Abstract
Though the association between follicular carcinoma and bone metastasis is well established, the site-wise distribution is not known. One hundred seventy-three patients of follicular carcinoma presenting between 2003 and 2011 were selected from 1093 patients of follicular lesions presenting at a single institution. Of these, 59 (34%) with bone metastasis were included in the study. Fifty of the 59 patients (84.7%) had metastasis at presentation, while 9 developed bone metastasis during follow-up. Sixty-one percent had solitary metastasis, 15 (25.4%) had multiple bone involvement, while 8 patients (13.6%) had synchronous lung metastasis. Overall, the spine was the commonest site of bone metastases, seen in 20 patients (33.9%), followed by the pelvis, skull, long bones and sternum. Bone metastasis is a known phenomenon in follicular carcinoma. The spine is the commonest site followed by the pelvis.
Keywords: Adenocarcinoma, Follicular, Thyroid neoplasm, Bone neoplasm
Introduction
Differentiated thyroid cancers (DTC) account for over 90% of follicular cell origin thyroid cancers. They are one of the few cancers of the head neck region with excellent prognosis with 10 year survival rates of up to 95% [1, 2]. The impact of metastases on survival is well established with 50% reduction in survival rates in metastatic disease. This is especially true for bone metastases, in which the 10 year survival rate drops down further to 13–21% [3]. However, compared to other malignancies with bone metastases, the prognosis is still superior and hence warrants aggressive treatment.
The association of bone metastases and follicular carcinoma is well documented [4]. However, there are no studies which elaborate on the pattern of bone metastases. Hence, we decided to undertake the present study to look into the pattern of bone metastases and the bones which are commonly afflicted by metastatic lesions.
Materials and Methods
One hundred and seventy-three patients of Follicular Carcinoma (FC) who presented for treatment at Tata Memorial Hospital, Mumbai from January 2003 to December 2011 were selected. These cases were identified from a total of 1093 patients referred to our centre as Follicular lesions. A review of the histology slides or blocks from the primary and/or metastatic lesion was performed by the Department of Pathology before commencing treatment, as a part of institutional policy. A diagnosis of FC was established when vascular and/or capsular invasion was confirmed histologically. These included per primum patients who presented for treatment at our centre, as well as patients who had undergone surgery elsewhere and were referred to us for further management. Patients of other histological subtypes including Follicular variant of Papillary carcinoma thyroid often mistaken for follicular carcinoma, were excluded from the study (Table 1).
Table 1.
Break-up of patients presenting with follicular lesions (n = 1093)
| Histology on review | n | % |
|---|---|---|
| Follicular carcinoma | 173 | 15.8 |
| Papillary carcinoma | 140 | 12.8 |
| Follicular variant papillary carcinoma | 689 | 63.1 |
| Poorly differentiated carcinoma | 38 | 3.5 |
| Follicular adenoma | 53 | 4.8 |
| Total | 1093 | 100 |
Patient details, tumour and treatment related details were retrieved by a retrospective review of patient files and cases of FC with bone metastases were isolated. In the absence of bone cytology or biopsies, Radioiodine uptake on whole body scan (WBS) was taken as confirmatory evidence of bone metastases. Of the 173 patients with confirmed Follicular carcinoma, 59 (34%) patients had bone metastases and formed the study population.
For the purpose of analysis, metastasis to the femur and humerus were considered as metastasis to long bones, to various skull bones as skull metastases, and to pelvic bones as pelvic metastases. SPSS Version 16 was used for statistical analysis.
Results and Analysis
Two-third patients (n = 44, 75%) of were females, while there were only 15 (25%) males in the study population, suggesting a female predilection of the disease. The mean age of patients was 58.4 ± 12.2 years (range 26–88 years). The mean age of female patients was comparable to that of males in the present study population 59 ± 11.5 versus 56.7 ± 14.4 years (p = 0.53).
There were 59 (34%) patients with bone metastases among 173 cases of follicular carcinoma. Of these, 50 (84.7%) patients had metastases at presentation, while the remaining 9 (15.3%) developed metastases during follow up. A total of 78 metastatic lesions were observed in these 59 patients. On reviewing the pattern of metastases, majority of patients i.e. 36 (61%) had solitary bony metastases. Fifteen patients (25.4%) had involvement of multiple bones, while 8 patients (13.6%) had multiorgan involvement (Table 2).
Table 2.
Pattern of bone metastases in follicular carcinoma (n = 59)
| Pattern | n | % |
|---|---|---|
| Solitary | 36 | 61 |
| Multiple bones | 15 | 25.4 |
| Multi-site | 8 | 13.6 |
| Total | 59 | 100 |
The most common site of solitary metastasis was the spine, involved in one-third of the patients (n = 12, 33.3%), followed by the skull in 9 patients (25%), and 6 patients each (16.7%) to the pelvis and long bones. There were 3 patients (8.3%) with solitary metastasis in the sternum (Table 3). In patients with simultaneous involvement of multiple bones (n = 15), the spine and pelvis were most frequently involved with 8 cases each, followed by 6 patients each with metastasis to the skull and spine. Four patients had metastasis to the ribs, while only 2 patients had sternal involvement (Table 4). All the 8 patients with multiorgan metastases had synchronous pulmonary metastases in addition to bone metastases.
Table 3.
Site-wise distribution of solitary metastases (n = 36)
| Site | n | % |
|---|---|---|
| Spine | 12 | 33.3 |
| Skull | 9 | 25 |
| Pelvis | 6 | 16.7 |
| Long bones | 6 | 16.7 |
| Sternum | 3 | 8.3 |
| Total | 36 | 100 |
Table 4.
Site-wise distribution of 34 metastatic lesions in 15 patients with multiple bone involvement (n = 34)
| Site | n | % |
|---|---|---|
| Spine | 8 | 23.5 |
| Pelvis | 8 | 23.5 |
| Skull | 6 | 17.6 |
| Long bones | 6 | 17.6 |
| Ribs | 4 | 11.9 |
| Sternum | 2 | 5.9 |
| Total | 34 | 100 |
On a review of all the bones affected, the spine was the commonest site affected, with 20 cases (33.9%), followed by the pelvis in 18 (30.5%), and skull in 16 (27.1%) patients. The long bones were involved in 10 (16.9%) patients, while the sternum and ribs were involved in 7 patients (11.9%) each (Table 5).
Table 5.
Overall site-wise distribution of bone metastases among 59 patients (n = 59)
| Site of metastasis | n | % |
|---|---|---|
| Spine | 20 | 33.9 |
| Pelvis | 18 | 30.5 |
| Skull | 16 | 27.1 |
| Long bones | 10 | 16.9 |
| Sternum | 7 | 11.9 |
| Ribs | 7 | 11.9 |
In the spine, the lumbar, cervical and dorsal vertebrae were involved in 5 (25%), 4 (20%), and 4 (20%) patients respectively. The ilium was the commonest bone of the pelvis to be affected with 7 patients (38.9%) of pelvic metastases present in the ilium. This was followed by the sacrum and acetabulum in 6 (33.3%) and 5 (27.8%) patients respectively. Among the long bones, 60% (6 patients) of the metastases were to the femur, while 4 of the 10 patients (40%) had metastases to the humerus. In the femur, the head was more often affected than the lower end, with 5 of the 6 cases (83.3%) harboring metastases in the head of femur.
Discussion
Differentiated thyroid cancers (DTC) encompass over 90% of follicular cell derived thyroid cancers (FCDTC), and comprise of the two most commonly encountered variants of thyroid cancer viz. Papillary carcinoma (70–75%) and Follicular carcinoma (15–20%). Hurthle cell carcinoma (2%) and anaplastic carcinoma (<5%) are also of follicular cell origin, but are poorly differentiated and undifferentiated variants respectively. Other histologies include Medullary carcinoma (5–10%) derived from parafollicular C cells, and others such as lymphomas and sarcoma which are rarely encountered [1, 5].
Follicular carcinoma is often confused with other malignancies of follicular cell origin such as papillary carcinoma, and especially so follicular variant of papillary carcinoma. It is important to differentiate between these various entities as each has a different clinical profile, pathogenesis, and hence different management and prognosis. In the present study too, of the 1093 patients referred to us as follicular lesions, only 15.8% had true follicular carcinoma on histological review. Of the rest, majority of the lesions i.e. 63.1% had follicular variant of papillary carcinoma, 12.8% were conventional papillary carcinoma, while 3.5% were poorly differentiated carcinoma. The remaining 4.8% were miscellaneous lesions including follicular adenoma and medullary carcinoma (Table 1).
A recent increase in the number of cases of DTC has been well documented. This could either be attributed to an increase in the incidence of thyroid cancer, or more accurately reflect an increase in the diagnosis due to higher awareness and improved diagnostics. Despite this, DTC is one of the few malignancies of the head and neck with an excellent prognosis as shown by a cause specific survival (CSS) of up to 90% [2, 6]. This however drops dramatically by around 50% in the presence of distant metastases to around 40%.
Pathogenesis of Bone Metastasis
The hallmark of follicular carcinoma and indeed one of the criteria differentiating it from follicular adenoma is the presence of capsular and/or vascular invasion [7]. Microscopically, for vascular invasion to be present, the vessel should be in, or immediately outside the capsule, and not located within the tumour. In addition, one or more tumour emboli should be adherent to the vessel wall and protruding into the lumen. This is the reason why hematogenous metastases are much more common than lymphatic metastasis in follicular carcinoma (40 vs. 10%) [7–9]. Prognostically, bone metastases are common in extensive vascular invasion involving more than 4 vessels. In minimal vascular invasion bone metastasis is seen in less than 5%, while less than 1% patients exhibit bone metastases with only minimal capsular invasion [10].
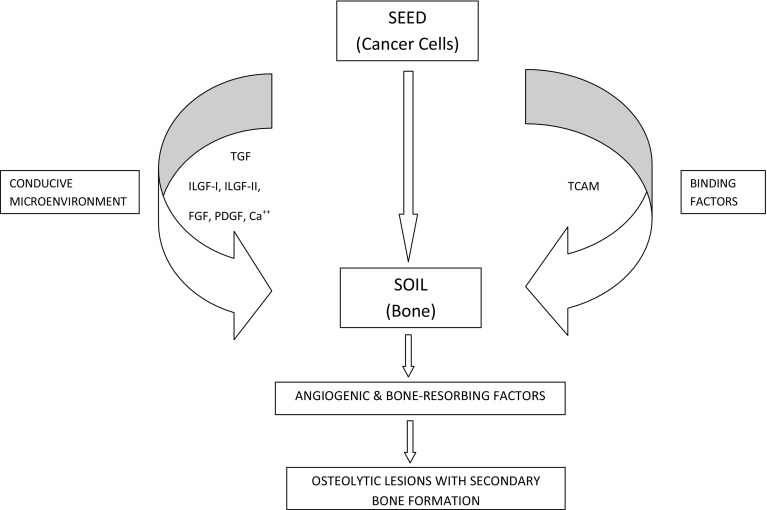
Hematogenous metastases are explained by Paget-s seed and soil theory. As per this hypothesis, migratory tumour cells will develop into metastases only in the presence of a conducive microenvironment with which they are able to exchange biological information [11, 12]. Various growth factors such as transforming growth factor (TGF), insulin-like growth factor -I and -II (ILGF), fibroblast growth factor (FGF), platelet-derived growth factors (PDGF), and calcium (Ca) are stored with the bone. These, along with tumour cell adhesive molecules (TCAM) help in binding the circulating tumour cells (seed) to the bone (soil). This contributes to the ability of the cancerous cells to not only survive, but to also proliferate by releasing angiogenic and bone resorbing factors (Fig. 1). This results in metastases manifesting as osteolytic lesions with secondary bone formation in response to bone destruction.
Fig. 1.
Figure illustrating the pathogenesis of bone metastases
Clinical Presentation and Prognosis
Distant metastases in DTC has been reported anywhere from 10 to 20% in various studies [4, 13, 14]. The most common site of hematogenous metastases from DTC is to the lungs (49%). Twenty-five percent of metastases are to bone, 15% have synchronous metastasis to the lungs and bone, and 11% to soft tissues [11, 15]. Bone metastases in DTC are thrice as common in Follicular carcinoma (7–28%) versus Papillary carcinoma (1–7%) [4, 16]. In our study, 34% of patients with Follicular carcinoma had bone metastases, while 8 patients (13.6%) had synchronous metastases to the lungs and bone. Although the overall incidence of bone metastases in DTC accounts for just 2–13% of patients, metastasis to this subsite is extremely significant as the 10 year CSS reduces by a further 50% to 13–21% [1, 11].
Distant metastases at presentation have been reported in 3–15% of patients by various authors [5, 13, 17]. The prognosis of this cohort of patients is undoubtedly worse than those who develop bone metastases later on follow-up [18]. In the present study, of the 59 patients with bone metastases, nearly 85% had metastases at the time of presentation, while 9 (15.3%) developed metastases during follow up. This could be since being a tertiary care hospital, many patients with advanced disease are referred to us for further management.
Other factors associated with poor prognosis in DTC include (i) age in males >40 years, females >50 years Lin 1 (ii) poor differentiation (iii) follicular carcinoma (iv) Hurthle cell variant (v) iodine avidity (vi) incomplete local control (vii) extrapulmonary distant metastases [5, 10].
The survival in DTC is directly related to the presence of bone metastases, with a 10 year overall survival rate of just 25% as compared to pulmonary metastases with a 10 year overall survival of 63% [16]. This is probably since bone metastases are not very radioactive iodine (RAI) responsive, from which we can infer that they are less differentiated, and hence more aggressive. In fact, the median life expectancy of undifferentiated anaplastic carcinoma is just 4–12 months [4, 5, 19]. Nixon et al. studied the impact of distant metastases at presentation on the prognosis of 52 patients with DTC and found the results to be closely associated with RAI avidity. The 5 year DSS in RAI avid metastases was 77%, which dropped to 66% in non-RAI avid metastases [13]. Besides an absence of RAI uptake, other adverse prognostic factors in metastatic DTC are multiplicity and a higher age at the time of diagnosis [1].
The axial skeleton is the site for bone metastases in over 80% of cases owing to the high blood flow [11]. In the present study too, 54 of the 78 metastatic lesions were present in the axial skeleton with involvement of the spine, skull, and pelvis in 20 (33.9%), 16 (27.1%) and 18 (30.5%) respectively. Metastases to the spine may cause pain, pathological fractures and spinal cord compression resulting in an overall deterioration of quality of life [20]. In fact, pathological fractures with or without signs of compression are often the presenting complaints in patients of follicular carcinoma. The other common bones to be involved are the long bones such as femur, and flat bones including pelvis [21]. The long bones were involved in 10 (16.9%) patients in our study. Among these, the femur harbored metastases in 6 (60%) and humerus in the remaining 4 (40%). In the femur, there was almost exclusive involvement of the head, seen in 5 of the 6 patients (83.3%), This has not been documented in any of the previous studies.
Conclusions
Metastatic thyroid disease is an indicator of aggressive advanced disease and poor prognosis, with 50% reduction in survival rates. This is especially true for bone metastases. Compared to other variants of Follicular cell derived thyroid cancer, bone metastases are commonly associated with follicular carcinoma. In the present study, we observed a high rate of bone metastases of 34%.
Being a tertiary referral centre, most of the patients in the present series (84.7%) had metastases at presentation. This is a known poor prognostic factor. Sixty-one percent of patients had solitary bone metastases, most often to the spine (33.3%). Multiple bones were affected in 25.4% patients, while 13.6% had synchronous lung metastases.
The spine was the commonest site of metastases, seen in 20 (33.9%) patients affecting lumbar, cervical and dorsal vertebrae. The second most often affected site was the pelvis in 30.5%, with metastases to the ilium, sacrum and acetabulum in 39, 33 and 28% respectively, followed by skull metastases in 27%.
Among the patients with long bone metastases (17%), the femur was affected in 60%, while the humerus was involved in 40%. In the femur, the head was almost exclusively the site of metastases. The sternum was involved in 12%.
Compliance with Ethical Standards
Conflict of interest
All authors declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. Being a retrospective study, for this type of study formal consent is not required.
References
- 1.Ramadan S, Ugas MA, Berwick RJ, Notay M, Cho H, Jerjes W, Giannoudis PV. Spinal metastasis in thyroid cancer. Head Neck Oncol. 2012;4:39. doi: 10.1186/1758-3284-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eustacia-Rutten CF, Romijri JA, Guijt MJ, Vielvoye GJ, van den Berg R, Corssmit EPM, Pereira AM, Smit JWA. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3184–3189. doi: 10.1210/jc.2003-030231. [DOI] [PubMed] [Google Scholar]
- 3.Schlumberger M, Tubiana JP, Fragu P, Lumbroso J, Caillou B, Parmentier C. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. doi: 10.1210/jcem-63-4-960. [DOI] [PubMed] [Google Scholar]
- 4.Satcher RL, Lin P, Harun N, Feng L, Moon BS, Lewis VO. Surgical management of appendicular skeletal metastases in thyroid carcinoma. Int J Surg Oncol. 2012;2012:417086. doi: 10.1155/2012/417086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol. 2013;20:1329–1335. doi: 10.1245/s10434-012-2711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Prognostic factors in papillary and follicular thyroid carcinoma: implications for cancer staging. Ann Surg Oncol. 2007;14:730–738. doi: 10.1245/s10434-006-9207-5. [DOI] [PubMed] [Google Scholar]
- 7.Sobrinho SM, Asa SL, Kroll TG, Nikifarav Y, DeLellis R, Farid P, Kitamura Y, Noguchi SU, Eng C, Harach HR (2004) Follicular carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU (eds) World Health Organization classification of tumors of endocrine organs. IARC Press, Lyon Eng C, pp 67–72
- 8.Tickoo SK, Pittas AG, Adler M, Fazzaari M, Larson SM, Robbins RJ, Rosai J. Bone metastases from thyroid carcinoma: a histopathologic study with clinical correlates. Arch Pathol Lab Med. 2000;124:1440–1447. doi: 10.5858/2000-124-1440-BMFTC. [DOI] [PubMed] [Google Scholar]
- 9.Rao RS, Parikh HK, Deshmane VH, Parikh DM, Shrikhande SS, Havaldar R. Prognostic factors in follicular carcinoma of the thyroid: a study of 198 cases. Head Neck. 1996;18:118–124. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<118::AID-HED2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Zhu B, Liu Y, Silverman JF. Follicular thyroid carcinoma invades venous rather than lymphatic vessels. Diagn Pathol. 2010;5:8. doi: 10.1186/1746-1596-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muresan MM, Oliver P, Leclere J, Sirveaux F, Brunaud L, Klein M, Zarnegar R, Weryha G. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008;15(1):37–49. doi: 10.1677/ERC-07-0229. [DOI] [PubMed] [Google Scholar]
- 12.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 13.Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, Patel SG, Ganly I. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. 2012;22(9):884–889. doi: 10.1089/thy.2011.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittas AG, Adler M, Fazzari M. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variable in one hundred forty-six patients. Thyroid. 2000;10(3):261–268. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 15.Kushchayev S, Kushchayev Y, Theodore N, Preul MC, Clark OH. Percutaneous vertebroplasty for thyroid cancer metastases to the spine. Thyroid. 2010;20:555–560. doi: 10.1089/thy.2009.0420. [DOI] [PubMed] [Google Scholar]
- 16.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Soh EY. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg. 2010;251:114–119. doi: 10.1097/SLA.0b013e3181b7faf6. [DOI] [PubMed] [Google Scholar]
- 18.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg. 2007;245:366–378. doi: 10.1097/01.sla.0000250445.92336.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orita Y, Sugitani I, Matsuura M. Prognostic factors and the therapeutic strategy for patients with bone metastasis from differentiated thyroid carcinoma. Surgery. 2010;147:424–431. doi: 10.1016/j.surg.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21:31–35. doi: 10.1089/thy.2010.0169. [DOI] [PubMed] [Google Scholar]
- 21.Nagamine Y, Suzuki J, Katakura R, Yoshimoto T, Matoba N, Takaya K. Skull metastasis of thyroid carcinoma, study of 12 cases. J Neurosurg. 1985;63:526–531. doi: 10.3171/jns.1985.63.4.0526. [DOI] [PubMed] [Google Scholar]