Abstract
Anxiety disorders and nicotine use are significant contributors to global morbidity and mortality as independent and comorbid diseases. Early-life stress, potentially via stress-induced hypothalamic-pituitary-adrenal axis (HPA) dysregulation, can exacerbate both. However, little is known about the factors that predispose individuals to the development of both anxiety disorders and nicotine use. Here, we examined the relationship between anxiety-like behaviors and nicotine responses following adolescent stress. Adolescent male and female BALB/cJ mice were exposed to either chronic variable social stress (CVSS) or control conditions. CVSS consisted of repeated cycles of social isolation and social reorganization. In adulthood, anxiety-like behavior and social avoidance were measured using the elevated plus-maze (EPM) and social approach-avoidance test, respectively. Nicotine responses were assessed with acute effects on body temperature, corticosterone production, locomotor activity, and voluntary oral nicotine consumption. Adolescent stress had sex-dependent effects on nicotine responses and exploratory behavior, but did not affect anxiety-like behavior or social avoidance in males or females. Adult CVSS males exhibited less exploratory behavior, as indicated by reduced exploratory locomotion in the EPM and social approach-avoidance test, compared to controls. Adolescent stress did not affect nicotine-induced hypothermia in either sex, but CVSS males exhibited augmented nicotine-induced locomotion during late adolescence and voluntarily consumed less nicotine during adulthood. Stress effects on male nicotine-induced locomotion were associated with individual differences in exploratory locomotion in the EPM and social approach-avoidance test. Relative to controls, adult CVSS males and females also exhibited reduced corticosterone levels at baseline and adult male CVSS mice exhibited increased corticosterone levels following an acute nicotine injection. Results suggest that the altered nicotine responses observed in CVSS males may be associated with HPA dysregulation. Taken together, adolescent social stress influences later-life nicotine responses and exploratory behavior. However, there is little evidence of an association between nicotine responses and prototypical anxiety-like behavior or social avoidance in BALB/cJ mice.
Keywords: adolescent, social stress, social avoidance, anxiety, nicotine, BALB/cJ
1. Introduction
Tobacco use is the leading cause of preventable death in the United States. Approximately 480,000 Americans die each year as a result of smoking cigarettes (1). This public health problem does not exist in isolation; a strong bi-directional link has been established between anxiety disorders and nicotine use. Affective disorders, which include anxiety and depression, are the second leading cause of global disease burden with an annual economic cost of $210 billion in the United States alone (2, 3). Individuals who are diagnosed with an anxiety disorder are at higher risk of smoking compared to those without (4, 5). Conversely, higher risk of anxiety diagnosis and greater symptom severity are reported in smokers compared to non-smokers (6–8). However, it is unclear what factors promote the development of anxiety disorders and nicotine use comorbidity. Use of tobacco products could precipitate dysregulated mood (9). However, acute nicotine use has anxiolytic effects whereas nicotine withdrawal has been associated with increased anxiety (10). Thus, smoking could serve as a form of self-medication to ameliorate anxiety symptoms (11). More research is needed to investigate factors that predispose individuals to the development of both anxiety disorders and nicotine use.
Adolescence is a developmental period that is associated with a number of vulnerabilities. For example, problem nicotine use almost always begins in adolescence when there is a concomitant rise in the incidence of anxiety disorders (12, 13). Chronic stress may be an important mediator of both anxiety disorders and nicotine use. Adolescent stress exposure can precipitate anxiety (14) and predicts the initiation, degree, and continuation of smoking (15, 16). Prospective clinical studies have attributed these stress effects to elevated glucocorticoid (GC) hormones which are released following stress-induced activation of the hypothalamic-pituitary-adrenal axis (HPA) (16). Aberrant HPA activity may be particularly detrimental in adolescence because GCs play an important role in developmental programming of brain regions mediating both emotional behavior and reward processing (i.e., the mesolimbic dopamine system) (17–19). Thus, adolescent stress could predispose individuals to develop anxiety disorders and nicotine use by altering the development of biological processes involved in both conditions.
Rodent models of social stress have proved useful to investigate neurobiological factors mediating stress-induced anxiety disorders. In rodents, adolescence is often defined as the period between postnatal days (PND) 21–59 (20). We recently reported that inbred male and female BALB/cJ mice exposed to adolescent chronic variable social stress (CVSS; repeated cycles of individual housing and exposure to novel social partner) exhibit increased anxiety-like behavior when assessed on the elevated plus-maze (EPM) during adulthood (21). Adolescent CVSS was further associated with sex-specific behavioral effects. Adult females, but not males, exposed to adolescent CVSS exhibited increased depression-like behavior in the sucrose preference test (21). These findings are in agreement with other studies in which rats and mice exposed to repeated social instability (e.g., repeated exposure to novel social partners) or social defeat stress exhibited increased anxiety/depression-like behavior and social avoidance (i.e., a social anxiety-like behavior (22)) in adolescence and adulthood (23–27). Adolescent stress exposure can also increase, decrease, or have no effect on nicotine responses under certain conditions. For instance, adolescent social instability, social defeat, or chronic restraint stress had no effect on nicotine-induced locomotion or nicotine self-administration in male rats (28–32). However, exposure to social instability during mid-adolescence blunted locomotor sensitization to repeated nicotine injections in late adolescent (PND 58) female rats (30), had no effect at a lower dose in adulthood (PND 69) ~3.5 weeks after stress exposure (29), and augmented sensitization in adult (PND 80) females tested ~5 weeks after stress exposure (28).
Long-lasting HPA abnormalities frequently accompany adolescent stress-induced alterations in anxiety-like behavior and nicotine responses. For example, adolescent and adult rats and mice exposed to social instability stress exhibit elevated basal corticosterone (CORT) levels (23, 33). Curiously, our lab and others have also reported reduced HPA activity in adult males following adolescent social stress which may represent a protective mechanism to limit the deleterious effects of prolonged GC exposure (21, 34). Stress-induced alterations in the expression of corticotropin releasing-hormone and vasopressin, which regulate anxiety, HPA activity, and many pharmacological effects of nicotine have also been reported (23, 33, 35). To date, few studies have simultaneously investigated the influence of adolescent social stress on anxiety-like behavior, HPA activity, and nicotine responses.
In the current study, we sought to replicate and extend prior findings (21) by systematically assessing the effects of adolescent CVSS on anxiety-like behaviors, social avoidance, and nicotine responses. The CVSS protocol was previously developed to investigate sex-specific effects of adolescent stress in inbred mice (i.e., animals with limited genetic variability) (21). Here, a within-subjects experimental design was used to assess the relationship between anxiety-like behaviors and nicotine responses. Based on previous findings, we hypothesized that exposure to adolescent CVSS would: 1) increase anxiety-like behaviors in male and female mice, 2) decrease nicotine responses during late adolescence or increase responses during adulthood in female mice, and 3) result in a strong positive correlation between anxiety-like behaviors and nicotine responses within individuals.
2. Materials and methods
2.1. Animals
Male and female BALB/cJ mice (The Jackson Laboratory, Bar Harbor, ME, USA) were bred at The Pennsylvania State University. A total of 117 mice (51 females and 66 males) from 18 litters were used in the current study. Pups remained with the dam until weaning on postnatal day (PND) 21 and then were housed with 3–4 same-sex cagemates. Mice were housed in polycarbonate cages (28 cm × 17 cm × 12 cm) with corn-cob bedding in a temperature-controlled vivarium. Mice were maintained on a reverse 12:12 h light:dark schedule (lights on 13:00 h) with ad libitum food and water. All procedures were approved by The Pennsylvania State University IACUC committee. Mice were randomly assigned to either CVSS or control (CON) conditions (see section 2.3). Littermates were evenly distributed between groups in order to avoid bias due to litter effects.
2.2. Experimental design
Experiment 1 (Fig. 1) was designed to determine whether adolescent CVSS would induce sex-specific changes in late adolescent nicotine responses as well as adult anxiety-like behavior, social avoidance, and nicotine intake (n = 11–15/group). Specifically, we assessed nicotine-induced hypothermia and locomotor activity during late adolescence (PND 56–59) and voluntary oral nicotine consumption in adulthood (PND 116–135). Adult anxiety-like behavior and social avoidance were tested in the EPM (PND 62–65) and the social approach-avoidance test (PND 140–144), respectively.
Figure 1.
Diagram illustrating experimental timelines. PND = Postnatal day; CVSS = Chronic Variable Social Stress
Experiment 2 (Fig. 1) was designed to determine whether adolescent CVSS would induce sex-specific changes in adult nicotine responses, anxiety-like behavior, and social avoidance (n = 9–12/group). In this experiment, acute nicotine responses were assessed in adulthood because prior studies reported that adolescent social stress can either reduce or augment nicotine locomotor sensitization when tested during late adolescence or adulthood, respectively (28, 30). Acute nicotine-induced hypothermia and corticosterone (CORT) production (PND 65–70) as well as voluntary oral nicotine consumption (PND 73–92) were measured during adulthood. As in Experiment 1, adult anxiety-like behavior and social avoidance were assessed in the EPM (PND 61–63) and social approach-avoidance test (PND 95–97), respectively.
2.3. Chronic variable social stress (CVSS) protocol
Mice in the stress condition were exposed to CVSS during adolescence (PND 25–59) (21). CVSS consisted of repeated cycles of individual housing for 3 days followed by re-socialization with 1–2 unfamiliar same-sex cagemates (i.e., social reorganization) for 4 days. The social reorganization schedule was designed such that the likelihood of a repeated encounter of the same mouse during the course of CVSS was minimized. CON mice remained housed with their original same-sex cagemates throughout the experiment. In order to limit differences in handling and husbandry between conditions, all CON mice were transferred to clean cages with their cagemates on days when CVSS mice were placed into individual housing or social reorganization. On PND 59, CVSS mice were re-housed with their original cagemates from weaning where they remained for the duration of the study unless otherwise specified (See section 2.4.2).
2.4. Behavioral testing
All behavioral testing except voluntary oral nicotine consumption (section 2.4.2) was performed in a room that was separate from the colony room. On the morning of testing, mouse tails were marked with Sharpie® marker for easy identification and to limit handling stress prior to testing. Mice were transported to the behavior room at least 1 h prior to testing to habituate to the environment.
2.4.1. Acute nicotine responses
The acute effects of nicotine on locomotor activity, body temperature, and HPA activity were examined using a within-subjects design as part of a modified test battery (36–38). Specifically, every animal in each experiment received intraperitoneal injections of both saline and nicotine (Experiment 1 – 0.5 mg/kg; Experiment 2 – 0.5 or 1 mg/kg; doses presented as freebase nicotine). In Experiment 1, a subset of mice (N = 11–12/group) were tested for acute nicotine responses. In both experiments, nicotine and saline injections were counter-balanced according to a Latin square design and testing sessions occurred 48 hours apart.
In Experiments 1 and 2, locomotor activity was examined under reduced anxiogenic conditions in a symmetrical Y-maze consisting of 3 red covered Plexiglas arms (27.5 L × 8 W × 10 H; cm). Testing was performed in a brightly lit room (~600 lux) between 13:00–16:00 h, but light intensity was low inside the Y-maze (~30 lux). Trials were recorded by an overhead camera and analyzed using an automated video tracking system (Any-maze v.4.60, Stoelting, Wood Dale, IL, USA). Immediately following injection, each mouse was placed into a Y-maze for 10 min. Locomotor activity was analyzed by measuring total distance traveled in the first 5 min of the test because near-maximal effects on Y-maze locomotion are observed 5 min after nicotine injection (36). Following locomotor activity testing, mice were returned to holding cages until rectal body temperature was measured 15 min after the injection. Body temperature was measured using a TH-5 Thermalert Monitoring Thermometer with a RET-3 mouse rectal probe (Physitemp Instruments Inc., Clifton, NJ, USA) lubricated by peanut oil.
The acute effects of nicotine on HPA activity were measured in Experiment 2. Mice were briefly restrained in a broom-style restrainer 30 and 90 min after the injection, a short segment (< 1 mm) of the tail tip was cut with a scalpel, and blood was collected into heparinized capillary tubes (RAM Scientific, Yonkers, NY, USA). Repeat blood samples were obtained by palpating or cutting the tail tip to re-stimulate blood flow. A final blood sample for baseline CORT was collected 3 days after the last saline/nicotine injection. Mice were transported in holding cages to a separate room for blood collection. All samples were collected within 3 minutes of initial cage disruption between 14:00–16:00 h.
Nicotine doses and testing times were based on published methods (36–38). Two primary dependent variables were used to assess acute nicotine responses: nicotine-induced change in locomotor activity (cm) and change in body temperature (°C). Both variables were calculated as a within subject change score by subtracting the saline response from the nicotine response. Thus, positive locomotion values represent greater nicotine-stimulated activity and negative temperature values represent nicotine-induced hypothermia. In Experiment 2, data for adult nicotine-induced locomotor activity was excluded due to a technical error. Three primary dependent variables were used to assess HPA activity: plasma CORT levels at 30 min post-injection, plasma CORT levels at 90 min post-injection, and baseline plasma CORT levels.
2.4.2. Two-bottle choice nicotine consumption
Nicotine intake was measured in a standard 2-bottle free choice paradigm (39, 40). Animals were singly housed in standard mouse cages for testing. Mice were provided access to two 25 ml graduated cylinders fitted with drinking spouts filled with water for the first two days to acclimate them to the test environment. At the start of nicotine testing, one tube of water was replaced with a tube containing 25 μg/ml nicotine. Nicotine drinking solutions were made of free-base nicotine (Sigma Aldrich, St. Louis, MO, USA) diluted in tap water (41). The volume of fluid in the tubes was recorded at approximately 14:00 h every day. The left/right location of the nicotine and water-containing bottles was switched every other day to control for side bias. Nicotine was presented in concentrations (25, 50, 100 and 200 μg/ml) that increased every 4 days. Mice were weighed every 4th day when a new concentration of nicotine was presented and consumption data were adjusted for body weight. Consumption data were adjusted for evaporation/leakage by measuring fluid loss from 4 empty cages that were handled the same as the experimental cages. Three primary dependent variables were obtained: nicotine consumption (mg/kg), nicotine preference (ml of nicotine/total ml of fluid), and total fluid consumed (ml). These dependent variables were derived from the average of days 2 and 4 of each nicotine concentration (i.e., the second full day after the bottle side or drug concentration was changed) (42).
2.4.3. Elevated plus-maze
Adult anxiety-like behavior was measured using the EPM as described previously (21). The 5 min test was conducted under dim red lights (~30 lux) between 9:00–12:00 h. The EPM was made from black Plexiglas and consisted of two open (30 L × 5 W; cm) and two closed arms (30 L × 14.5 H × 5 W; cm) elevated 42 cm off the ground. The maze was cleaned with 30% EtOH at the end of each trial. Three primary dependent variables were obtained open arm time, percent open arm entries, and number of closed arm entries. Reductions in percent time on and entries into the open arms represent unconditioned anxiety which is independent of locomotion (43, 44). A reduction in closed arm entries signifies reduced locomotor activity which may also be indicative of an anxiety-like state (43–45). Trials were recorded by an overhead camera and behavior was analyzed using an automated video tracking system (Any-maze v.4.60, Stoelting, Wood Dale, IL, USA). Arm entry was defined as 85% of the body passing the threshold of an arm. Percent open arm time was calculated as [Time on the open arms/(Time on open arms + Time on closed arms)] × 100. Percent open arm entries was calculated as [Total open arm entries/(Total open arm entries + Total closed arm entries)] × 100.
2.4.4. Social approach-avoidance test
Social avoidance was measured in the social approach-avoidance test during adulthood because social withdrawal is a common symptom of many human psychiatric disorders such as social anxiety disorder and depression (22). Adolescent social stress paradigms (e.g. social defeat, social isolation, and social instability) can induce social avoidance (22, 25–27) which can be reduced or prevented by anxiolytic drugs (22). Finally, prior studies have reported differences in the anxiogenic effects of adolescent social stress when animals were tested in a social interaction test compared to a non-social test (e.g. EPM or OFT) (22, 25, 27). In the current study, a modified version of the social approach-avoidance test (25, 26, 46) was employed. Briefly, trials were monitored by an overhead camera and a video tracking system (Any-maze v.4.60, Stoelting, Wood Dale, IL, USA) was used to analyze social avoidance and general locomotion in the arena (60 L × 60 L × 30 H; cm). Testing was performed under dim red lights (~30 lux) between 13:00–17:00 h. The test consisted of two consecutive 2.5 min trials during which mice were allowed to freely explore the arena. During the first trial (“Social Target Absent”) an empty circular wire mesh cage (9 cm in diameter) was located at one end of the field. During the second trial (“Social Target Present”) the empty cage was replaced with an identical cage containing a target mouse (an unfamiliar same-sex adult BALB/cJ mouse). In between trials, the test mouse was removed from the arena and placed back in the home cage for ~1 min. The primary dependent variables obtained were total distance traveled (cm), time spent in the interaction zone (5 cm corridor surrounding the cage), and time spent in the corner zones (8 × 8 cm) in the presence of the empty cage (“Social Target Absent”) or a novel conspecific (“Social Target Present”). Social avoidance was defined as a reduction in time spent in the social interaction zone during the second trial (“Social Target Present”) relative to the first trial (“Social Target Absent”).
2.5. Radioimmunoassay
Blood samples (20–50 μl) were obtained by tail cut (See section 2.4.1), collected into heparinized capillary tubes (RAM Scientific, Yonkers, NY, USA), and stored on ice. Samples were centrifuged at 4°C, plasma was collected and stored at −80°C until assayed. Plasma CORT levels were measured in duplicate using a commercially available [I125] radioimmunoassay kit (MP Biomedicals, Solon, OH, USA) according to the manufacturer’s instructions. Intra- and inter-assay coefficients of variation for low and high controls were 7.6 and 12.7, respectively. Additionally, area under the curve (AUC) (47) was calculated from the baseline, 30 min, and 90 min CORT levels to create an integrated measure of CORT response to each injection. CORT AUC was used for correlation analyses between EPM behavior, social approach-avoidance test behavior, and nicotine responses.
2.6. Statistical analysis
All statistical analyses were performed in R (v3.3.2). Dependent variables were analyzed using an analysis of variance (ANOVA) or a mixed factorial ANOVA with sex (male and female), stress condition (CVSS and CON), nicotine dose (Saline, 0.5 mg/kg, and 1.0 mg/kg), nicotine concentration (25 μg/ml, 50 μg/ml, 100 μg/ml, and 200 μg/ml), time since injection (30 min and 90 min), or social target presence (social target absent and social target present) as possible independent variables. Dependent variables were checked for normality and outliers removed (± 1.5 * interquartile range) or data were log transformed, where appropriate, to meet requirements for parametric statistical analyses. Continuous litter mean values were calculated for each dependent variable and included as covariates in all statistical models to control for litter effects. Data in Experiments 1 and 2 were combined for analysis of EPM behaviors, and social approach-avoidance test behaviors with experimental cohort included as a factor in the statistical model. Nicotine responses were analyzed separately for each experiment because previous studies have shown that the acute effects of nicotine differ considerably in adolescent and adult rodents and adolescent nicotine exposure can alter subsequent adult nicotine responses (48–51). Whenever a significant main effect or interaction was identified post hoc analyses were performed using Tukey’s HSD. α < 0.05 was considered significant for all statistical analyses including post hoc comparisons. Partial correlations, controlling for litter, were calculated separately for males and females in each experiment to test the relationship between anxiety-like behavior, social avoidance, and nicotine responses within individuals. For clarity, figures depict estimated marginal means calculated from untransformed data.
3. Results
3.1. Locomotion and body temperature
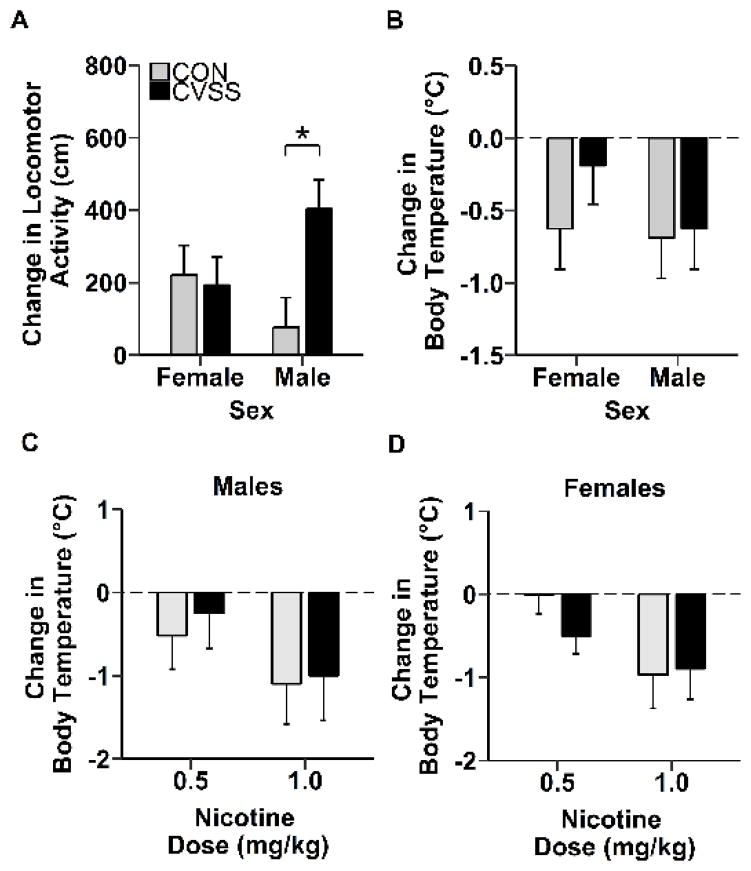
Exposure to adolescent social stress affected nicotine-induced locomotor activity during late adolescence (sex × stress condition interaction: F1,40= 5.1, p < 0.05). Following treatment with 0.5 mg/kg nicotine, late adolescent CVSS males exhibited significantly greater locomotor activity than CON males, but no difference was observed between CVSS and CON females (Fig. 2A; Tukey’s HSD, p = 0.01). There were no other main effects for nicotine-induced locomotor activity.
Figure 2.
Adolescent social stress altered nicotine-induced changes in locomotor activity in late-adolescent male mice. CVSS did not influence nicotine induced change in body temperature for males or females during late-adolescence or adulthood. Data (mean ± SEM) represent (A) nicotine-induced (0.5 mg/kg, i.p.) locomotor activity in late-adolescent mice, (B) nicotine-induced (0.5 mg/kg, i.p.) hypothermia in late-adolescent mice, and nicotine-induced (0.5 and 1.0 mg/kg, i.p.) hypothermia in adult (C) male and (D) female mice. Bars represent nicotine response minus saline response. Thus, higher positive locomotion values indicate locomotor stimulation and negative temperature values represent nicotine-induced hypothermia. *p < 0.05 depicting a significant sex × stress condition interaction (Experiment 1: N = 11–12/group, Experiment 2: N = 9–12/group).
Exposure to adolescent social stress did not affect nicotine-induced hypothermia (Fig. 2B–D). There were no effects of sex or stress condition on reduction in body temperature following a 0.5 mg/kg nicotine injection in late adolescent mice (Fig. 2B). During adulthood, there was a dose-dependent reduction in body temperature following nicotine 0.5 mg/kg and 1.0 mg/kg nicotine injections (Fig. 2C–D; Main effect of nicotine dose: F1,36= 7.4, p < 0. 01). There were no other main effects or interactions on nicotine-induced hypothermia.
3.2. Glucocorticoid production
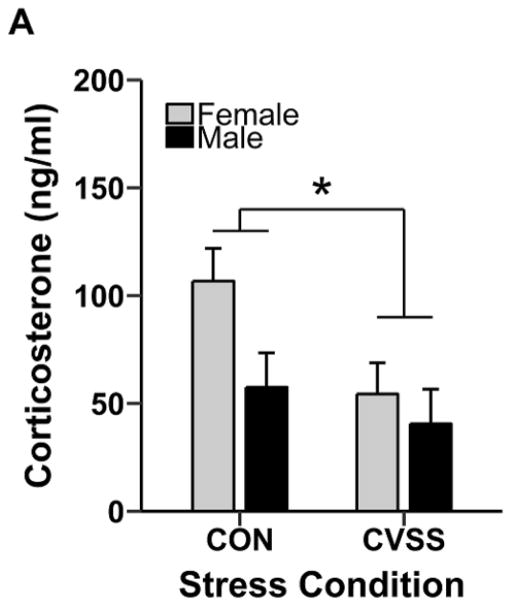
Exposure to adolescent social stress affected adult GC production at baseline and in response to nicotine (Fig. 3). Adult CVSS mice had lower baseline CORT levels than CON mice (Fig. 3A; Main effect of stress condition: F1,34= 5.0, p < 0.05). There was no main effect of sex or interaction for baseline CORT levels.
Figure 3.
Adolescent social stress influenced adult glucocorticoid production at baseline and following acute nicotine injections. Data (mean ± SEM) represent (A) baseline plasma corticosterone (CORT) levels in adult CVSS and CON mice, and plasma CORT responses to injections of saline, 0.5 mg/kg nicotine, and 1.0 mg/kg nicotine in male (B–D, respectively) and female (E–F, respectively) mice. * depicts a significant main effect stress condition (p < 0.05) on plasma CORT levels at (A) baseline and (D) in response to 1.0 mg/kg nicotine (N=9–12/group).
Exposure to adolescent social stress increased nicotine-induced CORT production in a sex- and dose-dependent manner (Fig. 3B–G). Analyses were performed separately at each dose for males and females due to a significant main effect of nicotine dose (F2,183= 5.7, p < 0.01) and a significant time × sex × stress condition interaction (F1,183= 4.48, p < 0.05). In males, plasma CORT levels were consistently higher at 30 min, relative to 90 min, for all doses (Fig. 3B–D; main effect of time for saline: F1,17= 19.4, p < 0.001, 0.5 mg/kg nicotine: F1,17= 96.7, p < 0.001, and 1.0 mg/kg nicotine: F1,16= 80.8, p < 0.001). There was no effect of stress condition on plasma CORT levels following saline or 0.5 mg/kg nicotine. However, adolescent social stress increased plasma CORT responses to the 1.0 mg/kg nicotine dose (time × stress condition interaction: F1,16= 6.4, p < 0.05). Specifically, CVSS males had significantly higher plasma CORT levels than CON males 30 min following injection, with no difference observed at 90 min (Fig. 3D; Tukey’s HSD, p = 0.02). In females, plasma CORT levels were consistently higher at 30 min, relative to 90 min, for all doses (Fig. 3E–G; main effect of time for saline: F1,20= 31.9, p < 0.001, 0.5 mg/kg nicotine: F1,20= 29.5, p < 0.001, and 1.0 mg/kg nicotine: F1,20= 22.4, p < 0.001). There was no effect of stress condition on plasma CORT levels following saline or nicotine injections in female mice.
3.3. Two-bottle choice nicotine consumption
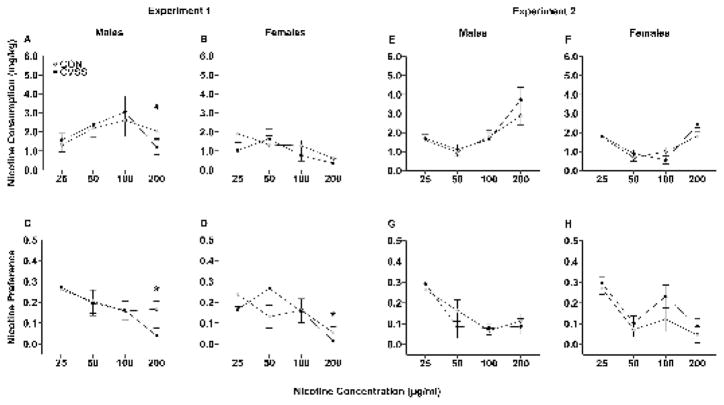
Exposure to adolescent social stress decreased adult nicotine consumption in male, but not female mice, during Experiment 1 (Fig. 4A–B). Analyses of nicotine consumption in Experiment 1 were performed separately for males and females due to a significant nicotine concentration × sex interaction (F3,141= 2.7, p < 0.05). In males, nicotine consumption followed an inverted U-shaped dose-response curve. Males consumed more nicotine when the 100 μg/ml concentration was available compared to the 25 μg/ml concentration (main effect of nicotine concentration: F3,60= 3.8, p < 0.05; 25 μg/ml: 0.7 ± 0.1, 50 μg/ml: 1.0 ± 0.1, 100 μg/ml: 1.2 ± 0.1, 200 μg/ml:1.0 ± 0.1). Furthermore, exposure to adolescent social stress decreased nicotine consumption in a concentration-dependent manner (nicotine concentration x stress condition interaction: F3,60= 4.0, p < 0.05). CVSS males consumed less nicotine than CON males when the 200 μg/ml concentration was available (Fig. 4A; Tukey’s HSD, p = 0.03), but there were no differences in consumption of the 25, 50, or 100 μg/ml concentrations. Females consumed less nicotine when the 200 μg/ml concentration was available compared to the 50 or 100 μg/ml concentrations (main effect of nicotine concentration: F3,81= 6.8, p < 0.001; 25 μg/ml: 0.7 ± 0.1, 50 μg/ml: 0.9 ± 0.1, 100 μg/ml: 1.1 ± 0.1, 200 μg/ml: 0.3 ± 0.1). There was no effect of stress condition on female nicotine consumption (Fig. 4B).
Figure 4.
Adolescent CVSS decreased adult nicotine consumption in male, but not female mice. Data (mean ± SEM) represent average 24 h nicotine consumption of male (A & E) and female (B & F) mice and preference for nicotine of male (C & G) and female (D & H) mice during experiment 1 (A–D) and 2 (E–H). # denotes a significant nicotine concentration × stress condition interaction in males (p < 0.05). * denotes a significant nicotine concentration x stress condition interaction in both sexes (p < 0.05). (Experiment 1: N=11–15/group, Experiment 2: N=9–12/group).
Exposure to adolescent social stress decreased adult nicotine preference during Experiment 1 (Fig. 4C–D). Analyses for males and females were performed together because there was no effect of sex and no interactions with sex for nicotine preference in Experiment 1. Nicotine preference decreased when 200 μg/ml nicotine was available compared to 25 μg/ml nicotine (main effect of nicotine concentration: F3,141= 16.4, p < 0.001; 25 μg/ml: 0.24 ± 0.03, 50 μg/ml: 0.21 ± 0.03, 100 μg/ml: 0.15 ± 0.02, 200 μg/ml: 0.06 ± 0.01). Adolescent social stress also decreased nicotine preference in a concentration dependent manner (Nicotine concentration × stress condition interaction: F3,141= 3.1, p < 0.05). CVSS mice preferred less nicotine than CON mice when 200 μg/ml nicotine was available (Fig. 4C–D; Tukey’s HSD, p = 0.01), but there were no differences in consumption of the 25, 50, or 100 μg/ml concentrations.
Exposure to adolescent social stress altered total fluid consumption in males, but not females, during Experiment 1. Analyses of total fluid consumption during Experiment 1 were performed separately for males and females due to a significant nicotine concentration × sex interaction (F3,141= 3.3, p < 0.05). Males consumed more total fluid when 200 μg/ml nicotine was available compared to 25 μg/ml nicotine (main effect of nicotine concentration: F3,60= 4.2, p < 0.01; 25 μg/ml: 6.0 ± 0.1, 50 μg/ml: 6.4 ± 0.1, 100 μg/ml: 6.5 ± 0.2; 200 μg/ml: 6.8 ± 0.3). Male CVSS mice also consumed more total fluid than CON mice (main effect of stress condition: F1,19= 5.2, p < 0.05; 6.7 ± 0.2 vs. 6.1 ± 0.2, respectively). There were no effects of nicotine concentration or stress condition on female total fluid consumption.
Exposure to adolescent social stress did not affect adult nicotine consumption, in Experiment 2 (Fig. 4E–F). Analyses of nicotine consumption were performed separately for males and females due to a significant nicotine concentration x sex interaction (F3,110= 3.3, p < 0.05). Males consumed more nicotine when the 200 μg/ml concentrations was available compared to all other nicotine concentrations (Fig. 4E; main effect of nicotine concentration: F3,50= 7.7, p < 0.001; 25 μg/ml: 1.6 ± 0.2; 50 μg/ml: 1.0 ± 0.2; 100 μg/ml: 1.7 ± 0.2, 200 μg/ml: 3.3 ± 0.4). In males, there was no effect of stress condition nicotine consumption (Fig. 4E). Females consumed more nicotine when the 25 and 200 μg/ml concentrations were available compared to 50 or 100 μg/ml concentrations (Fig. 4F; main effect of nicotine concentration: F3,59= 6.5, p < 0.001; 25 μg/ml: 1.8 ± 0.2; 50 μg/ml: 0.8 ± 0.1; 100 μg/ml: 0.8 ± 0.2, 200 μg/ml: 2.1 ± 0.3). Similar to males, there was no effect of stress condition on nicotine consumption in female mice (Fig. 4F).
Exposure to adolescent social stress did not affect adult nicotine preference, during experiment 2 (Fig. 4G–H). Analyses for males and females were performed together because there was no effect of sex and no interactions with sex for nicotine preference in Experiment 2. Nicotine preference decreased when the 50, 100, and 200 μg/ml nicotine concentrations were available compared to 25 μg/ml nicotine (Fig. 4G–H; main effect of nicotine concentration: F3,111= 13.9, p < 0.001; 25 μg/ml: 0.27 ± 0.03; 50 μg/ml: 0.11 ± 0.02; 100 μg/ml: 0.12 ± 0.02, 200 μg/ml: 0.08 ± 0.02). There was no effect of stress condition on nicotine preference.
Exposure to adolescent social stress did not affect total fluid consumption during Experiment 2. Mice consumed more total fluid when the 200 μg/ml nicotine concentration was available compared to 50 μg/ml nicotine (main effect of nicotine concentration: F3,111= 4.1, p < 0.01; 25 μg/ml: 5.8 ± 0.1; 50 μg/ml: 5.6 ± 0.1; 100 μg/ml: 6.0 ± 0.1, 200 μg/ml: 6.2 ± 0.1). There were no effects of sex or stress condition on total fluid consumption in Experiment 2.
3.4. Elevated plus-maze
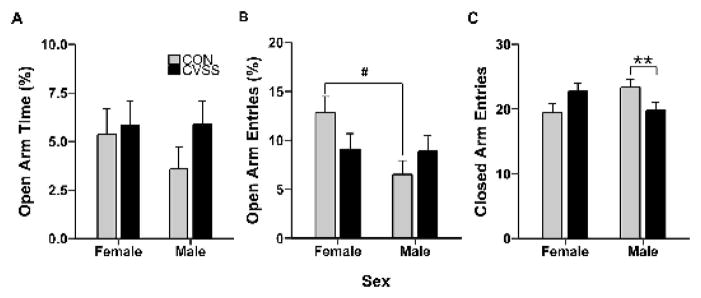
Exposure to adolescent social stress decreased closed arm entries on the EPM in male, but not female mice (Fig. 5C). There was no effect of sex or stress condition on percent time on the open arms of the EPM (Fig. 5A), but females exhibited greater percent open arm entries than males (Main effect of sex: F1,106= 4.4, p < 0.05; 10.7 ± 1.1 vs. 7.4 ± 1.0, respectively). Furthermore, adolescent social stress abrogated the sex difference in open arm entries (stress condition × sex interaction: F1,106= 3.9, p < 0.05). Post hoc analyses revealed that CON females entered the open arms significantly more than CON males (Fig. 5B; Tukey’s HSD; p = 0.005) whereas no sex difference was observed for CVSS mice (Fig. 5B). Finally, adolescent social stress reduced closed arm entries in a sex-dependent manner (stress condition × sex interaction: F1,110= 7.6, p < 0.01). Post hoc analyses revealed that CVSS males entered the closed arms less frequently than CON males (Fig. 5C; Tukey’s HSD; p = 0.04), but no difference was observed between CVSS and CON females (Fig. 5C).
Figure 5.
Adolescent CVSS reduced exploratory locomotion on the elevated plus-maze (EPM) in male, but not female mice. Data (mean ± SEM) represent (A) percent time spent on the open arms, (B) percent entries into the open arms, and (C) number of closed arm entries on the EPM. * denotes a significant sex × stress condition interaction (p < 0.05). ** denotes a significant sex × stress condition interaction (p < 0.01). Data from Experiment 1 (N=14–23/group) and 2 (N=9–12/group) are combined.
3.5. Social approach-avoidance test
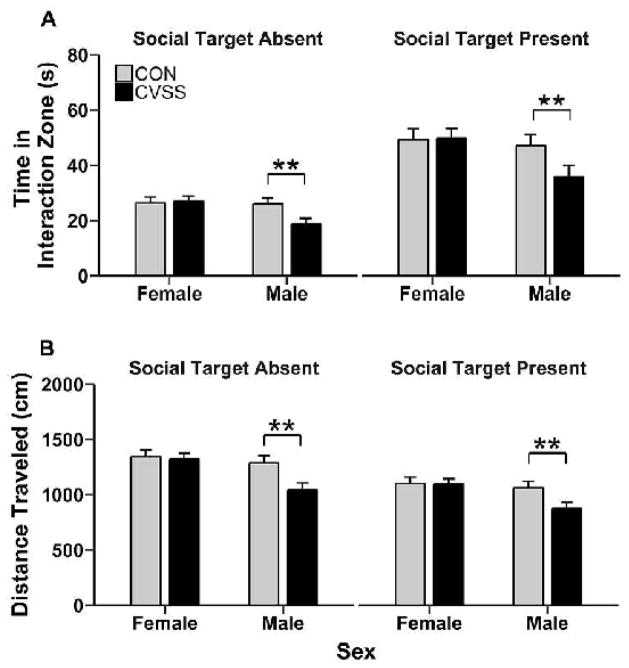
Exposure to adolescent social stress decreased time spent in the interaction zone and the total distance traveled during both trials of social approach-avoidance test for males, but not females (Fig. 6A–B). Distance traveled in the social approach-avoidance test was significantly different between mice in Experiments 1 and 2 (F1,84= 33.27, p < 0.001). Mice in Experiment 1 traveled larger distances than mice in Experiment 2 (1292.3 ± 35.3 vs. 989.7 ± 37.1, respectively). However, data from both experiments were combined for further analyses because there was no interaction of experimental cohort with social target presence, sex, or stress condition, and similar results were obtained when experimental cohorts were analyzed separately.
Figure 6.
Adolescent CVSS reduced exploratory behavior in the social approach-avoidance test for male, but not female mice. Data (mean ± SEM) represent (A) the time mice spent in the interaction zone and (B) total distance traveled. ** denotes a significant sex × stress condition interaction (p < 0.01). Data from Experiment 1 (N=11–15/group) and 2 (N=9–12/group) are combined. Trials 1 and 2 are depicted as “Social Target Absent” and Special Target Present”, respectively.
Overall, mice spent more time in the interaction zone when the social target was present compared to when the social target was absent (main effect of social target presence: F1,84= 110.0, p < 0.001; 45.5 ± 1.9 vs. 24.5 ± 1.0, respectively) and females spent more time in the interaction zone than males (main effect of sex: F1,85= 6.6, p < 0.05; 38.1 ± 1.5 vs. 31.9 ± 1.7). Additionally, adolescent social stress reduced time spent in the interaction zone in a sex-dependent manner (sex × stress condition interaction: F1,85= 4.9, p < 0.05). Post hoc analyses indicated that CVSS males spent less time in the interaction zone than CON males (Fig. 6A; Tukey’s HSD; p = 0.008), but there was no effect of stress condition in female mice (Fig. 6A). There were no significant main effects or interactions for time in the corner zones. Overall, mice traveled greater distances when the social target was absent compared to when the social target was present (main effect of social target presence: F1,75= 38.7, p < 0.001; 1248.8 ± 30.4 vs. 1033.2 ± 28.3, respectively), females also traveled further than males (main effect of sex: F1,84= 9.6, p < 0.01; 1215.2 ± 31.10 vs.1066.9 ± 34.8, respectively), and CON mice traveled greater distances than CVSS mice (main effect of stress condition: F1,84= 4.87, p < 0.05; 1199.0 ± 33.5 vs. 1083.1 ± 32.4, respectively). Exposure to adolescent social stress reduced the distance traveled in a sex-dependent manner (sex × stress condition interaction: F1,84= 4.61, p < 0.05). Post hoc analyses indicated that CON males traveled greater distances than CVSS males (Fig. 6B; Tukey’s HSD; p = 0.002), whereas no difference was observed between CVSS and CON females (Fig. 6B).
3.6. Correlation analyses
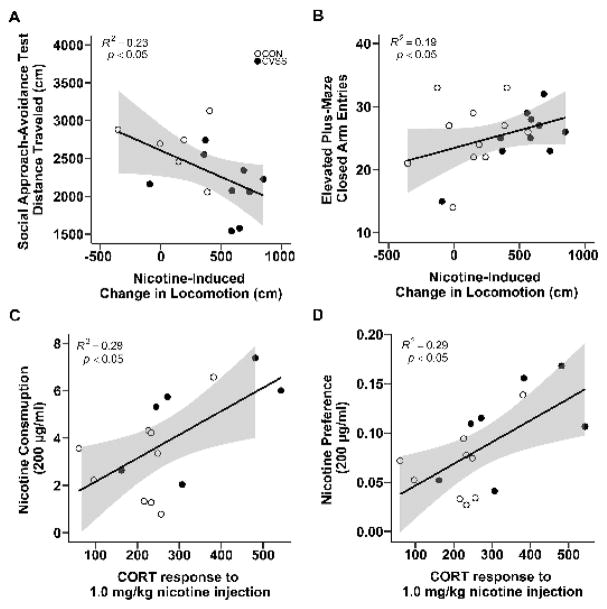
Correlation analyses were performed to investigate the relationship between individual differences in EPM, social approach-avoidance, and nicotine behaviors. Separate analyses were performed for males and females within each experiment because of sex-specific effects of adolescent social stress. Analyses were restricted to those behaviors that were influenced by adolescent social stress including closed arm entries on the EPM, distance traveled in the social approach-avoidance test, nicotine-induced locomotor activity, nicotine consumption and preference when the 200 μg/ml concentration was available, and CORT response to the 1 mg/kg nicotine injection. The relationships between all variables are included in the supplemental materials (Supplemental Figures S1–4).
Individual differences in exploratory behavior in the EPM (i.e., closed arm entries) and social approach-avoidance test (i.e., distance traveled) were associated with variation in nicotine responses among males. Males that exhibited increased nicotine-induced locomotion during late adolescence traveled less distance in the social approach-avoidance test (Fig. 7A; R2= 0.23, p < 0.05) and made more closed arm entries in the EPM (Fig. 7B; R2= 0.19, p < 0.05) during adulthood. There was no association between late adolescent nicotine-induced locomotion and distance traveled in the social approach-avoidance test or closed arm entries on the EPM for females (Supplementary Fig. S2). Adult males with higher CORT responses to the 1.0 mg/kg nicotine injection also consumed more nicotine (Fig. 7C; R2= 0.29, p < 0.05) and preferred more nicotine when 200 μg/ml concentration was available (Fig. 7D; R2= 0.29, p < 0.05). Furthermore, there were no significant associations between closed arm entries on the EPM or distance traveled in the social approach-avoidance test and consumption or preference for the 200 μg/ml nicotine solution (Supplementary Fig. S1–4).
Figure 7.
Correlations between nicotine responses and exploratory behavior in the social approach-avoidance test and EPM in male mice. Nicotine-induced locomotor activity during late adolescence was (A) negatively correlated with distance traveled in the social approach-avoidance (N = 15) and (B) positively correlated with closed arm entries in the EPM during adulthood (N = 21). Plasma CORT response to 1.0 mg/kg nicotine was positively correlated with (C) consumption (N = 16) and (D) preference for 200 μg/ml nicotine (N = 16). The shaded regions signify 95% confidence intervals.
Exploratory analyses revealed associations between nicotine responses during late adolescence and adulthood which were not predicted a priori. During Experiment 1, females that exhibited less nicotine-induced locomotor activity during late adolescence consumed more of the 200 μg/ml nicotine solution as adults (Supplementary Fig. S2; R2= 0.25, p < 0.05). Further, adult females that exhibited less nicotine-induced hypothermia also preferred more 200 μg/ml nicotine (Supplementary Fig. S2, R2= 0.20, p < 0.05).
4. Discussion
4.1. Overview
The current study simultaneously evaluated the impact of adolescent chronic variable social stress (CVSS) on anxiety-like behavior, social avoidance, and nicotine responses in inbred mice. The results presented here provide the most comprehensive assessment of adolescent stress effects on nicotine responses to date. Further, this is the first study, to our knowledge, that assessed the relationship between stress-induced changes in anxiety-like behavior, social avoidance, and nicotine responses within the same animals. Exposure to adolescent social stress led to altered nicotine responses and reduced exploratory behavior in the EPM (i.e., number of closed arm entries) and social approach-avoidance test (i.e., distance traveled) for male, but not female, BALB/cJ mice. There were no effects of stress on prototypical anxiety-like behavior or social avoidance. These results were unexpected given our previous findings and may be related to methodological differences between studies (21). However, mice exposed adolescent social stress in our prior study also exhibited reduced EPM exploratory behavior (21). In the current study, similar reductions in exploratory behavior on both the EPM and social approach-avoidance tests partially replicate and extend prior findings. Results suggest that male mice exposed to adolescent social stress exhibit persistent alterations in exploration of novel environments. Further, in males, the locomotor-stimulating effects of nicotine were associated with exploratory behavior, but there was little evidence that individual differences in prototypical anxiety-like behavior or social avoidance were associated with nicotine responses.
4.2. Adolescent social stress alters nicotine responses in males
The effects of adolescent social stress on acute nicotine responses were assessed in late adolescence and adulthood. Adolescent nicotine responses differ considerably from that of adults and adolescents are more susceptible to develop nicotine dependence (13, 48–51). Prospective clinical studies suggest that chronic stress exposure contributes to adolescent susceptibility as it predicts the initiation, degree, and continuation of smoking (15, 16). In the current study, we report a sex-specific impact of adolescent social stress on behavioral and physiological responses to nicotine. Male mice exposed to adolescent social stress displayed augmented nicotine-induced locomotor activity and HPA activity during late adolescence and adulthood, respectively. Alternatively, adolescent social stress reduced voluntary nicotine consumption in adult males, but not females. Finally, there was no effect of stress on nicotine-induced hypothermia for either sex. The current results are in agreement with findings from prospective human adolescent studies (15, 16). Furthermore, our findings indicate that the effects of adolescent stress can vary depending on the nicotine response measured.
There is scant evidence that adolescent stress impacts nicotine responses in rodent models. Most studies have investigated the impact of stress on nicotine’s locomotor effects and no previous studies have assessed stress effects on nicotine-induced hypothermia. The available literature suggests that females may be more susceptible than males. McCormick and colleagues found that adolescent social instability stress reduced locomotor sensitization to repeated nicotine (0.5 mg/kg) exposure when administered during adolescence while there was no effect on acute locomotor responses to the initial exposure (30). When nicotine (0.5 mg/kg) was administered in adulthood, the initial locomotor response and locomotor sensitization were augmented in stressed female rats. These effects were specific to a higher nicotine dose as stress did not affect locomotor responses at a lower dose (0.25 mg/kg) (28, 29). Neither social instability stress nor chronic restraint stress influenced nicotine-induced locomotor activity among adolescent and adult male rats (28–31).
There are a number of factors that could contribute to the differences observed between our results and those of previous studies. For example, nicotine-induced locomotor responses may be influenced by the types of testing apparatus used across studies. We measured nicotine-induced locomotor activity in a symmetrical Y-maze whereas previous studies measured locomotor responses in open field arenas. We also measured locomotor activity for 5 mins, which corresponds to the time when near-maximal effects of nicotine are observed (36, 48), whereas other studies have measured cumulative distance traveled over 30–60 min (24, 28, 29, 31). As such, it is difficult to directly compare our results with those of previous studies. Finally, nicotine responses are highly dependent on dose and genetic background (37), which varies across studies. We assessed locomotor responses to 0.5 mg/kg nicotine in inbred BALB/cJ mice, whereas previous studies evaluated responses to 0.25–0.5 mg/kg nicotine in outbred rats. Future studies which utilize a more thorough dose-response curve and/or multiple inbred strains would provide valuable insight into the potential factors contributing to the variability in results across studies.
Several studies have reported elevated CORT levels that are consistent with our results following nicotine administration in rodents (31, 52) and smoking in humans (53). GCs modulate many of the pharmacological effects of acute and chronic nicotine exposure, including locomotor activity, hypothermia, and development of tolerance (54, 55). Stress-induced GC production may also contribute to the reinforcing properties of nicotine (55, 56). Thus, nicotine’s ability to stimulate HPA activity influences risk for dependence. Furthermore, GCs program the development of HPA regulatory mechanisms during adolescence and chronic stress effects on human adolescent smoking have been attributed to elevated GC production (16, 17). Given previous reports of long-lasting stress-induced changes in HPA activity (23, 33, 52), we hypothesized that adolescent social stress would increase adult GC responses to nicotine. In support of our hypothesis, adult CVSS males exhibited greater plasma CORT responses to high dose nicotine (1.0 mg/kg – a dose previously shown to result in physiologically relevant plasma CORT levels resembling an acute stress response (52)). It is unlikely that this effect was a result of general enhancement in HPA responses to stress per se, because there was no effect of adolescent social stress on plasma CORT levels following injections of saline or 0.5 mg/kg nicotine. Thus, adolescent stress effects may only become apparent with higher nicotine doses.
Augmented HPA responses to nicotine were also observed in male C57BL/6J mice exposed to chronic stress during adulthood (52). However, our results are inconsistent with previous findings where adolescent chronic restraint stress did not influence nicotine-induced CORT production in rats (31). Experimental differences, including species or type and duration of stress paradigm, limit the ability to compare across studies. However, Cruz and colleagues (31) measured nicotine-induced HPA responses during mid-adolescence. Stress effects on nicotine-induced HPA activity could have been influenced by the fact that adolescents metabolize nicotine more rapidly than adults (57). Alternatively, the stress-related neurobiological changes that mediate augmented HPA responses to nicotine may develop over time. This view is in agreement with prior studies where the chronic stress produced delayed changes in brain regions that continue to mature during adolescence such as the hippocampus (14). Overall, the present results suggest that enhanced HPA responses to nicotine may contribute to the associations between adolescent stress and smoking risk reported in prospective clinical studies (15, 16).
Voluntary oral nicotine consumption was measured during adulthood to assess the long-lasting behavioral effects of stress. There are several advantages to voluntary oral nicotine consumption models, such as 24 h free access for extended periods of time that resembles human smoking behavior (58). Importantly, animals with a high preference for nicotine can achieve pharmacologically relevant blood nicotine concentrations comparable to human smokers (58). Furthermore, oral nicotine (200 μg/ml) consumption can result in locomotor activation and development of tolerance (59, 60). Neurochemical adaptations implicated in nicotine dependence, including nicotinic acetylcholine receptor (nAChR) upregulation and alterations in intracellular signaling pathways within the mesolimbic dopamine system, accompany these behavioral changes (59, 61).
In the current study, adolescent social stress reduced voluntary nicotine consumption and preference in adult males but not females. Interestingly, these stress-effects only emerged after mice had been exposed to nicotine during late-adolescence (Experiment 1) as opposed to those for which initial exposure occurred in adulthood (Experiment 2). Although these results should be interpreted cautiously, they suggest that adolescent nicotine exposure during social stress may interact to shape subsequent nicotine responses later in life. Brief nicotine exposure leads to a long-lasting enhancement of excitatory inputs into the mesolimbic dopamine system of young animals (62). Furthermore, nicotine injections administered in adolescence, but not adulthood, increase sensitivity to nicotine’s reinforcing properties when animals are re-exposed later in adulthood (63, 64). Even a single exposure to nicotine during adolescence has been shown to alter adult nicotine responses (51, 65), highlighting the importance of age at the initial exposure as a predictor of subsequent responses. The current results suggest that age at one’s initial exposure to nicotine may be an important mediator of adolescent stress effects on voluntary oral nicotine consumption during adulthood.
The only previous study, to our knowledge, which investigated adolescent stress effects on nicotine intake found no difference in the acquisition of intravenous (IV) self-administration between socially defeated and control male rats (32). However, comparisons of results between IV and oral self-administration experiments are complicated by a number differences between these models (e.g. whether voluntary oral consumption measures the reinforcing properties of nicotine is unclear) (58, 66). If chronic adolescent stress effects are similar to those previously reported following acute stress (e.g., stress enhances nicotine’s reinforcing properties (67)), CVSS males may consume less nicotine than CON males because the desired effect is acquired at a lower dose. Alternatively, we cannot rule out the possibility that CVSS altered sensitivity to the drug’s aversive properties, such as nicotine-induced seizures, which can also modulate voluntary oral consumption (66).
Stress-induced alterations in the development of distinct neurobiological circuits associated with dopamine transmission and/or nAChR function could influence nicotine responses. Increased locomotor response to nicotine observed in the absence of a stress effect on nicotine-induced hypothermia may reflect a change in mesolimbic dopamine transmission. The most well-known mediators of nicotine-induced locomotor activity, hypothermia, voluntary oral nicotine consumption, and HPA activation are nAChRs containing α4 and β2 subunits distributed across distinct neural circuits (58, 68, 69). Nicotine effects on locomotion may contribute to nicotine reinforcement and reflects, in part, activation of the mesolimbic dopamine system by α4β2-containing nAChR on dopaminergic neurons (69–71). However, nicotine-induced hypothermia is not mediated by dopamine neurons expressing α4β2-containing nAChR (70). While this is only one potential mechanism by which this effect may occur, the plausibility of this hypothesis is supported by the fact that dopamine systems (e.g., cortical and mesolimbic dopamine) and nAChR function actively develop during adolescence (19, 72). Furthermore, adolescent social stress reduces cortical dopamine levels which could indirectly enhance nicotine-induced dopamine release in the nucleus accumbens (14).
4.3. Adolescent social stress alters exploratory behavior in adult males
Adolescent social stress reduced exploratory behavior on the EPM, but did not influence prototypical anxiety-like behavior. BALB/cJ mice are characterized by high baseline anxiety-like behavior relative to other inbred mouse strains (e.g., low anxiety C57BL/6J mice) (73). This anxious phenotype may have limited our ability to detect an anxiogenic effect of adolescent social stress. We previously reported that adult BALB/cJ mice exposed to CVSS exhibited reduced open arm activity on the EPM in addition to less exploratory locomotion (21). Methodological differences between studies may explain these contrasting results. Mice in our previous study were isolated for 7 days, at the end of the CVSS protocol, and were rehoused 24h prior to EPM testing. Stress from isolation/re-socialization could have contributed to the reduction in open arm activity that we previously observed. In contrast, stable reductions in exploratory locomotion observed in adult CVSS males during the present study partially replicate our prior findings (21). However, a recent study found that several prototypical anxiolytics consistently increased exploratory locomotion (e.g., distance traveled in an open field test or total arm entries on the EPM) in C57BL/6J, BALB/cJ, and DBA/2J mice across 4 different tests of anxiety-like behavior. In contrast, no major changes were observed for prototypical anxiety-like behaviors (e.g., open arm time on the EPM) (45). Therefore, reductions in exploratory locomotion are difficult to distinguish from prototypical anxiety-like behaviors and may reflect an anxiety-like state.
Adolescent social stress has been shown to increase social avoidance which may reflect a social anxiety-like behavior (22). Furthermore, stress-induced anxiety-like behavior may be more evident when tested under social conditions than in the EPM (25, 27). In the current study, social avoidance was measured in the social approach-avoidance test. In this test, we found a stress-induced reduction in time spent in the social interaction zone for males, regardless of whether the social target was present. However, avoidance of the social interaction zone was most likely due to a stress-induced reduction in exploratory locomotion as indicated by reduced distance traveled. Thus, CVSS-induced behavioral changes observed in the social approach-avoidance test do not represent social avoidance as described in prior studies (25–27). On the other hand, the stress-induced reduction in exploratory locomotion during the social approach-avoidance test is similar to the results described for the EPM. These results suggest that adolescent social stress effects persist well into adulthood. Overall, adult CVSS males appear to exhibit a persistent low-exploration phenotype, perhaps when faced with a novel or anxiogenic situation.
Curiously, baseline plasma CORT levels were decreased by adolescent social stress. This effect was mainly driven by females and did not influence male HPA responses to nicotine discussed above. These findings replicate the results of our earlier study in which adolescent social stress reduced basal CORT production in male and female mice (21). Stress-induced HPA hypoactivity may reflect an adaptation meant to limit the deleterious effects of prolonged GC exposure. Our lab and others have reported associations between HPA hypoactivity and elevated anxiety- and depression-like behavior following adolescent social stress among both sexes (21, 34, 74). Taken together, these results suggest that the CVSS protocol elicits stress-related physiological changes in both sexes. It is unclear why no differences in female anxiety-like behavior, social avoidance, exploratory behavior, or nicotine responses were observed but this may be related to estrous cycle variation. The estrous cycle has been shown to moderate the effects of adolescent social instability stress on anxiety- and depression-like behavior (24). Future studies would be necessary to determine the impact of estrous cycle variation on CVSS-induced behavioral and physiological changes.
4.4. Associations between exploratory behavior and nicotine responses
Analyses of the association between stress-induced changes in exploratory behavior and nicotine responses revealed that individual differences in late adolescent nicotine-induced locomotor activity predicted adult exploratory locomotion in two unfamiliar test apparatus. Whereas females showed no association between nicotine responses and exploratory behavior, males exhibiting greater nicotine-induced locomotor activity were less exploratory in the social approach-avoidance test and more exploratory in the EPM. These results may indicate that a common underlying neurobiological mechanism influences both exploratory behavior in novel environments and nicotine-induced locomotor activity among males. However, we observed relatively weak associations between these behaviors. Replication will be essential if strong conclusions are to be made from these findings.
There are several potential explanations for these associations. Prior studies have indicated that hypoactive nucleus accumbens dopamine transmission causes reduced exploratory locomotion in novel environments such as the social approach-avoidance test arena (75). The nAChR subunits known to modulate nicotine-induced nucleus accumbens dopamine release (e.g., α4, α6, and β3 (76)) also regulate novelty-evoked exploratory locomotion. For example, mice that harbor genetic mutations in the α4, α6, or β3 subunit genes exhibit increased locomotion or reduced habituation of locomotion in unfamiliar test arenas (77–79). This overlap is notable in light of recent studies that demonstrate developmental changes in nAChR function and nicotine’s ability to stimulate dopamine release in the ventral striatum (19). Altered development of striatal nAChR function following adolescent stress may, therefore, contribute to correlated changes in exploration of a novel environment and nicotine responses.
4.5. Conclusion
The current study has provided evidence that adolescent CVSS can have sex-specific impacts on the development of nicotine responses and exploratory behaviors. There was little effect of CVSS on adult anxiety-like behavior or social avoidance in males. Alternatively, female mice exposed to adolescent CVSS exhibited long-lasting reductions in basal HPA axis activity with no change in anxiety-like behavior, social avoidance, exploratory behavior, or nicotine responses. The human clinical literature suggests that chronic stress and HPA hyperactivity may predispose individuals to develop both anxiety disorders and nicotine use because GCs program the development of brain regions mediating both emotion-related behavior and reward processing (17–19). However, the current results suggest that stress-induced anxiety disorders and elevated risk for nicotine use may also have unique biological mediators. Future research identifying mechanisms by which adolescent CVSS alters physiological and behavioral responses to nicotine may lead to a better understanding of the unique biological mediators of stress-induced nicotine use reported in the human literature.
Supplementary Material
Highlights.
Adolescent social stress altered nicotine responses in male but not female mice
Adolescent social stress altered exploratory behavior in male mice
Stress-effects on nicotine responses were associated with exploratory behavior
Adolescent stress may have sex-specific effects on development of nicotine responses
Acknowledgments
We thank T.B. Fetherston, L.W. Gallaher, C.N. Miller, C.J. Silva, T.L. Pascoe, G.M. Gavitt, A.L. Brittingham, K.M. Czajkowski, L.B. Cannon, N.Q. Singh, and C.E. Peck for their outstanding technical assistance during data collection. Funding for this research was provided by NIH grants K01 AA019447 (HMK), R21 MH092667 (SAC), The Broadhurst Career Development Professorship for the study of Health Promotion and Disease Prevention (HMK), The Penn State Institute for Neuroscience (SAC), and The Penn State Social Science Research Institute (SAC). The funding sources had no role in data collection, analysis and interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Center for Disease Control and Prevention. Smoking and Tobacco Use: Fast Facts. 2015 Retrieved February 20, 2017, from https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/
- 2.Greenberg PE, Fournier A, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) The Journal of clinical psychiatry. 2015;76:155–62. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, et al. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. (P. J. Hay, editor) PLoS Medicine. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Archives of General Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- 5.Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, et al. Mental disorders as risk factors for substance use, abuse and dependence: Results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105:1117–1128. doi: 10.1111/j.1360-0443.2010.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson A, He JP, Curry L, Merikangas K. Cigarette smoking and mood disorders in U.S. adolescents: Sex-specific associations with symptoms, diagnoses, impairment and health services use. Journal of Psychosomatic Research. 2012;72:269–275. doi: 10.1016/j.jpsychores.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grover KW, Goodwin RD, Zvolensky MJ. Does current versus former smoking play a role in the relationship between anxiety and mood disorders and nicotine dependence? Addictive Behaviors. 2012;37:682–685. doi: 10.1016/j.addbeh.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Jamal M, Willem Van der Does AJ, Cuijpers P, Penninx BWJH. Association of smoking and nicotine dependence with severity and course of symptoms in patients with depressive or anxiety disorder. Drug and Alcohol Dependence. 2012;126:138–146. doi: 10.1016/j.drugalcdep.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: Tests of causal linkages using a longitudinal birth cohort. British Journal of Psychiatry. 2010;196:440–446. doi: 10.1192/bjp.bp.109.065912. [DOI] [PubMed] [Google Scholar]
- 10.Molas S, DeGroot SR, Zhao-Shea R, Tapper AR. Anxiety and Nicotine Dependence: Emerging Role of the Habenulo-Interpeduncular Axis. Trends in pharmacological sciences. 2016;38:169–180. doi: 10.1016/j.tips.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 12.Kessler R, Berglund P, Demler O. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–768. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings 2011 [Google Scholar]
- 14.Burke AR, McCormick CM, Pellis SM, Lukkes JL. Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neuroscience and Biobehavioral Reviews. 2017;76:280–300. doi: 10.1016/j.neubiorev.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein DM, Kubzansky LD, Goodman E. Social Status, Stress, and Adolescent Smoking. Journal of Adolescent Health. 2006;39:678–685. doi: 10.1016/j.jadohealth.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Rao U, Hammen CL, London ED, Poland RE. Contribution of hypothalamic-pituitary-adrenal activity and environmental stress to vulnerability for smoking in adolescents. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:2721–32. doi: 10.1038/npp.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: Inter-species and intra-species differences. Brain Research Reviews. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Nestler EJ, Carlezon WA. The Mesolimbic Dopamine Reward Circuit in Depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. The Journal of Physiology. 2015;593:3397–3412. doi: 10.1113/JP270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience and Biobehavioral Reviews. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 21.Caruso MJ, Kamens HM, Cavigelli SA. Exposure to chronic variable social stress during adolescence alters affect-related behaviors and adrenocortical activity in adult male and female inbred mice. Developmental Psychobiology. 2017:1–9. doi: 10.1002/dev.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell and Tissue Research. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- 23.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Hormones and behavior. 2008;53:386–94. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 24.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural brain research. 2008;187:228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male long-evans rats. Developmental Psychobiology. 2013;55:849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- 26.Vidal J, de Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiology & behavior. 2007;92:824–30. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Saavedra-Rodríguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biological Psychiatry. 2013;73:44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Hormones and Behavior. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 29.McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Hormones and behavior. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.McCormick CM, Ibrahim FN. Locomotor activity to nicotine and Fos immunoreactivity in the paraventricular nucleus of the hypothalamus in adolescent socially-stressed rats. Pharmacology Biochemistry and Behavior. 2007;86:92–102. doi: 10.1016/j.pbb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Cruz FC, DeLucia R, Planeta CS. Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addiction Biology. 2008;13:63–69. doi: 10.1111/j.1369-1600.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- 32.Zou S, Funk D, Shram MJ, Lê AD. Effects of stressors on the reinforcing efficacy of nicotine in adolescent and adult rats. Psychopharmacology. 2014;231:1601–1614. doi: 10.1007/s00213-013-3314-3. [DOI] [PubMed] [Google Scholar]
- 33.McCormick CM, Merrick A, Secen J, Helmreich DL. Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. Journal of neuroendocrinology. 2007;19:116–26. doi: 10.1111/j.1365-2826.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- 34.Scharf SH, Sterlemann V, Liebl C, Müller MB, Schmidt MV. Chronic social stress during adolescence: Interplay of paroxetine treatment and ageing. Neuropharmacology. 2013;72:38–46. doi: 10.1016/j.neuropharm.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 35.Lutfy K, Aimiuwu O, Mangubat M, Shin CS, Nerio N, Gomez R, et al. Nicotine stimulates secretion of corticosterone via both CRH and AVP receptors. Journal of Neurochemistry. 2012;120:1108–1116. doi: 10.1111/j.1471-4159.2011.07633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marks MJ, Romm E, Bealer SM, Collins AC. A test battery for measuring nicotine effects in mice. Pharmacology Biochemistry & Behavior. 1985;23:325–330. doi: 10.1016/0091-3057(85)90577-5. [DOI] [PubMed] [Google Scholar]
- 37.Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacology, Biochemistry and Behavior. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- 38.Kamens HM, Miyamoto J, Powers MS, Ro K, Soto M, Cox R, et al. The β3 subunit of the nicotinic acetylcholine receptor: modulation of gene expression and nicotine consumption. Neuropharmacology. 2015;99:639–649. doi: 10.1016/j.neuropharm.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butt CM, King NM, Hutton SR, Collins AC, Stitzel Ja. Modulation of nicotine but not ethanol preference by the mouse Chrna4 A529T polymorphism. Behavioral neuroscience. 2005;119:26–37. doi: 10.1037/0735-7044.119.1.26. [DOI] [PubMed] [Google Scholar]
- 40.Wilking JA, Hesterberg KG, Crouch EL, Homanics GE, Stitzel JA. Chrna4 A529 knockin mice exhibit altered nicotine sensitivity. Pharmacogenetics and Genomics. 2010;20:121–130. doi: 10.1097/FPC.0b013e3283369347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 42.Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcoholism, clinical and experimental research. 1994;18:931–41. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 43.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behavioural Brain Research. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 44.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neuroscience and Biobehavioral Reviews. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 45.Thompson T, Grabowski-Boase L, Tarantino LM. Prototypical anxiolytics do not reduce anxiety-like behavior in the open field in C57BL/6J mice. Pharmacology Biochemistry and Behavior. 2015;133:7–17. doi: 10.1016/j.pbb.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berton O, McClung Ca, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science (New York, NY) 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 47.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 48.Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2010;96:82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61:189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilking JA, Hesterberg KG, Nguyen VH, Cyboron AP, Hua AY, Stitzel JA. Comparison of nicotine oral consumption and baseline anxiety measures in adolescent and adult C57BL/6J and C3H/Ibg mice. Behavioural Brain Research. 2012;233:280–287. doi: 10.1016/j.bbr.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicology and Teratology. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 52.Lutfy K, Brown MC, Nerio N, Aimiuwu O, Tran B, Anghel A, Friedman TC. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis. Journal of Neurochemistry. 2006;99:1321–1327. doi: 10.1111/j.1471-4159.2006.04217.x. [DOI] [PubMed] [Google Scholar]
- 53.Pomerleau OF, Pomerleau CS. Cortisol response to a psychological stressor and/or nicotine. Pharmacology, Biochemistry and Behavior. 1990;36:211–213. doi: 10.1016/0091-3057(90)90153-9. [DOI] [PubMed] [Google Scholar]
- 54.Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C, et al. The role of corticosteroids in nicotine’s physiological and behavioral effects. Psychoneuroendocrinology. 1998;23:143–159. doi: 10.1016/s0306-4530(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 55.Pauly JR, Grun EU, Collins AC. Tolerance to nicotine following chronic treatment by injections: A potential role for corticosterone. Psychopharmacology. 1992;108:33–39. doi: 10.1007/BF02245282. [DOI] [PubMed] [Google Scholar]
- 56.Pomerleau OF, Pomerleau CS. Research on stress and smoking: progress and problems. British Journal of Addiction. 1991;86:599–603. doi: 10.1111/j.1360-0443.1991.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 57.Lopez M, Simpson D, White N, Randall C. Age- and sex-related differences in alcohol and nicotine effects in C57BL/6J mice. Addiction biology. 2003;8:419–27. doi: 10.1080/13556210310001648176. [DOI] [PubMed] [Google Scholar]
- 58.Collins AC, Pogun S, Nesil T, Kanit L. Oral Nicotine Self-Administration in Rodents. Journal of Addiction Research & Therapy. 2012;29:997–1003. doi: 10.4172/2155-6105.S2-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57B1/6 mice. Psychopharmacology. 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- 60.King SL, Caldarone BJ, Picciotto MR. β2-Subunit-Containing Nicotinic Acetylcholine Receptors Are Critical for Dopamine-Dependent Locomotor Activation Following Repeated Nicotine Administration. Neuropharmacology. 2004;47:132–139. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 61.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57B1/6J mice. Journal of Neurochemistry. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 62.Mansvelder HD, McGehee DS. Long-Term Potentiation of Excitatory Inputs to Brain Reward Areas by Nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 63.Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology. 2006;184:382–390. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- 64.Kota D, Robinson SE, Damaj MI. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochemical Pharmacology. 2009;78:873–879. doi: 10.1016/j.bcp.2009.06.099. [DOI] [PubMed] [Google Scholar]
- 65.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-Dependent Effects of nicotine and epibatidine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 66.Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology. 1996;124:332–339. doi: 10.1007/BF02247438. [DOI] [PubMed] [Google Scholar]
- 67.Brielmaier JM, McDonald CG, Smith RF. Effects of acute stress on acquisition of nicotine conditioned place preference in adolescent rats: A role for corticotropin-releasing factor 1 receptors. Psychopharmacology. 2012;219:73–82. doi: 10.1007/s00213-011-2378-1. [DOI] [PubMed] [Google Scholar]
- 68.Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- 69.Tritto T, McCallum SE, Waddle Sa, Hutton SR, Paylor R, Collins AC, Marks MJ. Null mutant analysis of responses to nicotine: deletion of β2 nicotinic acetylcholine receptor subunit but not α7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine & Tobacco Research. 2004;6:145–58. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- 70.McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. α4β2 Nicotinic Acetylcholine Receptors on Dopaminergic Neurons Mediate Nicotine Reward and Anxiety Relief. Journal of Neuroscience. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 72.Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: A review. Behavior Genetics. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- 74.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 76.Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochemical Pharmacology. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, et al. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, et al. In Vivo Activation of Midbrain Dopamine Neurons via Sensitized, High-Affinity α6* Nicotinic Acetylcholine Receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, et al. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC, et al. Neuroanatomic Differences Associated with Stress Susceptibility and Resilience. Biological Psychiatry. 2016;79:840–849. doi: 10.1016/j.biopsych.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.