Abstract
The development of a self-tolerant and effective T cell receptor repertoire is dependent on interactions coordinated by various antigen presenting cells (APC) within the thymus. T cell receptor–self-peptide–MHC interactions are essential for determining T cell fate, however different cytokine and co-stimulatory signals provided by the diverse APCs within the thymus are also critical. Here, we outline the different localization and functional capabilities of thymic APCs. We also discuss how these distinct APCs work collectively to facilitate the establishment of a diverse T cell receptor repertoire that is tolerant to an array of different self-antigens.
Keywords: Tolerance, thymus, selection, dendritic cells, thymic epithelial cells, antigen presentation
Graphical abstract
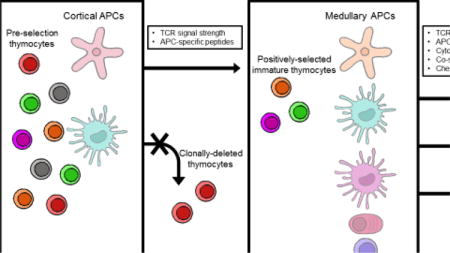
1. Introduction
Random rearrangement of the T cell receptor (TCR) α and β genes in the thymus enables the T cell repertoire to broadly react to a universe of potential antigens derived from pathogens. However, many TCRs will not be able to bind to those antigens when presented by the host’s limited set of major histocompatibility complex (MHC) molecules. Thus, a positive selection step that ensures that TCRs recognize antigens in the context of the host’s own MHC molecules is needed to guarantee that T cells are well equipped to respond when these pathogens are encountered. In addition, the ability to distinguish self-peptides from foreign peptides is essential to prevent the pathogenesis of autoimmune disease. Therefore, as T cells develop in the thymus, tolerance to self-peptides is acquired, in which autoreactive clones are pruned from the repertoire (clonal deletion) or directed to a regulatory lineage (Treg differentiation).
Antigen presenting cells (APC) orchestrate these selection events in the thymus. The strength of the interaction between the TCR and the self-peptide–MHC complexes presented by thymic APCs is crucial in determining the fate of a T cell. Weak interactions facilitated by cortical thymic epithelial cells (cTEC) promote positive selection, whereas stronger interactions drive both clonal deletion and Treg differentiation. But in addition to the strength of the TCR interaction, the specific peptides presented and the cytokine/costimulatory context of that recognition plays a critical role in the outcome of selection. In this review, we discuss the diverse thymic APCs and how they facilitate the generation of a safe and effective T cell repertoire.
2. Cortical Thymic Epithelial Cells
Double positive CD4+CD8+ (DP) thymocytes first express a surface αβ TCR in the cortex of the thymus. Weak TCR interactions with peptide-MHC complexes in this environment mediate positive selection and CD4 and CD8 lineage commitment [1]. Cortical thymic epithelial cells (cTEC) play an essential role in this process (Figure 1). In fact, cTECs are uniquely primed to drive positive selection, in part due to their ability to process and present antigens via machinery distinct from other antigen presenting cells.
Figure 1. Antigen presenting cells encountered in the thymus.
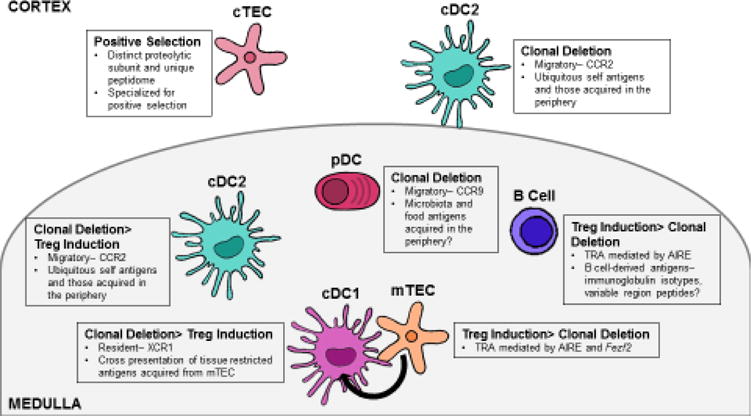
The thymus plays an essential role in mediating self-tolerance by selecting a T cell receptor repertoire based on self-antigens presented by thymic antigen presenting cells (APCs). Thymic APCs include cortical and medullary thymic epithelial cells (cTEC and mTEC), thymic dendritic cells (DC), and B cells. Self-antigens are presented in the thymus via multiple mechanisms. cTEC present antigens specialized for positive selection through their use of the unique proteolytic subunit, β5t. The autoimmune regulator, AIRE, promotes presentation of tissue-restricted self-antigens (TRA) on both mTECs and B cells. Additionally, thymic APCs rely on distinct chemotactic cues for localization within the thymus. These cues, at least in part, govern the unique self-peptides presented by distinct thymic APCs. cDC1 express XCR1, which allows them to localize next to mTEC and acquire mTEC-derived TRAs. cDC2 and plasmacytoid DC (pDC) can acquire antigen in the periphery and present that antigen in the thymus. The chemokine receptors expressed by cDC2 and pDC (CCR2 and CCR9 respectively) may determine the unique self-antigens presented by these two DC subsets. Although a mechanism is yet to be defined, current data suggest that specific medullary APCs favor distinct methods of T cell tolerance–clonal deletion vs. regulatory T cell differentiation.
2.1. Cortical Thymic Epithelial Cells and Antigen Processing
Proteasomes degrade cytosolic proteins, resulting in peptide fragments that can be loaded onto MHC I molecules in the endoplasmic reticulum (ER). The catalytic core of the proteasome includes the three β-subunits: β1, β2, and β5 [2]. While other cell types express either the β5- or β5i- subunits, cTECs have a specialized proteasome subunit that is critical for positive selection: β5t [3, 4]. The ‘thymoproteasome’, unique to cTECs, is specifically composed of β1i (Psmb9), β2i (Psmb10), and β5t (Psmb11) subunits. Mice that lack β5t, and therefore, the thymoproteasome, have a substantial defect in positive selection of CD8 T cells [3]. The number of CD8 T cells in β5t deficient mice is approximately 20% that of their wild type counterparts [5, 6]. In addition, the ensuing T cell pool has diminished responsiveness to infection, demonstrating that the thymoproteasome is critical for shaping the T cell repertoire [5, 6].
Since the proteasome plays a key role in producing peptides that are loaded onto MHC I molecules, it is reasonable to hypothesize that the β5t-containing thymoproteasomes may have proteolytic capabilities that lead to a unique peptide–MHC repertoire. Indeed, β5t promotes decreased chymotrypsin-like activity compared to proteasomes that use either β5 or β5i [3]. Therefore, it was hypothesized that β5t-containing thymoproteasomes would produce peptides that are enriched for basic residues at the C-terminus. Since a substantial portion of the binding energy of MHC to peptides is through the hydrophobic C-terminus of the peptide, the thymoproteasome could generate peptides that bind to MHC molecules more weakly. However, it has been recently shown that while the thymoproteasome does produce unique peptides compared to the β5i-containing immunoproteasome; both generate peptides with hydrophobic residues at the C-terminus [7].
The question then becomes whether cTECs promote positive selection merely because their peptides are different from those presented by other APCs during negative selection, or if the cTEC-exclusive peptides themselves are somehow specialized for inducing positive selection. Experimental results are mixed. When both the immunoproteasome and thymoproteasome subunits were removed, leaving just the constitutive proteasome in all thymic APCs, only 10% of mature CD8+ thymocytes developed [8]. This decrease in CD8 T cells was rescued in Bim-deficient mice, suggesting that negative selection was responsible for the death of most CD8+ thymocytes [8]. The authors concluded that the peptidome therefore needs to be distinct between cTEC and other thymic APC in order to generate a normal sized repertoire, and the role of β5t is to generate a distinct peptidome in cTEC. However, in another study, mice were engineered to express different peptidomes in cTEC versus other thymic APC, but without using β5t. In these mice, positive selection of CD8 T cells was still diminished [9], suggesting that the β5t generated peptidome is specialized for inducing positive selection. It is possible that both concepts are accurate, that thymoproteasome-dependent peptides are both distinct from other thymic APC and specialized for positive selection. Although the mechanism of this specialization is unclear, it may relate to preferentially producing low-affinity TCR ligands [7].
Another interesting possibility that has not yet been explored experimentally is that the thymoproteasome may change the extent to which the peptide repertoire consists of “spliced peptides.” CD8 T cells have been shown to recognize peptides formed from two noncontiguous fragments of a protein that have been spliced together [10]. This splicing is proteasome dependent; for example an immunoproteasome is not always capable of generating the same spliced peptide as a constitutive proteasome [11]. It was recently suggested that in humans, approximately one-third of the peptides presented by HLA I molecules may be spliced peptides [12], indicating that this subset is a non-trivial proportion of the overall peptide repertoire. As the catalytic mechanism proposed in peptide splicing includes the function of the β-subunit of the proteasome [11], this leads to the appealing question of whether cTECs present unique spliced peptides that somehow facilitate positive selection of an even more diverse T cell repertoire.
In an analogous fashion to the thymoproteasome, genes involved in the proteolysis of endosomal proteins are preferentially expressed in cTECs and play a role in the selection of the CD4 T cell repertoire. These include the thymus specific serine protease (TSSP) (Prss16), and cathepsin L (CatL) [13, 14]. As MHC II molecules bind to peptides from the endosomal pathway, the function of these genes appears to be to create unique peptides optimized for selecting the CD4 T cell repertoire. Indeed, deficiency in TSSP and CatL impact positive selection of CD4 T cells [13–15].
2.2. Cortical Thymic Epithelial Cells and Antigen Presentation
Particularly for MHC II molecules, cTEC utilize a distinct antigen presentation pathway in addition to unique antigen processing machinery. CD83 is a member of the immunoglobulin superfamily that is expressed by activated dendritic cells and a wide array of other cell types, including B and T cells [16]. In dendritic cells, CD83 stabilizes MHC II by preventing ubiquitination and subsequent internalization [17]. Importantly, CD83 is constitutively expressed in cTEC and has recently been suggested as a direct target of Foxn1, the lineage defining transcription factor required for thymic epithelial cell growth and differentiation [18].
Mice deficient in CD83 have a substantial defect in CD4 T cell selection [19–21]. Because selection of TCR transgenic CD4 T cells was markedly reduced in CD83-deficient bone marrow recipients, this defect in CD4 T cell selection is likely attributed to a failure of positive selection, rather than disproportionate clonal deletion [19]. CD83-deficient mice have increased turnover of MHC II at cTEC cell surfaces, suggesting that, like in other APCs, CD83 regulates the stability of MHC II on the surface of cTECs [19, 20]. CD83 does so by antagonizing the ubiquitination-dependent lysosomal degradation of MHC II, which is mediated by the ubiquitin ligase, March 8 [19, 22]. This mechanism parallels that of MHC II ubiquitination mediated by March 1 in dendritic cells [17]. The requirement for stable MHC II surface expression for CD4 T cell selection supports the notion that CD4 T cells may require prolonged interactions with selecting peptides for positive selection, based on the kinetic signaling model for T cell lineage commitment [23, 24].
It is unclear whether March 8 and March 1 evolved simultaneously in distinct cell types to mediate identical functions, or if March 8 plays a more specific role specialized for cTEC-mediated positive selection. However, March 8 expression does not appear to have a direct impact on the CD4 T cell receptor repertoire [22]. One separate function may lie in the ability to target distinct additional substrates, including CD86, which is targeted by March 1 in dendritic cells, but not by March 8 in thymic epithelial cells [17, 22].
Interestingly, Foxn1 controls the expression of both Cd83 and Psmb11, suggesting that Foxn1 may direct positive selection of both CD4 single positive (SP) and CD8SP thymocytes [18, 25]. For both MHC I and MHC II restricted thymocytes, positive selection triggers the upregulation of C-C chemokine receptors CCR4 and CCR7 that guide the cells toward the medulla and its dendritic cell (DC)-rich environment [26–31]. The APCs here have distinct roles in both clonal deletion and further differentiation of T cells with homeostatic functions.
3. Medullary Thymic Epithelial Cells
Medullary thymic epithelial cells (mTEC) play a critical role in mediating tolerance to self-antigens through ectopic expression of tissue restricted antigens (TRAs) (Figure 1). TRA expression by mTECs largely depends on AIRE (autoimmune regulator), a transcriptional regulator that controls the expression of antigens normally expressed in certain peripheral tissues [32]. Additionally, AIRE plays a role in mTEC development, promoting the expression of CD80 and MHC II on mature mTECs [33, 34]. Lineage tracing experiments have revealed that mTECs undergo discrete stages of development, eventually downregulating AIRE. These “post-AIRE” mTECs lose their mature phenotype and express decreased MHC II, CD80/86, and AIRE-dependent TRAs [35, 36]. Furthermore, post-AIRE mTECs preferentially migrate toward the center of the medulla, indicating that localization may dictate how mTECs facilitate central tolerance [35].
Recently, an additional transcription factor that promotes thymic expression of a subset of TRAs via an AIRE-independent mechanism was identified. Fezf2 plays an essential role in mediating immune tolerance to tissue restricted antigens [37]. Distinct pathways regulate the expression of Fezf2 and AIRE; Fezf2 is regulated by the lymphotoxin beta receptor (LTβR), whereas AIRE is regulated by receptor activator of nuclear factor-κB (RANK) and CD40, which are members of the tumor necrosis factor receptor superfamily (TNFRSF) [37]. Therefore, Fezf2 and AIRE may have emerged at different points in evolution and possibly cooperate by regulating distinct gene sets.
In addition to self-antigen presentation mediated by AIRE and Fezf2, mTECs produce AIRE-independent chemokine ligands, CCL19 and CCL21, which attract CCR7-expressing developing thymocytes to the medulla [29, 31, 38]. These ligands may facilitate interactions between these developing thymocytes and mTECs–reviewed in reference 39 – to drive thymocyte selection [39].
3.1. Medullary Thymic Epithelial Cells and Clonal Deletion
Classic studies showed that mTECs can facilitate tolerance by inducing clonal deletion of TRA-reactive T cells. In a model in which membrane-bound ovalbumin expression was driven by the rat insulin promoter (RIP-mOVA), and thus specifically expressed in an AIRE-dependent manner, AIRE knockout (KO) and mTEC-depleted mice had a small but significant increase in the number of OT-II transgenic CD4SP thymocytes, suggesting that AIRE plays a role in mediating clonal deletion [40, 41]. More recently Malhotra et al. used tetramer enrichment to show that rare polyclonal T cells specific for TRA were also modestly increased in AIRE KO mice [42]. Clonal deletion is now assumed to be the mechanism for eliminating a large number of TRA-specific clones as mTEC ablation leads to an increase in the proportion of polyclonal CD4SP thymocytes [35]. The role of Fezf2 in mediating clonal deletion is less clear. Fezf2 deficient animals do not have a difference in the CD4SP or CD8SP pool size compared to wild type animals, however differences in TCRVβ usage indicate Fezf2 shapes the CD4 and CD8 TCR repertoire [37].
Because direct MHC II-dependent interactions between thymocytes and mTECs are required for proper medullary architecture and organization [43], assessing the role of mTEC MHC II molecules in tolerance required the development of a method in which the class II transactivator (C2TA) was specifically knocked down (kd) in mTECs via AIRE promoter driven shRNA [44]. C2TAkd bone marrow recipients showed a moderate increase in the frequency of CD4SP thymocytes, indicating that mTECs mediate clonal deletion. Interestingly, CD4SP thymocytes further increased when donor bone marrow was also deficient in MHC II, suggesting that mTECs and DCs play non-redundant roles in mediating deletion of the polyclonal T cell repertoire [44]. A different group performed high throughput analysis of the TCR repertoire in animals lacking MHC II on bone marrow APCs or in C2TAkd animals. Repertoire analysis revealed fewer unique TCRs enriched in C2TAkd animals compared to animals with MHC II deficient bone marrow, suggesting that while mTECs are capable of mediating clonal deletion, their relative contribution is minimal compared to bone marrow APCs [45].
3.2. Medullary Thymic Epithelial Cells and Regulatory T cell Differentiation
A study in which mTECs were shown to induce development of Tregs specific for an AIRE-dependent model antigen gave the first indication that AIRE-expressing mTECs may also impact tolerance through shaping the Treg repertoire [46]. Indeed, it was recently shown that organ-specific Tregs required AIRE-mediated expression of the self-antigen [47, 48]. Although polyclonal Treg numbers are not dramatically altered in the absence of AIRE, at least in adult mice [40, 49], AIRE may play a major role in directing specific T cell clones into the Treg lineage. Therefore, strategies to analyze the impact of AIRE and mTECs at the TCR-level have been employed. One approach took advantage of using a fixed TCR-β chain to enable analysis of TCR specificities via sequence analysis of the TCRα chain [45, 50, 51]. Perry et al. performed high throughput analysis of the TCR Vα2 repertoire in animals lacking MHC II on bone marrow APCs or in C2TAkd animals. Like with clonal deletion, mTECs contributed less to Treg generation compared to bone marrow APCs. However, mTECs contributed substantially more to Treg induction than to clonal deletion at the repertoire level [45]. Further TCR analysis in AIRE deficient mice revealed that AIRE plays a major role in selecting the thymic Treg TCR repertoire, particularly on lower frequency TCRs [45].
Similarly, Malchow et al. performed deep sequencing of the complete TCRα repertoire in isolated peripheral Treg and conventional T cells in AIRE-sufficient or -deficient animals. This study found a large number of underrepresented Treg TCRs in AIRE-deficient animals. Interestingly, in the absence of AIRE, these clones were identified in the conventional T cell repertoire. A major proportion of the clonotypes mediating autoimmune pathology in AIRE deficient animals are preferentially expressed by Tregs in AIRE-sufficient animals, suggesting that a major mechanism by which AIRE enforces central tolerance is directing autoreactive conventional TCR clones into the regulatory T cell lineage [51].
Recent data also suggest that the AIRE-dependent Treg repertoire is distinct during different points in ontogeny and that these repertoires may be responsible for protecting separate tissues. Despite similar fractions of MHC IIhi mTEC and AIRE expression in perinatal and adult mice, the differences mediating this age-dependent selection of the Treg repertoire seem to lie in distinct antigen processing and presentation machinery capabilities. Perinatal mTECs had a decreased DO to DM ratio compared to adult mTECs. DM aids in the removal of the invariant chain derivative, CLIP, and other peptides from MHC II, while DO is known to inhibit this action. Therefore, perinatal mTECs had a corresponding decrease in the amount of CLIP, suggesting that perinatal mTECs are more efficient at replacing CLIP with other peptides and that the peptide repertoire presented by MHC II may therefore be more broad in perinatal mTECs [49]. These data support the notion that AIRE is essential to central tolerance during the neonatal period, but dispensable in adults [52].
Although AIRE’s role in mediating Treg differentiation has been more thoroughly investigated, Fezf2 may also play an essential role in Treg lineage commitment. Fezf2 deficient animals have decreased frequencies of thymic Tregs, indicating that Fezf2 may play an even more substantial role in mediating thymic Treg development [37]. Further analysis comparing the TCR repertoires of Tregs and conventional T cells in Fezf2 deficient animals will be necessary to determine if Fezf2 similarly directs TCRs from the conventional T cell pool into the Treg lineage.
While the relative importance of the contribution of AIRE-dependent clonal deletion and Treg induction to tolerance in not known, the studies discussed above suggest that AIRE-mediated Treg induction may be the crucial mechanism by which AIRE enforces tolerance. Several studies have recently suggested that clonal deletion of T cells specific for self-antigens and TRAs is incomplete [42, 53–56]. T cells specific to an epitope of the AIRE-dependent retina-specific protein interphotoreceptor retinoid binding protein (IRBP) were incompletely deleted via an AIRE-dependent mechanism [55]. Furthermore, through the use of a model utilizing Cre recombinase as a neo-self antigen, Legoux et al. were able to track Cre tetramer-specific CD4 T cells in various mouse strains where Cre expression was restricted to specific peripheral tissues. Although efficient deletional tolerance was found in animals which ubiquitously expressed Cre, when the epitope was restricted to peripheral tissues, Cre:I-Ab specific T cell numbers were not decreased in either the thymus or the periphery, suggesting a lack of deletional tolerance [54]. Furthermore, tolerance to some tissue specific antigens, such as lung and intestine self antigens, required antigen-specific Tregs [54]. Another study that used a similar model to investigate antigen-specific tolerance to fluorescent proteins expressed by various tissue specific promoters came to similar conclusions. Malhotra et al. defined three clusters of self-specific cells. The first cluster is characterized by wild type numbers of cells with relatively few regulatory T cells, suggesting that these cells did not encounter their epitope. Thus, the mechanism mediating tolerance for cluster 1 was likely ignorance. The second cluster resembled that of the lung and intestine-specific cells reported by Legoux et al., in which there were relatively large numbers of Treg cells and few effector cells. Finally, the mechanism mediating tolerance to the third cluster was deletion, which also included T cells specific for ubiquitously expressed proteins [42]. These data suggest that the mechanism of tolerance for a specific epitope may not be solely mediated by the APC type, but may also depend on its relative expression within the thymus.
4. Thymic Dendritic Cells
Three universally defined dendritic cell (DC) subsets have been described within the thymus [57]. These subsets include plasmacytoid DC (pDC) and two conventional DC (cDC) populations, which are delineated based on their expression of lineage-defining cell surface markers and transcription factors [57, 58] (Figure 1). cDC1 are defined based on their expression of the chemokine receptor, XCR1 (XC-chemokine receptor 1), and require the transcription factor IRF8. Alternatively, cDC2 express SIRPα (signal regulatory protein alpha, CD172a) and require the transcription factor IRF4 [58]. These DC subsets are uniquely primed to process and present distinct antigens based on their functional specialization and their ability to respond to different migratory cues.
cDC1 are specialized to cross-present mTEC-derived self-antigens and accumulate in the medulla in an XCR1-dependent manner. XCL1 (XC-chemokine ligand 1 or lymphotactin), the ligand for XCR1, is produced by mTEChi cells in an AIRE-dependent manner, indicating that XCR1 expression facilitates the transfer of mTEC derived TRAs for cross-presentation [59, 60]. Recent evidence suggests that these cDC1 undergo constant homeostatic maturation within the thymus; mature cDC1 express CCR7 and upregulate MHC II, CD40, CD83, and CD86. In contrast to immature (CCR7 negative) cDC1, only mature cDC1 are able to cross-present mTEC-derived antigens [61]. Therefore, this maturation process is likely essential for efficient self-antigen presentation in the thymus; immature cDC1 may not contribute to or may play a distinct role in driving central tolerance compared to their mature counterparts. The factors that control thymic DC maturation have not yet been defined.
In contrast to cDC1, cDC2 originate in the periphery and are capable of acquiring serum-antigens and/or transporting self-antigens into the thymus [57, 62, 63]. C-C chemokine receptor 2 (CCR2) dictates cDC2 migration into the thymus [63]. Although both cDC1 and cDC2 localize within the medulla, cDC2 may also accumulate within the perivascular region of the cortex [27, 63]. However, the specific site in which this subset accumulates within the thymus remains controversial and the specific chemotactic signals that are required for localization are unknown [1, 63]. pDCs also migrate to the thymus from peripheral sites, however the thymic homing of this subset is CCR9-dependent [64, 65]. Like cDC2, pDC are capable of acquiring self-antigens in the periphery and homing to the thymus to present antigens to developing thymocytes [65].
The distinct chemotactic requirements and antigen presenting capabilities of the DC subsets suggests that they may play unique functional roles in facilitating selection of the T cell repertoire.
4.1. Thymic Dendritic Cells and Clonal Deletion
Although the relative contribution of bone marrow APCs and mTECs to clonal deletion is unknown, recent studies examining negative selection at the individual TCR level suggest that bone marrow-derived APCs contribute more to clonal deletion than mTECs [45, 66]. This makes sense, given that the majority of clonal deletion occurs in the cortex compared to the medulla and that migratory cDC2 can localize in the cortex [63, 66–69].
Because cDC2 are capable of acquiring self-antigens in the periphery and transporting them to the thymus, it has been suggested that they mediate tolerance to extrathymic antigens [57, 62, 63]. Indeed, OVA-specific thymocytes underwent clonal deletion mediated by circulating DCs in a model in which OVA was expressed exclusively by cardiomyocytes [62]. Interestingly, cDC2 express the highest levels of the CCR4 ligands, CCL17 and CCL22 [70]. CCR4 is required for thymocyte migration from the cortex to the medulla [26]. Although thymic cellularity in CCR4 deficient animals is mostly unaffected compared to wild type animals, mixed CCR4 deficient and wild type bone marrow chimeras revealed a relative increase in CCR4 deficient CD69+ thymocyte populations compared to wild type thymocytes, implying that CCR4-mediated thymocyte–cDC2 interactions are required for efficient clonal deletion [27].
Recent evidence also suggests that there may be variability in the relative contribution of these dendritic cell subsets to clonal deletion over time. The frequency of both cDC2 and pDC populations was increased in 4-week-old non-obese diabetic (NOD) mice compared to their newborn counterparts [71]. Additionally, antigen processing and presentation was enhanced in cDC2 from four-week-old mice compared to cDC2 from newborns [71]. The frequency of cDC1 in the thymus may also be age-dependent. Perinatal NOD mice had nearly 1/3rd of the frequency of cDC1 compared to adults [49]. This is in direct contrast to findings reported by Kroger et al., who suggest that newborn NOD mice have a larger proportion of cDC1 compared to 4 week old NOD mice [71]. Although the explanation for this discrepancy is unclear, these contradictory findings might be justified by differences in gating strategies, housing conditions, or NOD strains. Additionally, neither group reported cDC1 cell numbers when comparing perinatal to adult mice, which may be more indicative of the temporal changes in this cell population. Overall, the idea of age dependent differences in DC composition is interesting and may consequently result in changes in clonal deletion and Treg cell development, however further studies are needed to more directly define these changes.
As mentioned above, thymic dendritic cells seem to contribute to a large proportion of the deletional tolerance to AIRE-dependent TRAs [42, 55]. Thymic DCs mediate AIRE-dependent deletion of T cells specific for IRBP epitope [55]. Additionally, in the cluster system described by Malhotra et al. (see Section 3.2), a major proportion of DCs expressed cluster 3 antigens, in which deletion was the major tolerance mechanism, compared to clusters 1 and 2, in which ignorance or Treg induction were the major mechanisms of tolerance [42].
Because of their capability to produce type I interferons during viral infections [72] and their poor antigen presenting capacity compared to cDC, it was largely believed that pDC assumed an immunomodulatory function within the thymus [73, 74]. However, evidence now suggests that pDC may play a role in mediating central tolerance as well. Wild type pDC loaded with OVA peptide and adoptively transferred, migrated to the thymus and promoted deletion of OVA-specific OT-II thymocytes, whereas CCR9-deficient pDC did not [65]. Furthermore, because CCR9 is also essential for pDC homing to the small intestine during both homeostatic and inflammatory conditions [75], pDC may mediate tolerance to commensal or food antigens within the thymus, however this hypothesis has not been tested to date.
4.2. Thymic Dendritic Cells and Regulatory T Cell Differentiation
Both cDC1 and cDC2 are capable of promoting Treg induction in vivo [74, 76, 77], and it has been suggested that cDC2 are more efficient at driving Treg differentiation in vitro [77]. Interestingly, several recent studies suggest that bone marrow APC play a critical role in promoting selection of AIRE-dependent Treg clones [45, 48, 78]. However, there are conflicting reports about whether cDC1 and cDC2 are redundant in their roles for AIRE-dependent Treg selection. Perry et al. demonstrated a non-redundant role with data showing that cDC1 were required for the selection of four AIRE-dependent Treg clones. However, utilizing high throughput TCR analysis comparing cDC1-sufficient and -deficient animals, Leventhal et al. concluded that cDC1 did not have an impact on the Treg TCR repertoire. One explanation may be related to the analysis method. Perry et al. showed that removal of the three most frequent TCR specificities from analysis was required to determine a difference in Treg TCR repertoires in C2TAkd and WT animals [45]. However, Leventhal et al. did not utilize this strategy to assess similarity between cDC1-dependent Treg TCRs.
pDC can also promote the development of Treg in vitro [79], however their role in mediating Treg differentiation in vivo is unclear. pDC did not have an effect on Treg selection of four bone marrow APC-dependent TCRs [45], however the precise role of pDC in mediating Treg selection of the polyclonal repertoire is unknown.
In addition to their role in mediating the TCR-dependent first step of Treg differentiation, DCs may also instruct the second step of Treg development, which requires interleukin (IL)-2 and IL-15 cytokine signals [80]. It was previously believed that thymocytes were the major producers of IL-2 in the thymus, however a recent study using thymic tissue slices found that thymic DCs provide a local source of IL-2 to developing Tregs [81]. Therefore, distinct APC subsets within the thymus may cooperate to drive thymic Treg differentiation.
The distinct contributions of cDC1 and cDC2 to central tolerance remain to be tested. Unfortunately, no current method exists to specifically deplete cDC2 [82]. Therefore, new tools are needed to assess the relative contribution of cDC1 and cDC2 to both clonal deletion and Treg differentiation. Additionally, the relative importance of antigens acquired in the periphery compared to those acquired in the thymus is unknown. Does the functional specialization of cDC2 change depending on the location of where antigen is acquired? Do intrathymic and extrathymic self-antigens favor different tolerance mechanisms? Is tolerance to commensal and food antigens mediated in the thymus as well as in the periphery?
5. Thymic B Cells
Although thymic B cells comprise a similar proportion of total thymic cells compared to DCs and mTECs, relatively little is known about their function in the thymus [83]. However, their localization in the medulla and cortico-medullary junction suggests that thymic B cells may play an integral role in mediating Treg development and deletion of autoreactive T cell clones (Figure 1) [84, 85]. In support of this notion, thymic B cells seem exceptionally primed to present antigens; compared to splenic B cells, thymic B cells have markedly increased expression of MHC II and co-stimulatory molecules, including CD80 and CD86 [83–85].
Whether thymic B cells arise and develop in the thymus or circulate from the periphery and adopt a new phenotype remains unclear, however it is likely that both occur [83, 85, 86]. It is evident that the thymic microenvironment is important for driving B cell functions that are distinct from those of the periphery. Notably, licensed thymic B cells express AIRE, whereas peripheral B cells do not [83]. Thymocyte interactions with B cells promote B cell licensing much in the way that they orchestrate mTEC maturation, in that in both cases, thymocytes provide TNFRSF stimulation to induce AIRE upregulation. CD40 is critical for the maintenance of thymic B cells and MHC II-restricted cognate interactions drive thymic B cell class switching and AIRE expression, indicating that cognate interactions between B cells and T cells may be essential for driving central tolerance [83, 87].
Recently, Nuñez et al. demonstrated that memory B cells accumulate within the perivascular space of the human thymus in an age-dependent manner [88]. However, the expression of molecules associated with antigen presentation decreased in B cells from older thymi, suggesting that B cells may become less integral to T cell selection over time, at least in humans [88]. Whether this represents a distinct niche from other thymic B cells is not clear, since a similar phenomenon is not seen in mice [87].
5.1. Thymic B Cells and Clonal Deletion
It is evident that thymic B cells can mediate clonal deletion, as B cells have been shown to delete T cells in the context of superantigen and in systems with model antigens [87, 89, 90]. More recently, it has been suggested that self-specific B cells present cognate antigen to autoreactive T cells [85]. KRN T cells, specific for a peptide from the self-protein glucose-6-phosphate isomerase (GPI), were deleted by B cells with a transgenic B cell receptor (BCR) specific for GPI and by wild-type I-Ag7 thymic B cells [85]. This would suggest that autoreactive B cells within the thymus acquire self-antigen via BCR-mediated endocytosis and mediate tolerance through cognate interactions [85]. However, BCR-independent presentation of endogenous self-antigens may also be an important mechanism by which B cells mediate central tolerance. Licensed B cells directly presented an endogenously expressed antigen and mediated clonal deletion of T cells specific for that antigen [83]. Interestingly, BCR cross-linking in the presence of CD40 signaling suppressed AIRE induction in thymic B cells, but not MHC II upregulation [83]. These findings suggest that both AIRE-expressing and non-expressing B cells in the thymus are capable of mediating clonal deletion. Although more studies are required to delineate a specific mechanism, this evidence indicates that AIRE-expressing B cells may drive tolerance to endogenous antigens, whereas B cells that do not express AIRE may direct tolerance to BCR-acquired antigens.
B cells may also play a critical role in driving tolerance to B cell-specific antigens; B cells present B cell-specific peptides on MHC II, including variable region peptides [91, 92]. Interestingly, recent evidence suggests that thymic B cells undergo class switching, but not somatic hypermutation within the thymus. Class switching is dependent on cognate interactions between B and T cells in the thymus. In the absence of activation induced cytidine deaminase (AID), which is required for class switching, the T cell repertoire is more autoreactive [86]. This implies that class switched B cells assist in mediating tolerance of the T cell repertoire. Although the mechanism by which class switching promotes deletion of autoreactive T cells is unclear, class switching may be important for B cells to function as antigen presenting cells, and the class switched B cell may display a distinct MHC II-bound self-peptidome that necessitates T cell tolerance. There is much to be learned in the future about how B cells shape the polyclonal T cell repertoire.
5.2. Thymic B Cells and Regulatory T Cell Differentiation
B cells also play a striking role in the development of thymic Tregs. The number and frequency of thymic Tregs is decreased by approximately one third in the absence of B cells [84, 93]. Additionally, in BAFF transgenic mice, which have an expansion of extrasplenic B cells, the number and frequency of thymic Treg is increased nearly two-fold [93]. This B cell-mediated induction of thymic Tregs is dependent on direct interactions with MHC II and co-stimulatory molecules [84, 93]. Additionally, in vitro experiments suggest that thymic B cells are capable of directing development of CD4+CD25+ Treg precursors, but are not required for the second stage of development into mature Tregs, which requires the cytokines IL-2 or IL-15 [84, 94]. Whether AIRE-dependent Tregs arise out of cognate interactions with B cells is unclear [78]. However, given the strong expression of TRA transcripts by AIRE-expressing thymic B cells, it will be interesting to see if AIRE expression in B cells contributes distinctly to the Treg TCR repertoire compared to mTECs [45, 83].
6. Thymocytes
In addition to the classical antigen presenting cells discussed here, thymocytes themselves play a unique role in mediating selection of distinct innate-like T cell populations, including natural killer T (NKT) and mucosa associated invariant T (MAIT) cells. The antigen-presenting molecule CD1d displays lipid based antigens to NKT cells [95]. Although CD1d is broadly expressed by many APCs, DP thymocytes in the thymus cortex play a critical role in positively selecting NKT cells [96–98]. How additional APC populations facilitate the positive selection and development of NKT cells remains an active area of investigation. MAIT cells are specialized to recognize vitamin B2 derivatives presented by the non-classical MHC Ib protein, MR1, and much like NKT cells, undergo positive selection and lineage commitment following interactions with MR1-expressing DP thymocytes [98–101].
Thymocytes may also play a role in selecting MHC II restricted CD4 T cells. Although mouse thymocytes do not express MHC II, human thymocytes do. In a mouse model in which MHC II was expressed solely on T cells, functionally competent CD4+ T cells developed [102, 103]. Further investigation of both mouse and human fetal thymocytes revealed that these cells express promyelocytic leukemia zinc finger protein (PLZF) and subsequently acquire an innate phenotype similar to NKT cells, suggesting a common developmental process driven by PLZF expression [104, 105].
7. Conclusions and Future Directions
Recent studies evaluating the non-redundant roles by which distinct APC subsets mediate thymocyte selection are providing new insights into how the TCR repertoire is shaped. Although the affinity between TCR and self-peptide–MHC may remain the driving factor in thymocyte selection, the context in which self-peptide is presented is becoming an increasingly important factor in determining thymocyte fate. APC subsets within the thymus are localized based on discrete stromal cues and shape the architecture in which thymocytes are selected. Furthermore, each subset provides a distinct framework in which thymocytes are selected, including chemokines, cytokines, and unique self-peptides (Figure 1).
Defining the non-redundant functional capabilities of distinct APC subsets remain major ambitions of future investigations (Box 1). Until we have a more complete understanding of the various roles of thymic APCs, we will not fully understand if and how the breakdown of central tolerance contributes to human autoimmune diseases. A comprehensive understanding of the thymic APCs required for appropriate selection of the T cell repertoire is also needed as the field seeks to develop methods of stem cell based T cell production for purposes of therapeutic T cell reconstitution.
Box 1. Future Questions.
Do specific APCs preferentially promote clonal deletion over Treg differentiation or vice-versa?
How do peripheral antigens differentially impact the TCR repertoire compared to intrathymically-derived self-antigens?
Do specific peptides or TCRs determine the fate of a T cell, or is the cytokine/co-stimulatory context the driving factor?
How does APC localization within the thymus drive thymocyte selection?
Does thymic APC composition/function change over time and does this change promote different tolerance mechanisms?
Acknowledgments
We thank Katharine Block and Tijana Martinov for their feedback on this manuscript. Research in the Hogquist laboratory is supported by the NIH (R37 AI39560 and PO1 AI35296) and T32 training grant support to ERB (T32 AI007313) and STL (T32 GM113846).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nature reviews Immunology. 2014;14(6):377–91. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annual review of biochemistry. 1996;65(1):801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 3.Murata S, Sasaki K, Kishimoto T, Niwa S-i, Hayashi H, Takahama Y, Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316(5829):1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 4.Florea BI, Verdoes M, Li N, van der Linden WA, Geurink PP, van den Elst H, Hofmann T, de Ru A, van Veelen PA, Tanaka K. Activity-based profiling reveals reactivity of the murine thymoproteasome-specific subunit β5t. Chemistry & biology. 2010;17(8):795–801. doi: 10.1016/j.chembiol.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, Koyasu S, Tanaka K, Takahama Y. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32(1):29–40. doi: 10.1016/j.immuni.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Takada K, Van Laethem F, Xing Y, Akane K, Suzuki H, Murata S, Tanaka K, Jameson SC, Singer A, Takahama Y. TCR affinity for thymoproteasome-dependent positively selecting peptides conditions antigen responsiveness in CD8+ T cells. Nature immunology. 2015 doi: 10.1038/ni.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki K, Takada K, Ohte Y, Kondo H, Sorimachi H, Tanaka K, Takahama Y, Murata S. Thymoproteasomes produce unique peptide motifs for positive selection of CD8+ T cells. Nature communications. 2015;6 doi: 10.1038/ncomms8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kincaid EZ, Murata S, Tanaka K, Rock KL. Specialized proteasome subunits have an essential role in the thymic selection of CD8+ T cells. Nature immunology. 2016;17(8):938–945. doi: 10.1038/ni.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing Y, Jameson SC, Hogquist KA. Thymoproteasome subunit-β5T generates peptide-MHC complexes specialized for positive selection. Proceedings of the National Academy of Sciences. 2013;110(17):6979–6984. doi: 10.1073/pnas.1222244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vigneron N, Stroobant V, Chapiro J, Ooms A, Degiovanni G, Morel S, van der Bruggen P, Boon T, Van den Eynde BJ. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304(5670):587–590. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- 11.Dalet A, Robbins PF, Stroobant V, Vigneron N, Li YF, El-Gamil M, Hanada K-i, Yang JC, Rosenberg SA, Van den Eynde BJ. An antigenic peptide produced by reverse splicing and double asparagine deamidation. Proceedings of the National Academy of Sciences. 2011;108(29):E323–E331. doi: 10.1073/pnas.1101892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liepe J, Marino F, Sidney J, Jeko A, Bunting DE, Sette A, Kloetzel PM, Stumpf MP, Heck AJ, Mishto M. A large fraction of HLA class I ligands are proteasome-generated spliced peptides. Science. 2016;354(6310):354–358. doi: 10.1126/science.aaf4384. [DOI] [PubMed] [Google Scholar]
- 13.Gommeaux J, Gregoire C, Nguessan P, Richelme M, Malissen M, Guerder S, Malissen B, Carrier A. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur J Immunol. 2009;39(4):956–64. doi: 10.1002/eji.200839175. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280(5362):450–3. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 15.Honey K, Nakagawa T, Peters C, Rudensky A. Cathepsin L regulates CD4+ T cell selection independently of its effect on invariant chain: a role in the generation of positively selecting peptide ligands. The Journal of experimental medicine. 2002;195(10):1349–58. doi: 10.1084/jem.20011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breloer M, Fleischer B. CD83 regulates lymphocyte maturation, activation and homeostasis. Trends in immunology. 2008;29(4):186–194. doi: 10.1016/j.it.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Tze LE, Horikawa K, Domaschenz H, Howard DR, Roots CM, Rigby RJ, Way DA, Ohmura-Hoshino M, Ishido S, Andoniou CE. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10–driven MARCH1-mediated ubiquitination and degradation. The Journal of experimental medicine. 2011;208(1):149–165. doi: 10.1084/jem.20092203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Žuklys S, Handel A, Zhanybekova S, Govani F, Keller M, Maio S, Mayer CE, Teh HY, Hafen K, Gallone G. Foxn1 regulates key target genes essential for T cell development in postnatal thymic epithelial cells. Nature immunology. 2016;17(10):1206–1215. doi: 10.1038/ni.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Rohrscheidt J, Petrozziello E, Nedjic J, Federle C, Krzyzak L, Ploegh HL, Ishido S, Steinkasserer A, Klein L. Thymic CD4 T cell selection requires attenuation of March8-mediated MHCII turnover in cortical epithelial cells through CD83. The Journal of experimental medicine. 2016;213(9):1685–1694. doi: 10.1084/jem.20160316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwano Y, Prazma CM, Yazawa N, Watanabe R, Ishiura N, Kumanogoh A, Okochi H, Tamaki K, Fujimoto M, Tedder TF. CD83 influences cell-surface MHC class II expression on B cells and other antigen-presenting cells. International immunology. 2007;19(8):977–992. doi: 10.1093/intimm/dxm067. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto Y, Tu L, Miller AS, Bock C, Fujimoto M, Doyle C, Steeber DA, Tedder TF. CD83 expression influences CD4+ T cell development in the thymus. Cell. 2002;108(6):755–767. doi: 10.1016/s0092-8674(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Jain R, Guan J, Vuong V, Ishido S, La Gruta NL, Gray DH, Villadangos JA, Mintern JD. Ubiquitin ligase MARCH 8 cooperates with CD83 to control surface MHC II expression in thymic epithelium and CD4 T cell selection. The Journal of experimental medicine. 2016;213(9):1695–1703. doi: 10.1084/jem.20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura MY, Thomas J, Tai X, Guinter TI, Shinzawa M, Etzensperger R, Li Z, Love P, Nakayama T, Singer A. Timing and duration of MHC I positive selection signals are adjusted in the thymus to prevent lineage errors. Nature immunology. 2016 doi: 10.1038/ni.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4-versus CD8-lineage choice. Nature Reviews Immunology. 2008;8(10):788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uddin MM, Ohigashi I, Motosugi R, Nakayama T, Sakata M, Hamazaki J, Nishito Y, Rode I, Tanaka K, Takemoto T, Murata S, Takahama Y. Foxn1-beta5t transcriptional axis controls CD8+ T-cell production in the thymus. Nature communications. 2017;8:14419. doi: 10.1038/ncomms14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowan JE, McCarthy NI, Parnell SM, White AJ, Bacon A, Serge A, Irla M, Lane PJ, Jenkinson EJ, Jenkinson WE. Differential requirement for CCR4 and CCR7 during the development of innate and adaptive αβT cells in the adult thymus. The Journal of Immunology. 2014;193(3):1204–1212. doi: 10.4049/jimmunol.1400993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Z, Lancaster JN, Sasiponganan C, Ehrlich LI. CCR4 promotes medullary entry and thymocyte–dendritic cell interactions required for central tolerance. The Journal of experimental medicine. 2015;212(11):1947–1965. doi: 10.1084/jem.20150178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24(2):165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. Journal of immunology. 2004;172(7):3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 30.Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proceedings of the National Academy of Sciences. 2009;106(40):17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex–medulla migration of developing thymocytes. The Journal of experimental medicine. 2004;200(4):493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 33.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. The Journal of experimental medicine. 2007;204(11):2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gäbler J, Arnold J, Kyewski B. Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. European journal of immunology. 2007;37(12):3363–3372. doi: 10.1002/eji.200737131. [DOI] [PubMed] [Google Scholar]
- 35.Metzger TC, Khan IS, Gardner JM, Mouchess ML, Johannes KP, Krawisz AK, Skrzypczynska KM, Anderson MS. Lineage tracing and cell ablation identify a post-Aire-expressing thymic epithelial cell population. Cell reports. 2013;5(1):166–179. doi: 10.1016/j.celrep.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishikawa Y, Nishijima H, Matsumoto M, Morimoto J, Hirota F, Takahashi S, Luche H, Fehling HJ, Mouri Y, Matsumoto M. Temporal lineage tracing of Aire-expressing cells reveals a requirement for Aire in their maturation program. The Journal of Immunology. 2014;192(6):2585–2592. doi: 10.4049/jimmunol.1302786. [DOI] [PubMed] [Google Scholar]
- 37.Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, Kodama T, Takayanagi H. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell. 2015;163(4):975–987. doi: 10.1016/j.cell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Lkhagvasuren E, Sakata M, Ohigashi I, Takahama Y. Lymphotoxin β receptor regulates the development of CCL21-expressing subset of postnatal medullary thymic epithelial cells. The Journal of Immunology. 2013;190(10):5110–5117. doi: 10.4049/jimmunol.1203203. [DOI] [PubMed] [Google Scholar]
- 39.Hu Z, Lancaster JN, Ehrlich LI. The contribution of chemokines and migration to the induction of central tolerance in the thymus. Frontiers in immunology. 2015;6 doi: 10.3389/fimmu.2015.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23(2):227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Anderson MS, Su MA. AIRE expands: new roles in immune tolerance and beyond. Nature reviews Immunology. 2016;16(4):247–58. doi: 10.1038/nri.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, Nelson RW, Fife BT, Orr HT, Anderson MS. Tolerance is established in polyclonal CD4+ T cells by distinct mechanisms, according to self-peptide expression patterns. Nature immunology. 2016;17(2):187–195. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29(3):451–63. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nature immunology. 2010;11(6):512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 45.Perry JS, Lio CWJ, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41(3):414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature immunology. 2007;8(4):351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 47.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, Savage PA. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339(6124):1219–24. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin J, Yang L, Silva HM, Trzeciak A, Choi Y, Schwab SR, Dustin ML, Lafaille JJ. Increased generation of Foxp3+ regulatory T cells by manipulating antigen presentation in the thymus. Nature communications. 2016;7 doi: 10.1038/ncomms10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348(6234):589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21(2):267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA. Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity. 2016;44(5):1102–1113. doi: 10.1016/j.immuni.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. The Journal of experimental medicine. 2009;206(6):1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moon JJ, Dash P, Oguin TH, McClaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3− subsets of self-peptide/MHC class II-specific CD4+ T cells. Proceedings of the National Academy of Sciences. 2011;108(35):14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, Sparwasser T, Way SS, Moon JJ. CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity. 2015;43(5):896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, Anderson MS. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):7847–52. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu W, Jiang N, Ebert PJ, Kidd BA, Muller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, Newell EW, Wilson DM, Grotenbreg GM, Valitutti S, Quake SR, Davis MM. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity. 2015;42(5):929–41. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. The Journal of experimental medicine. 2009;206(3):607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity. 2016;45(3):669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bösl MR, Holländer GA, Hayashi Y, de Waal Malefyt R. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. The Journal of experimental medicine. 2011;208(2):383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. The Journal of experimental medicine. 2004;200(8):1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ardouin L, Luche H, Chelbi R, Carpentier S, Shawket A, Sanchis FM, Santa Maria C, Grenot P, Alexandre Y, Grégoire C. Broad and Largely Concordant Molecular Changes Characterize Tolerogenic and Immunogenic Dendritic Cell Maturation in Thymus and Periphery. Immunity. 2016;45(2):305–318. doi: 10.1016/j.immuni.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 62.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nature immunology. 2006;7(10):1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 63.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirpα+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. The Journal of Immunology. 2009;183(5):3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 64.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nature immunology. 2008;9(11):1253–60. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36(3):438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonald BD, Bunker JJ, Erickson SA, Oh-Hora M, Bendelac A. Crossreactive αβ T Cell Receptors Are the Predominant Targets of Thymocyte Negative Selection. Immunity. 2015 doi: 10.1016/j.immuni.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, Jameson SC, Hogquist KA. Murine thymic selection quantified using a unique method to capture deleted T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4679–84. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. The Journal of experimental medicine. 2013;210(2):269–85. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. The Journal of experimental medicine. 2008;205(11):2575–84. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrlich LIR, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31(6):986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kroger CJ, Wang B, Tisch R. Temporal increase in thymocyte negative selection parallels enhanced thymic SIRPalpha+ DC function. Eur J Immunol. 2016;46(10):2352–2362. doi: 10.1002/eji.201646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nature Reviews Immunology. 2011;11(8):558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Atibalentja DF, Murphy KM, Unanue ER. Functional redundancy between thymic CD8alpha+ and Sirpalpha+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. Journal of immunology. 2011;186(3):1421–31. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Förster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proceedings of the National Academy of Sciences. 2007;104(15):6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. Journal of immunology. 2009;183(12):7909–18. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proceedings of the National Academy of Sciences. 2008;105(50):19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leventhal DS, Gilmore DC, Berger JM, Nishi S, Lee V, Malchow S, Kline DE, Kline J, Vander Griend DJ, Huang H. Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells. Immunity. 2016;44(4):847–859. doi: 10.1016/j.immuni.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proceedings of the National Academy of Sciences. 2009;106(25):10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–11. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nature immunology. 2015;16(6):635–41. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loschko J, Rieke GJ, Schreiber HA, Meredith MM, Yao KH, Guermonprez P, Nussenzweig MC. Inducible targeting of cDCs and their subsets in vivo. Journal of immunological methods. 2016;434:32–38. doi: 10.1016/j.jim.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, Gerdes N, Lutgens E, Ishimaru N, Busslinger M. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity. 2015;42(6):1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Lu FT, Yang W, Wang YH, Ma HD, Tang W, Yang JB, Li L, Ansari AA, Lian ZX. Thymic B cells promote thymus-derived regulatory T cell development and proliferation. Journal of autoimmunity. 2015;61:62–72. doi: 10.1016/j.jaut.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 85.Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proceedings of the National Academy of Sciences. 2013;110(42):17011–17016. doi: 10.1073/pnas.1313001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perera J, Zheng Z, Li S, Gudjonson H, Kalinina O, Benichou JI, Block KE, Louzoun Y, Yin D, Chong AS. Self-Antigen-Driven Thymic B Cell Class Switching Promotes T Cell Central Tolerance. Cell Reports. 2016;17(2):387–398. doi: 10.1016/j.celrep.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujihara C, Williams JA, Watanabe M, Jeon H, Sharrow SO, Hodes RJ. T cell–B cell thymic cross-talk: maintenance and function of thymic B cells requires cognate CD40–CD40 ligand interaction. The Journal of Immunology. 2014;193(11):5534–5544. doi: 10.4049/jimmunol.1401655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nuñez S, Moore C, Gao B, Rogers K, Hidalgo Y, Pedro J, Restaino S, Naka Y, Bhagat G, Madsen JC. The human thymus perivascular space is a functional niche for viral-specific plasma cells. Science Immunology. 2016;1(6) doi: 10.1126/sciimmunol.aah4447. eaah4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frommer F, Waisman A. B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PloS one. 2010;5(10):e15372–e15372. doi: 10.1371/journal.pone.0015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kleindienst P, Chretien I, Winkler T, Brocker T. Functional comparison of thymic B cells and dendritic cells in vivo. Blood. 2000;95(8):2610–6. [PubMed] [Google Scholar]
- 91.Munthe LA, Corthay A, Os A, Zangani M, Bogen B. Systemic autoimmune disease caused by autoreactive B cells that receive chronic help from Ig V region-specific T cells. The Journal of Immunology. 2005;175(4):2391–2400. doi: 10.4049/jimmunol.175.4.2391. [DOI] [PubMed] [Google Scholar]
- 92.Detanico T, Heiser RA, Aviszus K, Bonorino C, Wysocki LJ. Self-tolerance checkpoints in CD4 T cells specific for a peptide derived from the B cell antigen receptor. The Journal of Immunology. 2011;187(1):82–91. doi: 10.4049/jimmunol.1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walters SN, Webster KE, Daley S, Grey ST. A role for intrathymic B cells in the generation of natural regulatory T cells. The Journal of Immunology. 2014;193(1):170–176. doi: 10.4049/jimmunol.1302519. [DOI] [PubMed] [Google Scholar]
- 94.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nature reviews Immunology. 2012;12(3):157–67. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 95.Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small-molecule display by CD1 and MR1. Nature reviews Immunology. 2015;15(10):643–54. doi: 10.1038/nri3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. Journal of immunology. 2000;164(5):2412–8. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 97.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. The Journal of experimental medicine. 2005;202(2):239–48. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nature immunology. 2015;16(11):1114–23. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 99.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–23. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 100.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. The Journal of experimental medicine. 1999;189(12):1907–21. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S, Soudais C, Lantz O. Double-positive thymocytes select mucosal-associated invariant T cells. Journal of immunology. 2013;191(12):6002–9. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 102.Choi EY, Jung KC, Park HJ, Chung DH, Song JS, Yang SD, Simpson E, Park SH. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005;23(4):387–96. doi: 10.1016/j.immuni.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Kim MG, Gourley TS, McCarthy BP, Sant’Angelo DB, Chang CH. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005;23(4):375–86. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 104.Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY, Jung KC, Park SH. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. The Journal of experimental medicine. 2010;207(1):237–46. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee YJ, Jung KC, Park SH. MHC class II-dependent T-T interactions create a diverse, functional and immunoregulatory reaction circle. Immunology and cell biology. 2009;87(1):65–71. doi: 10.1038/icb.2008.85. [DOI] [PubMed] [Google Scholar]


