Abstract
Application of high-content immune profiling technologies has enormous potential to advance medicine. Whether these technologies reveal pertinent biology when implemented in interventional clinical trials is an important question. The beneficial effects of pre-operative arginine-enriched supplements (AES) are highly context-specific as they reduce infection rates in elective surgery, but possibly increase morbidity in critically ill patients. This study combined single-cell mass cytometry with the multiplex analysis of relevant plasma cytokines to comprehensively profile the immune-modifying effects of this much-debated intervention in patients undergoing surgery. An EN algorithm applied to the high-dimensional mass cytometry dataset identified a cross-validated model consisting of twenty interrelated immune features that separated patients assigned to AES from controls. The model revealed wide-ranging effects of AES on innate and adaptive immune compartments. Notably, AES increased STAT1 and STAT3 signaling responses in lymphoid cell subsets after surgery, consistent with enhanced adaptive mechanisms that may protect against postsurgical infection. Unexpectedly, AES also increased ERK and P38 MAPKs signaling responses in M-MDSCs, which was paired with their pronounced expansion. These results provide novel mechanistic arguments as to why AES may exert context-specific beneficial or adverse effects in patients with critical illness. This study lays out an analytical framework to distill high dimensional datasets gathered in an interventional clinical trial into a fairly simple model that converges with known biology and provides insight into novel and clinically relevant cellular mechanisms.
INTRODUCTION
Recent developments in multiplexed, high-content immune profiling technologies have enormous potential to advance our understanding of the biology that drives disease processes and restores human health. Among these technologies, mass cytometry is increasingly implemented in clinical studies for the high-resolution surveillance of human circulating immune cells in response to clinically relevant perturbations (1–6). A pertinent study recently revealed immune signatures that predicted the rate of clinical recovery in patients undergoing surgery (1,7,8). With the emerging promise of mass cytometry (and other high-parameter flow cytometry platforms) to discover novel molecular metrics for advancing precision medicine, demonstrating the utility of these technologies to comprehensively profile therapeutic interventions becomes paramount.
We applied mass cytometry to immune-profile a pharmaconutrient that is widely used in patients undergoing surgery. Specifically, we studied a commercially available arginine-enriched dietary supplement (AES) that reduces the risk of infection in patients undergoing elective surgery, but possibly increases morbidity in critically ill patients (9,10). Arginine plays a critical role in T cell proliferation, differentiation, and function (11–13), and in a murine model of surgical injury, surgery-induced arginine depletion has been linked to T cell dysfunction and an increased infection risk (14).
Previous studies in patients receiving perioperative AES have provided important insight on the effects of immuno-nutrition on aspects of the human immune response to surgery. These studies have focused on certain circulating factors (15), the distribution of pooled immune cell subsets (e.g., T cells, B cells, neutrophils) (16), changes in the expression of select surface markers (e.g. CD3 expression, HLA-DR expression), or the functional analysis of isolated immune cells in ex vivo assays (e.g. phagocytosis of neutrophils) (17,18). However, technological limitations did not allow for the comprehensive phenotyping of all major immune cell subsets or the functional analysis of intracellular signaling activities as they occur in vivo. Furthermore, the statistical interpretation of high-dimensional immunological data presents an analytical challenge that has thus far precluded a system-wide characterization of the immune-modifying properties of AES in patients undergoing surgery.
Chosen experimental and clinical setting were therefore appealing to examine two major questions, i.e., whether “bedside” application of mass cytometry would allow detecting expected and potentially novel immunological effects of an accepted clinical intervention with proven benefits, and whether application of a machine learning algorithm particularly adapted to the analysis of highly correlated and complex immunological data would capture known biology and provide novel insight into cellular mechanisms that may explain the context-specific clinical effects of AES (9,10).
MATERIALS AND METHODS
Study design
The aim of this prospective, randomized clinical trial was to comprehensively characterize the molecular effect of AES on the human inflammatory response to surgical trauma from the combined proteomic and mass cytometry analysis of peripheral blood samples from patients undergoing abdominal surgery. The study was conducted between 08/19/2013 and 06/03/2015 at Stanford Medicine. The study used a randomized, controlled, and open-label design as only objective outcomes were assessed. Research Randomizer (https://www.randomizer.org) was used for patient treatment allocation. Patients randomized to arginine-rich supplements were asked to drink 4 containers (237 ml) of Impact® every day for 5 days before surgery. One container of impact contains 4.2 g of L-arginine. Randomization was performed by a study nurse. No adverse effects attributable to the intervention were observed. Exclusion and inclusion criteria and consort chart are available in the supplementary materials (Supplemental Fig. 1).
Anesthesia protocol
Anesthesia care was standardized to the use of fentanyl and hydromorphone as intravenous analgesics. Analgesics were dosed to maintain the blood pressure and heart rate within 20% of baseline during surgery, and keep pain levels ≤ 4/10 on a 10-point numerical pain rating scale after emergence from anesthesia. Anesthesia was induced with propofol and rocuronium and maintained with the volatile anesthetic sevoflurane. Medications with potential immune-modulatory effects including steroids, ketamine, and intravenous local anesthetics were not allowed. No violations of this protocol were noted.
Deviations from study protocol
One patient in the AIN group took the supplement for 4 rather than 5 days. One patient in the AIN group and on adalimumab (40 mg once every 2 weeks) stopped therapy 2 rather than 4 weeks before surgery, and one patient in the control group on mercaptopurine (50 mg every other day) stopped therapy 1 rather than 4 weeks before surgery.
Mass cytometry analysis of peri-operative whole blood samples
Serial blood samples collected at all peri-operative time points (5 days and 1h before surgery, then 1h, 1 day, and 3 days after surgery) were processed using a standardized protocol for fixation (Smart Tube Inc., San Carlos, CA), storage, and antibody staining of whole blood samples for mass cytometry analysis (1,7,19). Extracellular and intracellular antibodies used in the analysis are described in Supplementary Table 2. To minimize experimental variability, samples corresponding to an entire time series were barcoded, stained, and run simultaneously on the mass cytometry instrument (20,21). To maximize the sensitivity of the assay for detection of differences between the AES and control group, sample time series from patients in the AES group were randomly paired with samples from patients in the control group, and paired sample time series were barcoded and run using the same barcode plate. Barcoded samples were analyzed at a flow rate of approximately 500 cells/sec on a CyTOF 2.0 mass cytometer (Fluidigm). Samples were normalized and de-barcoded as described previously (20,74).
Multiplex cytokine analysis of peri-operative plasma samples
Multiplex analyses of plasma cytokines were performed in the Human Immune Monitoring Center (Institute for Immunity, Transplantation, and Infection, Stanford Medicine) using Luminex Human 63-plex kits from eBiosciences/Affymetrix according to the manufacturer’s recommendations.
Derivations of immune features
Mass cytometry data from each sample were manually gated into 23 immune-cell types of interest (Supplemental Fig. 2). Immune cell subsets were selected on the basis that immune features included in the current analysis would capture at least all innate and adaptive immune responses previously detected in our mass cytometry analysis of patients’ undergoing surgery (1).
Cell frequency features
Cell frequencies were expressed as a percentage of gated singlets in the case of granulocytes, and as a percentage of mononuclear cells (CD45+CD66−) in the case of all other cell types. For each cell type, frequency features were calculated as the difference in cell frequency between each post-operative time point and the 1h pre-operative time point.
Cell signaling features
The signal intensity of the following functional markers was simultaneously quantified per single cell: phospho (p) STAT1, pSTAT3, pSTAT5, pNF-κB, total IκB, pMAPKAPK2, pP38, prpS6, pERK1/2, and pCREB. For each cell type, signaling immune features were calculated as the difference in median signal intensity (arcsinh transformed value) of each signaling protein between each post-operative time points and the 1h pre-operative time point.
Cytokine features
For each of the 63 plasma analytes, cytokine features were calculated as the difference in mean fluorescence intensity between each post-operative time point and the 1h pre-operative time point.
Identification of Myeloid-Derived Suppressor Cells
A number of studies in mouse and human models have documented the accumulation of MDSCs (both granulocytic and monocytic MDSCs) during acute inflammatory processes such as traumatic stress, burn injury, and sepsis (22–26). In this study, we followed recent recommendations aiming to standardize MDSC nomenclature to define monocytic MDSCs (M-MDSCs) (27). M-MDSCs were defined 1) phenotypically as lineage negative (CD66−CD15−CD3−CD19−CD7−), CD11b+CD33+CD14+HLA-DRlow; and 2) functionally by their ability to suppress CD4+ and CD8+ T cell proliferation in a standard MDSC suppression assay (see Supplemental Fig. 3). The antibody panel utilized for the gating of immune-cell from whole-blood samples did not allow Granulocytic MDSCs (G-MDSCs) to be distinguished from neutrophils, which are also CD66+CD15+CD11b+ and CD14−HLA-DRlow. G-MDSCs were therefore not included in the analysis.
T cell suppression assays
As previously shown in the context of orthopedic surgery (1), M-MDSC frequency increased over 5-fold after abdominal surgery and peaked at the 24h time point (Supplemental Fig. 3B). T cell suppression assays were therefore performed with T cells isolated from samples collected before surgery and M-MDSCs isolated from samples collected 24h after surgery. Briefly, peripheral blood mononuclear cells (PBMC) were isolated before and 24h after surgery from blood samples collected from five patients undergoing abdominal surgery. Fresh PBMCs isolated before surgery were used as source for responder T cells. CD2+ T cells were subsequently enriched from carboxyfluorescien succinimdyl ester (CFSE)-labeled PBMCs using a CD2 positive selection kit (Stem Cell Technologies) according to the manufacturer’s protocol. Fresh PBMCs isolated 24h after surgery were used as source of M-MDSCs. PBMCs were incubated in the presence of fluorescent mAbs (Supplemental Table 3). M-MDSCs were identified as lin−CD14+CD11b+HLA-DRlow cells and sorted on a FACSAria sorter (BD Biosciences). Enriched T cells were cultured for 5 days at 37°C in the presence of anti-CD3/CD28 microbeads (Dynalbeads, Thermo Fisher Scientific) either alone or in the presence of M-MDSCs (1 suppressor cell per 2 T cells). Cells were then collected and stained with fluorescent antibodies (Supplemental Table 3) to identify CD4+ and CD8+ T cells and analyzed by flow cytometry on an LSRII. Proliferation was quantified for each gated cell type as percent CFSEdim cells.
Statistical analysis
Sample size: Based on previous data documenting the activation of STAT3 and MAPK signaling pathways in M-MDSCs and their rapid expansion after surgery (1), a sample size of 10 patients in each group provided 80% power at p<0.05 to detect an intervention-related change in STAT3 phosphorylation in M-MDSCs ≥ 40%.
Correlation network
The correlation network consists of a minimum spanning tree of a graph on which the weight of each edge is the inverse of the absolute value of the Spearman correlation between the two respective immune features (Fig. 1). To visualize the modularity of the network, edges with a significant correlation p-value (after Bonferroni adjustment) were added to the graph. The graph layout was calculated using the Large Graph Layout algorithm (28) as implemented by the R package (3.2.2) igraph (1.0.1).
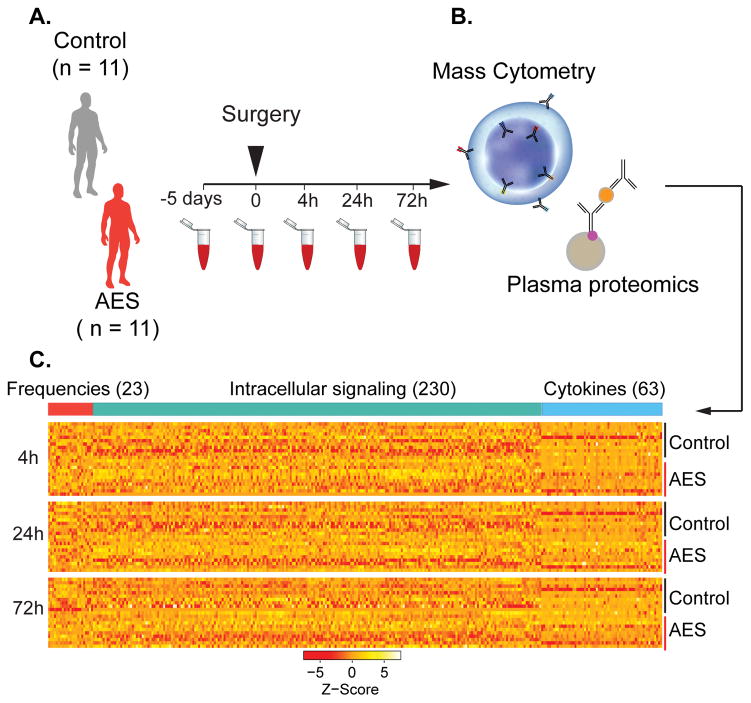
FIGURE 1. Experimental flowchart and analytical approach.
(A) Whole blood was obtained 5d and 1h before surgery, and 4h, 24h, and 72h after surgery. (B) Aliquots were stained with cell surface and intra-cellular antibodies and analyzed with mass cytometry. Plasma proteins were measured using a Luminex 63-plex assay. (C) Assays produced three data layers providing information about cell frequencies (red bar), cell signaling (green bar), and plasma protein concentrations (blue bar).
Elastic net analysis
Elastic net analysis was performed using the R package (3.2.2) glmnet (2.0). All parameters were set to default except for alpha = 0.5 (to limit the number of selected features but account for most important components of each intracorrelated module) and standardize = FALSE to enable the modifications described above.
Handling of missing values
Two samples (the 4h sample from one patient and the 24h sample from another patient) did not contain a sufficient number of cells for analysis. Immune feature values for these two samples were set to the average of the respective values from the entire cohort.
Data resources
Raw data, manually gated cell-types, and plasma cytokines are available for download from http://flowrepository.org/experiments/1021.
RESULTS
Subjects, surgical parameters, and arginine plasma concentrations
A representative sample of patients undergoing major abdominal surgery was randomized to a five-day preoperative intervention with arginine-enriched dietary supplements (AES, Impact®, Nestle HealthCare Nutrition Inc., Florham Park, NJ) or routine preoperative care without supplement. The study was registered at ClinicalTrials.gov on June 18, 2013 (NCT01885728). Abdominal surgery was chosen because most compelling evidence for beneficial effects of AES on postoperative infection rates exists for this type of surgery (10).
Participant flow is summarized in Supplemental Fig. 1 according to CONSORT recommendations (Consolidated Standards of Reporting Trials). Two hundred and forty-one patients were screened for eligibility and 135 patients were eligible; ultimately 22 patients (16%) were randomized and included in the final analysis. Of the 135 eligible patients 33% declined or withdraw early during the study, while an unexpectedly high percentage of patients (43%) could not be randomized due to logistic challenges. These predominantly included late scheduling for the first preoperative visit or the final date of surgery, which precluded preoperative treatment with AES for five days. Of the 22 patients completing the study, eleven patients received AES before surgery (AES group), and 11 patients served as controls (control group). Study groups were evenly matched except for age (control group 47.7 ± 12.9 years, AES group 61.8 ± 7.9 years). Complications including infections within 30 days after surgery were nominally twice as high in the control group compared to the AES group (29). Patient demographics, clinical diagnoses, surgical procedures, surgical and anesthetic parameters, and postoperative complications are listed in Table 1.
Table 1.
Patient and procedural characteristics
| Demographics (% of patients) | Control (n = 11) | Arginine (n = 11) | t-test/chi square |
|---|---|---|---|
| Gender | |||
| Female (%) | 36.4 | 27.3 | ns |
| Male (%) | 63.6 | 72.7 | |
| Race | |||
| African American (%) | 9.1 | 9.1 | ns |
| Asian (%) | 27.3 | 0.0 | ns |
| Caucasian (%) | 45.4 | 63.6 | ns |
| Unknown (%) | 18.2 | 27.3 | ns |
| Ethnicity (% Hispanic/Latino) | 18.2 | 18.2 | ns |
| Age (year; mean±SD) | 47.7±12.9 | 61.8±7.9 | −3.08** |
| Body mass index (kg/m2; mean±SD) | 24.8±5.4 | 28.7±3.3 | ns |
|
| |||
| Diagnosis (% of patients) | |||
|
| |||
| Malignancy (%) | 63.6 | 81.8 | ns |
| Colon Carcinoma (%) | 27.3 | 36.4 | |
| Rectal Carcinoma (%) | 36.4 | 45.5 | |
| Non-cancerous conditions (%) | 36.4 | 18.2 | ns |
| Diverticulitis/colitis | 27.2 | 18.2 | |
| Partial obstruction | 9.1 | 0.0 | |
| History of chemo-radiation >1 month (%) | 27.3 | 27.3 | ns |
|
| |||
| Surgical procedure (% of patients) | |||
|
| |||
| Segmental colectomy (%) | 36.4 | 45.5 | ns |
| Proctectomy (%) | 45.5 | 54.5 | ns |
| Small bowel operations (%) | 18.2 | 0.0 | ns |
| Laparoscopic (%) | 63.6 | 81.8 | ns |
| Open (%) | 36.4 | 18.2 | ns |
| Stoma (%) | 36.4 | 45.5 | ns |
|
| |||
| Surgical and anesthetic parameters | |||
|
| |||
| Patient ASA class (median; range) | 2 (2–3) | 3 (2–3) | ns |
| Surgery Duration (min; mean±SD) | 183±101 | 159±59 | ns |
| Anesthesia Duration (min; mean±SD) | 228±100 | 208±56 | ns |
| Blood loss (ml; mean±SD) | 81±61 | 82±60 | ns |
| Urine output (ml; mean±SD) | 239±143 | 269±162 | ns |
| Fluids | |||
| Crystalloids (ml; mean±SD) | 1160±425 | 1261±307 | ns |
| Colloids (ml; mean±SD) | 100±211 | 73±168 | ns |
| Blood products (ml; mean±SD) | 0 | 0 | ns |
|
| |||
| Postoperative complications1 (% of patients) | |||
|
| |||
| Any complication (%) | 54.5 | 27.3 | ns |
| Grade 1 | 0.0 | 9.1 | |
| Grade 2 | 27.3 | 0.0 | |
| Grade 3 | 27.3 | 18.2 | |
| Grade 4 | 0.0 | 0.0 | |
| Grade 5 | 0.0 | 0.0 | |
| Infection (%) | 36.4 | 18.2 | ns |
| Abscess | 9.1 | 18.2 | |
| Wound | 18.2 | 0.0 | |
| Pouch | 9.1 | 0.0 | |
p = 0.007
Clavien-Dindo classification: Grade 1 = minor deteriorations without need of specific treatment, grade 2: treatment with drugs, blood transfusion, or physiotherapy, grade 3: require interventional or operative treatment, grade 4 = life-threatening requiring management in intensive care unit management, grade 5 = death
The median plasma concentration of arginine increased significantly from 91 nmol/mL (IQR 87–121) five days before surgery to 138 nmol/mL (IQR 111–142) 1 hour before surgery in the AES group, indicating successful pre-surgical arginine-enrichment in this patient group (p-value = 0.01). Corresponding plasma concentrations in the control group were 87 nmol/mL (IQR 80–137) and 97 nmol/mL (IQR 71–128). Observed increase in plasma arginine concentration after AES supplementation (averaging 51%) was consistent with observed increases in previous studies demonstrating clinical benefits of AES (18,30).
Deep cellular and proteomic profiling of patients’ immune response to surgery
Serial whole blood and plasma samples were collected starting 5 days before surgery and ending three days after surgery (Fig. 1A). Deep immune and proteomic profiling of patient samples with single-cell mass cytometry and a multiplexed proteomic platform revealed surgery-induced changes in immune cell frequency, immune cell signaling, and plasma cytokine concentrations (Fig. 1B, see Supplemental Fig. 2 for gating strategy). Three hundred and sixteen immune features were captured per time point including the frequency of 23 cell types, the activity of 10 signaling proteins in each cell type, and the plasma concentration of 63 cytokines (Fig. 1C).
Examination of select immune features in samples from the control group indicated that abdominal surgery produced profound cell frequency, cell signaling, and cytokine response patterns (Fig. 2A–C) that recapitulated patterns previously described in the context of surgical and traumatic injury (1,31,32). The three-layered dataset built a correlation network that characterized each patient’s immune response to surgery (Fig. 2D). A minimum spanning tree algorithm juxtaposed immune features that were most tightly correlated. While many correlations were observed within the same data layer, a significant number of correlations were also observed between data layers. These findings highlight the complexity and inter-connectivity of surgery-induced immune changes. Construction of this network provided the structural basis for further computational analysis.
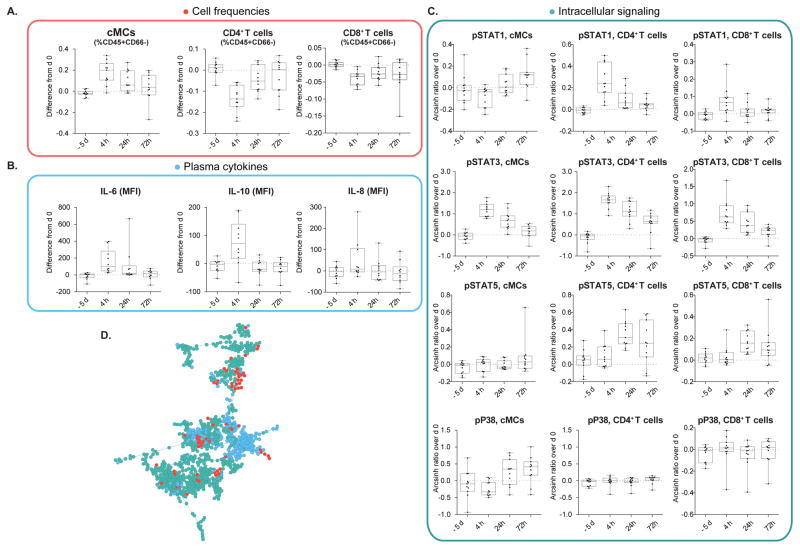
FIGURE 2. Abdominal surgery elicits canonical immune responses.
Representative changes in cell frequency, intracellular signaling, and cytokine plasma concentration are shown. The same changes have previously been described in patients undergoing other types of surgery (1,31). Depicted are changes observed in patients from the control group (n = 11). Changes are calculated as the difference in cell frequency (%CD45+CD66− cells), intracellular signaling activity (Arcsinh transform of mass cytometry signal), and cytokine plasma concentration (mean fluorescence intensity, MFI) between peri-operative time points (−5d, 4h, 24h, 72h) and day 0 (1h before surgery). Box plots represent median and interquartile range. (A) Classical monocytes (cMCs) increased, CD4+ T decreased, and CD8+ T cells decreased in frequency after surgery. (B) Increased signal transducer and activator of transcription (STAT) 1, STAT3, and STAT5 phosphorylation in cMCs, CD4+ T, and CD8+ T cell subsets (4h, 24h, or 72h depending on cell type) and increased phosphorylation of mitogen-activated protein kinase P38 (24h, 72h) in cMCs (but not in CD4+, or CD8+T cells) also recapitulate sentinel cell-type specific signaling changes previously observed in patients undergoing orthopedic surgery (1). (C) Plasma concentrations of IL-6, IL-8, and IL-10 increased after surgery. (D) The entire dataset composed of 316 immune features is represented by a minimum spanning tree emphasizing the correlations between the tree categories of immune features (red dots, cell frequencies, green dots, cell signaling; blue dots, plasma cytokines).
An elastic net analysis reveals specific immune features that are modulated by arginine-enriched supplements
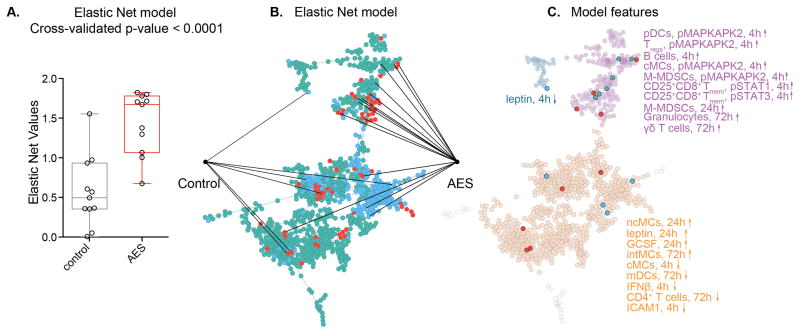
An elastic net (EN) algorithm (33) was applied to extract the components of the correlation network that best differentiated the immune response to surgery between the AES and control groups. The EN is a penalized regression method particularly adapted to the analysis of highly correlated data as it eliminates redundant parameters, while retaining inter-related parameters (34–37). This approach identified a cross-validated model that separated the AES from the control group (leave-one-out cross validated p-value = 0.0001, Fig. 3A). The model consisted of 20 immune features including 9 differences in cell frequency, 6 differences in cell signaling, and 5 differences in plasma cytokine concentrations. Fourteen features were increased and 6 features were decreased in the AES group (Fig. 3B).
FIGURE 3. An Elastic Net analysis identifies a cross-validated model of interrelated immune features separating patients treated with AES from controls.
(A) An elastic net (EN) algorithm extracted 20 immune features from the correlation network that differentiated the immune response to surgery between patients randomized to arginine-enriched supplements (AES) from controls (cross validated, p = 0.0001). (B) The EN features were extracted from 3 visually segregated modules. Fourteen EN features were higher and 6 features were lower in the arginine group. (C) Ten EN features projected onto module 1 (purple dots) 9 features projected onto module 2 (orange dots), and one single feature projected onto module 3 (light blue dots).
EN features resided within 3 modules that visually segregated the correlation network into distinct sets of correlated parameters (Fig. 3C). All EN features within module 1 (purple) were increased in the AES group. They included changes in the frequency of B cells (4h), M-MDSCs (1d), granulocytes (3d), and γδ T cells (3d). They also included changes in signaling activity of MAPKAPK2 (4h) in M-MDSCs, cMCs, pDCs, and Tregs, and changes in signaling activity of STAT1 and STAT3 (4h) in CD25+CD8+ Tmem cells. EN features within module 2 (orange) included increased frequencies of ncMCs (24h), intMCs (72h), mDCs (72h), and CD4+ T cells (72h), as well as a decreased frequency of cMCs (4h) in the AES group. They also included higher concentrations of the plasma cytokines leptin (24h) and G-CSF (24h), and decreased concentrations of IFNβ (4h) and ICAM1 (4h) in the AES group. Module 3 (blue) contained the plasma protein leptin (4h) as the only parameter, which was decreased in the AES group.
Several broad themes became apparent when examining the EN model. First, high-dimensional mass cytometry sensitively captured the modifying effects of the pre-operative intervention (arginine-enriched supplements) on endogenous cellular immune response in patients undergoing surgery. Second, the components of the EN model separating the AES group from the control group were embedded in a larger correlation network emphasizing that changes in cell frequency, cell signaling activity, and plasma cytokine concentrations are highly inter-related rather than isolated events. Third, the nutritional intervention modulated a wide array of cell types and signaling events encompassing the innate and adaptive branch of the immune system. These findings highlight the utility of high-parameter single-cell immune profiling and an EN approach to comprehensively characterize the complex modulation of the immune system with a common clinical nutritional intervention.
Elastic net features are proxies reflecting broader immunological changes
The EN analysis provided a list of 20 inter-related immune features. However, the biological interpretation of this multivariate output requires further consideration. EN analysis is a statistical approach that markedly reduced the high-dimensional correlation network to a set of stringent and inter-related but not redundant parameters that differentiate the two study groups. However, the EN may not reveal all biologically meaningful parameters that separate the two groups. As such, EN parameters can be viewed as particularly stringent proxies that can reveal broader biology upon further examination.
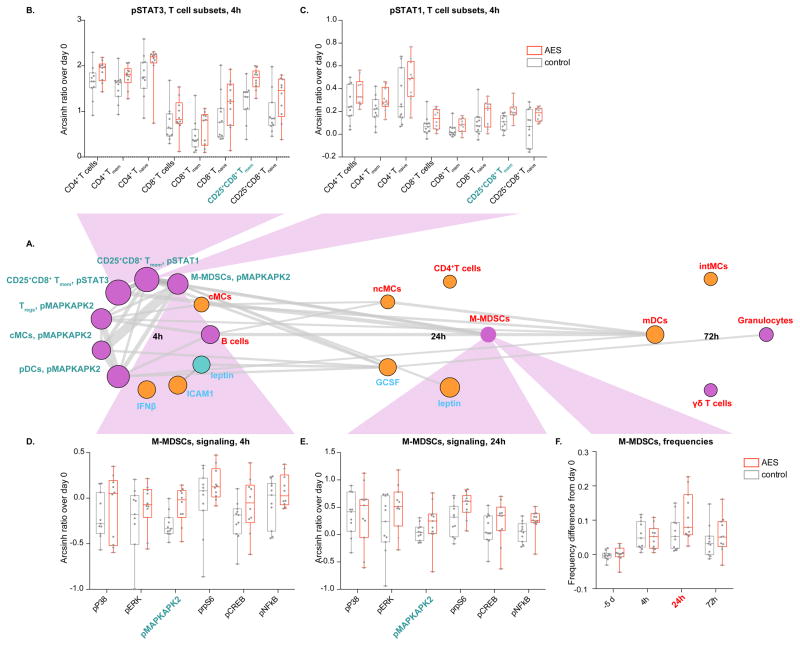
From a biological perspective, intracellular signaling changes are particularly informative as they are intimately associated with cell function. All signaling changes captured by the EN occurred early (4 hours) after surgery (Fig. 4A). They were prominent for STAT1 and STAT3 signaling in CD25+CD8+ Tmem cells and MAPKAPK2 signaling in M-MDSCs, cMCs, pDCs, and Tregs. These signaling changes were tightly interlinked but also correlated with changes in cell frequency (including M-MDSC frequencies) that occurred later in the postoperative course, i.e., 24 and 72 hours after surgery.
FIGURE 4. Elastic net features are proxies that reveal broader immunological effects of arginine-enriched supplements (AES).
(A) EN features are grouped chronologically (color-coded according to correlation network). Size of circle indicates relative statistical strength. Thickness of grey lines indicates correlation strength between features (Spearman’s coefficient). Signaling changes occurred 4h after surgery and were prominent for pSTAT1/3 in CD25+CD8+ T memory cells (Tmem cells), and pMAPKAPK2 in monocytic myeloid-derived suppressor cells (M-MDSCs), classical monocytes (cMCs), plasmacytoid dendritic cells (pDCs), and regulatory T cells (Tregs). (B) Increased STAT3 signaling in CD25+CD8+ Tmem cells (AES group) was reflected in several CD4+ and CD8+ T-cell subsets indicating that the EN parameter “STAT3 signaling in CD25+CD8+ Tmem cells” acted as a proxy revealing broad effects of AES on STAT3 signaling in T cells. (C) The same findings applied to STAT1. (D) Increased MAPKAPK2 signaling in M-MDSCs 4h after surgery (AES group) was reflected along the P38 and ERK1/2 MAPK signaling pathways (pP38, pERK, pS6, pCREB, and NF-kB) indicating that the net parameter “MAPKAPK2 signaling in M-MDSCs” acted as a proxy revealing consistent changes along this pathway. (E) Increased signaling in M-MDSCs along the MAPK pathway was also present at 24h. (F) Accentuated expansion of M-MDSCs (AES group) 24h after surgery was echoed at 72h.
While the EN specifically captured differences in STAT1 and STAT3 signaling in CD25+CD8+ Tmem cells, consistent directional differences were found across all phenotyped CD4+, and CD8+ T cells (Fig. 4B and C). These results indicate that the EN features “STAT1 and STAT3 signaling in CD25+CD8+ Tmem cells” acted as proxy that revealed broad effects of AES on STAT1 and STAT3 signaling in multiple adaptive cell subsets. Similarly, while the EN specifically captured differences in MAPKAPK2 signaling in M-MDSCs 4 hours after surgery, such differences were reflected across many components of the P38 and ERK MAPK signaling pathways including P38, ERK, rpS6, CREB, and NF-κB 4 hours and/or 24 hours after surgery (Fig. 4D and E). These results indicate that the EN parameter “MAPKAPK2 signaling in M-MDSCs” was a proxy revealing consistent directional differences along the MAPK pathway, which in turn corroborated the biological significance of the EN parameter. Interestingly, increased MAPKAPK2 signaling in M-MDSCs was linked to the expansion of M-MDSCs at 24 hours, and such expansion was also seen at 72 hours (Fig. 4F).
Taken together, the EN analysis identified an immune signature that specified the effect of AES on innate and adaptive immune responses to surgery. A post-hoc multiple linear regression analysis estimating whether demographic (age, sex, race), clinical (preoperative diagnosis of malignancy), or surgical (open vs. laparoscopic surgery) variables confounded the effect of AES indicated that the EN model remained significant after controlling for these variables (residual p-value = 0.001, Supplemental Table 1). Similarly, after controlling for these demographic, clinical and surgical variables the effect of AES treatment remained significant as an independent predictor of key EN model components, including the pSTAT1 and pSTAT3 signal in CD8+ T cell subsets, and the pMAPKAPK2 signal in M-MDSCs.
DISCUSSION
This study combined the high-resolution functional profiling of circulating immune cells from patients undergoing surgery with multiplex analysis of plasma factors to capture the immunological fingerprint of arginine-enriched nutritional supplements (AES), a pre-operative intervention that decreases infectious complications after abdominal surgery. An EN algorithm extracted inter-related immune features that separated patients randomized to AES from controls and pointed to biologically relevant innate and adaptive mechanisms modified by the pre-operative intervention.
EN features should be viewed as statistically stringent proxies that can be linked to broader biological information. Notably, in the absence of prior knowledge EN proxies pointed to signaling responses and frequency changes in immune cell subsets that are widely discussed in the context of AES. As such, our results integrate well with previous findings highlighting the interplay between arginine, MDSCs, and T cells in trauma and surgery: within hours of trauma, plasma arginine levels decrease (38,39) leading to multiple T-cell dysfunctions including the downregulation of operational T-cell receptors, decreased cell proliferation, and cytokine production (40). A sentinel role for these changes has been attributed to MDSCs. MDSCs are a heterogeneous population of immune cells with immunosuppressive properties, consisting of immature granulocytes (G-MDSCs) and monocytes (M- and early stage MDSCs) that accumulate in the context of malignancies, sepsis, and severe trauma (26,27,41,42), and metabolize arginine at a high rate (24,43). Counteracting the surgery-induced depletion of arginine stores has been proposed as an important mechanism for the clinical observation that AES reduces infection rates in patients undergoing surgery (10,44).
In our analysis, the proxies “STAT1 and STAT3 signaling in CD25+CD8+ Tmem cells” (elevated in the AES group) revealed that the same directional signaling changes occurred in many CD4+, and CD8+ T cell subsets. STAT1 and STAT3 regulate numerous T cell functions downstream of type I and type II cytokine receptors, including cell proliferation, survival, effector functions, and differentiation into specific helper T cell subsets (45–47). The simultaneous increase in MAPKAPK2 signaling observed in dendritic cells and classical monocytes (Fig. 3C) indicates that the nutritional intervention may also upregulate shared mechanisms activated downstream of pattern recognition receptors in the setting of acute inflammation (48). Together these enhanced innate and adaptive immune responses may be protective against postsurgical infections by improving the host’s ability to mount an efficient pathogen response (49).
Interestingly, changes in plasma cytokines engaging the JAK/STAT1 (Type I interferons and interferon-gamma) or the JAK/STAT3 pathway (including IL-6, IL-8, and IL-10) could not account for increased STAT1 or STAT3 signaling in the AES group. While plasma concentrations of IL-6, IL-8, and IL-10 increased after surgery (Fig. 2B), these increases were not different between the AES and control groups. These results suggest that other factors modulated by AES likely contributed to increases in STAT1/3 signaling. One possibility is that AES activated the mechanistic target of rapamycin (mTOR), a key metabolic regulator and sensor of amino acids (50,51). Availability of intracellular arginine is critical for T cell survival, and function (13). Recent work highlights cross-talk between mTOR and JAK/STAT pathways – particularly the JAK/STAT3 pathway – as they synergistically regulate T cell differentiation (52,53). Peri-operative AES may therefore alter T cell function by enhancing JAK/STAT signaling via mTOR activation. This hypothesis derived from an agnostic analysis of the high-dimensional dataset will guide further investigation.
The EN model also pointed to enhanced activation and expansion of M-MDSCs in surgical patients receiving AES, a novel and surprising finding. Further examination of signaling responses in M-MDSCs suggested that AES increased the phosphorylation of multiple members of the MAPK signaling pathway (including P38, ERK, MAPKAPK2, rpS6, CREB, and NF-κB), a critical component of the MyD88-mediated Toll-like receptor (TLR) signal transduction (54). TLR2 and TLR4 in particular are primed and activated in response to surgical trauma by endogenous ligands released from damaged tissue (55–57). In rodent models, arginine supplementation has been shown to facilitate MAPK activation downstream of TLR4 (58). Results thus indicate that AES may exacerbate the activation of the MAPK pathway downstream of TLRs in response to tissue injury, thereby facilitating the expansion of M-MDSCs after surgery (Fig. 2). Of note, the more pronounced expansion of M-MDSCs in patients receiving AES seems to contradict findings of a recent study suggesting that AES decreased M-MDSC frequency after surgery (16). However, expression of HLA-DR, a critical phenotypical marker of human M-MDSCs, was not assessed in this recent study. As such, reported decrease in cell frequency cannot clearly be attributed to M-MDSCs.
MDSCs arise as a conserved response to acute inflammatory processes (such as trauma or sepsis) to protect the organism from an uncontrolled immune response (11). However, prolonged expansion of MDSCs can drive pathological states in chronic illness and cancer (25,42). The observed expansion of M-MDSCs provides a mechanistic argument in response to the clinical dilemma surrounding the benefits or harm of arginine supplementation in critically ill patients (59). A review emphasizing studies of high methodological quality suggested that AES may increase mortality in critically ill patients (60). However, studies in septic patients with only moderate illness reported decreased mortality and infection rates in patients receiving AES (61), a finding further supported by animal experiments (62,63). By contrast a recent study in patients with severe sepsis linked the persistent elevation of MDSCs to increased infection, prolonged intensive care treatments, and poor functional status (25). It has become clear that the biological role of MDSCs is highly contextual, and dependent on the type, severity, and chronicity of a disease (26). A better understanding of the interplay between AES, MDSCs, and potential beneficial or adverse clinical outcomes will require clinical trials that carefully describe the functional properties of MDSCs and clinical characteristics of the studied patient population.
The role of MDSCs in facilitating tumor growth and metastasis is well established. Ample evidence supports a close association between MDSCs and clinical outcomes in cancer patients (64,65). The increased abundance of M-MDSCs observed in samples from patients treated with AES raises the question whether such expansion could have negative consequences in cancer patients undergoing surgery. To address this question, future studies will need to examine migration properties and functional state of MDSCs as the sole expansion of MDSCs in peripheral blood does not necessarily imply that this cell type accumulates in tissue compartments and contributes to tumor growth and metastasis (66,67). Nevertheless, it is noteworthy that critical functional attributes that are linked to the accumulation and immune suppressive function of MDSCs such as the activation of STAT3 and N are prominent features of M-MDSCs retrieved from patients undergoing surgery (1,68).
This study has several limitations. This proof-of-concept study enrolled a relatively small number of subjects, which limits the generalizability of the results and by design, did not provide sufficient power to consolidate that observed nominal increase in postoperative infection rate in the control group was significant. Despite this limitation, reported findings integrate well with results from previous studies, are directly pertinent to human biology, and generate several hypotheses worthy of future examination. While the role of arginine in regulating immune cell function is well established, the reported immune-modulating effects may not solely be attributed to the use of arginine. The AES supplement contained other components with potential immune-modulating properties, such as Omega-3 fatty acid and glutamine. These nutritional supplements may also modulate the inflammatory response to surgery through several mechanisms, including alteration in plasma membrane composition, and modulation of eicosanoid production, cytokine biosynthesis, and immune cell signaling responses (69–72). However, the primary purpose of this study was to comprehensively monitor the immune response to a widely used nutritional intervention that has been linked to beneficial and potentially adverse clinical outcomes. While mass cytometry allows for the phenotyping of major immune cell subsets and the functional characterization of major signaling pathways with a panel of up to 50 antibodies, this number of antibodies precludes deep phenotyping of all cell subsets (e.g., Th1, Th2, and Th17 CD4+ T cells) and in-depth evaluation of all pertinent signaling pathways (e.g., mTOR) in a given blood sample. In particular, the antibody panel – specifically developed for phenotyping of whole blood immune cell subsets – did not allow G-MDSCs to be distinguished from other CD66+ neutrophil subsets. This technical limitation may have undermined the effect of AES on G-MDSCs and biased the analysis towards more readily identifiable M-MDSCs. It is therefore unlikely that all immune modulating effects of AES have been captured. In subsequent studies, it will be interesting to introduce an antibody that recognizes the Lectin-type oxidized LDL receptor-1 (LOX-1), which was recently identified as a specific marker distinguishing G-MDSCs from neutrophils in human peripheral blood samples (73). Finally, alternative predictive algorithms including other machine leaning methods could have been used for the analysis of our high-dimensional data set. However, the systematic comparison of different algorithm for interrogating highly modular immunological data will require formal evaluation of multiple data sets from various clinical settings.
Implementing high-content immune profiling strategies to guide the development of effective therapies is the subject of substantial interest. This study provides the analytical framework needed to comprehensively survey the peripheral immune system of patients randomized to a therapeutic intervention in the perioperative setting, and offers a strategy generalizable to the analysis of other interventional clinical studies. Future applications in larger clinical trials will allow biology extracted from complex networks of inter-related immune features to be linked to pertinent clinical outcomes, to detect off target immune responses, and to identify patient-specific immune signatures associated with response to therapy.
Supplementary Material
Acknowledgments
We thank Astraea Jager and Angelica Trejo for technical assistance with CyTOF experiments. William Magruder for critically editing this manuscript.
Funding: This work was primarily supported by the US National Institutes of Health 1K23GM111657 (BG) and the Department of Anesthesiology, Perioperative and Pain Medicine at Stanford University (B.G., M.A.). The study was also supported by the National Institutes of Health U19AI057229 (G.P.N.), 1U19AI100627 (G.P.N.), and the Food and Drug Administration HHSF223201210194C (G.P.N.).
Abbreviations used in this article
- AES
arginine-enriched dietary supplement
- cMCs
classical monocytes
- cDCs
classical dendritic cells
- MDSC
myeloid-derived suppressor cell
- NK
nature killer
- pDCs
plasmacytoid dendritic cells
- Tregs
regulatory T cells
- Tnaive cells
naïve T cells
- Tmem cells
memory T cells
- p
phosphorylated
- CREB
cAMP response element-binding protein
- STAT
Signal transducer and activator of transcription
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- IkB
NF-kB inhibitor alpha
- MAPK
Mitogen-activated protein kinases
- MAPKAPK2
MAPK activated protein kinase 2
- rp-S6
ribosomal protein-S6
- Tbet
T box transcription factor
- FOXP3
forkhead box P3
- ERK
extracellular signal–regulated kinase 1/2
- IL
Interleukin
- G-CSF
Granulocyte colony stimulating factor
- IFNβ
Interferon beta
- ICAM1
intercellular adhesion molecule 1
Footnotes
Author contributions: B.L.G. conceived and supervised the execution of the study, and wrote the manuscript. N.A. developed and implemented the computational methods for statistical data analysis. E.A.G and H.L.L., A.T. and B.C. assisted in the fabrication of reagents and sample processing for mass cytometry analysis. K.P.J. developed, executed and assisted in the analysis of experiments related to T cell suppression assays. M.T. L.S.M. and R.O. assisted in patient recruitment and clinical data collection. D.K.G. assisted in clinical data collection and interpretation. C.K. and A.A.S. assisted in clinical study design and patient recruitment. M.S.A and G.P.N. assisted in interpreting mass cytometry data and writing the manuscript.
Competing interests: G.P.N. has personal financial interest in the companies Fluidigm and Becton Dickinson, the manufacturers that produce the reagents or instrumentation used in this manuscript.
Data and materials availability: All data are publicly available at http://flowrepository.org/experiments/1021
References
- 1.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Science translational medicine. 2014;6:255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kordasti S, Costantini B, Seidl T, Perez Abellan P, Martinez Llordella M, McLornan D, Diggins KE, Kulasekararaj A, Benfatto C, Feng X, Smith A, Mian SA, Melchiotti R, de Rinaldis E, Ellis R, Petrov N, Povoleri GA, Chung SS, Thomas NS, Farzaneh F, Irish JM, Heck S, Young NS, Marsh JC, Mufti GJ. Deep phenotyping of Tregs identifies an immune signature for idiopathic aplastic anemia and predicts response to treatment. Blood. 2016;128:1193–1205. doi: 10.1182/blood-2016-03-703702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine JH, Simonds EF, Bendall SC, Davis KL, Amir el AD, Tadmor MD, Litvin O, Fienberg HG, Jager A, Zunder ER, Finck R, Gedman AL, Radtke I, Downing JR, Pe’er D, Nolan GP. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Gorman WE, Hsieh EW, Savig ES, Gherardini PF, Hernandez JD, Hansmann L, Balboni IM, Utz PJ, Bendall SC, Fantl WJ, Lewis DB, Nolan GP, Davis MM. Single-cell systems-level analysis of human Toll-like receptor activation defines a chemokine signature in patients with systemic lupus erythematosus. The Journal of allergy and clinical immunology. 2015;136:1326–1336. doi: 10.1016/j.jaci.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kling J. Cytometry: Measure for measure. Nature. 2015;518:439–443. doi: 10.1038/518439a. [DOI] [PubMed] [Google Scholar]
- 6.Nair N, Mei HE, Chen SY, Hale M, Nolan GP, Maecker HT, Genovese M, Fathman CG, Whiting CC. Mass cytometry as a platform for the discovery of cellular biomarkers to guide effective rheumatic disease therapy. Arthritis research & therapy. 2015;17:127. doi: 10.1186/s13075-015-0644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fragiadakis GK, Gaudilliere B, Ganio EA, Aghaeepour N, Tingle M, Nolan GP, Angst MS. Patient-specific Immune States before Surgery Are Strong Correlates of Surgical Recovery. Anesthesiology. 2015;123:1241–1255. doi: 10.1097/ALN.0000000000000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarnok A. Revisiting the crystal ball--high content single cells analysis as predictor of recovery. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2015;87:97–98. doi: 10.1002/cyto.a.22635. [DOI] [PubMed] [Google Scholar]
- 9.Heyland DK, Novak F. Immunonutrition in the critically ill patient: more harm than good? JPEN. Journal of parenteral and enteral nutrition. 2001;25:S51–55. doi: 10.1177/014860710102500211. discussion S55–56. [DOI] [PubMed] [Google Scholar]
- 10.Drover JW, Dhaliwal R, Weitzel L, Wischmeyer PE, Ochoa JB, Heyland DK. Perioperative use of arginine-supplemented diets: a systematic review of the evidence. Journal of the American College of Surgeons. 2011;212:385–399. 399 e381. doi: 10.1016/j.jamcollsurg.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature reviews. Immunology. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, Zamboni N, Sallusto F, Lanzavecchia A. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167:829–842. e813. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Pribis JP, Rodriguez PC, Morris SM, Jr, Vodovotz Y, Billiar TR, Ochoa JB. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury. Annals of surgery. 2014;259:171–178. doi: 10.1097/SLA.0b013e31828611f8. [DOI] [PubMed] [Google Scholar]
- 15.Weimann A, Bastian L, Bischoff WE, Grotz M, Hansel M, Lotz J, Trautwein C, Tusch G, Schlitt HJ, Regel G. Influence of arginine, omega-3 fatty acids and nucleotide-supplemented enteral support on systemic inflammatory response syndrome and multiple organ failure in patients after severe trauma. Nutrition. 1998;14:165–172. doi: 10.1016/s0899-9007(97)00429-2. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton-Reeves JM, Bechtel MD, Hand LK, Schleper A, Yankee TM, Chalise P, Lee EK, Mirza M, Wyre H, Griffin J, Holzbeierlein JM. Effects of Immunonutrition for Cystectomy on Immune Response and Infection Rates: A Pilot Randomized Controlled Clinical Trial. European urology. 2015 doi: 10.1016/j.eururo.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braga M, Gianotti L, Cestari A, Vignali A, Pellegatta F, Dolci A, Di Carlo V. Gut function and immune and inflammatory responses in patients perioperatively fed with supplemented enteral formulas. Arch Surg. 1996;131:1257–1264. doi: 10.1001/archsurg.1996.01430240011001. discussion 1264–1255. [DOI] [PubMed] [Google Scholar]
- 18.Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n−3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132:805–814. doi: 10.1067/msy.2002.128350. [DOI] [PubMed] [Google Scholar]
- 19.Gaudilliere B, Ganio EA, Tingle M, Lancero HL, Fragiadakis GK, Baca QJ, Aghaeepour N, Wong RJ, Quaintance C, El-Sayed YY, Shaw GM, Lewis DB, Stevenson DK, Nolan GP, Angst MS. Implementing Mass Cytometry at the Bedside to Study the Immunological Basis of Human Diseases: Distinctive Immune Features in Patients with a History of Term or Preterm Birth. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2015;87:817–829. doi: 10.1002/cyto.a.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zunder ER, Finck R, Behbehani GK, Amir el AD, Krishnaswamy S, Gonzalez VD, Lorang CG, Bjornson Z, Spitzer MH, Bodenmiller B, Fantl WJ, Pe’er D, Nolan GP. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nature protocols. 2015;10:316–333. doi: 10.1038/nprot.2015.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behbehani GK, Thom C, Zunder ER, Finck R, Gaudilliere B, Fragiadakis GK, Fantl WJ, Nolan GP. Transient partial permeabilization with saponin enables cellular barcoding prior to surface marker staining. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2014 doi: 10.1002/cyto.a.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiwai H, Kumamaru H, Ohkawa Y, Kubota K, Kobayakawa K, Yamada H, Yokomizo T, Iwamoto Y, Okada S. Ly6C+ Ly6G− Myeloid-derived suppressor cells play a critical role in the resolution of acute inflammation and the subsequent tissue repair process after spinal cord injury. Journal of neurochemistry. 2013;125:74–88. doi: 10.1111/jnc.12135. [DOI] [PubMed] [Google Scholar]
- 23.Bryk JA, Popovic PJ, Zenati MS, Munera V, Pribis JP, Ochoa JB. Nature of myeloid cells expressing arginase 1 in peripheral blood after trauma. The Journal of trauma. 2010;68:843–852. doi: 10.1097/TA.0b013e3181b026e4. [DOI] [PubMed] [Google Scholar]
- 24.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 25.Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, Efron PA the Sepsis, C. I. R. C. I. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Annals of surgery. 2016 doi: 10.1097/SLA.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nature communications. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adai AT, Date SV, Wieland S, Marcotte EM. LGL: creating a map of protein function with an algorithm for visualizing very large biological networks. Journal of molecular biology. 2004;340:179–190. doi: 10.1016/j.jmb.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 29.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of surgery. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 30.Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122:1763–1770. doi: 10.1053/gast.2002.33587. [DOI] [PubMed] [Google Scholar]
- 31.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117–126. doi: 10.1067/msy.2000.101584. [DOI] [PubMed] [Google Scholar]
- 32.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG Inflammation, and Host Response to Injury Large-Scale Collaborative Research, P. A genomic storm in critically injured humans. The Journal of experimental medicine. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou H, Hastie T. Regularization and Variable Selection via the Elastic Net. Journal of the Royal Statistical Society. Series B (Methodological) 2005;67:301–320. [Google Scholar]
- 34.Zou H, Hastie T, Tibshirani R. Sparse Principal Component Analysis. Journal of Computational and Graphical Statistics. 2006;15:265–286. [Google Scholar]
- 35.Tibshirani R. Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society. Series B (Methodological) 1996;58:267–288. [Google Scholar]
- 36.Aghaeepour N, Finak G, Flow CAPC, Hoos H, Mosmann TR, Brinkman R, Gottardo R, Scheuermann RH Consortium D. Critical assessment of automated flow cytometry data analysis techniques. Nature methods. 2013;10:228–238. doi: 10.1038/nmeth.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aghaeepour N, Chattopadhyay PK, Ganesan A, O’Neill K, Zare H, Jalali A, Hoos HH, Roederer M, Brinkman RR. Early immunologic correlates of HIV protection can be identified from computational analysis of complex multivariate T-cell flow cytometry assays. Bioinformatics. 2012;28:1009–1016. doi: 10.1093/bioinformatics/bts082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochoa JB, Bernard AC, O’Brien WE, Griffen MM, Maley ME, Rockich AK, Tsuei BJ, Boulanger BR, Kearney PA, Morris SM., Jr Arginase I expression and activity in human mononuclear cells after injury. Annals of surgery. 2001;233:393–399. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pribis JP, Zhu X, Vodovotz Y, Ochoa JB. Systemic arginine depletion after a murine model of surgery or trauma. JPEN. Journal of parenteral and enteral nutrition. 2012;36:53–59. doi: 10.1177/0148607111414579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, Park HJ, Zabaleta J, Ochoa AC. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cellular immunology. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature reviews. Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI. Regulatory myeloid suppressor cells in health and disease. Cancer research. 2009;69:7503–7506. doi: 10.1158/0008-5472.CAN-09-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 44.Zhu X, Herrera G, Ochoa JB. Immunosupression and infection after major surgery: a nutritional deficiency. Critical care clinics. 2010;26:491–500. ix. doi: 10.1016/j.ccc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews. Immunology. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, Douek DC, Fahle GH, Cohen JI, Holland SM, Milner JD. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 49.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 50.Chapman NM, Chi H. mTOR Links Environmental Signals to T Cell Fate Decisions. Frontiers in immunology. 2014;5:686. doi: 10.3389/fimmu.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carroll B, Maetzel D, Maddocks OD, Otten G, Ratcliff M, Smith GR, Dunlop EA, Passos JF, Davies OR, Jaenisch R, Tee AR, Sarkar S, Korolchuk VI. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. eLife. 2016:5. doi: 10.7554/eLife.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saleiro D, Platanias LC. Intersection of mTOR and STAT signaling in immunity. Trends in immunology. 2015;36:21–29. doi: 10.1016/j.it.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. The Journal of experimental medicine. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 56.Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. Alarmins: awaiting a clinical response. The Journal of clinical investigation. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 58.Mieulet V, Yan L, Choisy C, Sully K, Procter J, Kouroumalis A, Krywawych S, Pende M, Ley SC, Moinard C, Lamb RF. TPL-2-mediated activation of MAPK downstream of TLR4 signaling is coupled to arginine availability. Science signaling. 2010;3:ra61. doi: 10.1126/scisignal.2000934. [DOI] [PubMed] [Google Scholar]
- 59.Zhou M, Martindale RG. Arginine in the critical care setting. The Journal of nutrition. 2007;137:1687S–1692S. doi: 10.1093/jn/137.6.1687S. [DOI] [PubMed] [Google Scholar]
- 60.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA : the journal of the American Medical Association. 2001;286:944–953. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 61.Galban C, Montejo JC, Mesejo A, Marco P, Celaya S, Sanchez-Segura JM, Farre M, Bryg DJ. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Critical care medicine. 2000;28:643–648. doi: 10.1097/00003246-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Derive M, Bouazza Y, Alauzet C, Gibot S. Myeloid-derived suppressor cells control microbial sepsis. Intensive care medicine. 2012;38:1040–1049. doi: 10.1007/s00134-012-2574-4. [DOI] [PubMed] [Google Scholar]
- 63.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. The Journal of experimental medicine. 2010;207:1453–1464. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annual review of medicine. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA, Sanchez-Pino D, Hernandez C, Wyczechowska DD, Ochoa AC, Rodriguez PC. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer research. 2015;75:275–283. doi: 10.1158/0008-5472.CAN-14-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ortiz ML, Lu L, Ramachandran I, Gabrilovich DI. Myeloid-derived suppressor cells in the development of lung cancer. Cancer immunology research. 2014;2:50–58. doi: 10.1158/2326-6066.CIR-13-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends in immunology. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 70.Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends in immunology. 2015;36:81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, Oburoglu L, Mongellaz C, Floess S, Fritz V, Matias MI, Yong C, Surh N, Marie JC, Huehn J, Zimmermann V, Kinet S, Dardalhon V, Taylor N. Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Science signaling. 2015;8:ra97. doi: 10.1126/scisignal.aab2610. [DOI] [PubMed] [Google Scholar]
- 72.Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? The Journal of nutrition. 2001;131:2515S–2522S. doi: 10.1093/jn/131.9.2515S. discussion 2523S–2514S. [DOI] [PubMed] [Google Scholar]
- 73.Condamine T, Dominguez GA, Youn J, Kossenkov AV, Alicea-Torres SMK, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Science Immunology. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, Pe’er D, Nolan GP, Bendall SC. Normalization of mass cytometry data with bead standards. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2013;83:483–494. doi: 10.1002/cyto.a.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.