Abstract
Local treatment with corticosteroids results in side effects involving the upper respiratory tract including candidiasis, sore throat, and dysphonia. Although these effects are well known, they have not been evaluated using a histopathological approach. This study investigated the histopathological aspects of steroid-induced dysphonia. A total of 16 female Wistar albino rats were divided into two groups. The eight rats in the experimental group were given an inhaled dose of mometasone furoate daily for 4 weeks. The control group was kept at room temperature for 4 weeks. The vocal cords were evaluated histopathologically using hematoxylin and eosin staining. Both groups had typical epithelial lining and basal membranes. Inflammation differed between the two groups (P = 0.024). There were no differences in squamous metaplasia and hyperplasia (P = 0.302 and 0.302, respectively). This study revealed that inhaled corticosteroids inhibit mucosal immunity, and may result in reversible mucosal changes.
Keywords: Inhaled corticosteroid, Dysphonia, Histopathology, Vocal cord, Inflammation
Introduction
Asthma is a chronic disease characterized by bronchoconstriction, bronchial hypersensitivity, and inflammation [1, 2]. Due to their anti-inflammatory effects, inhaled corticosteroids (ICS) are commonly used alone or in combination with bronchodilator agents for the treatment of asthma attacks and for maintenance therapy in asthmatic patients [3–5]. Although this type of medication is widely used, ICS cause side effects including dysphonia [6].
Studies have shown that dysphonia associated with ICS is caused by mucosal changes and chemical laryngopharyngitis in a dose-dependent manner. These studies were performed using methods such as laryngostroboscopy, endoscopic laryngeal examination, and questionnaires, but histopathological verification is lacking [7–9]. The goal of this study was to characterize the histopathological aspects of steroid-induced laryngitis.
Materials and Methods
International Review Board approval was obtained from the Istanbul University Experimental Animal Research Ethics Committee. Sixteen healthy adult female Wistar albino rats (200–250 g; 7–8 months old) were used.
The rats were divided into two groups; the eight rats in the experimental group were administered a dose of mometasone furoate as an oral inhalation powder once daily (four puffs) for 4 weeks. The control group was kept in room air. After 4 weeks, the subjects were sacrificed under high-dose ketamine anesthesia. Each glottis was removed intact and stored in 10 % (v/v) buffered formalin solution. Specimens were cut into 5 mm thick sections. Standard tissue-processing methods were used, and the sections were stained with hematoxylin and eosin prior to analysis with light microscopy. An expert pathologist blinded to group assignment evaluated epithelial distribution, inflammation, hyperplasia, and metaplasia.
Inflammation was graded based on cellular density: grade 1 represented scattered inflammatory cells, grade 3 represented inflammatory cell density that forms lymphoid follicles, and grade 2 represented cellular density between grades 1 and 3. Statistical analysis was performed using SPSS software, version 17. Pearson’s Chi squared test was used for data analysis. Because the epithelial distribution and cellular reactivity of the mucosa were identical in both groups, these data were not analyzed. P values less than 0.05 were accepted as statistically significant.
Results
There were no macroscopic differences evident between the groups. Microscopically, three types of epithelial cells were detected in the control group: (1) The ventral portion of the glottis was composed of striated ciliary epithelium adjacent to loose connective tissue; (2) The free margins of the vocal cords and adjacent areas were covered with striated nonciliated columnar epithelium; (3) The arytenoid region and adjacent areas were covered with striated squamous epithelium, which was thinner on the arytenoid cartilages. The study group exhibited similar histopathological features. Both groups had typical epithelial lining and basal membranes. Inflammation differed between the two groups (P = 0.024; Fig. 1; Table 1). There were no differences in squamous metaplasia and hyperplasia (P = 0.302 and 0.302, respectively; Fig. 2; Tables 2, 3).
Fig. 1.
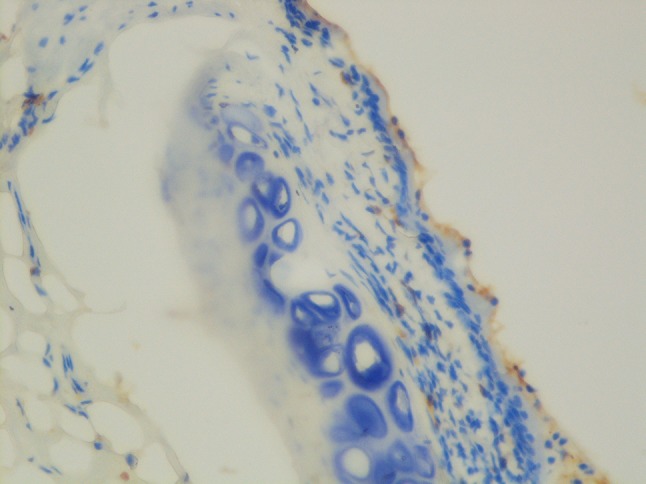
Decreased inflammatory response
Table 1.
Inflammation
| Inflammation | 1+ | 2+ | 3+ | Total |
|---|---|---|---|---|
| Control | 1 (12.5 %) | 5 (62.5 %) | 2 (25 %) | 8 (100 %) |
| Steroid | 4 (50.0 %) | 0 (0 %) | 4 (50 %) | 8 (100 %) |
| Total | 5 (31.3 %) | 5 (31.3 %) | 6 (37.5 %) | 16 (100 %) |
Pearson chi square P = 0.024
Bolditalic values indicate (P < 0.05) as significant
Fig. 2.
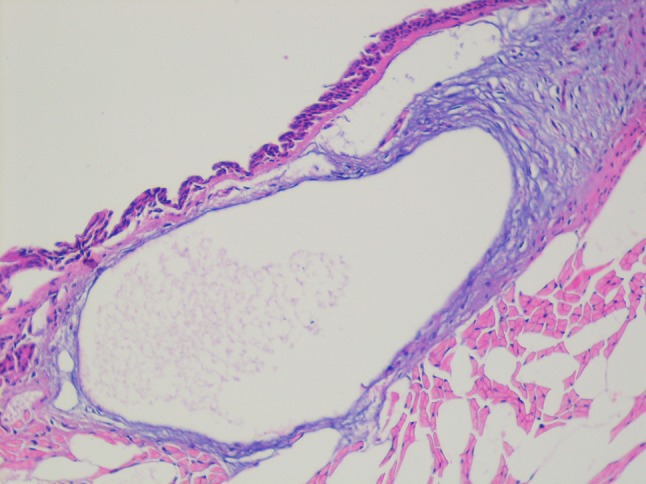
Mild hyperplasia
Table 2.
Metaplasia
| Metaplasia | Negative | Positive | Total |
|---|---|---|---|
| Control | 7 (87.5 %) | 1 (12.5 %) | 8 (100 %) |
| Steroid | 8 (100 %) | 0 (0 %) | 8 (100 %) |
| Total | 15 (93.8 %) | 1 (6.3 %) | 16 (100 %) |
Pearson chi square P = 0.302
Bolditalic values indicate (P < 0.05) as significant
Table 3.
Hyperplasia
| Hyperplasia | Negative | Positive | Total |
|---|---|---|---|
| Control | 8 (100 %) | 0 (0 %) | 8 (100 %) |
| Steroid | 7 (87.5 %) | 1 (12.5 %) | 8 (100 %) |
| Total | 15 (93.8) | 1 (6.3 %) | 16 (100 %) |
Pearson chi square P = 0.302
Bolditalic values indicate (P < 0.05) as significant
Discussion
Local treatment with corticosteroids results in side effects involving the upper respiratory tract including candidiasis, sore throat, and dysphonia [10]. Williamson et al. [6] concluded that two-thirds of patients using ICS had at least one symptom arising from the oropharyngeal or laryngeal region. It has been proposed that non-specific mucosal irritation caused by freon propellants and drying action of the spray may cause dysphonia [11, 12]. Williams et al. [10] claimed that dysphonia was related to ICS, because after cessation of steroids, symptoms resolved despite continued propellant exposure. In addition, patients who used inhaled beclomethasone experienced dysphonia five times more often than patients treated with propellant only [13].
Babu and Samuel et al. [14] evaluated the upper aerodigestive tracts of 48 consecutive patients who underwent ICS therapy (with beclomethasone); 20 % of patients experienced voice changes, 46 % had congested erythematous vocal cords, and 8.5 % had adductor palsy with vocal cord bowing. Videolaryngostroboscopic analysis revealed mucosal edema, erythema, thickening, adduction deficits, nodules, irregularity, and supraglottic hyperfunction [15, 16]. Our study revealed no macroscopic differences between groups. This study had a limited duration, and we were unable to use stroboscopic analysis. Williams et al. [10] initially described adduction deformity. They found that the cords bowed or had an elliptical appearance during phonation. Lavy et al. [16] found that mucosal changes, apposition abnormalities, and supraglottic hyperfunction were noted in 58, 43, and 40 % of patients, respectively. A mean of 39 % cycle-to-cycle irregularity was detected along with a reduced maximum phonation time in 73 % of cases. Ozbilen et al. [15] evaluated the larynx using electromyography, and found that the cricothyroid muscle was more affected than the thyroarytenoid muscle. However, it was concluded that steroid myopathy or mucosal inflammatory theory alone is insufficient to explain the etiopathogenesis of dysphonia in asthmatic patients, because these problems were not seen in all patients using ICS with dysphonia.
Williams et al. [10] proposed that the total dosage of steroids was more important than the type of steroid, because changing the steroid did not influence dysphonia. A study focusing on short-term side effects showed that there were no significant voice changes in patients using corticosteroid inhalers over 3 months; however, minor mucosal changes were detected [17].
Several studies have addressed the clinical aspects of steroid-induced dysphonia, but none have analyzed the histopathological changes. Our study found a decreased inflammatory response and no hyperplasia or metaplasia. Despite the lack of moderate inflammation, the incidence of mild and severe inflammation did not differ. Due to the small sample size, it is impossible to comment on whether severe inflammation is related to irritating factors such as reflux or infection. These results may indicate that the mucosa of the larynx is prone to infections. Recurrent infections and laryngeal exposure to infected sputum may cause mucosal changes in humans. Further studies of a longer duration are required to evaluate the regenerative ability of the mucosa. No atrophic changes were detected at the end of the study period.
Conclusions
This study revealed that ICS inhibit mucosal immunity, and may result in reversible mucosal changes. Further studies combining mucosal and electrophysiological data are required to address the possibility that the etiology of dysphonia is due to steroid myopathy [15, 16].
References
- 1.Murphy KR. Asthma: versatile treatment for a variable disease. J Asthma. 2005;42:149–157. doi: 10.1081/JAS-54649. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein DI. ABCs of asthma. Clin Cornerstone. 2008;8:9–25. doi: 10.1016/S1098-3597(08)80010-5. [DOI] [PubMed] [Google Scholar]
- 3.Maspero JF, Nolte H, Cherrez-Ojeda I. Long term safety of mometasone furoate/formeterol combination for treatment of patients with persistent asthma. J Asthma. 2010;47:1106–1115. doi: 10.3109/02770903.2010.514634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global initiative for asthma (2010) Global strategy for asthma management and prevention. http://www.ginasthma.com/Guidelineitem.asp??11=2&12=1&intld=1561. Accessed 29 July 2010
- 5.National Hearth Lung and Breath Institute-National Asthma Prevention and Education Program (2010) Expert panel report 3: guidelines for the diagnosis and management of asthma. http://www.nhlbi.nih.gov/guideline/asthma/asthgdln.pdf. Accessed 29 July 2010
- 6.Williamson IJ, Matusiewicz SP, Brown PH, Greening AP, Crompton GK. Frequency of voice problems and cough in patients using pressurized aerosol inhaled steroid preparations. Eur Respir J. 1995;8:590–592. [PubMed] [Google Scholar]
- 7.Mirza N, Kasper Schwartz S, Antin-Ozerkis D. Laryngeal findings in users of combination corticosteroid and bronchodilator therapy. Laryngoscope. 2004;114:1566–1569. doi: 10.1097/00005537-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 8.DelGaudio JM. Steroid inhaler laryngitis: dysphonia caused by inhaled fluticasone therapy. Arch Otolaryngol Head Neck Surg. 2002;128:677–681. doi: 10.1001/archotol.128.6.677. [DOI] [PubMed] [Google Scholar]
- 9.Gallivan GJ, Gallivan KH, Gallivan HK. Inhaled corticosteroids: hazardous effects on voice-an update. J Voice. 2007;21:101–111. doi: 10.1016/j.jvoice.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Williams AJ, Baghat MS, Stableforth DE, Cayton RM, Shenoi PM, Skinner C. Dysphonia caused by inhaled steroids: recognition of a characteristic laryngeal abnormality. Thorax. 1983;38:813–821. doi: 10.1136/thx.38.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiley RF, Milne LPR, Crompton GK, et al. Beclomethasone dipropionate aerosol and oropharyngeal candidiasis. Chest. 1976;70:32–38. doi: 10.1016/0007-0971(76)90004-8. [DOI] [PubMed] [Google Scholar]
- 12.Williams MH, Kane C, Shim CS. Treatment asthma with triamcinolone acetonide delivered by aerosol. Am Rev Respir Dis. 1974;109:538–543. doi: 10.1164/arrd.1974.109.5.538. [DOI] [PubMed] [Google Scholar]
- 13.Toogood JH, Jennings B, Greenway RW, Chuang L. Candidiasis and dysphonia complicating beclomethasone treatment of asthma. J Allergy Clin Immunol. 1980;65:145–153. doi: 10.1016/0091-6749(80)90200-6. [DOI] [PubMed] [Google Scholar]
- 14.Babu S, Samuel P. The effect of inhaled steroids on the upper respiratory tract. J Laryngol Otol. 1988;102:592–594. doi: 10.1017/S0022215100105808. [DOI] [PubMed] [Google Scholar]
- 15.Ozbilen Acar G, Uzun Adatepe N, Kaytaz A, et al. Evaluation of laryngeal findings in users of inhaled steroids. Eur Arch Otorhinolaryngol. 2010;267:917–923. doi: 10.1007/s00405-009-1141-2. [DOI] [PubMed] [Google Scholar]
- 16.Lavy JA, Wood G, Rubin JS, Harries M. Dysphonia associated with inhaled steroids. J Voice. 2000;14:581-8.2-term investigation of dysphonia in asthmatic patients using inhaled budesonide. J Voice 2011. 2000;25:88–93. doi: 10.1016/s0892-1997(00)80014-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Moon JW, Chung SM, Lee JH. A short-term investigation of dysphonia in asthmatic patients using inhaled budesonide. J Voice. 2011;25:88–93. doi: 10.1016/j.jvoice.2009.07.003. [DOI] [PubMed] [Google Scholar]


