Abstract
Target volume delineations for prostate cancer (PCa) salvage radiotherapy (SRT) after radical prostatectomy are usually drawn in the absence of visibly recurrent disease. 68Ga-labeled prostate-specific membrane antigen (PSMA-11) PET/CT detects recurrent PCa with sensitivity superior to standard-of-care imaging at serum prostate-specific antigen (PSA) values low enough to affect target volume delineations for routine SRT. Our objective was to map the recurrence pattern of PCa early biochemical recurrence (BCR) after radical prostatectomy with 68Ga-PSMA-11 PET/CT in patients with serum PSA levels of less than 1 ng/mL, determine how often consensus clinical target volumes (CTVs) based on the Radiation Therapy Oncology Group (RTOG) guidelines cover 68Ga-PSMA-11 PET/CT-defined disease, and assess the potential impact of 68Ga-PSMA-11 PET/CT on SRT. Methods: This was a post hoc analysis of an intention-to-treat population of 270 patients who underwent 68Ga-PSMA-11 PET/CT at 4 institutions for BCR after prostatectomy without prior radiotherapy at a PSA level of less than 1 ng/mL. RTOG consensus CTVs that included both the prostate bed and the pelvic lymph nodes were contoured on the CT dataset of the PET/CT image by a radiation oncologist masked to the PET component. 68Ga-PSMA-11 PET/CT images were analyzed by a nuclear medicine physician. 68Ga-PSMA-11–positive lesions not covered by planning volumes based on the consensus CTVs were considered to have a potential major impact on treatment planning. Results: The median PSA level at the time of 68Ga-PSMA-11 PET/CT was 0.48 ng/mL (range, 0.03–1 ng/mL). One hundred thirty-two of 270 patients (49%) had a positive 68Ga-PSMA-11 PET/CT result. Fifty-two of 270 (19%) had at least one PSMA-11–positive lesion not covered by the consensus CTVs. Thirty-three of 270 (12%) had extrapelvic PSMA-11–positive lesions, and 19 of 270 (7%) had PSMA-11–positive lesions within the pelvis but not covered by the consensus CTVs. The 2 most common 68Ga-PSMA-11–positive lesion locations outside the consensus CTVs were bone (23/52, 44%) and perirectal lymph nodes (16/52, 31%). Conclusion: Post hoc analysis of 68Ga-PSMA-11 PET/CT implied a major impact on SRT planning in 52 of 270 patients (19%) with PCa early BCR (PSA < 1.0 ng/mL). This finding justifies a randomized imaging trial of SRT with or without 68Ga-PSMA-11 PET/CT investigating its potential benefit on clinical outcome.
Keywords: prostate cancer, PSMA, PET/CT, recurrence, salvage radiotherapy
See an invited perspective on this article on page 228.
Prostate cancer (PCa) biochemical recurrence (BCR) takes place in 20%–80% of patients within 10 y after radical prostatectomy, with the risk of failure dependent on National Comprehensive Cancer Network risk group, pathologic features, and genomic classification (1,2). After BCR, salvage radiotherapy (SRT) is the main curative option (3). Overall, SRT offers long-term biochemical control in about 50% of patients (4), depending on pre-SRT prostate-specific antigen (PSA) level (5), radiotherapy dose (6), and risk group (7). For high-risk patients, 5-y BCR after SRT reaches 70% (8,9). Intuitively, SRT is curative only if recurrent disease is completely encompassed by the irradiated volumes. Therefore, accurate estimation of the location of recurrent disease is critical.
In practice, SRT is commonly initiated in patients with serum PSA levels of less than 1 ng/mL, a threshold at which standard-of-care imaging is insensitive for detecting recurrence (10). As such, SRT target volumes are usually drawn in the absence of radiographically visible disease (gross disease). The Radiation Therapy Oncology Group (RTOG) published contouring guidelines for both prostate bed and pelvic lymph node (LN) clinical target volumes (CTVs) (areas with potential microscopic occult tumor) based on a consensus panel of experienced genitourinary radiation oncologists (11,12). These consensus CTVs are applied in ongoing clinical trials and guide routine care.
68Ga-labeled (Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)]) prostate-specific membrane antigen (PSMA-11) PET/CT is superior to standard-of-care imaging for detecting regional and distant metastatic recurrent PCa at low PSA levels (13–16) and is also highly specific (16) and reproducible (17). Detection rates of about 50% are reported even at PSA levels of less than 0.5 ng/mL (15,16). Therefore, 68Ga-PSMA-11 PET/CT has the potential to guide and improve target volume delineations for SRT.
The potential impact of 68Ga-PSMA-11 PET/CT on radiotherapy planning has been assessed in several inhomogeneous patient groups with primary and recurrent disease. These studies established that 68Ga-PSMA-11 PET/CT can image PCa at low serum PSA values and potentially affect radiotherapy planning. Limitations include inconsistent descriptions of anatomic relapse patterns and the pooling of patients with a wide range of serum PSA values and clinical disease states (18–24).
We present a large cohort of patients who underwent 68Ga-PSMA-11 PET/CT at a PSA level of less than 1 ng/mL after prior prostatectomy. This cohort of patients is representative of those who are routinely offered SRT in the absence of radiographically visible disease. We map the 68Ga-PSMA-11 PET/CT recurrence pattern of early BCR after prostatectomy, evaluate how often SRT based on consensus contouring guidelines fails to cover PSMA-11–expressing disease, and assess the potential impact of 68Ga-PSMA-11 PET/CT on SRT planning for patients with PCa early BCR.
MATERIALS AND METHODS
Patients and Data Management
We first identified 270 consecutive and well-documented patients from databases established at 4 institutions (Technical University of Munich [n = 147], University of California at Los Angeles [UCLA, n = 47; clinicaltrial.gov identifier NCT02940262, Institutional Review Board approval 16-001095], Ludwig-Maximilians-University of Munich [n = 40], and University of Essen [n = 36]). All patients underwent radical prostatectomy, had BCR without prior radiotherapy, and underwent 68Ga-PSMA-11 PET/CT at a serum PSA level of less than 1 ng/mL between August 2013 and May 2017 to detect the sites of recurrence. All patients gave written consent to undergo the procedures. The clinical data and DICOM files of all patients were anonymized and imported onto a dedicated radiotherapy contouring workstation at UCLA (MIM, version 6.7.5; MIM Software Inc.). The UCLA Institutional Review Board approved this anonymized post hoc retrospective analysis (approval #17-001340), and the requirement to obtain informed consent was waived.
68Ga-PSMA-11 PET/CT Image Acquisition
68Ga-PSMA-11 PET/CT imaging was performed according to recent guidelines (25). Images were acquired on different PET/CT devices: the Siemens Biograph 128 mCT (n = 183, 68%), Siemens Biograph 64 (n = 50, 19%), Siemens Biograph 64 mCT (n = 24, 9%), or GE Healthcare Discovery 690 (n = 13, 5%). The 68Ga-PSMA-11 compound was used at all sites (26). The median injected dose was 154 MBq (range, 65–267 MBq). To reduce bladder activity, patients received 20 mg of furosemide at the time of tracer injection if there was no contraindication. The median uptake period was 59 min (range, 37–132 min). A diagnostic CT scan (200–240 mAs, 120 kV) was performed after intravenous injection of contrast agent (if no contraindication existed), followed by the whole-body PET image acquisition (2–4 min/bed position).
Simulation of Consensus SRT Planning
SRT consensus CTVs were contoured on the CT dataset of the PET/CT scan for all 270 patients by an experienced radiation oncologist who was masked to the PET findings. Consensus RTOG contouring guidelines were used (Fig. 1A) (11,12), except that the common iliac nodes were contoured beginning inferior to L4/L5 (rather than L5/S1). Briefly, the prostate bed CTV included the anatomic prostatic fossa and the seminal vesicle remnants. The pelvic nodal CTV included presacral, common iliac, internal iliac, external iliac, and obturator LNs. Although the addition of pelvic LN irradiation in SRT is controversial (27,28) and under investigation (RTOG 0534, NCT00567580), we included pelvic LN coverage along with the prostate bed for all patients to establish a generous estimate of how often SRT based on consensus CTVs fails to cover PSMA-11–expressing recurrent disease.
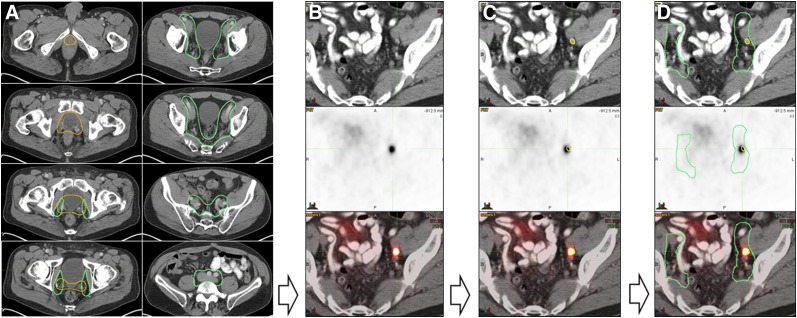
FIGURE 1.
Study methodology. (A) Experienced radiation oncologist masked to PET findings contoured RTOG CTVs onto CT dataset of PET/CT scan for all 270 patients (prostate bed CTV in orange and pelvic LN CTV in green). (B) All 68Ga-PSMA-11 PET/CT images were analyzed by an experienced nuclear medicine physician. (C) PSMA-11–positive lesions were contoured in yellow on CT images. (D) Consensus CTVs were coregistered with 68Ga-PSMA-11 PET/CT images and PSMA-11–positive lesion contours (yellow) to assess, for each patient, whether PSMA-11–positive lesions were localized inside or outside consensus CTVs.
68Ga-PSMA-11 PET/CT Image Analysis
Next, all 68Ga-PSMA-11 PET/CT images were analyzed by an experienced nuclear medicine physician according to recent recommendations (25,29): any focal uptake of 68Ga-PSMA-11 above the surrounding background level and not associated with physiologic uptake or known pitfalls (30) was considered suggestive of malignancy (Fig. 1B). Distinction between malignant and inflammatory LNs (e.g., reactive or granuloma) was based on degree of PSMA-11 uptake, typically intermediate and low for inflammation, and location, typically perihilar, axillary, or inguinal for inflammatory nodes. On the basis of TNM staging, the following regions were systematically analyzed for recurrence: prostate bed/seminal vesicle remnants (T), pelvic LNs (N) (internal iliac, obturator, external iliac, perirectal, presacral, common iliac), extrapelvic LNs (M1a) (retroperitoneal, inguinal, chest, other), bone (M1b), and other visceral organs (M1c).
68Ga-PSMA-11 PET Lesion Contouring
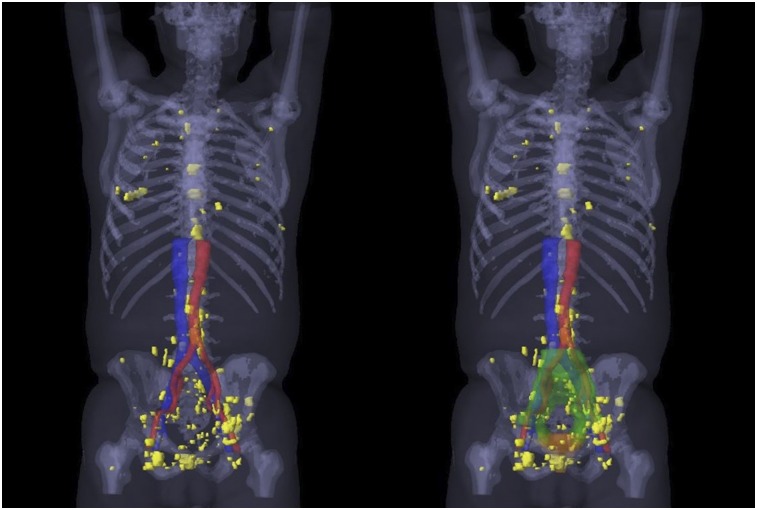
In a third step, 68Ga-PSMA-11–positive lesions were contoured on the CT images (Fig. 1C). These contours were subsequently used to define 68Ga-PSMA-11–based target volumes. Moreover, we generated a 3-dimensional map of all 68Ga-PSMA-11–positive lesion contours across the entire study population on a template patient (Fig. 2). This was achieved by rigid image registration of each patient’s CT image to the template patient image. Then, the 68Ga-PSMA-11–based contours were transferred to the template patient image through this registration (MIM, version 6.7.5).
FIGURE 2.
A 3-dimensional map of the PSMA-11–positive lesions (yellow) of all 52 patients with recurrence outside consensus CTVs (23 patients with recurrence outside only and 29 patients with recurrence outside and inside consensus CTVs), created by rigid registration of each patient’s CT image to template patient’s CT image, followed by transfer of each PSMA-11–positive lesion contour to template patient CT image (MIM, version 6.7.5; MIM Software Inc.). On right side, 3-dimensional prostate bed consensus CTV is shown in orange and 3-dimensional pelvic LN consensus CTV in green.
Coregistration of Consensus CTVs with 68Ga-PSMA-11 PET/CT Images
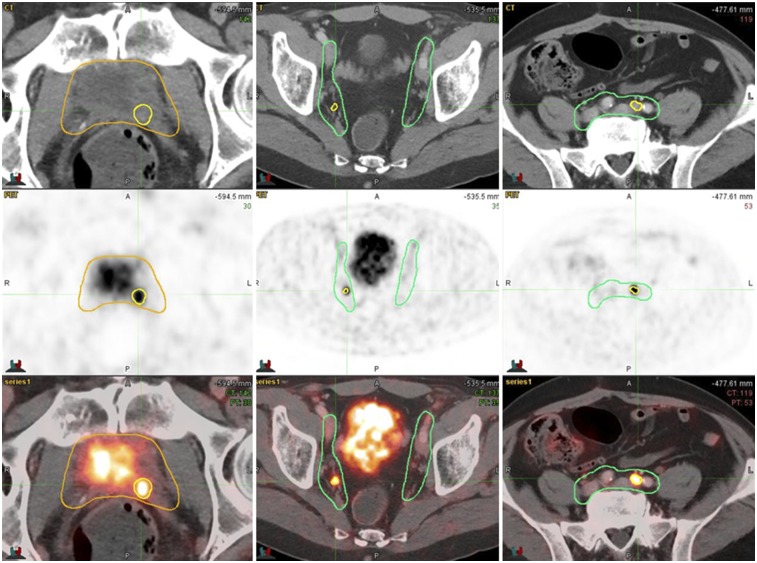
In a final step, the consensus CTVs were coregistered with the 68Ga-PSMA-11 PET/CT images (Fig. 1D). Contours including the PSMA-11–positive lesions were then compared with the consensus CTVs for each patient to assess whether PSMA-11–positive lesions were localized inside (Fig. 3) or outside (Fig. 4) the consensus CTVs. To take into consideration the final planning target volumes, only PSMA-11–positive lesion contours at least 10 mm away from the CTVs were considered inadequately covered. Because many modern centers use CTVs to plan target volume expansions of less than 10 mm, this analysis should yield a generous estimate of how often planning based on consensus CTVs offers adequate coverage. PSMA-11–positive lesions within either the prostate bed or the pelvic LN consensus CTVs were considered covered.
FIGURE 3.
PSMA-11–positive lesions (yellow contours) inside prostate bed CTV (gold contours) and nodal CTV (green contours).
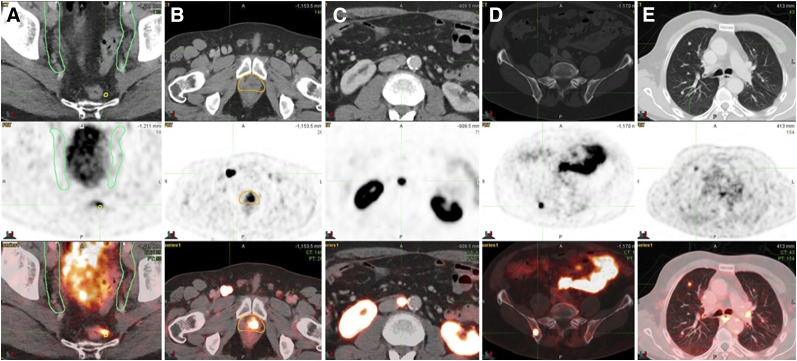
FIGURE 4.
Examples of PSMA-11–positive lesions outside consensus CTVs: perirectal LN (A), inguinal LN (B), lumboaortic LN (C), bone (D), and lung (E).
Potential Impact of 68Ga-PSMA-11 PET/CT on Radiotherapy Planning
PSMA-11–positive lesions not covered by the consensus CTVs were considered to have a potential major impact on treatment planning. Potential major impact was further subclassified as extension of the CTVs to cover PSMA-11–positive lesions within the pelvis; superior extension of the CTVs to cover paraaortic LNs; addition of metastasis-directed stereotactic body radiation therapy for extrapelvic oligometastatic disease (1–5 extrapelvic sites that are M1a or M1b); or radiotherapy not indicated (futile) because of the presence of visibly polymetastatic (>5 M1a or M1b) or visceral (M1c) metastatic disease. If PSMA-positive lesions were covered by the CTVs, the potential impact of 68Ga-PSMA-11 PET/CT on treatment planning was defined as minor (potential for dose escalation to gross disease (visibly PSMA-11–positive). Negative 68Ga-PSMA-11 PET/CT findings were considered to have no impact on SRT planning.
Statistical Analysis
We performed a post hoc analysis of this intention-to-treat population and simulated the impact of 68Ga-PSMA-11 PET/CT on SRT planning. Descriptive statistics were used (median, range). The comparisons of clinical and pathologic characteristics between positive and negative 68Ga-PSMA-11 PET/CT patients were conducted using the t test along with the Wilcoxon test as a verification for continuous variables and the χ2 test for categoric variables. The serum PSA level before PET/CT was considered first as continuous variable and converted into categoric-variable—low PSA (0.5–1.0 ng/mL) and very low PSA (<0.5 ng/mL)—groups for comparison. These analyses were conducted in R (31).
RESULTS
Patient Characteristics
Table 1 summarizes the clinical and pathologic characteristics of the 270 patients. In brief, the median age was 68 y (range, 43–90 y), and the median serum PSA level was 0.44 ng/mL (range, 0.03–1 ng/mL). Thirty-three of 270 patients (12.5%) underwent androgen deprivation therapy within 6 mo before the 68Ga-PSMA-11 PET/CT study. Thirty-six of 270 (13.5%) were National Comprehensive Cancer Network–defined intermediate-risk. One hundred sixty-three of 270 (60.5%) were National Comprehensive Cancer Network–defined high-risk, 142 of 270 (52.5%) were pT3, and 54 of 270 (20%) were pN1. Sixty-seven of 270 patients (25%) had positive surgical margins (R1). Overall, the cohort was at high risk for treatment failure after prostatectomy.
TABLE 1.
Clinical and Pathologic Characteristics of the 270 Patients
| Characteristic | Data |
| Age at PET/CT, median (y) | 68 (range, 43–90) |
| Initial PSA level before surgery, median (ng/mL) | 8.3 (range, 0.4–200) |
| 10 | 130 (48%) |
| ≥10 < 20 | 46 (17%) |
| ≥20 | 38 (14%) |
| Unknown | 56 (21%) |
| Gleason score | |
| ≤6 | 33 (12%) |
| 7 | 135 (50%) |
| ≥8 | 86 (32%) |
| Unknown | 16 (6%) |
| Pathologic primary tumor stage | |
| pT2 | 99 (36.5%) |
| pT3 | 142 (52.5%) |
| pT4 | 2 (0.7%) |
| Unknown | 27 (10%) |
| Pathologic regional LN stage | |
| pN0 | 166 (61.5%) |
| pN1 | 54 (20%) |
| pNx | 50 (18.5%) |
| Positive margin | |
| R0 | 152 (56.5%) |
| R1 | 67 (25%) |
| Unknown | 51 (19%) |
| National Comprehensive Cancer Network risk group | |
| Low | 4 (1.5%) |
| Intermediate | 36 (13.5%) |
| High | 163 (60.5%) |
| N1 | 54 (20%) |
| Unknown | 13 (5%) |
| Androgen deprivation therapy within 6 mo before imaging | 33 (12.5%) |
| Time between surgery and PET/CT, median (mo) | 25 (range, 2–272) |
| Last PSA value before PET/CT, median (ng/mL) | 0.44 (range, 0.03–1) |
68Ga-PSMA-11 PET/CT Findings and Consensus CTVs
Tables 2 and 3 depict the 68Ga-PSMA-11 PET/CT findings. One hundred thirty-two of 270 patients (49%) had a positive 68Ga-PSMA-11 PET/CT result. Fifty-two of 132 patients (39%) had at least one PSMA-11–positive lesion not covered by consensus CTVs: 33 of 132 (25%) had extrapelvic metastases whereas 19 of 132 (14%) had PSMA-11–positive pelvic lesions not covered by consensus CTVs, without extrapelvic metastases. The 3 most common 68Ga-PSMA-11–positive lesion locations outside the consensus CTVs were bone (23/52, 44%), perirectal LNs (16/52, 31%), and distal external iliac LNs (9/52, 17%). Figure 2 displays a 3-dimensional map of all 68Ga-PSMA-11 PET recurrences outside the consensus CTVs coregistered on a template patient’s CT image.
TABLE 2.
68Ga-PSMA-11 PET/CT Patterns of Relapse
| Pattern | Number of patients |
| PSMA-11 PET/CT+ | 132 (49%) |
| Prostate bed (T+) | 47 (17.5%) |
| Pelvic LN (N1) | 83 (30.5%) |
| Extrapelvic LN (M1a) | 9 (3.5%) |
| Bone (M1b) | 23 (8.5%) |
| Visceral (M1c) | 3 (1%) |
| PSMA-11 T+ N0 M0 | 32 (12%) |
| PSMA-11 T0 N1 M0 | 59 (22%) |
| PSMA-11 T+ N1 M0 | 8 (3%) |
| PSMA-11 T+ N0 M1 | 2 (0.7%) |
| PSMA-11 T0 N0 M1 | 15 (5.5%) |
| PSMA-11 T0 N1 M1 | 11 (4%) |
| PSMA-11 T+ N1 M1 | 5 (2%) |
Total population = 270. Percentages do not add up to 100 because multiple disease localizations per patient were possible.
TABLE 3.
Anatomic Repartition of 68Ga-PSMA-11–Positive Findings and Outside Planning Volumes Based on RTOG Consensus CTVs
| Site | PSMA-11–positive patients (n) | Outside CTV patients (n) | PSMA-11–positive lesions (n) | Outside CTV lesions (n) | Median size (mm)* | Median SUVmax* |
| Overall | 132 (49%) | 52 (19.5%) | 304 | 119 | 6.0 (3.0–23.0) | 5.7 (0.5–86.9) |
| Prostate bed (T+) | 47 (17.5%) | 1 (0.003%) | 52 | 1 | 7.0 (4.0–23.0) | 6.4 (2.2–86.9) |
| Pelvic LN (N+) | 83 (30.5%) | 30 (11%) | 174 | 39 | 6.0 (3.0–17.0) | 5.8 (1.5–69.7) |
| Internal iliac | 27 (10%) | 2 (0.7%) | 32 | 2 | 6.0 (3.0–10.0) | 7.3 (2.3–55.0) |
| External Iliac | 38 (14%) | 9 (3.5%) | 45 | 9 | 7.0 (3.5–15.0) | 5.9 (1.5–69.7) |
| Obturator | 19 (7%) | 2 (0.7%) | 24 | 2 | 6.0 (4.0–17.0) | 3.5 (2.1–17.4) |
| Perirectal | 18 (6.5%) | 16 (6%) | 25 | 19 | 5.0 (4.0–10.0) | 5.2 (1.50–57.7) |
| Presacral | 13 (5%) | 3 (1%) | 22 | 4 | 6.0 (4.0–10.0) | 7.5 (1.5–45.7) |
| Common iliac | 16 (6%) | 2 (0.7%) | 26 | 3 | 6.0 (3.0–15.0) | 5.9 (2.0–33.3) |
| Extrapelvic LN (M1a) | 9 (3.5%) | 9 (3.5%) | 28 | 28 | 8.0 (3.0–12.0) | 13.6 (2.7–38.9) |
| Inguinal | 2 (0.7%) | 2 (0.7%) | 7 | 7 | — | — |
| Retroperitoneal | 6 (2%) | 6 (2.2%) | 15 | 15 | — | — |
| Upper diaphragm | 2 (0.7%) | 2 (0.7%) | 6 | 6 | — | — |
| Bone (M1b) | 23 (8.5%) | 23 (8.5%) | 39 | 39 | — | 5.3 (2.7–28.8) |
| Lung (M1c) | 3 (1%) | 3 (1%) | 11 | 11 | 5.0 (4.0–7.0) | 1.0 (0.5–2.6) |
Data in parentheses are range.
Percentages do not add up to 100 because multiple disease localizations per patient were possible.
68Ga-PSMA-11 PET/CT Findings and Clinicopathologic Characteristics
The 132 positive 68Ga-PSMA-11 PET/CT patients had significantly higher PSA levels (median, 0.5 vs. 0.36 ng/mL; P < 0.001) and shorter times to recurrence (median, 21.3 vs. 30.4 mo; P = 0.05) than the 138 negative ones. The detection rate (at least one PSMA-11–positive finding) was significantly higher in patients with a Gleason score of more than 7 than in those with a Gleason score of 7 or less (56/86 [65%] vs. 68/168 [40%]; P < 0.001), in N1 patients than in N0 patients (35/54 [65%] vs. 75/166 [45%]; P = 0.02), and in T3 patients than in T2 patients (82/144 [57%] vs. 34/99 [34%]; P < 0.001). One hundred fifty-three patients had serum PSA levels of less than 0.5 ng/mL (very low PSA group), and 117 had levels of between 0.5 and 1.0 ng/mL (low PSA group). The detection rate was significantly higher in the low PSA group than in the very low PSA group: (70/117 [60%] vs. 62/153 [40.5%]; P = 0.003). The frequency of PSMA-11–positive lesions not covered by the consensus CTVs had a borderline-significant dissimilar pattern in the low and the very low PSA groups (29/117 [25%] vs. 23/153 [15%]; P = 0.06).
Verification of PSMA-11–Positive Lesions Outside Consensus CTVs
Lesions not covered by the consensus CTVs were verified in 24 of 52 patients (46%). This was done by biopsy (n = 1), surgery (n = 3), bone scanning (n = 1), MRI (n = 1), follow-up imaging (CT or PET/CT) showing progression at the site (n = 8), follow-up imaging (CT or PET/CT) showing a response to treatment (n = 5), or a decrease in serum PSA after focal treatment (stereotactic body radiation therapy; n = 5).
Potential Impact of 68Ga-PSMA-11 PET/CT on SRT Planning
Table 4 summarizes the potential impact of 68Ga-PSMA-11 PET/CT on SRT planning.
TABLE 4.
Potential Impact of 68Ga-PSMA-11 PET/CT Imaging on SRT Planning for Early BCR After Primary Prostatectomy
| Impact | Data |
| Major impact on SRT planning—outside RTOG CTV recurrence | 52 (19%) |
| Extension of pelvic consensus CTVs | 19 (7%) |
| Superior extension to cover paraaortic LNs | 5 (2%) |
| Oligometastasis-directed stereotactic body radiation therapy (≤5 M1a or M1b) | 22 (9.5%) |
| Radiotherapy futile because of polymetastatic or visceral disease (>5 M1a, M1b, or M1c) | 6 (2.5%) |
| Minor impact on SRT planning—covered by planning based on consensus CTVs; dose escalation to gross disease (68Ga-PSMA-11–positive disease) | 80 (29.5%) |
| No impact on SRT planning—negative 68Ga-PSMA-11 PET/CT results | 138 (51%) |
Total population = 270.
Potential Major Impact
Fifty-two patients had at least one PSMA-11–positive lesion not covered by the consensus CTVs (19% of all 270 patients, 39% of 132 PSMA-11–positive patients). SRT based on consensus CTVs would not be curative for these patients. Nineteen patients with pelvic LN metastasis outside the consensus CTVs (7% of all 270 patients, 14% of 132 PSMA-11–positive patients) could have experienced extension of consensus CTVs to cover PSMA-11–expressing disease. Twenty-two of the 33 patients with extrapelvic metastases (67%) were oligometastatic (≤5 metastatic sites), potentially eligible for metastasis-directed stereotactic body radiation therapy; 5 of 33 (15%) could have experienced superior extension of the nodal CTVs to encompass the paraaortic LNs, and 6 of 33 (18%) had visceral or diffuse metastatic disease (3 with multiple lung metastasis and 3 with ≥5 metastatic sites) and would be unlikely to benefit from local or metastasis-directed therapy.
Potential Minor Impact
Eighty patients (29.5% of all 270 patients, 61% of 132 PSMA-11–positive patients) had PSMA-11–positive lesions covered by the consensus CTVs and thus could have experienced focal dose escalation, which is often customary for irradiation of areas known to harbor gross disease.
DISCUSSION
The lack of sensitivity of standard-of-care imaging for recurrent PCa combined with a sensitive and specific biomarker of early disease recurrence (PSA) generates a unique challenge for local treatment of PCa BCR: we know there is cancer, but we do not know where it is. There is thus an unmet clinical need to improve target delineation in patients with potentially curable PCa with early BCR.
We report in this post hoc analysis of 270 patients with early BCR after prostatectomy that 68Ga-PSMA-11 PET/CT would have had a major impact on 19% of patients imaged (39% of PSMA-11–positive patients) and a minor impact on 30% (61%). Overall, the addition of 68Ga-PSMA-11 PET/CT may affect SRT planning in half the patients with a PSA level of less than 1 ng/mL. Prospective clinical trials are necessary to assess the clinical value of a restaging 68Ga-PSMA-11 PET/CT study before SRT.
Somewhat encouragingly, although most patients are at high-risk, treatment volumes based on consensus CTVs covered all PSMA-11–positive lesions for 61% of patients with a positive 68Ga-PSMA-11 PET/CT result. This frequency is consistent with the historical success rate of SRT. However, consensus CTVs were inadequate to cover all PSMA-11–positive lesions in 39% of patients with a positive 68Ga-PSMA-11 PET/CT result.
The detection rate of 49% for PSMA-11–positive lesions in this cohort with BCR after surgery and a low PSA level (<1.0 ng/mL) is consistent with previous reports (15,16). The anatomic distribution of PSMA-11–positive lesions is consistent with previous PET studies using choline and PSMA-11 ligands in the setting of BCR (9,19,24,32–35). The most common nodal regions outside the CTVs in patients in this study were the perirectal (n = 16), distal external iliac (n = 9), and paraaortic (n = 5), which are neither assessed by routine LN dissections at prostatectomy nor targeted by routine SRT. It is unlikely that a uniform expansion of the consensus CTVs to cover these regions would be feasible, given the risks of additional toxicity. Notably, most PSMA-11–positive nodes were subcentimeter (median LN short axis: 6 mm [range, 3–17 mm]). Most PSMA-11–positive lesions in the prostate bed were covered. Furthermore, 32 patients (24% of the 132 PSMA-11–positive patients) had PSMA-positive lesions isolated to the prostate bed alone, whereas 67 of 132 patients (51%) had PSMA-11–positive lesions within the pelvis but without distant metastasis. This underscores the potential benefit of including pelvic nodal CTVs, which is currently under investigation in prospective trials (RTOG 0534, NCT00567580).
Importantly, the most common PSMA-11–positive lesion location outside the consensus CTVs was bone (23/52, 44%). No expansion of current CTVs would successfully cover these recurrences. Most M1 patients (67%) in this study were oligometastatic M1a or M1b (1–5 extrapelvic sites). Currently, most patients with M1 PCa receive palliative hormonal therapy as primary treatment. The use of image-guided, metastasis-directed ablative therapy (such as stereotactic body radiation therapy) to distant lesions is an attractive strategy (35–38) being investigated in prospective trials (NCT01558427, NCT02274779) (39,40). The success of this approach, however, depends on accurate staging. 68Ga-PSMA-11 PET/CT is probably the most sensitive imaging modality for selecting patients who might benefit.
The impact of choline PET/CT imaging on SRT planning has been assessed in several retrospective studies (9,32,33,41–45) and has ranged from 13.5% to 81.3%, with a median of 32%. Taken together, these studies found that the addition of choline PET/CT to SRT planning changed the initial plan in 357 of 1,083 patients (33%). However, 68Ga-PSMA-11 PET/CT is superior to choline PET/CT, as shown in several studies (13,46–48), with a more favorable tumor-to-background ratio and better sensitivity for lesion detection at low PSA values (<2 ng/mL). Therefore, a higher impact of 68Ga-PSMA-11 PET/CT on SRT planning would be expected. Several prior studies did report on the potential impact of 68Ga-PSMA-11 PET/CT imaging on radiotherapy planning (18–22,24,35). The impact ranged from 34% to 87%, with a median of 57%. However, these studies had limitations such as inhomogeneous patient groups, with primary and recurrent disease, and a wide range of serum PSA values and clinical disease states (18–24). Therefore, strengths unique to our study include the large number of patients (270), the fact that all patients had BCR after radical prostatectomy without prior radiotherapy, and the fact that all patients had a PSA level of less than 1 ng/mL at the time of 68Ga-PSMA-11 PET/CT imaging. This is the most relevant patient cohort to assess the impact of 68Ga-PSMA-11 PET/CT on SRT.
This was a post hoc retrospective analysis of a well-controlled patient cohort. The design of this study precludes analysis of the impact of 68Ga-PSMA-11 PET/CT on clinical outcomes. To minimize bias, consensus CTVs were drawn with masking of the 68Ga-PSMA-11 PET images. Another limitation was the absence of lesion verification in all patients, but lesion confirmation in recurrent patients is frequently not feasible.
CONCLUSION
This multicenter-study post hoc analysis of 270 patients with PCa early BCR (PSA < 1.0 ng/mL) after radical prostatectomy implied a major impact (19%) of 68Ga-PSMA-11 PET/CT on SRT. This finding justifies a randomized prospective trial to determine whether 68Ga-PSMA-11 PET/CT can improve outcomes in patients with PCa early BCR after radical prostatectomy.
DISCLOSURE
Jeremie Calais is the recipient of grant SAE20160604150 from the Fondation ARC pour la recherche sur le cancer. Wolfgang Fendler received a scholarship (grant 807122) from the Deutsche Forschungsgemeinschaft. Matthias Eiber was supported by Sonderforschungsbereich 824, Project B11, from the Deutsche Forschungsgemeinschaft. Nicholas Nickols is a Prostate Cancer Foundation Young Investigator and a recipient of VA Career Development Award 5IK2BX002520, UCLA Prostate SPORE Career Enhancement Award 4P50CA092131, a STOP Cancer Foundation career development award, and a UCLA JCCC seed grant. Johannes Czernin is the recipient of grant DE SC0012353 from the U.S. Department of Energy, 2017 Challenge Award 17CHAL02 from the Prostate Cancer Foundation, and NIH–NCI Cancer Center Support Grant P30 CA016042 from the Jonsson Comprehensive Cancer Center and is a founder and board member and holds equity in Sofie Biosciences and Trethera Therapeutics. Intellectual property has been patented by the University of California and has been licensed to Sofie Biosciences and Trethera Therapeutics. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kishan AU, Shaikh T, Wang P-C, et al. Clinical outcomes for patients with Gleason score 9-10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: a multi-institutional comparative analysis. Eur Urol. 2017;71:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190:441–449. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King CR. Adjuvant versus salvage radiotherapy for high-risk prostate cancer patients. Semin Radiat Oncol. 2013;23:215–221. [DOI] [PubMed] [Google Scholar]
- 6.King CR. The dose-response of salvage radiotherapy following radical prostatectomy: a systematic review and meta-analysis. Radiother Oncol. 2016;121:199–203. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. [DOI] [PubMed] [Google Scholar]
- 8.Goenka A, Magsanoc JM, Pei X, et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int J Radiat Oncol Biol Phys. 2012;84:112–118. [DOI] [PubMed] [Google Scholar]
- 9.Sobol I, Zaid HB, Haloi R, et al. Contemporary mapping of post-prostatectomy prostate cancer relapse with 11C-choline positron emission tomography and multiparametric magnetic resonance imaging. J Urol. 2017;197:129–134. [DOI] [PubMed] [Google Scholar]
- 10.Kane CJ, Amling CL, Johnstone PAS, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–611. [DOI] [PubMed] [Google Scholar]
- 11.Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawton CAF, Michalski J, El-Naqa I, et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 14.Rauscher I, Maurer T, Beer AJ, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–1719. [DOI] [PubMed] [Google Scholar]
- 15.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 16.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. [DOI] [PubMed] [Google Scholar]
- 17.Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–1623. [DOI] [PubMed] [Google Scholar]
- 18.Shakespeare TP. Effect of prostate-specific membrane antigen positron emission tomography on the decision-making of radiation oncologists. Radiat Oncol. 2015;19:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Leeuwen PJ, Stricker P, Hruby G, et al. 68 Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–739. [DOI] [PubMed] [Google Scholar]
- 20.Sterzing F, Kratochwil C, Fiedler H, et al. 68Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albisinni S, Artigas C, Aoun F, et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. [DOI] [PubMed] [Google Scholar]
- 22.Bluemel C, Linke F, Herrmann K, et al. Impact of 68Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res. 2016;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habl G, Sauter K, Schiller K, et al. 68Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: individualized medicine or new standard in salvage treatment. Prostate. 2017;77:920–927. [DOI] [PubMed] [Google Scholar]
- 24.Schiller K, Sauter K, Dewes S, et al. Patterns of failure after radical prostatectomy in prostate cancer? Implications for radiation therapy planning after 68Ga-PSMA-PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:1656–1662. [DOI] [PubMed] [Google Scholar]
- 25.Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 26.Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. [DOI] [PubMed] [Google Scholar]
- 27.Moghanaki D, Koontz BF, Karlin JD, et al. Elective irradiation of pelvic lymph nodes during postprostatectomy salvage radiotherapy. Cancer. 2013;119:52–60. [DOI] [PubMed] [Google Scholar]
- 28.Zumsteg ZS, Daskivich TJ, Sandler HM. Salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy. J Clin Oncol. 2016;34:3829–3833. [DOI] [PubMed] [Google Scholar]
- 29.Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzenboeck SM, Rauscher I, Bluemel C, et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med. 2017;58:1545–1552. [DOI] [PubMed] [Google Scholar]
- 31.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 32.Souvatzoglou M, Krause BJ, Pürschel A, et al. Influence of 11C-choline PET/CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiother Oncol. 2011;99:193–200. [DOI] [PubMed] [Google Scholar]
- 33.Würschmidt F, Petersen C, Wahl A, Dahle J, Kretschmer M. [18F]fluoroethylcholine-PET/CT imaging for radiation treatment planning of recurrent and primary prostate cancer with dose escalation to PET/CT-positive lymph nodes. Radiat Oncol. 2011;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegemann N-S, Wenter V, Spath S, et al. Distribution of prostate nodes: a PET/CT-derived anatomic atlas of prostate cancer patients before and after surgical treatment. Radiat Oncol. 2016;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habl G, Straube C, Schiller K, et al. Oligometastases from prostate cancer: local treatment with stereotactic body radiotherapy (SBRT). BMC Cancer. 2017;17:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guler OC, Engels B, Onal C, et al. The feasibility of prostate-specific membrane antigen positron emission tomography (PSMA PET/CT)-guided radiotherapy in oligometastatic prostate cancer patients. Clin Transl Oncol. August 9, 2017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Muldermans JL, Romak LB, Kwon ED, Park SS, Olivier KR. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12. [DOI] [PubMed] [Google Scholar]
- 39.Decaestecker K, De Meerleer G, Ameye F, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer. 2014;14:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Supiot S, Rio E, Pacteau V, Mauboussin M-H, Campion L, Pein F. OLIGOPELVIS–GETUG P07: a multicentre phase II trial of combined salvage radiotherapy and hormone therapy in oligometastatic pelvic node relapses of prostate cancer. BMC Cancer. 2015;15:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceci F, Herrmann K, Castellucci P, et al. Impact of 11C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. Eur J Nucl Med Mol Imaging. 2014;41:2222–2231. [DOI] [PubMed] [Google Scholar]
- 42.Castellucci P, Fanti S. Prostate cancer: identifying sites of recurrence with choline-PET-CT imaging. Nat Rev Urol. 2015;12:134–135. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein J, Even-Sapir E, Ben-Haim S, et al. Does choline PET/CT change the management of prostate cancer patients with biochemical failure? Am J Clin Oncol. 2017;40:256–259. [DOI] [PubMed] [Google Scholar]
- 44.Jereczek-Fossa BA, Rodari M, Bonora M, et al. [11C]choline PET/CT impacts treatment decision making in patients with prostate cancer referred for radiotherapy. Clin Genitourin Cancer. 2014;12:155–159. [DOI] [PubMed] [Google Scholar]
- 45.Lamanna G, Tabouret-Viaud C, Rager O, et al. Long-term results of a comparative PET/CT and PET/MRI study of 11C-acetate and 18F-fluorocholine for restaging of early recurrent prostate cancer. Clin Nucl Med. 2017;42:e242–e246. [DOI] [PubMed] [Google Scholar]
- 46.Bluemel C, Krebs M, Polat B, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-choline-PET/CT. Clin Nucl Med. 2016;41:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwenck J, Rempp H, Reischl G, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. [DOI] [PubMed] [Google Scholar]