Wilson, & Slaughter, 1969; Richardson & Akil, 1977; Satpute et al., 2013; Tovote et al., 2016). It also encompasses a number of cortical regions implicated in the expression and regulation of fear and anxiety, including the anterior insula, dorsolateral prefrontal cortex, mid‐cingulate cortex (MCC), and OFC (Birn et al., 2014; Buhle et al., 2014; Cavanagh & Shackman, 2015; de la Vega, Chang, Banich, Wager, & Yarkoni, 2016; Fox et al., 2015, 2010b; Grupe & Nitschke, 2013; Mobbs et al., 2009, 2007, 2010; Shackman, McMenamin, Maxwell, Greischar, & Davidson, 2009; Shackman et al., 2011; Stout, Shackman, Pedersen, Miskovich, & Larson, 2017; Uddin, Kinnison, Pessoa, & Anderson, 2014). While it is widely believed that the synchronized flow of information across this network underlies the human capacity for flexibly regulating fear and anxiety, the functional architecture of the EAc network and the degree to which the BST and the Ce are characterized by distinct patterns of functional connectivity remains incompletely understood.
Building on prior work (Table 1), we used a combination of imaging approaches to trace and compare the intrinsic functional connectivity of the BST and the Ce. Whole‐brain “resting‐state” functional MRI (fMRI) data were acquired from a relatively large (n = 130) sample of psychiatrically healthy, racially diverse, community‐dwelling adults, providing increased statistical power and generalizability. Given the challenges of imaging the EAc (Fox et al., 2015a; Shackman & Fox, 2016; Fox & Shackman, in press), several techniques were used to maximize effective spatial resolution, including a multiband imaging sequence with 2‐mm3 nominal resolution, boundary‐based co‐registration (Greve & Fischl, 2009), a novel brain‐extraction (“skull‐stripping”) approach, and diffeomorphic normalization (Avants, Epstein, Grossman, & Gee, 2008; Avants et al., 2011, 2010; Klein et al., 2009). To further enhance anatomical specificity, analyses were conducted using spatially unsmoothed data and newly developed extended amygdala seeds. Collectively, these techniques enabled us to compare the intrinsic functional connectivity of the BST and the Ce with enhanced statistical sensitivity and anatomical precision (Table 1). Understanding these functional networks is important: it would provide a baseline against which to compare a range of special populations—including individuals at risk for developing mental illness and patients suffering from psychiatric disorders—and it would inform our understanding of the EAc's role in normal and pathological fear and anxiety.
Table 1.
Intrinsic functional connectivity of the human central extended amygdala
| Citation | Population | N | Coverage | Native EPI resolution | Smoothing | Normalization | Ce seed | BST seed |
|---|---|---|---|---|---|---|---|---|
| Present study | Adults | 130 | Whole brain | 2 × 2 × 2 mm | N/A | FSL‐BBR, ANTS/SyN | Prescribed by an experienced neuroanatomist using a specially processed, ultra‐high‐resolution, multi‐modal probabilistic template (CITI168) | Prescribed by 2 raters using T2 images acquired from 10 young adults and normalized using ANTS/SyN; thresholded at 25% (Theiss, Ridgewell, McHugo, Heckers, & Blackford, 2017) |
| Avery et al., 2014 | Midlife adults | 99 | Whole brain | 3 × 3 × 4 mm | 3 mm | SPM8 | Prescribed using a single ultra‐high‐resolution T2 image acquired from a 42‐year‐old male | N/A |
| Gorka et al., 2017 | Young adults | 27 | Partial | 1.3 × 1.3 × 1.3 mm | 2.6 mm | 3dAllineate, 3dQWarp | Prescribed by 2 raters for the left hemisphere using 8 study‐specific, ultra‐high‐resolution, multi‐modal probabilistic templates; thresholded at 20% (Tyszka & Pauli, 2016) | Prescribed by 3 raters using each subject's T1 image; thresholded at 66.67% (Torrisi et al., 2015) |
| Motzkin et al., 2015 | Older adults | 17 | Whole‐brain | 3.5 × 3.5 × 3 mm | 4 mm | ANTS/SyN | N/A | Prescribed by an experienced neuroanatomist using the 1‐mm MNI152 T1 template |
| Oler et al., 2012 | Adolescents | 105 | Whole‐brain |
3 × 3 × 3 or 3.75 × 3.75 × 5 mm |
6 mm | Affine | Prescribed by an experienced neuroanatomist using the 1‐mm MNI152 T1 template | N/A |
| Torrisi et al., 2015 | Young adults | 27 | Partial | 1.3 × 1.3 × 1.3 mm | 2.6 mm | 3dAllineate, 3dQWarp | N/A | Prescribed by 3 raters using each subject's T1 image; thresholded at 66.67% (Torrisi et al., 2015) |
2. MATERIALS AND METHODS
2.1. Subjects
Data were extracted from the publicly available Nathan Kline Institute‐Rockland Sample (NKI‐RS) (http://fcon_1000.projects.nitrc.org/indi/enhanced; Nooner et al., 2012) for 185 adults (18–40 years old). Exclusionary criteria included: positive drug urine screen (n = 12); self‐reported lifetime bipolar disorder, neurological disorder, pervasive developmental disorder, or psychosis/schizophrenia (n = 14); incomplete MRI data (n = 15); and incomplete demographic data (n = 5). Using procedures detailed below, 18 additional subjects were excluded due to excessive motion artifact (n = 8), susceptibility artifact (n = 9), or unusable T1 scans (n = 1). The final sample consisted of 130 subjects (59 males, M = 25.3 years, SD = 6.1). Additional demographic details can be found in the Supporting Information.
2.2. Data acquisition
MRI data were acquired using a Siemens Magnetom Trio Tim 3 T scanner and 32‐channel head‐coil (http://fcon_1000.projects.nitrc.org/indi/enhanced/mri_protocol.html). T1‐weighted anatomical images were acquired using a magnetization‐prepared, rapid‐acquisition, gradient‐echo sequence (inversion time: 900 ms; repetition time: 1,900 ms; echo time: 2.52 ms; flip angle: 9°; field‐of‐view: 250 250; matrix: 256 256; number of slices: 176 sagittal; slice thickness: 1 mm). Building on prior work with partial‐brain coverage (Gorka, Torrisi, Shackman, Grillon, & Ernst, 2017; Torrisi et al., 2015), functional scans were obtained using a T2*‐weighted echo‐planar image (EPI) sequence (multiband acceleration: 4; repetition time: 1,400 ms; echo time: 30 ms; flip angle: 65°; number of excitations: 1; field‐of‐view: 224 224 mm; number of slices: 64 oblique‐axial; matrix: 112 112; slice thickness: 2 mm; gap: ∼0 mm; volumes: 404), enabling us to survey the entire brain.
2.3. Data processing pipeline
2.3.1. Brain extraction and normalization
Given our focus on the BST and the Ce, methods were optimized to minimize spatial normalization error and incidental spatial blurring. Consistent with other work (Acosta‐Cabronero, Williams, Pereira, Pengas, & Nestor, 2008; Fein et al., 2006; Fischmeister et al., 2013), unpublished observations by our group demonstrate that the quality of spatial normalization is enhanced by using a brain‐extracted (i.e., “skull‐stripped” or “de‐skulled”) template and brain‐extracted T1 images. This advantage is particularly evident for publicly available datasets, such as the NKI‐RS, where portions of the skull and tissue in the region of the face have been manually removed (“de‐faced”) by the curators to mitigate risks to subject confidentiality (i.e., “anonymized” or “de‐identified”). However, this benefit is only realized when the quality of the extraction is sufficiently high and consistent, as with images that have been manually extracted by an experienced neuroanatomist. To ensure consistently high‐quality extractions, we implemented a multi‐tool strategy (for a similar approach, see Meyer et al., 2017; Najafi, Kinnison, & Pessoa, 2017). For each inhomogeneity‐corrected (using N4; Tustison et al., 2014) T1 image, six extraction masks were generated. Five masks were generated using BET (Smith, 2002), BSE (Shattuck, Sandor‐Leahy, Schaper, Rottenberg, & Leahy, 2001), 3dSkullstrip (Cox, 1996), ROBEX (Iglesias, Liu, Thompson, & Tu, 2011), and SPM unified segmentation (Ashburner & Friston, 2005), respectively. The sixth mask was generated by applying the inverse spatial transformation (see below) to the MNI152 brain mask distributed with FSL. Specifically, for each subject: (a) the defaced T1 image was spatially normalized to the MNI152 template using the unified segmentation approach implemented in SPM12; (b) the 1‐mm MNI152 template was defaced to match the idiosyncratic defacing of the T1 image; (c) the original T1 image was normalized to the individually defaced 1‐mm template using SyN; and (d) the inverse transformation was used to “reverse‐normalize” the MNI152 brain mask distributed with FSL to native space. Next, a best‐estimate extraction mask was determined by consensus, requiring agreement across four or more extraction techniques. Using this mask, each T1 image was extracted and spatially normalized to the 1‐mm MNI152 template using the high‐precision diffeomorphic approach implemented in SyN (mutual information cost function; Avants et al., 2008, 2011, 2010; Klein et al., 2009). The average of the 130 normalized T1 images is depicted in Supporting Information, Figure S1.
2.3.2. EPI data
The first 3 volumes of each EPI scan were removed and the remaining volumes were de‐spiked and slice‐time corrected using default settings in AFNI (Cox, 1996). Recent methodological work indicates that de‐spiking is more effective than “scrubbing” (Jo et al., 2013; Power, Schlaggar, & Petersen, 2015; Siegel et al., 2014) for attenuating motion‐related artifacts in intrinsic functional connectivity. Spike‐ and slice‐time‐corrected EPI data were co‐registered to the corresponding brain‐extracted, native‐space T1 image using the boundary‐based registration technique implemented in FSL (Greve & Fischl, 2009) and converted to a compatible file format using Convert3d (https://sourceforge.net/p/c3d). Motion correction was then performed using ANTS (https://stnava.github.io/ANTs). The maximum value of the frame‐to‐frame displacement was calculated for each subject and z‐transformed. Subjects with a z‐score >1.96 (p = .05) were excluded (n = 8). Residual displacement in final dataset was negligible (median = 0.11 mm, SD = 0.07 mm, maximum = 0.43 mm). To minimize incidental spatial blurring, the transformation matrices for motion correction, co‐registration, and spatial normalization were concatenated and applied to the EPI data in a single step. Normalized EPI data were resampled to 2‐mm3 voxels using fifth‐order splines. To maximize spatial resolution, no additional spatial filters were applied, consistent with recent recommendations (Stelzer, Lohmann, Mueller, Buschmann, & Turner, 2014; Turner & Geyer, 2014). Each EPI and T1 dataset was visually inspected before and after processing for quality assurance. To quantify susceptibility artifact in the medial temporal lobe (MTL), we computed the ratio of mean signal in the amygdala relative to the caudate and putamen separately for each hemisphere and subject and then standardized across subjects (i.e., z‐transformed). Preliminary visual inspection indicated that values >∼2.50 were associated with substantial signal loss (“drop‐out”) in the MTL. Accordingly, subjects with z‐scores <−2.50 were excluded (n = 9) (for a similar approach, see Birn et al., 2014). To attenuate physiological noise, white matter (WM) and cerebrospinal fluid (CSF) time‐series were identified by thresholding the tissue prior images distributed with FSL, as in prior work by our group (Birn et al., 2014) and others (Coulombe, Erpelding, Kucyi, & Davis, 2016). The EPI time‐series was orthogonalized with respect to the first 3 right eigenvectors of the data covariance matrix from the WM and CSF compartments (Behzadi, Restom, Liau, & Liu, 2007), a Legendre polynomial series (first‐ to fifth‐order), and motion estimates (6 parameters lagged by 0, 1, and 2 volumes), consistent with recent recommendations (Hallquist, Hwang, & Luna, 2013). Orthogonalized time‐series were bandpass filtered (0.009–0.10 Hz) using AFNI and rescaled to zero‐mean unit variance in MATLAB. Using 3dFWHMx, the mean spatial smoothness of the orthogonalized data was estimated to be ∼2.28 mm3.
2.3.3. Seed regions
The BST seed was implemented using a previously published probabilistic region of interest thresholded at 25% (Theiss, Ridgewell, McHugo, Heckers, & Blackford, 2017). Building on prior work by our group (Birn et al., 2014; Nacewicz, Alexander, Kalin, & Davidson, 2014; Najafi et al., 2017; Oler et al., 2012, 2017), the Ce was manually prescribed by an experienced neuroanatomist (B.M.N.) using a specially processed version of the CITI168 high‐resolution (0.7 mm), multimodal (T1/T2) probabilistic template (http://evendim.caltech.edu/amygdala-atlas; Tyszka & Pauli, 2016) and guided by the atlas of Mai et al. (2007). The methods used for processing the template and prescribing the Ce seed are detailed in the Supporting Information, Figures S2–S5. Consistent with prior reports (Birn et al., 2014; Entis, Doerga, Barrett, & Dickerson, 2012; Hrybouski et al., 2016), visual inspection indicated that this approach provides enhanced anatomical sensitivity and selectivity compared to the more widely used centromedial amygdala region‐of‐interest distributed with FSL (Amunts et al., 2005) (Supporting Information, Figure S5). The BST and Ce seeds are depicted in Figure 1b and Supporting Information, Figure S6. To minimize partial volume artifacts, seeds were decimated to the 2‐mm MNI template using an iterative procedure that maintained a consistent seed volume across templates. Specifically, each seed was minimally smoothed (2.24 mm FWHM Gaussian) and the voxel size was dilated by 0.1 mm and resliced (linear interpolation), enabling us to identify a threshold that approximated the original seed volume and better preserved anatomical boundaries (Left BST: 96 mm3; Right BST: 96 mm3; Left Ce: 152 mm3; Right Ce: 152 mm3).
2.4. Analytic plan
We adopted a standard a priori seed‐based approach to quantifying intrinsic functional connectivity (Biswal, Yetkin, Haughton, & Hyde, 1995; Fox et al., 2005). For each subject, SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12) and in‐house MATLAB code was used to perform a voxelwise regression between the artifact‐attenuated, average seed time series and voxel times series throughout the brain. Single‐subject regression analyses were performed using the Cochrane–Orcutt procedure for estimating autoregressive error, which is more efficient and potentially less biased than ordinary least‐squares (Stocker, 2007). In order to identify regions showing consistent functional connectivity with the BST or Ce seeds across subjects, we tested the intercept in regression models, equivalent to a single‐sample t test (t > 5.47, p < .05, whole‐brain Šidák corrected for 228,483 voxels) (Birn et al., 2014; Oler et al., 2010; Šidák, 1967). At this threshold, clusters of negative connectivity were only identified in regions of deep white matter and gray matter adjacent to ventricles and, so, are neither reported nor interpreted. A minimum conjunction (Boolean “AND”) was used to identify regions showing significant coupling with both seeds (Nichols, Brett, Andersson, Wager, & Poline, 2005) and a paired t test was used to assess differential functional connectivity. For ease of interpretation, differential connectivity was only examined in the subset of 12,004 voxels where functional connectivity was significant for one or both seeds (t > 4.80, p < .05, Šidák corrected for the 12,004 voxel region‐of‐interest). This approach circumvents the need to interpret significant differences (e.g., BST > Ce) in regions where neither seed shows significant functional connectivity. For both analyses, we imposed an arbitrary 80 mm3 (i.e., 10 native EPI voxels) minimum‐extent criterion—in addition to the intensity‐based thresholds (p < .05, Šidák corrected)—to suppress noise. Exploratory analyses yielded no reliable sex differences in Ce or BST functional connectivity. As an additional check on the integrity of the data and our approach, we confirmed our ability to identify the default mode network (Supporting Information, Figure S7). Clusters were labeled using a combination of the Mai and Harvard–Oxford atlases (Desikan et al., 2006; Frazier et al., 2005; Goldstein et al., 2007; Mai, Majtanik, & Paxinos, 2015; Makris et al., 2006). Some figures were created using MRIcron (http://people.cas.sc.edu/rorden/mricron).
3. RESULTS
3.1. Subcortical regions
As shown in Figure 2 and Supporting Information, Figure S8, whole‐brain regression analyses revealed robust coupling between the BST and the Ce regions (p < .05, whole‐brain Šidák corrected; Tables 2, 3, 4). Analyses seeded in the BST showed significant functional connectivity with neighboring regions of the basal forebrain and basal ganglia and distal voxels in the region of the Ce. The complementary pattern was observed for the Ce seed—significant functional connectivity with neighboring regions of the dorsal amygdala and with distal voxels located in the region of the BST. Consistent with invasive tracing studies (Oler et al., 2017), the BST and Ce also showed robust coupling with anatomically intermediate voxels located in the SLEA, the ribbon of subcortical gray matter (substantia innominata) encompassing the ventral amygdalofugal pathway (Figure 3). Finally, both seeds showed significant functional connectivity with the amygdalohippocampal area and anterior hippocampus (Figure 2).
Figure 2.
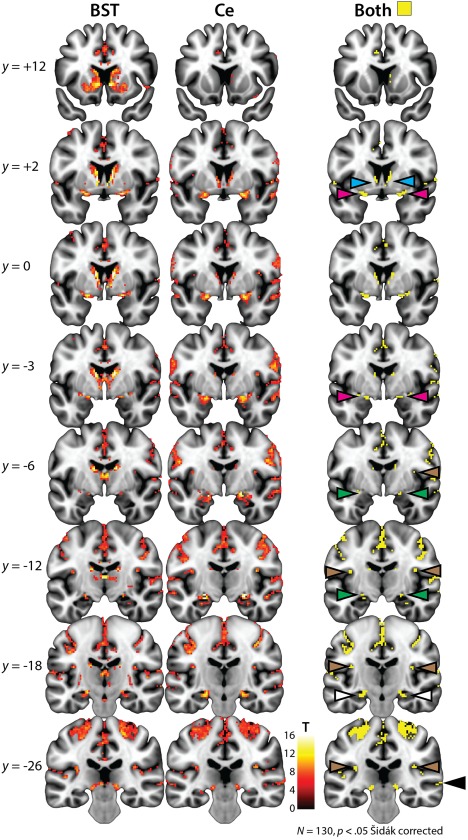
Intrinsic functional connectivity of the EAc. Left and center columns depict the results of whole‐brain regression analyses for the BST and the Ce seed regions, respectively, conservatively thresholded at p < .05 whole‐brain Šidák corrected. The right column depicts the intersection or conjunction (Boolean “AND”) of the two thresholded maps (Nichols et al., 2005). The BST seed showed significant functional connectivity with neighboring voxels in the basal forebrain (cyan arrowheads) and voxels in the region of the Ce (green arrowheads), while the Ce seed showed significant coupling with neighboring voxels in the dorsal amygdala and distal voxels in the region of the BST. Analyses also demonstrated that the BST and Ce exhibit robust functional connectivity with intermediate voxels located along the path of the ventral amygdalofugal pathway in the sublenticular extended amygdala (magenta arrowheads). Finally, both regions showed significant coupling with the amygdalohippocampal area and anterior hippocampus (white arrowheads), posterior insula (brown arrowheads), and superior temporal sulcus (black arrowheads). Note: Results are depicted here and reported in the accompanying tables for clusters of at least 80 mm3. See Figures 3 and 5 for additional views of these contrasts. Abbreviations: BST = bed nucleus of the stria terminalis; Ce = central nucleus of the amygdala; EAc = central division of the extended amygdala; L = left hemisphere; R = right hemisphere [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Regions showing significant functional connectivity with the BSTa
| x | y | z | t | mm3 | Hemisphere | Region(s)/subregions |
|---|---|---|---|---|---|---|
| 11 | 45 | 1 | 7.65 | 176 | B | Cingulate sulcus, pregenual |
| −21 | 41 | 29 | 8.55 | 352 | L | Superior frontal sulcus, anterior |
| −25 | 33 | 49 | 10.03 | 896 | L | Superior frontal sulcus, anterior |
| 27 | 32 | 35 | 8.75 | 888 | B | Superior frontal sulcus, anterior |
| −42 | 23 | −5 | 7.86 | 272 | L | Orbitofrontal cortex, basal operculum |
| −5 | 3 | 0 | 21.04 | 49,072 | B | Midlineb |
| −6 | 4 | −1 | 21.04 | 9,128 | B | Basal forebrain: caudate, putamen, globus pallidus, nucleus accumbens, rostrodorsal hypothalamus, piriform cortex, sublenticular extended amygdala (ventral amygdalofugal pathway), dorsal amygdala (central and medial nuclei), amygdalohippocampal area and anterior hippocampus, thalamus, brainstem |
| −6 | −43 | 5 | 12.96 | 7,648 | B | Posterior cingulate/Precuneus |
| 1 | 19 | 37 | 11.7 | 3,072 | L | Cingulate: cingulate sulcus, midcingulate; cingulate sulcus, posterior; juxtapositional lobule |
| 11 | 18 | 33 | 10.27 | 480 | R | Cingulate: Cingulate sulcus, pregenual; Cingulate sulcus, midcingulate |
| 1 | 53 | −5 | 9.67 | 328 | B | Ventromedial prefrontal cortex: OP10r/mc; inferior frontopolar gyrus; rostral gyrus; anterior cingulate cortex, pregenual |
| −3 | −25 | −3 | 9.48 | 80 | L | Periaqueductal gray, dorsolateral |
| −53 | 2 | −1 | 7.97 | 136 | L | Superior temporal gyrus, planum polare |
| −39 | 1 | 59 | 7.12 | 136 | L | Precentral sulcus |
| 1 | −13 | −23 | 8.81 | 88 | R | Cerebellum |
| −37 | −15 | 17 | 10.79 | 1,648 | L | Posterior insula: central operculum, parietal operculum, posterior insula (dorsal portion of the long gyri), Heschl's gyrus |
| 53 | −16 | 5 | 9.57 | 2,224 | R | Posterior insula: central operculum, parietal operculum, posterior insula (dorsal portion of the long gyri), Heschl's gyrus |
| 31 | −17 | 3 | 7.43 | 184 | R | Putamen |
| 13 | −17 | 39 | 8.01 | 160 | B | Cingulate sulcus, posterior |
| −27 | −19 | 5 | 7.91 | 112 | L | Putamen |
| 7 | −21 | −1 | 10.85 | 152 | R | Thalamus |
| 69 | −22 | −3 | 8.19 | 544 | R | Superior temporal sulcus |
| −20 | −29 | 57 | 11.7 | 3,144 | L | Central sulcus |
| 21 | −29 | 57 | 13.12 | 3,024 | R | Central sulcus |
| 26 | −37 | 57 | 8.98 | 360 | B | Postcentral sulcus |
| −19 | −37 | 65 | 8.04 | 272 | L | Postcentral gyrus |
| 57 | −57 | 21 | 7.53 | 176 | R | Angular gyrus |
| 54 | −62 | 31 | 7.04 | 176 | R | Lateral occipital cortex |
| −9 | −69 | 5 | 8.29 | 256 | L | Calcarine sulcus |
| 31 | −72 | −37 | 8.41 | 344 | B | Cerebellum |
| −31 | −80 | −37 | 8.17 | 504 | L | Cerebellum |
| −7 | −81 | 1 | 7.94 | 384 | L | Calcarine sulcus |
| −35 | −83 | −19 | 7.03 | 96 | L | Lateral occipital cortex/fusiform, occipital |
| 25 | −85 | −19 | 8.08 | 328 | R | Fusiform, occipital |
| 15 | −93 | 1 | 7.9 | 80 | R | Occipital pole |
Note. Abbreviations: B, bilateral; Ce, central nucleus of the amydala; L, left hemisphere; R, right hemisphere.
Whole‐brain regression analysis (p < .05, whole‐brain Šidák corrected, k ≥ 80 mm3).
For large clusters, subregions were identified using T ≥ 7 and are shown in italics.
Areas 10r/m and 11 as described by Ongur, Ferry, and Price (2003).
Table 3.
Regions showing significant functional connectivity with the Cea
| x | y | z | t | mm3 | Hemisphere | Region(s)/subregions |
|---|---|---|---|---|---|---|
| 1 | 59 | 19 | 8.29 | 504 | B | Dorsomedial prefrontal cortex: BA10 |
| 1 | 53 | −13 | 8.7 | 600 | B | Ventromedial prefrontal cortex: OP10r/mc; inferior frontopolar gyrus; rostral gyrus |
| 8 | 39 | −15 | 7.27 | 112 | R | Ventromedial prefrontal cortex: inferior frontopolar gyrus, straight gyrus |
| 34 | 37 | −13 | 7.15 | 96 | R | Orbitofrontal cortex: OP11,c anterior orbitalgyrus |
| −19 | 37 | 43 | 7.92 | 392 | L | Superior frontal sulcus, anterior |
| 39 | 9 | −15 | 7.84 | 176 | R | Anterior insula: transverse insular gyrus |
| 9 | 3 | 3 | 10.11 | 424 | R | Basal forebrain: caudate, bed nucleus of the stria terminalis, rostrodorsal hypothalamus |
| −5 | 1 | 1 | 10.56 | 376 | L | Basal forebrain: caudate, bed nucleus of the stria terminalis, rostrodorsal hypothalamus |
| 57 | −5 | 23 | 12.64 | 12,736 | B | Central cortexb |
| 57 | −5 | 23 | 12.64 | 3,024 | R | Central sulcus |
| −3 | −22 | 45 | 10.87 | 1,096 | B | Cingulate sulcus, posterior; Cingulate sulcus, midcingulate |
| −1 | −31 | 57 | 8.44 | 160 | B | Precentral gyrus |
| −52 | −7 | 25 | 11.53 | 6,912 | L | Central sulcus |
| 23 | −9 | −13 | 22.02 | 2,696 | R | Basal forebrain: piriform cortex, sublenticular extended amygdala (ventral amygdalofugal pathway), amygdala (amygdalohippocampal area, basolateral, basomedial, cortical, lateral, and medial), anterior hippocampus, brainstem |
| −19 | −11 | −13 | 20.91 | 2,720 | L | Basal forebrain: putamen, piriform cortex, sublenticular extended amygdala (ventral amygdalofugal pathway), amygdala (amygdalohippocampal area, basolateral, basomedial, cortical, lateral, and medial), anterior hippocampus, brainstem |
| 51 | −12 | −13 | 10.6 | 4,904 | R | Temporal lobe: superior temporal gyrus, planum polare; parietal operculum; superior temporal sulcus |
| −37 | −15 | 17 | 10.73 | 6,400 | L | Posterior insula: central operculum, parietal operculum, posterior insula (dorsal portion of the long gyri), planum temporale, Heschl's gyrus, superior temporal sulcus |
| 39 | −15 | 17 | 10.89 | 1,096 | R | Posterior insula: central operculum, parietal operculum, posterior insula (dorsal portion of the long gyri) |
| 53 | −23 | 45 | 6.38 | 80 | R | Postcentral sulcus |
| 53 | −27 | 57 | 6.73 | 104 | R | Postcentral gyrus |
| 25 | −37 | 59 | 8.3 | 592 | R | Postcentral sulcus |
| −44 | −50 | −17 | 8.1 | 192 | L | Temporal lobe: inferior temporal gyrus, temporooccipital; fusiform, temporooccipital |
| 37 | −52 | −21 | 7.99 | 208 | R | Temporal lobe: inferior temporal gyrus, temporooccipital; fusiform, temporooccipital |
| −1 | −53 | 17 | 13.43 | 7,632 | B | Posterior cingulate/precuneus |
| 57 | −63 | 11 | 9.33 | 2,272 | R | Lateral occipital cortex |
| 29 | −83 | −19 | 6.39 | 88 | R | Fusiform, occipital |
Note. Abbreviations: B, bilateral; BA, Brodmann area; Ce, central nucleus of the amydala; L, left hemisphere; R, right hemisphere.
Whole‐brain regression analysis (p < .05, whole‐brain Šidák corrected, k ≥ 80 mm3).
For large clusters, subregions were identified using T ≥ 7 and are shown in italics.
Areas 10r/m and 11 as described by Ongur et al. (2003).
Table 4.
Regions showing significant functional connectivity with both the BST and the Cea
| x | y | z | mm3 | Hemisphere | Region(s)/subregions |
|---|---|---|---|---|---|
| 1 | 61 | 21 | 48 | R | Dorsomedial prefrontal cortex: BA10 |
| 3 | 59 | 17 | 16 | R | Dorsomedial prefrontal cortex: BA10 |
| 1 | 57 | 13 | 24 | R | Dorsomedial prefrontal cortex: BA10 |
| 1 | 53 | 19 | 80 | R | Dorsomedial prefrontal cortex: BA10 |
| −1 | 49 | 27 | 24 | L | Dorsomedial prefrontal cortex: BA10 |
| 1 | 39 | −15 | 296 | R | Ventromedial prefrontal cortex: OP10r/mb; inferior frontopolar gyrus; rostral gyrus |
| −21 | 27 | 37 | 304 | L | Superior frontal sulcus, anterior |
| 55 | 7 | −3 | 8 | R | Temporal pole |
| 63 | 7 | −1 | 664 | R | Planum temporale |
| 9 | 5 | −1 | 384 | R | Basal forebrain: caudate, bed nucleus of the stria terminalis, rostrodorsal hypothalamus |
| −9 | 5 | 35 | 3,448 | L | Cingulate: cingulate sulcus, posterior midcingulate; cingulate sulcus, posterior |
| −5 | 3 | −1 | 312 | L | Basal forebrain: caudate, bed nucleus of the stria terminalis |
| 5 | 1 | −3 | 8 | R | Bed nucleus of the stria terminalis |
| −53 | 1 | −1 | 40 | L | Planum polare |
| 53 | 1 | −1 | 24 | R | Planum polare |
| −1 | 1 | 47 | 8 | L | Juxtapositional lobule |
| −17 | −3 | −15 | 376 | L | Dorsal amygdala: amygdalohippocampal area, central, cortical, medial |
| 63 | −3 | 17 | 2,640 | R | Central sulcus |
| 61 | −5 | −13 | 392 | R | Superior temporal sulcus |
| 29 | −11 | −23 | 976 | R | Hippocampus |
| −41 | −15 | 31 | 2,648 | L | Central sulcus |
| 5 | −15 | 73 | 8 | R | Precentral gyrus |
| 63 | −17 | −7 | 8 | R | Superior temporal sulcus |
| −53 | −17 | 9 | 8 | L | Heschl's gyrus |
| −21 | −19 | −17 | 616 | L | Hippocampus/dorsal amygdala: basolateral, basomedial, central, medial |
| −57 | −19 | 9 | 152 | L | Planum temporale |
| 13 | −19 | 39 | 40 | R | Cingulate sulcus, posterior |
| 3 | −19 | 67 | 16 | R | Precentral gyrus |
| −47 | −25 | 3 | 880 | L | Planum temporale |
| 47 | −25 | 7 | 728 | R | Planum temporale |
| −25 | −31 | 67 | 96 | L | Postcentral gyrus |
| 3 | −33 | 49 | 16 | R | Posterior cingulate |
| 27 | −37 | 55 | 232 | R | Postcentral sulcus |
| −21 | −39 | 63 | 128 | L | Postcentral gyrus |
| 3 | −39 | 63 | 8 | R | Postcentral gyrus |
| 11 | −53 | 1 | 6,792 | R | Posterior cingulate/precuneus |
| 55 | −57 | 19 | 136 | R | Angular gyrus |
| 45 | −59 | 29 | 8 | R | Lateral occipital cortex |
| 31 | −85 | −19 | 88 | R | Occipital fusiform |
Note. Abbreviations: B, bilateral; BA, Brodmann area; BST, bed nucleus of the stria terminalis; Ce, central nucleus of the amydala; L, left hemisphere; R, right hemisphere.
Minimum conjunction (Boolean “AND”) analysis (p < .05, whole‐brain Šidák corrected, k ≥ 80 mm3).
Area 10r/m as described by Ongur et al. (2003).
Figure 3.
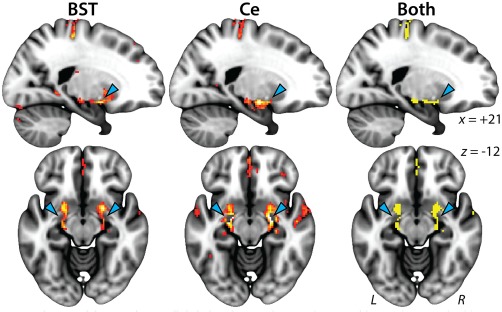
The BST and the Ce are functionally linked via the SLEA. Clusters in the region of the SLEA (cyan arrowheads). Conventions are similar to Figure 2. Abbreviations: BST = bed nucleus of the stria terminalis; Ce = central nucleus of the amygdala; L = left hemisphere; R = right hemisphere; SLEA = sublenticular extended amygdala. See Figures 2 and 5 for additional views of these contrasts [Color figure can be viewed at http://wileyonlinelibrary.com]
Compared to the Ce, the BST showed significantly stronger coupling with several subcortical regions, including the basal ganglia (i.e., nucleus accumbens, caudate, and putamen), thalamus, and the brainstem in the region of the dorsal periaqueductal gray (PAG) (Figure 4, Supporting Information, Figure S9, and Table 5). The only subcortical regions showing stronger functional connectivity with the Ce were located in the dorsal amygdala and anterior hippocampus, and included the amygdalohippocampal area and basolateral, basomedial, cortical, and medial nuclei.
Figure 4.
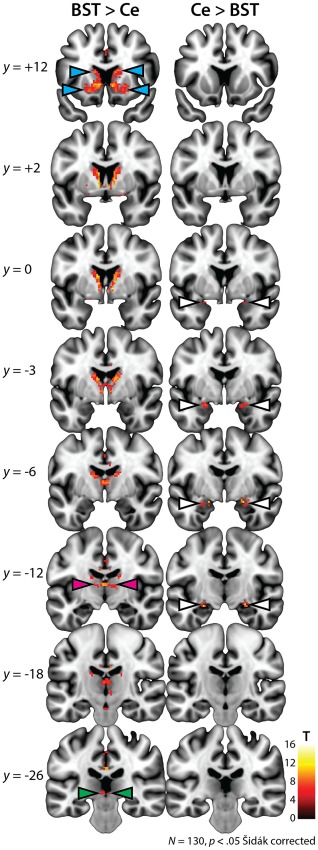
Differential functional connectivity of the BST versus Ce. Results of a paired t test comparing the intrinsic functional connectivity of the BST and Ce. The left and right columns depict regions showing significantly stronger coupling with the BST and Ce, respectively. For ease of interpretation, differences were only examined in the subset of 12,004 voxels, where functional connectivity was significant for the BST, the Ce, or both seeds (Figures 2 and 3). Consistent with other analyses, results were thresholded at p < .05 Šidák corrected for the extent of the 12,004‐voxel mask. Results revealed significantly stronger coupling between the BST and the basal ganglia, including the caudate, putamen, and nucleus accumbens (cyan arrowheads). The BST also showed significantly stronger connectivity with the thalamus (magenta arrowheads) and a region of the brainstem consistent with the dorsal periaqueductal gray (green arrowheads; see also Supporting Information, Figure S9). The only regions showing stronger connectivity with the Ce were neighboring regions of the amygdala (white arrowheads), including voxels in the region of the amygdalohippocampal area, anterior hippocampus (not depicted) and the basolateral, basomedial, cortical, and medial nuclei. Note: Results are depicted here and reported in the accompanying tables for clusters of at least 80 mm3. See Figure 5 for additional views of the BST > Ce contrast. Abbreviations: BST = bed nucleus of the stria terminalis; Ce = central nucleus of the amygdala; L = left hemisphere; R = right hemisphere [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 5.
Regions showing significant differences in intrinsic functional connectivity between the BST and the Cea
| Effect | x | y | z | t | mm3 | Hemisphere | Region(s)/subregions |
|---|---|---|---|---|---|---|---|
| BST > Ce | −25 | 55 | 31 | 6.8 | 80 | L | Frontal pole: BA9/BA10 |
| 2 | 45 | −1 | 8.43 | 344 | B | Ventromedial prefrontal cortex: OP10r/mb; inferior frontopolar gyrus; rostral gyrus; anterior cingulate, pregenual | |
| 21 | 41 | 31 | 7.12 | 96 | R | Superior frontal sulcus, anterior | |
| −25 | 41 | 35 | 5.69 | 112 | L | Superior frontal sulcus, anterior | |
| 11 | 37 | −3 | 7.11 | 96 | R | Cingulate: cingulate sulcus, pregenual | |
| 7 | 36 | 25 | 9.94 | 3,504 | B | Cingulate: cingulate sulcus, pregenual; cingulate sulcus, anterior midcingulate | |
| 49 | 23 | −9 | 7.53 | 80 | R | Orbitofrontal cortex: OP47, Basal operculum | |
| 6 | 5 | −2 | 17.15 | 10,472 | B | Basal forebrain: caudate, putamen, globus pallidus, nucleus accumbens, rostrodorsal hypothalamus, sublenticular extended amygdala (ventral amygdalofugal pathway), thalamus | |
| 3 | −11 | 35 | 6.73 | 128 | R | Posterior cingulate | |
| −1 | −17 | −21 | 7.06 | 80 | L | Brainstem ventral to the interpeduncular cistern | |
| −3 | −23 | −1 | 7.34 | 112 | L | Periaqueductal gray, dorsolateral | |
| 5 | −24 | −3 | 8.38 | 136 | R | Periaqueductal gray, dorsolateral | |
| 3 | −27 | 25 | 10.17 | 968 | B | Posterior cingulate | |
| 4 | −35 | 47 | 8.45 | 800 | B | Posterior cingulate | |
| 13 | −47 | 31 | 5.94 | 104 | R | Posterior cingulate | |
| −7 | −69 | 33 | 8.82 | 288 | L | Precuneus | |
| 1 | −75 | 43 | 6.89 | 232 | B | Precuneus | |
| −8 | −81 | 3 | 6.86 | 216 | L | Calcarine sulcus | |
| 9 | −87 | 1 | 7.59 | 488 | R | Calcarine sulcus | |
| Ce > BST | 25 | −9 | −15 | −14.31 | 536 | R | Anterior hippocampus and amygdala: amygdalohippocampal area, anterior hippocampus, basolateral, basomedial, cortical, medial |
| −21 | −10 | −15 | −11.19 | 504 | L | Amygdala: amygdalohippocampal area, anterior hippocampus, basolateral, basomedial, cortical, medial |
Note. Abbreviations: B, bilateral; BST, bed nucleus of the stria terminalis; Ce, central nucleus of the amygdala; L, left hemisphere; R, right hemisphere.
Paired t test for the subset of 12,004 voxels showing significant functional connectivity with the BST, Ce, or both seeds (p < .05, Šidák corrected for the extent of the 12,004‐voxel mask).
Area 10r/m as described by Ongur et al. (2003).
3.2. Cortical regions
As shown in Figures 2 and 5, the BST and the Ce showed significant functional connectivity with several cortical regions, including the ventromedial prefrontal cortex (vmPFC), posterior MCC, posterior insula, posterior cingulate/precuneus, and parts of the ventral visual processing stream (e.g., superior temporal sulcus, fusiform cortex) (Tables 2, 3, 4). As shown in Figure 5, relative to the Ce, the BST displayed significantly stronger coupling with a cluster centered on the anterior MCC that extends into the pregenual anterior cingulate cortex (pgACC) and vmPFC (Figure 5, far‐right panels, and Table 5). As detailed in the Supporting Information, Figure S10, control analyses indicated that these effects could not be attributed to regional differences in signal quality, as indexed by several widely used metrics (e.g., the temporal signal‐to‐noise ratio [tSNR]).
Figure 5.
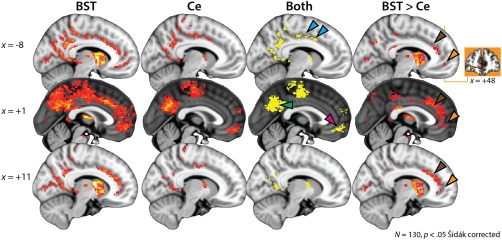
Intrinsic functional connectivity of the EAc and midline cortical regions. The first two columns depict the results of whole‐brain regression analyses for the BST and Ce seed regions, respectively (p < .05, whole‐brain Šidák corrected). The third column depicts the intersection (Boolean “AND”) of the two thresholded maps. The fourth column depicts the results of a paired t test comparing the intrinsic functional connectivity of the BST and Ce (p < .05, small‐volume Šidák corrected). Both seeds show significant functional connectivity with the posterior cingulate/precuneus (green arrowhead), posterior MCC (cyan arrowheads), and vmPFC (magenta arrowhead). Relative to the Ce, the BST shows significantly stronger coupling with the anterior MCC and pgACC (brown arrowheads) and the vmPFC (orange arrowheads). Orange inset depicts a coronal slice through the vmPFC cluster, which extends along the rostral–caudal axis from area 10r/m and the inferior frontopolar gyrus to the rostral gyrus and pgACC. Conventions are similar to Figure 2 (first three columns) and Figure 4 (fourth column). See Figures 2, 3, 4 for additional views of these contrasts. Abbreviations: BST = bed nucleus of the stria terminalis; Ce = central nucleus of the amygdala; EAc = central divisions of the extended amygdala; L = left hemisphere; MCC = midcingulate cortex; pgACC = pregenual anterior cingulate cortex; R = right hemisphere; vmPFC = ventromedial prefrontal cortex [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
The EAc plays a central role in assembling states of fear and anxiety and is implicated in the development, maintenance, and recurrence of a range of debilitating psychiatric disorders. The present findings provide new insights into the normative architecture of the EAc functional network. Our results indicate that the BST and the Ce are robustly interconnected via the SLEA (Figure 3 and Supporting Information, Figure S8), consistent with anatomical and functional tracing studies in monkeys (Birn et al., 2014; Oler et al., 2012, 2017). By and large, the BST and the Ce showed patterns of functional connectivity that were similar to one another and concordant with prior human imaging research (Table 6). Both regions showed significant coupling with subcortical and cortical regions implicated in fear and anxiety—including the anterior hippocampus, insula, MCC, and vmPFC (Figures 2 and 5)—reinforcing the hypothesis that these regions represent a functionally coherent macro‐circuit (Alheid & Heimer, 1988; Fox et al., 2015a; Fudge et al., 2017; Oler et al., 2012; Shackman & Fox, 2016; Fox & Shackman, in press).
Table 6.
| Seed | Citation | NAcc | Cd | Putamen | GP | BST | SLEA | Amygdala | Hippocampus | Thalamus | PAG | vmPFC/OFC | pgACC | MCC | Insula | Precuneus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BST | Present study | + | + | + | + | N/A | + | + | + | + | + | + | + | + | +g | + |
| Avery et al., 2014, c | + | + | + | + | N/A | + | + | + | + | + | + | +a, g | + | |||
| Torrisi et al., 2015 | + | + | + | N/A | + | + | + | + | + | + | + | +g | + | |||
| 3/3 | 3/3 | 3/3 | 2/3 | N/A | 2/3 | 3/3 | 3/3 | 3/3 | 2/3 | 3/3 | 3/3 | 2/3 | 3/3 | 3/3 | ||
| Ce | Present study | + | + | + | + | + | + | + | +a, g | + | ||||||
| Gorka et al., 2017, d | + | + | + | + | + | + | + | + | + | + | + | +a, g | + | |||
| Oler et al., 2012, e | + | + | + | + | + | + | + | |||||||||
| 2/3 | 2/3 | 2/3 | 0/3 | 3/3 | 2/3 | 3/3 | 3/3 | 2/3 | 1/3 | 2/3 | 1/3 | 2/3 | 3/3 | 2/3 |
Note. Abbreviations: BST = bed nucleus of the stria terminalis; Cd = caudate; Ce = central nucleus of the amygdala; GP = globus pallidus; MCC = midcingulate cortex; NAcc = nucleus accumbens; OFC = orbitofrontal cortex; PAG = periaqueductal gray; pgACC = pregenual anterior cingulate cortex; SLEA = sublenticular extended amygdala; vmPFC = ventromedial prefrontal cortex.
Anterior.
This table is not meant to be comprehensive and some regional labels (vmPFC/OFC) encompass multiple subdivisions. Plus signs (+) indicate significant clusters. Empty cells indicate an absence of positive evidence in the published report. In some cases this reflects the absence of significant functional connectivity at the chosen threshold. In other cases, it simply indicates the omission of a specific label (e.g., SLEA). Regardless, empty cells should not be interpreted as indicating an absence of coupling (Fox et al., 2018). Motzkin et al. (2015) do not provide a detailed table of significant clusters and so are not included here, although it merits comment that they do report significant BST connectivity clusters in the pgACC and the vmPFC/OFC. McMenamin et al. (2014) do not provide a detailed table and are also not included, although they too provide visual evidence of a significant BST cluster at the intersection of anterior MCC and pgACC and extending into the edge of vMPFC (rostral gyri). Finally, although Birn et al. (2014) do provide detailed results, their study focused on a large (n = 89) sample of monkeys and so are not included. Nonetheless, it merits comment that they observed significant coupling between the Ce and several relevant regions, including the pgACC, insula, BST, thalamus, and neighboring regions of the amygdala. They also report a significant negative association between Ce–vmPFC functional connectivity and somatomotor responses to human intruder threat, with the cluster encompassing parts of areas 10m, 11, and 14.
Although Avery et al. (2014) also do not provide a detailed table of significant clusters, they do provide a dense montage of sagittal slices and a brief verbal summary and so are included.
Gorka et al. (2017) only provide a detailed table for clusters showing significant functional connectivity with both the BST and the Ce. Relative to the Ce, they report significantly greater coupling between the BST and several regions, including the MCC, posterior cingulate, caudate, and NAcc. Conversely, they report significantly greater coupling between the Ce, insula, and neighboring regions of the amygdala.
Oler et al. (2012) and Birn et al. (2014) did observe significant functional connectivity between the Ce and SLEA in a large sample of anesthetized monkeys.
From the perspective of generating cumulative knowledge, this table underscores the need to provide detailed cluster tables for every key contrast and/or share data using http://NeuroVault.org.
posterior.
Despite their many similarities, it is unlikely that the BST and the Ce are interchangeable (Fox & Shackman, [Link]; Shackman & Fox, 2016). Indeed, the BST showed significantly stronger connectivity with anterior cortical regions (anterior MCC, pgACC, and vmPFC), with the posterior cingulate/precuneus, with the medial temporal lobe (striatum and SLEA), and with the brainstem in the region of the dorsal PAG (Supporting Information, Figure S9), whereas the Ce showed stronger connectivity with neighboring regions of the amygdala, amygdalohippocampal area, and anterior hippocampus (Figures 4 and 5)—observations that largely align with recent high‐resolution fMRI research (Gorka et al., 2017) (cf. Table 1). Supplementary analyses indicated that these effects were not a consequence of regional differences in signal quality (e.g., tSNR).
We also observed significant coupling between the BST, the Ce, and the vmPFC (i.e., inferior frontopolar gyrus, rostral gyrus, and area OP10), although this effect was stronger for the BST seed region (Figure 5). This pattern is consistent with other work leveraging the enhanced resolution afforded by 7T fMRI (Gorka et al., 2017; their figure 2e) and is particularly interesting in light of several recent observations in nonhuman primate models of fear and anxiety. First, intrinsic coupling between the Ce and vmPFC covaries with the intensity of defensive behaviors and neuroendocrine activity elicited by exposure to human intruder threat in monkeys (Birn et al., 2014). Second, metabolic activity in the Ce, BST, and vmPFC, as well as the anterior hippocampus and PAG, covaries with these same anxiety‐related responses (Fox et al., 2015b). Third, vmPFC lesions have been shown to reduce these defensive responses and imaging research suggests that this anxiolytic effect is likely to be mediated by “downstream” alterations in BST metabolism (Fox et al., 2010; Kalin, Shelton, & Davidson, 2007; Motzkin et al., 2015; Rudebeck, Saunders, Prescott, Chau, & Murray, 2013). These and other observations (e.g., Grayson et al., 2016; Kalin et al., 2016, 2004; Meyer et al., 2017; Mobbs et al., 2009, 2007, 2010) motivate the hypothesis that fear and anxiety partially reflect a core neural system encompassing the BST, Ce, vmPFC, anterior hippocampus, and PAG (Fox et al., 2015b; Oler et al., 2016; Shackman et al., 2016b).
Our results revealed evidence of robust coupling between the BST, Ce, and rostral cingulate and they hint at a rostro‐caudal gradient: both seeds showed coupling with the posterior MCC, while the BST showed significantly stronger coupling with a cluster centered on the anterior MCC (Figure 5). Notably, the MCC and a region consistent with the BST are frequently co‐activated in imaging studies of Pavlovian fear conditioning (Fullana et al., 2016; Mechias, Etkin, & Kalisch, 2010) and uncertain threat anticipation (Alvarez et al., 2011, 2015; Choi et al., 2012; Grupe et al., 2013; Herrmann et al., 2016; Klumpers et al., 2015; McMenamin et al., 2014; Meyer et al., 2017; Somerville et al., 2010). We have previously hypothesized that the MCC uses information about pain, negative feedback, punishment, and threat to bias responding in situations where the optimal course of action is uncertain or risky (Cavanagh & Shackman, 2015; Shackman et al., 2011) (see also de la Vega et al., 2016) and the present results highlight the potential importance of communication between the MCC and the EAc, particularly the BST, for this kind of adaptive control. A key challenge for future research will be to more formally characterize the nature of task‐related interactions among these three key regions using graph‐theoretic or related analytic techniques (McMenamin et al., 2014; Najafi et al., 2017).
Clearly, a number of other important challenges remain. As with most brain imaging studies, our analyses do not permit mechanistic inferences and like other studies focused on functional connectivity, our conclusions are tempered by questions about the origins and significance of correlated fluctuations in the blood‐oxygen‐level‐dependent (BOLD) fMRI signal (Akam & Kullmann, 2014; Cabral, Kringelbach, & Deco, 2014; Logothetis, 2008). A key challenge for future research will be to use a combination of mechanistic (e.g., optogenetic) and whole‐brain imaging techniques to clarify the specific causal contributions of the regions highlighted here and more precisely delineate the nature of their functional interactions (Fox & Shackman, in press; Shackman & Fox, 2016; Wiegert, Mahn, Prigge, Printz, & Yizhar, 2017).
Existing treatments for anxiety disorders are inconsistently effective or associated with significant adverse effects (Bystritsky, 2006; Cloos & Ferreira, 2009; Craske et al., 2017; Cuijpers, Cristea, Karyotaki, Reijnders, & Huibers, 2016; James, James, Cowdrey, Soler, & Choke, 2015), highlighting the need to identify and understand the neural mechanisms controlling the experience and expression of fear and anxiety. Building on prior mechanistic and imaging research, the present study indicates that the BST and the Ce are marked by broadly similar patterns of intrinsic functional connectivity, with both regions showing significant coupling with the EAc, anterior hippocampus, insula, MCC, and vmPFC. Despite these similarities, the BST displayed significantly stronger connectivity with the rostral cingulate and vmPFC. These observations provide a baseline against which to compare a range of special populations—including individuals at risk for developing mental illness and patients suffering from psychiatric disorders—and inform our understanding of the role of the EAc in normal and pathological fear and anxiety. The use of a relatively large sample increases our confidence in the robustness of these results (Cremers, Wager, & Yarkoni, 2017; Fox, Lapate, Davidson, & Shackman, 2018; Poldrack et al., 2017). Finally, from a methodological perspective, these results highlight the value of several new techniques for EAc seed prescription and image registration/normalization. The former is likely to be useful for other investigators focused on the BST and Ce, while the latter will be advantageous for any investigator confronted with the problem of spatially normalizing structural images that have been modified—anatomically “anonymized” or “de‐identified”—prior to public release (Holmes et al., 2015; Nooner et al., 2012).
5. CONTRIBUTIONS
R.M.T, A.J.S., and J.F.S. designed the study. M.D.S. coordinated data extraction. B.M.N. developed and implemented the protocol for segmenting the Ce seed and the HyperEdge method. J.F.S. developed and implemented the novel image registration/normalization pipeline. R.M.T. and J.F.S. processed data. J.F.S. and A.J.S. analyzed data. R.M.T., A.J.S., and A.S.F. interpreted data. R.M.T., A.J.S., A.S.F., and B.M.N. wrote the article. A.J.S., R.M.T., B.M.N., and A.S.F. created figures. R.M.T. and A.J.S. created tables. S.T. provided theoretical guidance. A.J.S. funded and supervised all aspects of the study. All authors contributed to reviewing and revising the article and approved the final version.
DATA AVAILABILITY/SHARING
Key statistical maps are available in NeuroVault.org. Raw data are publicly available (http://fcon_1000.projects.nitrc.org/indi/enhanced/).
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Table 1
ACKNOWLEDGMENTS
Authors acknowledge assistance from J. Blackford, K. DeYoung, L. Friedman, M. Milham, and D. Tromp and critical feedback from N. Fox, L. Pessoa, and E. Redcay. They also wish to thank Drs R. Poldrack and A. Holmes for guidance on the signal quality analyses. This work was supported by the University of California, Davis; University of Maryland, College Park; University of Wisconsin—Madison; and National Institutes of Health (DA040717 and MH107444). Authors declare no conflicts of interest.
Tillman RM, Stockbridge MD, Nacewicz BM, et al. Intrinsic functional connectivity of the central extended amygdala. Hum Brain Mapp. 2018;39:1291–1312. 10.1002/hbm.23917
Funding information University of California, Davis; University of Maryland, College Park; University of Wisconsin—Madison; National Institutes of Health, Grant/Award Numbers: DA040717, MH107444
REFERENCES
- Acosta‐Cabronero, J. , Williams, G. B. , Pereira, J. M. , Pengas, G. , & Nestor, P. J. (2008). The impact of skull‐stripping and radio‐frequency bias correction on grey‐matter segmentation for voxel‐based morphometry. Neuroimage, 39, 1654–1665. [DOI] [PubMed] [Google Scholar]
- Akam, T. , & Kullmann, D. M. (2014). Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nature Reviews Neuroscience, 15, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid, G. F. , & Heimer, L. (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27, 1–39. [DOI] [PubMed] [Google Scholar]
- Alvarez, R. P. , Chen, G. , Bodurka, J. , Kaplan, R. , & Grillon, C. (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage, 55, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, R. P. , Kirlic, N. , Misaki, M. , Bodurka, J. , Rhudy, J. L. , Paulus, M. P. , & Drevets, W. C. (2015). Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Translational Psychiatry, 5, e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano, K. , Tanikawa, T. , Kawamura, H. , Iseki, H. , Notani, M. , Kawabatake, H. , … Kitamura, K. (1982). Endorphins and pain relief. Further observations on electrical stimulation of the lateral part of the periaqueductal gray matter during rostral mesencephalic reticulotomy for pain relief. Applied Neurophysiology, 45, 123–135. [PubMed] [Google Scholar]
- Amunts, K. , Kedo, O. , Kindler, M. , Pieperhoff, P. , Mohlberg, H. , Shah, N. J. , … Zilles, K. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology, 210, 343–352. [DOI] [PubMed] [Google Scholar]
- Antoniadis, E. A. , Winslow, J. T. , Davis, M. , & Amaral, D. G. (2007). Role of the primate amygdala in fear‐potentiated startle: effects of chronic lesions in the rhesus monkey. Journal of Neuroscience, 27(28), 7386–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Assareh, N. , Sarrami, M. , Carrive, P. , & McNally, G. P. (2016). The organization of defensive behavior elicited by optogenetic excitation of rat lateral or ventrolateral periaqueductal gray. Behavioral Neuroscience, 130, 406–414. [DOI] [PubMed] [Google Scholar]
- Avants, B. B. , Epstein, C. L. , Grossman, M. , & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross‐correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants, B. B. , Tustison, N. J. , Song, G. , Cook, P. A. , Klein, A. , & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants, B. B. , Yushkevich, P. , Pluta, J. , Minkoff, D. , Korczykowski, M. , Detre, J. , & Gee, J. C. (2010). The optimal template effect in hippocampus studies of diseased populations. Neuroimage, 49, 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, S. N. , Clauss, J. A. , & Blackford, J. U. (2016). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 41, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, S. N. , Clauss, J. A. , Winder, D. G. , Woodward, N. , Heckers, S. , & Blackford, J. U. (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler, R. , Price, J. L. , & Keay, K. A. (2000). Brain mediation of active and passive emotional coping. Progress in Brain Research, 122, 333–349. [DOI] [PubMed] [Google Scholar]
- Banihashemi, L. , Sheu, L. K. , Midei, A. J. , & Gianaros, P. J. (2015). Childhood physical abuse predicts stressor‐evoked activity within central visceral control regions. Social Cognitive and Affective Neuroscience, 10, 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara, A. , Tranel, D. , Damasio, H. , Adolphs, R. , Rockland, C. , & Damasio, A. R. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269, 1115–1118. [DOI] [PubMed] [Google Scholar]
- Behzadi, Y. , Restom, K. , Liau, J. , & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn, R. M. , Shackman, A. J. , Oler, J. A. , Williams, L. E. , McFarlin, D. R. , Rogers, G. M. , … Kalin, N. H. (2014). Evolutionarily conserved dysfunction of prefrontal‐amygdalar connectivity in early‐life anxiety. Molecular Psychiatry, 19, 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Yetkin, F. Z. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Brinkmann, L. , Buff, C. , Feldker, K. , Neumeister, P. , Heitmann, C. Y. , Hofmann, D. , … Straube, T. (2018). Inter‐individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. Neuroimage, 166, 110–116. [DOI] [PubMed] [Google Scholar]
- Brinkmann, L. , Buff, C. , Feldker, K. , Tupak, S. V. , Becker, M. P. I. , Herrmann, M. J. , & Straube, T. (2017a). Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychological Medicine, 47, 2675–2688. [DOI] [PubMed] [Google Scholar]
- Brinkmann, L. , Buff, C. , Neumeister, P. , Tupak, S. V. , Becker, M. P. , Herrmann, M. J. , & Straube, T. (2017b). Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post‐traumatic stress disorder patients. Human Brain Mapping, 38, 2190–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buff, C. , Brinkmann, L. , Bruchmann, M. , Becker, M. P. I. , Tupaka, S. , Herrmann, M. J. , & Straube, T. (2017). Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in Generalized Anxiety Disorder. Social Cognitive and Affective Neuroscience, 12, 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky, A. (2006). Treatment‐resistant anxiety disorders. Molecular Psychiatry, 11, 805–814. [DOI] [PubMed] [Google Scholar]
- Cabral, J. , Kringelbach, M. L. , & Deco, G. (2014). Exploring the network dynamics underlying brain activity during rest. Progress in Neurobiology, 114C, 102–131. [DOI] [PubMed] [Google Scholar]
- Calhoon, G. G. , & Tye, K. M. (2015). Resolving the neural circuits of anxiety. Nature Neuroscience, 18, 1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive, P. , & Morgan, M. M. (2012). Periaqueductal gray In Mai J. K. & Paxinos G. (Eds.), The human nervous system (3rd ed., pp. 367–400. New York: Academic Press. [Google Scholar]
- Cavanagh, J. F. , & Shackman, A. J. (2015). Frontal midline theta reflects anxiety and cognitive control: Meta‐analytic evidence. Journal of Physiology, Paris, 109, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L. J. , Gianaros, P. J. , Manuck, S. B. , Krishnan, A. , & Wager, T. D. (2015). A sensitive and specific neural signature for picture‐induced negative affect. PLoS Biology, 13, e1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Zhou, H. , Guo, S. , Zhang, J. , Qu, Y. , Feng, Z. , … Zheng, X. (2015). Optogenetics based rat–robot control: Optical stimulation encodes “stop” and “escape” commands. Annals of Biomedical Engineering, 43, 1851–1864. [DOI] [PubMed] [Google Scholar]
- Cheng, D. T. , Knight, D. C. , Smith, C. N. , & Helmstetter, F. J. (2006). Human amygdala activity during the expression of fear responses. Behavioral Neuroscience, 120, 1187–1195. [DOI] [PubMed] [Google Scholar]
- Cheng, D. T. , Richards, J. , & Helmstetter, F. J. (2007). Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory, 14, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. M. , Padmala, S. , & Pessoa, L. (2012). Impact of state anxiety on the interaction between threat monitoring and cognition. NeuroImage, 59, 1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. S. , & Kim, J. J. (2010). Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences of the United States of America, 107, 21773–21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi, S. , Herry, C. , Grenier, F. , Wolff, S. B. , Letzkus, J. J. , Vlachos, I. , … Luthi, A. (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468, 277–282. [DOI] [PubMed] [Google Scholar]
- Cloos, J. M. , & Ferreira, V. (2009). Current use of benzodiazepines in anxiety disorders. Current Opinion in Psychiatry, 22(1), 90–95. [DOI] [PubMed] [Google Scholar]
- Costafreda, S. G. , Brammer, M. J. , David, A. S. , & Fu, C. H. (2008). Predictors of amygdala activation during the processing of emotional stimuli: a meta‐analysis of 385 PET and fMRI studies. Brain Research Reviews, 58, 57–70. [DOI] [PubMed] [Google Scholar]
- Coulombe, M. A. , Erpelding, N. , Kucyi, A. , & Davis, K. D. (2016). Intrinsic functional connectivity of periaqueductal gray subregions in humans. Human Brain Mapping, 37, 1514–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Craske, M. G. , Stein, M. B. , Eley, T. C. , Milad, M. R. , Holmes, A. , Rapee, R. M. , & Wittchen, H. U. (2017). Anxiety disorders. Nature Reviews. Disease Primers, 3, 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers, H. R. , Wager, T. D. , & Yarkoni, T. (2017). The relation between statistical power and inference in fMRI. PLoS One, 12, e0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers, P. , Cristea, I. A. , Karyotaki, E. , Reijnders, M. , & Huibers, M. J. (2016). How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry, 15, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. , Walker, D. L. , Miles, L. , & Grillon, C. (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 35, 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. , & Whalen, P. J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- de la Vega, A. , Chang, L. J. , Banich, M. T. , Wager, T. D. , & Yarkoni, T. (2016). Large‐scale meta‐analysis of human medial frontal cortex reveals tripartite functional organization. The Journal of Neuroscience, 36, 6553–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCampo, D. M. , & Fudge, J. L. (2013). Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: comparison with ventral striatal afferents. Journal of Comparative Neurology, 521, 3191–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R. S. , Ségonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31, 968–980. [DOI] [PubMed] [Google Scholar]
- DiLuca, M. , & Olesen, J. (2014). The cost of brain diseases: a burden or a challenge?. Neuron, 82, 1205–1208. [DOI] [PubMed] [Google Scholar]
- Ehrlich, I. , Humeau, Y. , Grenier, F. , Ciocchi, S. , Herry, C. , & Luthi, A. (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron, 62, 757–771. [DOI] [PubMed] [Google Scholar]
- Entis, J. J. , Doerga, P. , Barrett, L. F. , & Dickerson, B. C. (2012). A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra‐high resolution MRI. Neuroimage, 60, 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok, J. P. , Krabbe, S. , Markovic, M. , Courtin, J. , Xu, C. , Massi, L. , … Luthi, A. (2017). A competitive inhibitory circuit for selection of active and passive fear responses. Nature, 542, 96–100. [DOI] [PubMed] [Google Scholar]
- Faull, O. K. , & Pattinson, K. T. (2017). The cortical connectivity of the periaqueductal gray and the conditioned response to the threat of breathlessness. Elife, 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein, G. , Landman, B. , Tran, H. , Barakos, J. , Moon, K. , Di Sclafani, V. , & Shumway, R. (2006). Statistical parametric mapping of brain morphology: sensitivity is dramatically increased by using brain‐extracted images as inputs. Neuroimage, 30, 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein, J. S. , Adolphs, R. , Damasio, A. , & Tranel, D. (2011). The human amygdala and the induction and experience of fear. Current Biology, 21, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein, J. S. , Adolphs, R. , & Tranel, D. (2016). A tale of survival from the world of Patient S.M In Amaral D. G. & Adolphs R. (Eds.), Living without an amygdala. New York: Guilford. [Google Scholar]
- Fischmeister, F. P. , Hollinger, I. , Klinger, N. , Geissler, A. , Wurnig, M. C. , Matt, E. , … Beisteiner, R. (2013). The benefits of skull stripping in the normalization of clinical fMRI data. Neuroimage. Clinical, 3, 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. S. , & Kalin, N. H. (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry, 171, 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. S. , Lapate, R. C. , Davidson, R. J. , & Shackman, A. J. (2018). The nature of emotion: A research agenda for the 21st century In Fox A. S., Lapate R. C., Shackman A. J., & Davidson R. J. (Eds.), The nature of emotion. Fundamental questions (2nd ed). New York: Oxford University Press. [Google Scholar]
- Fox, A. S. , Oler, J. A. , Shackman, A. J. , Shelton, S. E. , Raveendran, M. , McKay, D. R. , … Kalin, N. H. (2015). Intergenerational neural mediators of early‐life anxious temperament. Proceedings of the National Academy of Sciences Usa, 112, 9118–9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. S. , Oler, J. A. , Tromp, D. P. , Fudge, J. L. , & Kalin, N. H. (2015). Extending the amygdala in theories of threat processing. Trends in Neurosciences, 38, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. S. , & Shackman, A. J. in press. The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. S. , Shelton, S. E. , Oakes, T. R. , Converse, A. K. , Davidson, R. J. , & Kalin, N. H. (2010). Orbitofrontal cortex lesions alter anxiety‐related activity in the primate bed nucleus of stria terminalis. Journal of Neuroscience, 30, 7023–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. S. , Shelton, S. E. , Oakes, T. R. , Davidson, R. J. , & Kalin, N. H. (2008). Trait‐like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE, 3, e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, J. A. , Chiu, S. , Breeze, J. L. , Makris, N. , Lange, N. , Kennedy, D. N. , … Biederman, J. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry, 162, 1256–1265. [DOI] [PubMed] [Google Scholar]
- Freese, J. L. , & Amaral, D. G. (2009). Neuroanatomy of the primate amygdala In Whalen P. J. & Phelps E. A. (Eds.), The human amygdala (pp. 3–42. NY: Guilford. [Google Scholar]
- Fudge, J. L. , Kelly, E. A. , Pal, R. , Bedont, J. L. , Park, L. , & Ho, B. (2017). Beyond the classic VTA: Extended amygdala projections to DA‐striatal paths in the primate. Neuropsychopharmacology, 42, 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana, M. A. , Harrison, B. J. , Soriano‐Mas, C. , Vervliet, B. , Cardoner, N. , Avila‐Parcet, A. , & Radua, J. (2016). Neural signatures of human fear conditioning: an updated and extended meta‐analysis of fMRI studies. Molecular Psychiatry, 21, 500–508. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli, P. , Placentino, A. , Carletti, F. , Landi, P. , Allen, P. , Surguladze, S. , … Politi, P. (2009). Functional atlas of emotional faces processing: a voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34, 418–432. [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Collaborators . (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388, 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, J. M. , Seidman, L. J. , Makris, N. , Ahern, T. , O'brien, L. M. , Caviness, V. S., Jr. , … Tsuang, M. T. (2007). Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biological Psychiatry, 61, 935–945. [DOI] [PubMed] [Google Scholar]
- Goode, T. D. , & Maren, S. (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learning &Amp; Memory (Cold Spring Harbor, N.Y.), 24, 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka, A. X. , Torrisi, S. , Shackman, A. J. , Grillon, C. , & Ernst, M. (2017). Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson, D. S. , Bliss‐Moreau, E. , Machado, C. J. , Bennett, J. , Shen, K. , Grant, K. A. , … Amaral, D. G. (2016). The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron, 91, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N. , & Fischl, B. (2009). Accurate and robust brain image alignment using boundary‐based registration. Neuroimage, 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel, G. , & Holmes, A. (2013). 50 years of hurdles and hope in anxiolytic drug discovery. Nature Reviews. Drug Discovery, 12, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe, D. W. , & Nitschke, J. B. (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14, 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe, D. W. , Oathes, D. J. , & Nitschke, J. B. (2013). Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex, 23, 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor, N. Z. , & Paré, D. (2016). Functional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience, 36, 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist, M. N. , Hwang, K. , & Luna, B. (2013). The nuisance of nuisance regression: spectral misspecification in a common approach to resting‐state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage, 82, 208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. , Soleiman, M. T. , Soden, M. E. , Zweifel, L. S. , & Palmiter, R. D. (2015). Elucidating an affective pain circuit that creates a threat memory. Cell, 162, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, M. J. , Boehme, S. , Becker, M. P. , Tupak, S. V. , Guhn, A. , Schmidt, B. , … Straube, T. (2016). Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping, 37, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, A. J. , Hollinshead, M. O. , O'keefe, T. M. , Petrov, V. I. , Fariello, G. R. , Wald, L. L. , … Buckner, R. L. (2015). Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Scientific Data, 2, 150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrybouski, S. , Aghamohammadi‐Sereshki, A. , Madan, C. R. , Shafer, A. T. , Baron, C. A. , Seres, P. , … Malykhin, N. V. (2016). Amygdala subnuclei response and connectivity during emotional processing. Neuroimage, 133, 98–110. [DOI] [PubMed] [Google Scholar]
- Iglesias, J. E. , Liu, C. Y. , Thompson, P. , & Tu, Z. (2011). Robust brain extraction across datasets and comparison with publicly available methods. IEEE Transactions on Medical Imaging, 30, 1617–1634. [DOI] [PubMed] [Google Scholar]
- Izquierdo, A. , Suda, R. K. , & Murray, E. A. (2005). Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience, 25(37), 8534–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, A. C. , James, G. , Cowdrey, F. A. , Soler, A. , & Choke, A. (2015). Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev, CD004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, H. J. , Gotts, S. J. , Reynolds, R. C. , Bandettini, P. A. , Martin, A. , Cox, R. W. , & Saad, Z. S. (2013). Effective preprocessing procedures virtually eliminate distance‐dependent motion artifacts in resting state FMRI. Journal of Applied Mathematics, 2013, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, J. B. (1923). Further contributions to the study of the evolution of the forebrain. The Journal of Comparative Neurology, 35, 337–481. [Google Scholar]
- Kaczkurkin, A. N. , Moore, T. M. , Ruparel, K. , Ciric, R. , Calkins, M. E. , Shinohara, R. T. , … Satterthwaite, T. D. (2016). Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biological Psychiatry, 80, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin, N. H. (2017). Mechanisms underlying the early risk to develop anxiety and depression: A translational approach. European Neuropsychopharmacology, 27, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin, N. H. , Fox, A. S. , Kovner, R. , Riedel, M. K. , Fekete, E. M. , Roseboom, P. H. , … Oler, J. A. (2016). Overexpressing corticotropin‐releasing hormone in the primate amygdala increases anxious temperament and alters its neural circuit. Biological Psychiatry, 80, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin, N. H. , Shelton, S. E. , & Davidson, R. J. (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 24, 5506–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin, N. H. , Shelton, S. E. , & Davidson, R. J. (2007). Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry, 62, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin, N. H. , Shelton, S. E. , Fox, A. S. , Oakes, T. R. , & Davidson, R. J. (2005). Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry, 58, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali, A. , Sair, H. I. , Blitz, A. M. , Riascos, R. F. , Mirbagheri, S. , Keser, Z. , & Hasan, K. M. (2016). Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Structure and Function, 221, 3561–3569. [DOI] [PubMed] [Google Scholar]
- Kamali, A. , Yousem, D. M. , Lin, D. D. , Sair, H. I. , Jasti, S. P. , Keser, Z. , … Hasan, K. M. (2015). Mapping the trajectory of the stria terminalis of the human limbic system using high spatial resolution diffusion tensor tractography. Neuroscience Letters, 608, 45–50. [DOI] [PubMed] [Google Scholar]
- Klein, A. , Andersson, J. , Ardekani, B. A. , Ashburner, J. , Avants, B. , Chiang, M. C. , … Parsey, R. V. (2009). Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage, 46, 786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers, F. , Kroes, M. C. , Heitland, I. , Everaerd, D. , Akkermans, S. E. , Oosting, R. S. , … Baas, J. M. (2015). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry, 78, 582–589. [DOI] [PubMed] [Google Scholar]
- Knight, D. C. , Nguyen, H. T. , & Bandettini, P. A. (2005). The role of the human amygdala in the production of conditioned fear responses. NeuroImage, 26, 1193–1200. [DOI] [PubMed] [Google Scholar]
- Korn, C. W. , Vunder, J. , Miró, J. , Fuentemilla, L. , Hurlemann, R. , & Bach, D. R. (2017). Amygdala lesions reduce anxiety‐like behavior in a human benzodiazepine‐sensitive approach‐avoidance conflict test. Biological Psychiatry, 82, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel, P. A. , Knodt, A. R. , Hariri, A. R. , & LaBar, K. S. (2016). Decoding Spontaneous Emotional States in the Human Brain. PLoS Biology, 14, e2000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel, P. A. , & LaBar, K. S. (2015). Multivariate neural biomarkers of emotional states are categorically distinct. Social Cognitive and Affective Neuroscience, 10, 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar, K. S. , Gatenby, J. C. , Gore, J. C. , LeDoux, J. E. , & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixed‐trial fMRI study. Neuron, 20, 937–945. [DOI] [PubMed] [Google Scholar]
- Lange, M. D. , Daldrup, T. , Remmers, F. , Szkudlarek, H. J. , Lesting, J. , Guggenhuber, S. , … Pape, H. C. (2017). Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Molecular Psychiatry, 22, 1422–1430. [DOI] [PubMed] [Google Scholar]
- LeDoux, J. E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux, J. E. (2007). The amygdala. Current Biology : Cb, 17, R868–R874. [DOI] [PubMed] [Google Scholar]
- Li, H. , Penzo, M. A. , Taniguchi, H. , Kopec, C. D. , Huang, Z. J. , & Li, B. (2013). Experience‐dependent modification of a central amygdala fear circuit. Nature Neuroscience, 16, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, K. A. , Satpute, A. B. , Wager, T. D. , Weber, J. , & Barrett, L. F. (2016). The brain basis of positive and negative affect: Evidence from a meta‐analysis of the human neuroimaging literature. Cerebral Cortex, 26, 1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453, 869–878. [DOI] [PubMed] [Google Scholar]
- Mai, J. K. , Majtanik, M. , & Paxinos, G. (2015). Atlas of the human brain (4th ed). San Diego, CA: Academic Press. [Google Scholar]
- Mai, J. K. , Paxinos, G. , & Voss, T. (2007). Atlas of the human brain (3rd ed.). San Diego, CA: Academic Press. [Google Scholar]
- Makris, N. , Goldstein, J. M. , Kennedy, D. , Hodge, S. M. , Caviness, V. S. , Faraone, S. V. , … Seidman, L. J. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research, 83, 155–171. [DOI] [PubMed] [Google Scholar]
- Mason, W. A. , Capitanio, J. P. , Machado, C. J. , Mendoza, S. P. , & Amaral, D. G. (2006). Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): generality and individual consistency of effects. Emotion, 6, 73–81. [DOI] [PubMed] [Google Scholar]
- McMenamin, B. W. , Langeslag, S. J. , Sirbu, M. , Padmala, S. , & Pessoa, L. (2014). Network organization unfolds over time during periods of anxious anticipation. Journal of Neuroscience, 34, 11261–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias, M. L. , Etkin, A. , & Kalisch, R. (2010). A meta‐analysis of instructed fear studies: implications for conscious appraisal of threat. NeuroImage, 49, 1760–1768. [DOI] [PubMed] [Google Scholar]
- Meyer, C. H. , Padmala, S. , & Pessoa, L. (2017). Tracking dynamic threat imminence. bioRxiv.
- Mobbs, D. , Marchant, J. L. , Hassabis, D. , Seymour, B. , Tan, G. , Gray, M. , … Frith, C. D. (2009). From threat to fear: the neural organization of defensive fear systems in humans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29, 12236–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs, D. , Petrovic, P. , Marchant, J. L. , Hassabis, D. , Weiskopf, N. , Seymour, B. , … Frith, C. D. (2007). When fear is near: threat imminence elicits prefrontal‐periaqueductal gray shifts in humans. Science, 317, 1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs, D. , Yu, R. , Rowe, J. B. , Eich, H. , FeldmanHall, O. , & Dalgleish, T. (2010). Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Acadademy of Sciences Usa, 107, 20582–20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta, S. C. , Carobrez, A. P. , & Canteras, N. S. (2017). The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neuroscience and Biobehavioral Reviews, 76, 39–47. [DOI] [PubMed] [Google Scholar]
- Motzkin, J. C. , Philippi, C. L. , Oler, J. A. , Kalin, N. H. , Baskaya, M. K. , & Koenigs, M. (2015). Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex, 64, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münsterkötter, A. L. , Notzon, S. , Redlich, R. , Grotegerd, D. , Dohm, K. , Arolt, V. , … Dannlowski, U. (2015). Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depression and Anxiety, 32, 656–663. [DOI] [PubMed] [Google Scholar]
- Nacewicz, B. M. , Alexander, A. L. , Kalin, N. H. , & Davidson, R. J. (2014). The neurochemical underpinnings of human amygdala volume including subregional contributions. Biological Psychiatry, 75, S222. [Google Scholar]
- Najafi, M. , Kinnison, J. , & Pessoa, L. (2017). Intersubject brain network organization during dynamic anxious anticipation. Frontiers in Human Neuroscience, 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold, B. S. , Wilson, W. P. , & Slaughter, D. G. (1969). Sensations evoked by stimulation in the midbrain of man. Journal of Neurosurgery, 30, 14–24. [DOI] [PubMed] [Google Scholar]
- Nauta, W. J. (1961). Fibre degeneration following lesions of the amygdaloid complex in the monkey. Journal of Anatomy, 95, 515–531. [PMC free article] [PubMed] [Google Scholar]
- Nichols, T. , Brett, M. , Andersson, J. , Wager, T. , & Poline, J. B. (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25(3), 653–660. [DOI] [PubMed] [Google Scholar]
- Nooner, K. B. , Colcombe, S. J. , Tobe, R. H. , Mennes, M. , Benedict, M. M. , Moreno, A. L. , … Milham, M. P. (2012). The NKI‐Rockland sample: A model for accelerating the pace of discovery science in psychiatry. Frontiers in Neuroscience, 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa, L. , & Saarimaki, H. (2017). Emotions as discrete patterns of systemic activity. Neuroscience Letters. [DOI] [PubMed] [Google Scholar]
- Oler, J. A. , Birn, R. M. , Patriat, R. , Fox, A. S. , Shelton, S. E. , Burghy, C. A. , … Kalin, N. H. (2012). Evidence for coordinated functional activity within the extended amygdala of non‐human and human primates. Neuroimage, 61, 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler, J. A. , Fox, A. S. , Shackman, A. J. , & Kalin, N. H. (2016). The central nucleus of the amygdala is a critical substrate for individual differences in anxiety In Amaral D. G. & Adolphs R. (Eds.), Living without an amygdala. NY: Guilford. [Google Scholar]
- Oler, J. A. , Fox, A. S. , Shelton, S. E. , Rogers, J. , Dyer, T. D. , Davidson, R. J. , … Kalin, N. H. (2010). Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature, 466, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler, J. A. , Tromp, D. P. , Fox, A. S. , Kovner, R. , Davidson, R. J. , Alexander, A. L. , … Fudge, J. L. (2017). Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non‐human primate: neuronal tract tracing and developmental neuroimaging studies. Brain Structure and Function, 222, 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur, D. , & Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10, 206–219. [DOI] [PubMed] [Google Scholar]
- Ongur, D. , Ferry, A. T. , & Price, J. L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology, 460, 425–449. [DOI] [PubMed] [Google Scholar]
- Pare, D. , & Duvarci, S. (2012). Amygdala microcircuits mediating fear expression and extinction. Current Opinion in Neurobiology, 22, 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, W. S. , Balderston, N. L. , Miskovich, T. A. , Belleau, E. L. , Helmstetter, F. J. , & Larson, C. L. (2017). The effects of stimulus novelty and negativity on BOLD activity in the amygdala, hippocampus, and bed nucleus of the stria terminalis. Soc Cogn Affect Neurosci, 12, 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo, M. A. , Robert, V. , & Li, B. (2014). Fear conditioning potentiates synaptic transmission onto long‐range projection neurons in the lateral subdivision of central amygdala. Journal of Neuroscience, 34, 2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo, M. A. , Robert, V. , Tucciarone, J. , De Bundel, D. , Wang, M. , Van Aelst, L. , … Li, B. (2015). The paraventricular thalamus controls a central amygdala fear circuit. Nature, 519, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa, L. (2017). A network model of the emotional brain. Trends in Cognitive Sciences, 21, 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack, R. A. , Baker, C. I. , Durnez, J. , Gorgolewski, K. J. , Matthews, P. M. , Munafo, M. R. , … Yarkoni, T. (2017). Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience, 18, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Schlaggar, B. L. , & Petersen, S. E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, D. E. , & Akil, H. (1977). Pain reduction by electrical brain stimulation inman. Part 1: Acute administration in periaqueductal and periventricular sites. Journal of Neurosurgery, 47, 178–183. [DOI] [PubMed] [Google Scholar]
- Roesch, M. R. , Esber, G. R. , Li, J. , Daw, N. D. , & Schoenbaum, G. (2012). Surprise! Neural correlates of Pearce‐Hall and Rescorla‐Wagner coexist within the brain. European Journal of Neuroscience, 35, 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck, P. H. , Saunders, R. C. , Prescott, A. T. , Chau, L. S. , & Murray, E. A. (2013). Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience, 16, 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli, D. , Fortune, E. E. , Li, Q. , Siddiqui, A. , Krafft, C. , Oliver, W. T. , … Jeffries, J. (2011). Emotional perception: Meta‐analyses of face and natural scene processing. NeuroImage, 54, 2524–2533. [DOI] [PubMed] [Google Scholar]
- Salomon, J. A. , Haagsma, J. A. , Davis, A. , de Noordhout, C. M. , Polinder, S. , Havelaar, A. H. , … Vos, T. (2015). Disability weights for the Global Burden of Disease 2013 study. The Lancet. Global Health, 3, e712–e723. [DOI] [PubMed] [Google Scholar]
- Sato, M. , Ito, M. , Nagase, M. , Sugimura, Y. K. , Takahashi, Y. , Watabe, A. M. , & Kato, F. (2015). The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Molecular Brain, 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute, A. B. , Wager, T. D. , Cohen‐Adad, J. , Bianciardi, M. , Choi, J. K. , Buhle, J. T. , … Barrett, L. F. (2013). Identification of discrete functional subregions of the human periaqueductal gray. Proceedings of the National Academy of Sciences of the United States of America, 110, 17101–17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie, K. , Chochol, C. , & Armony, J. L. (2008). The role of the amygdala in emotional processing: a quantitative meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32, 811–830. [DOI] [PubMed] [Google Scholar]
- Shackman, A. J. , & Fox, A. S. (2016). Contributions of the central extended amygdala to fear and anxiety. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 36, 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , & Fox, A. S. (2018). How are emotions organized in the brain?. In Fox A. S., Lapate R. C., Shackman A. J., & Davidson R. J. (Eds.), The nature of emotion. Fundamental questions (2nd ed). New York, NY: Oxford University Press. [Google Scholar]
- Shackman, A. J. , Fox, A. S. , Oler, J. A. , Shelton, S. E. , Davidson, R. J. , & Kalin, N. H. (2013). Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America, 110, 6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , Fox, A. S. , Oler, J. A. , Shelton, S. E. , Oakes, T. R. , Davidson, R. J. , & Kalin, N. H. (2017). Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety‐related psychopathology. Molecular Psychiatry, 22, 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , Fox, A. S. , & Seminowicz, D. A. (2015). The cognitive‐emotional brain: Opportunities and challenges for understanding neuropsychiatric disorders. Behavioral and Brain Sciences, 38, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , Kaplan, C. M. , Stockbridge, M. D. , Tillman, R. M. , Tromp, D. P. M. , Fox, A. S. , & Gamer, M. (2016). The neurobiology of anxiety and attentional biases to threat: Implications for understanding anxiety disorders in adults and youth. Journal of Experimental Psychopathology, 7, 311–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , McMenamin, B. W. , Maxwell, J. S. , Greischar, L. L. , & Davidson, R. J. (2009). Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science, 20, 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , Salomons, T. V. , Slagter, H. A. , Fox, A. S. , Winter, J. J. , & Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , Tromp, D. P. M. , Stockbridge, M. D. , Kaplan, C. M. , Tillman, R. M. , & Fox, A. S. (2016). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142, 1275–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck, D. W. , Sandor‐Leahy, S. R. , Schaper, K. A. , Rottenberg, D. A. , & Leahy, R. M. (2001). Magnetic resonance image tissue classification using a partial volume model. Neuroimage, 13, 856–876. [DOI] [PubMed] [Google Scholar]
- Šidák, Z. K. (1967). Rectangular confidence regions for the means of multivariate normal distributions. Journal of the American Statistical Association, 62, 626–633. [Google Scholar]
- Siegel, J. S. , Power, J. D. , Dubis, J. W. , Vogel, A. C. , Church, J. A. , Schlaggar, B. L. , & Petersen, S. E. (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high‐motion data points. Human Brain Mapping, 35, 1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky, R. , Geissberger, N. , Pfabigan, D. M. , Kraus, C. , Tik, M. , Woletz, M. , … Windischberger, C. (2017). Unsmoothed functional MRI of the human amygdala and bed nucleus of the stria terminalis during processing of emotional faces. Neuroimage, [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, L. H. , Wagner, D. D. , Wig, G. S. , Moran, J. M. , Whalen, P. J. , & Kelley, W. M. (2013). Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex, 23, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, L. H. , Whalen, P. J. , & Kelley, W. M. (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry, 68, 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer, J. , Lohmann, G. , Mueller, K. , Buschmann, T. , & Turner, R. (2014). Deficient approaches to human neuroimaging. Frontiers in Human Neuroscience, 8, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, J. S. , Kim, Y. J. , Galatzer‐Levy, I. R. , Reddy, R. , Ely, T. D. , Nemeroff, C. B. , … Ressler, K. J. (2017). Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biological Psychiatry, 81, 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, T. (2007). On the asymptotic bias of OLS in dynamic regression models with autocorrelated errors. Statistical Papers, 48, 81–93. [Google Scholar]
- Stout, D. M. , Shackman, A. J. , Pedersen, W. S. , Miskovich, T. A. , & Larson, C. L. (2017). Neural circuitry governing anxious individuals’ mis‐allocation of working memory to threat. Scientific Reports, 7, 8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss, J. D. , Ridgewell, C. , McHugo, M. , Heckers, S. , & Blackford, J. U. (2017). Manual segmentation of the human bed nucleus of the stria terminalis using 3T MRI. NeuroImage, 146, 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi, S. , O'connell, K. , Davis, A. , Reynolds, R. , Balderston, N. , Fudge, J. L. , … Ernst, M. (2015). Resting state connectivity of the bed nucleus of the stria terminalis at ultra‐high field. Human Brain Mapping, 36, 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote, P. , Esposito, M. S. , Botta, P. , Chaudun, F. , Fadok, J. P. , Markovic, M. , … Luthi, A. (2016). Midbrain circuits for defensive behaviour. Nature, 534, 206–212. [DOI] [PubMed] [Google Scholar]
- Tovote, P. , Fadok, J. P. , & Luthi, A. (2015). Neuronal circuits for fear and anxiety. Nature Reviews. Neuroscience, 16, 317–331. [DOI] [PubMed] [Google Scholar]
- Turner, R. , & Geyer, S. (2014). Comparing like with like: the power of knowing where you are. Brain Connectivity, 4, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison, N. J. , Cook, P. A. , Klein, A. , Song, G. , Das, S. R. , Duda, J. T. , … Avants, B. B. (2014). Large‐scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage, 99, 166–179. [DOI] [PubMed] [Google Scholar]
- Tyszka, J. M. , & Pauli, W. M. (2016). In vivo delineation of subdivisions of the human amygdaloid complex in a high‐resolution group template. Human Brain Mapping, 37, 3979–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q. , Kinnison, J. , Pessoa, L. , & Anderson, M. L. (2014). Beyond the tripartite cognition‐emotion‐interoception model of the human insular cortex. Journal of Cognitive Neuroscience, 26, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Well, S. , Visser, R. M. , Scholte, H. S. , & Kindt, M. (2012). Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear‐potentiated startle, and US‐expectancy data. Cognitive, Affective, & Behavioral Neuroscience, 12, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Kang, J. , Johnson, T. D. , Nichols, T. E. , Satpute, A. B. , & Barrett, L. F. (2015). A Bayesian model of category‐specific emotional brain responses. PLoS Computational Biology, 11, e1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert, J. S. , Mahn, M. , Prigge, M. , Printz, Y. , & Yizhar, O. (2017). Silencing neurons: Tools, applications, and experimental constraints. Neuron, 95, 504–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, L. E. , Oler, J. A. , Fox, A. S. , McFarlin, D. R. , Rogers, G. M. , Jesson, M. A. , … Kalin, N. H. (2015). Fear of the unknown: Uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology, 40, 1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, R. A. , & Koob, G. F. (2014). The development and maintenance of drug addiction. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 39, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, K. H. , Ver Hoef, L. W. , & Knight, D. C. (2014). The amygdala mediates the emotional modulation of threat‐elicited skin conductance response. Emotion (Washington, D.C.), 14, 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer‐Hanke, D. M. (2012). Amygdala In Mai J. K. & Paxinos G. (Eds.), The human nervous system (pp. 759–834. San Diego: Academic Press. [Google Scholar]
- Yu, K. , Ahrens, S. , Zhang, X. , Schiff, H. , Ramakrishnan, C. , Fenno, L. , … Li, B. (2017). The central amygdala controls learning in the lateral amygdala. Nature Neuroscience, 20, 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Table 1
Data Availability Statement
Key statistical maps are available in NeuroVault.org. Raw data are publicly available (http://fcon_1000.projects.nitrc.org/indi/enhanced/).


