Abstract
AIM
To assess the seroprevalence of hepatitis B virus (HBV) immunity among previously vaccinated pediatric liver transplant recipients and present a case report of de novo hepatitis B infection after liver transplantation.
METHODS
This study focused on children with chronic liver diseases who received primary hepatitis B immunization and had a complete dataset of anti-HBs before and after liver transplantation between May 2001 and June 2017. Medical records were retrospectively reviewed for potential factors relating to HBV immunity loss.
RESULTS
In total, 50 children were recruited. The mean time from liver transplantation to anti-HBs testing was 2.53 ± 2.11 years. The mean anti-HBs levels before and after liver transplantation were 584.41 ± 415.45 and 58.56 ± 6.40 IU/L, respectively. The rate of non-immunity (anti-HBs < 10 IU/L) in the participants was 46% (n = 26) at one year, 57% (n = 7) at two years and 82% (n = 17) at > three years following liver transplantation. The potential factors relating to HBV immunity loss after liver transplantation were identified as anti-HBs (P = 0.002), serum albumin (P = 0.04), total bilirubin (P = 0.001) and direct bilirubin (P = 0.003) before liver transplantation. A five-year-old boy with biliary cirrhosis received 4 doses of HBV vaccine with an anti-HBs titer of > 1000 IU/L and underwent liver transplantation; his anti-HBc-negative father was the donor. After liver transplantation, the boy had stenosis of the hepatic artery up to the inferior vena cava anastomosis and underwent venoplasty three times. He also received subcutaneous injections of enoxaparin for 5 mo and 20 transfusions of blood components. Three years and ten months after the liver transplantation, transaminitis was detected with positive tests for HBsAg, HBeAg, and anti-HBc (2169.61, 1706 and 8.45, respectively; cutoff value: < 1.00) and an HBV viral load of 33212320 IU/mL.
CONCLUSION
The present study showed that loss of hepatitis B immunity after liver transplantation is unexpectedly common. In our case report, despite high levels of anti-HBs prior to transplantation, infection occurred at a time when, unfortunately, the child had lost immunity to hepatitis B after liver transplantation.
Keywords: Hepatitis B vaccine, Liver transplantation, De novo hepatitis B infection, Anti-HBs antibody, Immunity
Core tip: Despite the completion of hepatitis B vaccination, loss of hepatitis B immunity in children after liver transplantation is common and we encountered a case of de novo hepatitis B virus (HBV) infection following liver transplantation. Serum anti-HBs, albumin, total bilirubin, and direct bilirubin prior to liver transplantation were identified as potential factors related to HBV immunity loss after liver transplantation. A booster dose of hepatitis B vaccine and raising serum albumin to normal levels could delay the rapid loss of HBV immunity after liver transplantation but may not prevent de novo hepatitis B. Consequently, strategies are required to maintain anti-HBs antibody above the protective level after liver transplantation. Regular assessment of anti-HBs after liver transplantation should also be considered along with revaccination to guarantee long-term protection from HBV infection.
INTRODUCTION
Hepatitis B virus (HBV) infection is considered a great burden worldwide owing to its chronicity and the increased risk of hepatocellular carcinoma. Moreover, antiviral therapy might not completely eradicate HBV from the human liver[1]. Since the 1980s, primary HBV immunization has been implemented to reduce HBV transmission and has shown high efficacy and good serological correlates for protective immunity[2,3]. The rapid and robust response, which usually develops 5-8 d after re-exposure to the HBsAg and peaks after approximately 14 d, indicates the long-lasting protective property of the vaccine despite the undetectable anti-HBs titer[4-6]. In contrast, a more rapid decline of anti-HBs antibody level has been observed in children post-liver transplantation compared to healthy children[7,8], which corresponds to a loss of protection as de novo HBV infection was evident in some cases[9-11]. This evidence implied that these immunocompromised patients might need a higher protective level of anti-HBs antibody with which to prevent HBV infection following liver transplantation[11-14].
The assessment of anti-HBs titers is recommended in patients who have undergone liver transplantation[12-14], but there is a lack of data regarding an appropriate schedule for revaccination. Moreover, there is insufficient data supporting the disease burden of de novo hepatitis B infection after liver transplantation, especially if the liver is from an antiHBc-negative donor[9,10].
The present study aimed to assess anti-HBs immunity loss in children who received primary vaccination and also possessed anti-HBs immunity above the protective level prior to liver transplantation. In addition, we also present a case of de novo hepatitis B infection after liver transplantation despite the fact that the patient had high titers of pre-transplantation anti-HBs and received an anti-HBc-negative liver from his father.
MATERIALS AND METHODS
Recruitment of participants
All children who underwent liver transplantation and received ≥ 3 doses of hepatitis B vaccine prior to transplantation between May 2001 and June 2017 were invited to participate in this study. Participants over 18 years of age at the time of the study and with no history of anti-HBs or anti-HBs < 10 IU/L before liver transplantation were excluded. Medical records were retrospectively reviewed to collate the following information: (1) demographic data [gender, age, body weight (BW), height, body mass index (BMI)] and (2) history of hepatitis B vaccination and booster prior to liver transplantation. Furthermore, the pediatric end-stage liver disease (PELD) score, or the model for end-stage liver disease (MELD) score, was calculated in children aged ≤ 13 years old and > 13 years old at the time of liver transplantation, respectively, every 3 mo after children were placed on the transplant waiting list. The current PELD/MELD score at the time of albumin infusion has not been initiated was used for data analysis. Laboratory data that might reflect immune status and disease severity was collated, including complete blood count, albumin, total bilirubin (TB), direct bilirubin (DB), immunosuppressant use and hepatitis B profiles before/after transplantation (HBsAg, anti-HBs, and anti-HBc). Follow-up time and donor characteristics were also collated, including gender; hepatitis B profiles (HBsAg, anti-HBs, and anti-HBc), and also whether the donor was alive or cadaveric. Samples were obtained from all participants who did not undergo hepatitis B profile testing after transplantation. Nutritional status (BW, height, BMI) and immunosuppressant use at the same time of hepatitis B profile testing were also recorded. Participants were classified into two subgroups: immune and non-immune. Hepatitis B immunity was defined as when anti-HBs level > 10 IU/L. De novo hepatitis B infection was defined as positive HBsAg and HBV DNA serological tests after liver transplantation despite a negative test prior to transplantation. A high anti-HBs titer was defined as when anti-HBs > 1000 IU/L.
Ethical considerations
Routine history taking and physical examination, including weight and height measurement, were carried out by physicians. Anti-HBs, anti-HBc, and HBsAg were tested concurrent with routine laboratory testing during the follow-up visit to the transplant clinic. Verbal consent was obtained from the caregivers. Ethical approval was granted by the Ethics Committee, Faculty of Medicine, Chulalongkorn University (IRB number: 614/60).
Statistical analysis
Continuous and categorical data were presented as mean ± SD/median (range) and proportion or percentage, respectively. The Mann-Whitney U test and unpaired t-test were used to compare continuous data, as appropriate. The Chi-square test was used to compare categorical data. Analysis of variance (ANOVA) was used to compare more than 2 continuous variables. Multiple linear regression was performed to investigate the influence of significant parameters on the loss of anti-HBs after liver transplantation. A P-value > 0.05 was regarded as being statistically significant. Data analyses were performed using SPSS version 24.0.0 (SPSS, Inc., Chicago, IL, United States).
RESULTS
Recipient and donor characteristics
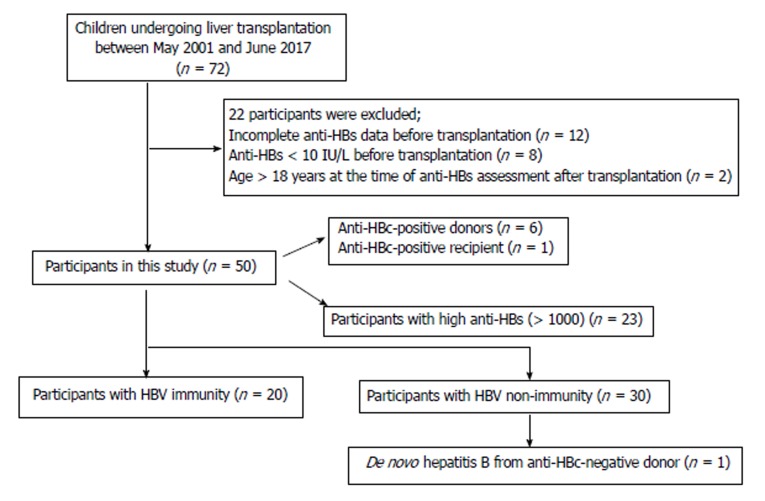
Seventy-two children underwent liver transplantation between May 2001 and June 2017. All children were negative for HBsAg. Twenty-two cases were excluded for the following reasons: incomplete anti-HBs data (n = 12), anti-HBs < 10 IU/L (n = 8) before transplantation, and age > 18 years (n = 2). Finally, 50 children were recruited into the present study with a mean age of 6.67 ± 4.63 years; 54% were female (Figure 1). The indications for liver transplantation were biliary atresia (n = 39), Alagille syndrome (n = 2), progressive familial intrahepatic cholestasis (PFIC) (n = 2), primary bile acid deficiency (n = 1), fulminant Wilson’s disease (n = 1), hepatoblastoma (n = 1), glycogen storage disease (n = 1) and cryptogenic cirrhosis (n = 3). All children received primary hepatitis B vaccination at birth and at 1 and 6 mo of age. Twenty-three subjects received one booster dose 1-2 mo before transplantation. Twenty-three subjects had high anti-HBs (> 1000 IU/L) before liver transplantation. Most subjects received a living donor liver transplantation from their parents (n = 44, 88%), while 6 (12%) received a liver from a cadaveric donor. Children who received an anti-HBc-positive liver from the 6 cadaveric donors were required to take lifelong lamivudine. The severity of chronic liver disease, or PELD score and MELD score, were 18.38 ± 8.47 (n = 47) and 15 (range: 11-19; n = 3), respectively. All children received at least two immunosuppressants early after transplantation and only one immunosuppressant subsequently, with the exception of immunosuppressive agent-withdrawal in one female patient. This particular patient developed post-transplantation lymphoproliferative disease (PTLD) and ongoing cholestasis, and is thus awaiting re-transplantation. The clinical and demographic characteristics of all participants and donors are shown in Table 1.
Figure 1.
Algorithm showing our study participants. HBV: Hepatitis B virus.
Table 1.
Patients’ demographic data and characteristics mean ± SD or n (%)
| Characteristics | All participants (n = 50) |
| Age (yr) | 6.67 ± 4.63 |
| Age at liver transplantation (yr) | 3.06 ± 3.97 |
| Gender - female | 27 (54) |
| Before liver transplantation | |
| Anti-HBs level (IU/L) | 584.41 ± 415.45 |
| Anti-HBc positive | 1 (2) |
| After liver transplantation | |
| Anti-HBs level (IU/L) | 58.56 ± 6.40 |
| Length of stay in hospital after transplant (d) | 44.10 ± 29.30 |
| ABO incompatibility | 3 (6) |
| PELD/MELD score | 18.38 ± 8.47/15 (11-19) |
| Time since transplantation (yr) | 2.53 ± 2.11 |
| Medical complications | |
| Acute rejection | 22 (44) |
| Cytomegalovirus infection | 15 (30) |
| Posttransplant lymphoproliferative disorder | 14 (28) |
| De novo hepatitis B infection | 1 (2) |
| De novo food allergy | 9 (18) |
| Surgical complications | |
| Vascular stricture | 20 (40) |
| Biliary stricture | 12 (24) |
| Chylous ascites/chylothorax | 7 (14) |
| Donor characteristics | |
| Gender - female | 22 (44) |
| Cadaveric | 6 (12) |
| Living | 44 (88) |
| Anti-HBs | |
| Negative | 13 (26) |
| Positive | |
| 1-9 IU/L | 30 (60) |
| > 10 IU/L | 7 (14) |
| Anti-HBc positive | 6 (12) |
PELD: Pediatric end-stage liver disease; MELD: Model for end-stage liver disease.
Recipient anti-HBs before and after liver transplantation
Anti-HBs titers before and after liver transplantation were 584.41 ± 415.45 and 58.56 ± 6.40 IU/L, respectively. Thirty participants (60%) experienced the loss of HBV immunity after transplantation. The rates of non-immunity (anti-HBs < 10 IU/L) in the participants were 46% (n = 26) at one year, 57% (n = 7) at two years and 82% (n = 17) at > three years following liver transplantation. The age at liver transplantation and the time from transplant to anti-HBs testing were 3.06 ± 3.97 years and 2.53 ± 2.11 years, respectively. One patient was diagnosed with de novo hepatitis B infection three years after liver transplantation.
Nutritional status, disease severity and the immune status of subjects before and after liver transplantation
After transplantation, the body weight and height, not including BMI, were increased compared to pre-transplant status (P < 0.001). TB, DB, and albumin levels, which were reflective of disease severity, improved after liver transplantation in both the short-term (at 3 mo) and long-term (2.53 ± 2.11 years; P < 0.001). With respect to immune status, white blood cell count (P = 0.002), neutrophils (P < 0.001) and lymphocytes (P = 0.01) were reduced, while platelet count (P = 0.005) increased after liver transplantation, in both the short-term (at 3 mo) and long-term (2.53 ± 2.11 years). The number of prescribed immunosuppressants decreased in the long-term post-liver transplantation (P < 0.001; Table 2).
Table 2.
Nutritional and laboratory results of pre and post liver transplantation mean ± SD or n (%)
| Pre-transplantation |
Post-transplantation |
P value | ||
| Short-term (3 mo) | Long-term | |||
| Body weight (kg) | 11.66± 8.01 | - | 21.32 ± 12.07 | < 0.001 |
| Height (meter) | 0.81 ± 0.23 | - | 1.07 ± 0.28 | < 0.001 |
| BMI (kg/m2) | 16.59 ± 1.67 | - | 16.63 ± 2.71 | 0.76 |
| Complete blood count | ||||
| White blood cell (/μL) | 10492 ± 4627 | 8559 ± 2495 | 7729 ± 3843 | 0.002 |
| Neutrophil (/μL) | 4938 ± 2565 | 3385 ± 1679 | 3418 ± 1912 | < 0.001 |
| Lymphocyte (/μL) | 4263 ± 2222 | 3982 ± 1722 | 3109 ± 1762 | 0.01 |
| Platelet (/μL) | 172880 ± 94557 | 199720 ± 82777 | 233957 ± 94136 | 0.005 |
| Liver function test | ||||
| Total bilirubin(mg/dL) | 18.50 ± 12.68 | 2.41 ± 0.91 | 0.84 ± 0.91 | < 0.001 |
| Direct bilirubin (mg/dL) | 13.41 ± 10.28 | 0.83 ± 0.71 | 0.38 ± 0.51 | < 0.001 |
| Albumin (g/dL) | 3.29 ± 0.97 | 3.94 ± 0.48 | 4.08 ± 0.35 | < 0.001 |
| Number of immunosuppressant | ||||
| 0 | 0 | 1 (2) | ||
| 1 | 5 (10) | 27 (54) | < 0.001 | |
| 2 | 23 (46) | 17 (34) | ||
| 3 | 22 (44) | 5 (10) | ||
BMI: Body mass index.
Comparing participants with HBV immunity and loss of immunity
The age at transplantation and the time of anti-HBs testing after transplantation were lower in participants with HBV immunity (5.27 ± 3.74 years and 2.09 ± 2.03 years, respectively) than in those with loss of HBV immunity (7.60 ± 4.98 years and 3.71 ± 4.78 years, respectively; P = 0.082). No significant differences were observed in terms of BW, height, and BMI that might reflect the nutritional status of either of the groups. In terms of disease severity, participants with HBV immunity had a lower PELD score (P = 0.086), TB (P = 0.003), DB (P < 0.001), and higher albumin (P = 0.04) levels. There were no differences in terms of white blood cell, neutrophil or lymphocyte count before and in the short- or long-term since liver transplantation. Even the number of prescribed immunosuppressants decreased in the long-term after liver transplantation, and there was no difference between the two groups in this respect. We further studied the effect of a booster vaccine before liver transplantation, as twenty-three patients received the booster vaccine. Of these, 14 (61%) still had HBV immunity while 9 (39%) showed a loss in HBV immunity. Twenty-seven patients did not receive the booster vaccine before liver transplantation; of these, six (22%) still had HBV immunity while 21 (78%) did not. We compared all potential factors associated with the loss of HBV immunity between patients who received a booster dose with (n = 14) and without HBV immunity (n = 9), and there were no significant differences with respect to any of the parameters tested (Table 2). One patient who received a booster dose, and whose anti-HBs was more than 1000 IU/L before liver transplantation, was diagnosed with de novo hepatitis three years and ten months after liver transplantation (Table 3).
Table 3.
Potential factors associated with immunity in participants with HBV immunity and immunity loss mean ± SD or n (%)
| Nonimmunity (n = 30) | Immunity (n = 20) | P value | |
| Age (yr) | 7.60 ± 4.98 | 5.27 ± 3.74 | 0.08 |
| Age at liver transplantation (yr) | 3.71 ± 4.78 | 2.09 ± 2.03 | 0.11 |
| Gender:Female | 15 (50) | 12 (60) | 0.58 |
| Body weight (kg) | 13.07 ± 9.91 | 9.56 ± 2.74 | 0.07 |
| Height (m) | 0.85 ± 0.28 | 0.75 ± 0.12 | 0.09 |
| Body mass index (kg/m2) | 16.45 ± 1.62 | 16.45 ± 1.62 | 0.47 |
| Anti-HBs level before transplantation (IU/L) | 441.39 ± 408.88 | 798.94 ± 330.47 | 0.24 |
| Length of hospital stay after transplantation (d) | 47.77 ± 34.89 | 38.80 ± 17.45 | 0.24 |
| PELD/MELD score | 20.19 ± 8.50 (n = 28) | 15.90 ± 7.98 (n = 19) | 0.09 |
| Time since transplant to anti-HBs testing (yr) | 2.83 ± 2.00 | 2.06 ± 2.22 | 0.22 |
| Donor characteristics | |||
| Gender: Female | 14 (47) | 8 (40) | 0.43 |
| Living | 26 (87) | 18 (90) | 0.54 |
| Medical complication | |||
| Acute rejection | 11 (37) | 11 (55) | 0.16 |
| CMV infection | 6 (20) | 9 (45) | 0.11 |
| PTLD | 7 (23) | 7 (35) | 0.28 |
| De novo hepatitis B | 1 (3) | 0 (0) | 0.65 |
| De novo food allergy | 5 (16) | 4 (20) | 0.52 |
| Surgical complication | |||
| Vascular stricture | 13 (43) | 7 (35) | 0.39 |
| Biliary stricture | 8 (27) | 4 (20) | 0.46 |
| Chylous ascites/chylothorax | 5 (17) | 2 (10) | 0.41 |
| Laboratory data before transplantation | |||
| White blood cell (/µL) | 10237 ± 4167 | 10875 ± 5334 | 0.63 |
| Neutrophil (/µL) | 4780 ± 2137 | 5174 ± 3145 | 0.60 |
| Lymphocyte (/µL) | 4372 ± 2515 | 4100 ± 1742 | 0.65 |
| Platelet (/µL) | 159366 ± 99431 | 193156 ± 85132 | 0.22 |
| Total bilirubin (mg/dL) | 22.80 ± 13.72 | 12.04 ± 7.37 | 0.003 |
| Direct bilirubin (mg/dL) | 16.51 ± 11.37 | 8.73 ± 6.08 | 0.001 |
| Albumin (g/dL) | 3.07 ± 0.65 | 3.63 ± 1.25 | 0.04 |
| Laboratory data 3 mo after transplantation | |||
| White blood cell (/µL) | 8312 ± 2596 | 8931 ± 3440 | 0.64 |
| Neutrophil (/µL) | 3221 ± 1307 | 3630 ± 2136 | 0.45 |
| Lymphocyte (/µL) | 3879 ± 1851 | 4136 ± 1542 | 0.61 |
| Platelet (/µL) | 193300 ± 86221 | 208600 ± 78591 | 0.53 |
| Albumin (g/dL) | 3.96 ± 0.51 | 3.91 ± 0.44 | 0.72 |
| Total bilirubin (mg/dL) | 0.99 ± 1.12 | 0.59 ± 0.35 | 0.07 |
| Direct bilirubin (mg/dL) | 0.37 ± 0.15 | 0.32 ± 0.17 | 0.12 |
| Laboratory data after long-term transplantation | |||
| White blood cell (/µL) | 7114 ± 2845 | 8815 ± 5083 | 0.22 |
| Neutrophil (/µL) | 3196 ± 1412 | 3809 ± 2576 | 0.38 |
| Lymphocyte (/µL) | 3039 ± 1553 | 3232 ± 2128 | 0.72 |
| Platelet (/µL) | 217033 ± 87395 | 263823 ± 100739 | 0.12 |
| Albumin (g/dL) | 4.12 ± 0.35 | 4.00 ± 0.35 | 0.26 |
| Total bilirubin (mg/dL) | 0.98 ± 1.10 | 1.10 ± 0.31 | 0.08 |
| Direct bilirubin (mg/dL) | 0.45 ± 0.62 | 0.25 ± 0.13 | 0.20 |
| Immunosuppressant 3 mo after transplantation | |||
| None | 0 | 0 | |
| 1 | 3 (10) | 2 (10) | 0.41 |
| 2 | 16 ((53) | 7 (35) | |
| 3 | 11 (37) | 11 (50) | |
| Immunosuppressant after long-term liver transplantation | |||
| None | 1 (3) | 0 | |
| 1 | 17 (56) | 10 (50) | 0.77 |
| 2 | 9 (30) | 8 (40) | |
| 3 | 3 (10) | 2 (10) |
PELD: Pediatric end-stage liver disease; MELD: Model for end-stage liver disease; PTLD: Post-transplantation lymphoproliferative disease.
A patient with de novo hepatitis B
A five-year-old boy with biliary cirrhosis and an unsuccessful Kasai’s operation underwent liver transplantation at 14 mo of age; his anti-HBc-negative father was the donor. He received 4 doses of HBV vaccine prior to liver transplantation, and his pre-existing anti-HBs antibody titer was > 1000 IU/L. His parent’s viral profiles were negative for HBV infection (HBsAg, anti-HBc, and anti-HBs were all negative). After liver transplantation, he developed stenosis at the hepatic artery leading to the inferior vena cava anastomosis and underwent venoplasty with balloon dilatation three times. Furthermore, he was given subcutaneous enoxaparin injections every 12 h for 5 mo and transfused 20 times with blood components. Three years and ten months after the liver transplantation, transaminitis was detected, with positive tests for HBsAg, HBeAg, and anti-HBc (2169.61, 1706, and 8.45, respectively; cutoff value: < 1.00), and an HBV viral load of 33212320 IU/mL. The timeline of this patient is shown in Figure 2.
Figure 2.
Timeline of the case report from pre-liver transplantation to diagnosis of HBV infection. HBV: Hepatitis B virus.
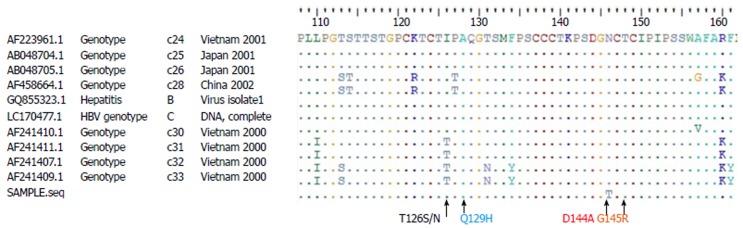
Several possible routes of HBV infection after liver transplantation were investigated. His father and mother were both tested for HBsAg, anti-HBc, and anti-HBs titers and serum HBV viral load; all results were negative. There were no apparent HBsAg carriers in the patient’s family. Another strong possibility might be from blood transfusion, in which the blood component was partly derived from a recently infected donor. However, the handling of blood and blood products in Thailand is extremely safe, as we employ the universally-accepted nucleic acid amplification test (NAT) to screen for HBV, hepatitis C virus, and HIV from all blood donors. All 15 donors who donated blood to this patient had undergone repeat NAT-based HBV testing and none had any evidence of HBV occult infection. The de novo activation of HBV with escape mutations from hepatitis B surface antibody after living donor liver transplantation has been documented previously, however, no vaccine escape mutants were found when the HBs gene was screened in our patient (Figure 3).
Figure 3.
Amino acid sequence of “a” determinant of the HBs gene (position 110-160) showing no significant escape mutants in common regions (T126S/N, Q129H, D144A, and G145R).
DISCUSSION
Transmission of the HBV core from hepatitis B core antibody-positive donors was first reported in 1998 by Uemoto et al[15]. In Uemoto et al’s, HBV existing in the liver of healthy donors who were hepatitis B core antibody-positive, but not in the blood, was shown to be transmitted to recipients by liver grafts following liver transplantation. Moreover, livers from hepatitis B core antibody-positive donors exerted influence on graft survival as this was lower in the recipients of hepatitis B core antibody-positive tissue compared to those receiving tissue from hepatitis B core antibody-negative donors, especially among HBsAg-negative recipients[16]. As a result, robust strategies have been developed to prevent viral activation and de novo hepatitis B infection in recipients receiving liver grafts from hepatitis B core antibody-positive donors. These strategies involve passive immunization with hyper-immune hepatitis B immunoglobulin (HBIG), with or without antiviral agent, or the administration of hepatitis B vaccine[17-20]. In contrast, the prevention of de novo hepatitis B infection in recipients of tissue from hepatitis B core antibody-negative donors has generally been disregarded because evidence to support de novo hepatitis B infection from the loss of HBV immunity after liver transplantation is scarce[9,10].
In this study, we report a high prevalence of the loss of HBV immunity following liver transplantation, and its association with disease severity and anti-HBs titer levels before liver transplantation. Our study demonstrated that 60% of pediatric liver transplant patients who were previously immunized were non-immune after their transplants. This was comparable to the 67% of patients reported in a previous study who also showed a loss of immunity[8]. While patients with HBV immunity had a significantly higher titer of anti-HBs before liver transplantation than participants showing a loss of HBV immunity after liver transplantation, a higher titer of anti-HBs before liver transplantation cannot guarantee protective HBV immunity following liver transplantation, as evidenced by the case report described in the present study. This finding contrasts with a previous study by Su et al[9] who reported that an anti-HBs titer of > 200 IU/L before liver transplantation might be sufficient to prevent de novo HBV infection in an HBsAg-negative recipient. One possible cause of HBV infection in our patient is that the loss of HBV immunity could have occurred after liver transplantation at a time when the patient was most likely exposed to HBV. Our data also showed a rapid reduction in the level of anti-HBs after liver transplantation with titers below the protective threshold. Regular assessment of anti-HBs, and revaccination after liver transplantation, should therefore be considered to maintain the anti-HBs titer level above the protective threshold and therefore prevent de novo hepatitis B. Lin et al[10] recommended maintaining a high level of anti-HBs (> 1000 IU/L), which may prevent de novo HBV infection in pediatric patients undergoing liver transplantation who had efficient primary vaccination. A booster vaccine appears to be the most simple and effective regimen with which to maintain high titers of anti-HBs. However, this previous study reported that patients had to receive 1-19 injections, within a period of 4-42 mo after liver transplantation to maintain adequate titers[10]. Potential factors for the rapid loss of anti-HBs loss should therefore be considered in order to avoid patients undergoing multiple injections.
In the present study, anti-HBs level, cholestasis, and low albumin levels before liver transplantation were identified to be significant factors contributing to the loss of immunity after liver transplantation. Contrast to a previous study that found statistical significance in terms of age or time since liver transplantation instead[8]. Anti-HBs level before liver transplantation may represent one of the main factors with which to predict the loss of anti-HBs after liver transplantation; age and time since liver transplantation should not be relied upon. Although the hepatitis B vaccine is highly immunogenic and very effective, there is a gradual reduction of anti-HBs titer to below the protective threshold after one or two decades of life, even in healthy individuals[21,22]. In children with chronic liver diseases, anti-HBs immunity decreases more rapidly than in healthy children, as demonstrated in the present study; indeed, at least 8 of the 72 children on the waiting list for liver transplantation in our institution had anti-HBs < 10 IU/L and were excluded from the present study. In a previous study, Leung et al[8] stated that a major limitation of their study was that they could not definitively state whether the loss of HBV immunity in their study was due to a loss of immunity before or after transplantation. We believe that the present study has addressed this shortfall by focusing on anti-HBs level before liver transplantation and then divided patients into high- and low-titer groups. A booster vaccine before liver transplantation is necessary in order to maintain a higher titer of anti-HBs in children following liver transplantation. However, the cutoff for a protective anti-HBs level that is more than 10 IU/L after vaccination might not be enough to protect these vulnerable patients from HBV[23], owing to the rapid decline of anti-HBs over time. Further functional cellular studies are now required to address the most appropriate cutoff level for the protective threshold of anti-HBs in children following liver transplantation.
However, it is not just strategies to delay HBV immunity loss that are needed; we also need to develop methods to re-establish active immunity against HBV after liver transplantation. Lu et al[20] reported the improved feasibility of vaccination combined with nucleoside analogues in the prevention of HBV reinfection after orthotopic liver transplantation compared to regular HBIG administration. In this previous study, long-term and repetitive vaccine stimulation was shown to be an important method with which to create and cultivate an enhanced immune response in these immunocompromised patients. Similar to this study, a few pediatric studies have reported the use of intermittent vaccination reinforcement, or booster vaccination, to maintain spontaneous anti-HBs production in children after liver transplantation[10,24,25]. For example, Ni[25] studied both the humoral and cellular immunity of booster hepatitis B vaccines in children after liver transplantation and demonstrated that the immunological response following a booster dose appeared to be adequate, at least over the short term (2 mo assessment period). However, Bauer et al[26] conducted a pilot study of cellular immune response investigating HBsAg-specific T and B cells in adults after liver transplantation compared with controls and highlighted the role of the strong inhibitory effect of regulatory T cells upon immunological response after hepatitis B revaccination over a period of long term assessment (> one year). The best rationale for HBV revaccination in liver transplantation patients has yet to be elucidated. Future studies are required to identify an appropriate HBV immunization protocol for children after liver transplantation which will effectively re-establish both cellular and humoral immunities to HBV.
Other predictive parameters responsible for rapid anti-HBs loss are cholestasis and low albumin levels. Low albumin levels might reflect the poor synthetic function of the liver, or severe malnutrition, or both. A previous study confirmed that albumin infusion could restore the immunological function of patients with decompensated cirrhosis by increasing circulating PGE2, a potent immunomodulator, both in vitro and in vivo[27-29]. However, a study by Leung et al[8], and this present study, could not demonstrate a significant difference in terms of disease severity when comparing PELD score between patients in immune and non-immune groups. While the mean PELD score in Leung et al’s[8] study was higher than the present study, the mean serum albumin level, which is one of the parameters used to calculate the PELD score, was in the upper normal level in Leung et al’s[8] study compared to the low albumin level in the present study. This upper normal level of serum albumin might imply that the subjects involved in this previous study had already received albumin infusion at the time of data collection. In the present study, we evaluated the PELD score every three months from when the patients joined the waiting list for transplantation and chose the most recent PELD score, at which point, albumin infusion had not been initiated. One limitation of our method is that our PELD score might be lower than the actual PELD score prior to liver transplantation; however, we obtained actual serum albumin data for analysis and that might be why our data showed lower PELD scores, with significantly low albumin levels in patients with anti-HBs loss, than the data reported by Leung et al[8]. As a result, while waiting for liver transplantation, albumin infusion is an effective treatment option to treat not only hepatorenal syndrome and spontaneous bacterial peritonitis, but also gain better immunity; this practice represents common practice for most chronic liver diseases in children.
In the present study, we were unable to demonstrate a significant effect of nutritional status upon anti-HBs immunity after liver transplantation. In our transplant unit, children were not routinely tested for lipid soluble vitamins A, D, and E. Furthermore, body weight and BMI are not the best parameters to perform nutritional assessment, as some children suffered from edema and huge abdominal distension as a result of ascites. Moreover, the immunosuppressants and complications after liver transplantation were not shown to be significantly associated with the loss of anti-HBs; in this respect, our study was consistent with Leung et al[8].
Our study has some limitations which need to be considered, particularly the low number of participants, inadequate data reflect the nutritional status, and the heterogeneity of immunosuppressant-use among patients. A larger, well-designed, multicenter study, using the same protocol of care after liver transplantation, is now needed to validate our present results.
In summary, significant loss of anti-HBs after liver transplantation is unexpectedly common. An anti-HBs level above 1000 IU/L before liver transplantation cannot prevent de novo hepatitis B. Boosters or a full-series vaccination is required for children after liver transplantation, concurrent with close monitoring of anti-HBs level. Further studies should aim to identify the best rationale for a HBV re-immunization program based upon strong clinical evidence. The potential factors that can affect anti-HBs levels, and which can be modulated prior to liver transplantation and therefore delay the rapid loss of anti-HBs include booster hepatitis B vaccines and the early administration of albumin.
ARTICLE HIGHLIGHTS
Research background
A more rapid decline of anti-HBs antibody was observed in children after liver transplantation compared to healthy children who had been previously immunized. The loss of anti-HBs might not indicate a loss of hepatitis B virus (HBV) immunity in healthy subjects. However, viral reactivation and de novo hepatitis B infection were clearly demonstrated in an HBsAg-negative recipient who received a liver from hepatitis B core antibody-positive or negative donors, suggesting the loss of HBV protection in such immunocompromised subjects. The present study provided strong evidence of HBV immunity loss in 60% of children after liver transplantation and one case of de novo hepatitis B infection. This was despite a high titer of anti-HBs prior to transplantation and the receipt of a hepatitis B core-negative liver. The present study highlighted the importance of developing strategies to re-establish active immunity to HBV following liver transplantation.
Research motivation
HBV infection in patients after liver transplantation can lead to chronic hepatitis, shorter graft survival and graft loss. However, a routine strategy for HBV reimmunization after liver transplantation, and an appropriate cutoff for the prevention of de novo hepatitis B infection, have yet to be elucidated. This study demonstrated a decline of anti-HBs level after liver transplantation and provided valuable data relating to the factors which can affect the rapid loss of anti-HBs and which can be modulated before liver transplantation in order to delay rapid anti-HBs loss. Such factors include booster hepatitis B vaccines and the early administration of albumin. A revaccination program is recommended for children after liver transplantation in order to re-establish active immunity to HBV.
Research objectives
Regular assessment of anti-HBs and revaccination after liver transplantation to maintain an anti-HBs titer level above the protective threshold should be considered to prevent de novo hepatitis B. Further studies should aim to identify the best rationale for a reimmunization program to effectively re-establish active immunity to HBV.
Research methods
The authors enrolled a total of 50 children who had undergone liver transplantation between May 2001 and June 2017. Demographic data, types of donor and liver transplant, anti-HBs level, time since liver transplantation, complications and immunosuppressant-use were collated and analyzed using SPSS version 24.0.0 software. To this end, the authors reported an observational study of a five-year-old boy who had received full HBV vaccination previously, underwent liver transplantation with his father’s anti-HBc-negative liver, but was then diagnosed with de novo hepatitis B three years after transplantation.
Research results
The authors found that the loss of hepatitis B immunity after liver transplantation was unexpectedly common and that 60% of subjects had an anti-HBs level < 10 IU/L after a mean period of 2.53 years after transplantation. The potential factors relating to the loss of HBV immunity were anti-HBs (P = 0.002), serum albumin (P = 0.04), total bilirubin (P = 0.001) and direct bilirubin (P = 0.003) prior to liver transplantation. We also report a case of de novo hepatitis B, who received a hepatitis B core antibody-negative liver from his father, and had a high titer of anti-HBs (> 1000 IU/L) prior to transplantation. Future studies should aim to develop strategies to re-establish active immunity to HBV after liver transplantation.
Research conclusions
The new findings of this study are the high prevalence of hepatitis B immunity loss, and a case report of de novo hepatitis B, in a previously immunized recipient who received a liver from a hepatitis B core antibody-negative donor. De novo hepatitis B in a previously immunized recipient who received a liver from a hepatitis B core antibody-negative donor, could have been initiated when exposed to HBV during the period following transplantation coincident with the loss of HBV immunity. High anti-HBs loss after liver transplantation is unexpectedly common. High anti-HBs (> 1000 IU/L) prior to liver transplantation cannot prevent de novo hepatitis. Serum anti-HBs, albumin, total bilirubin and direct bilirubin prior to liver transplantation were the potential factors associated with the loss of HBV immunity after liver transplantation. Anti-HBs levels below the protective level in children after liver transplantation might reflect the loss of immunity or the loss of protection against HBV. A re-immunization program for all liver-transplanted children in order to prevent de novo hepatitis B. Disease severity, nutritional status, immune status and HBV immunity as a result of HBV immunization might represent potential factors to consider for re-establishing active HBV immunity following liver transplantation. De novo hepatitis B could have occurred after liver transplantation at a point when the recipient was likely to have been exposed to HBV.
Research perspectives
Re-assessment of anti-HBs levels and vaccination to maintain levels of anti-HBs above the protective level might prevent de novo hepatitis B after liver transplantation. Studying the immunological response of HBsAg-specific T and B cells following HBV exposure in order to establish an appropriate cutoff for the protective level of anti-HBs to prevent HBV infection in children after liver transplantation will be merit. Moreover, setting up an appropriate HBV immunization protocol to re-establish active HBV immunity by assessing cellular and humoral immunity response after HBV immunization protocols over short- and long-term follow-up periods also should be considered.
ACKNOWLEDGEMENTS
We thank the staff, fellows and nurses in our liver transplant team at King Chulalongkorn Memorial Hospital, Bangkok 10330, Thailand for excellent patient care.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Supported by the Development of New Faculty Staff, Ratchadaphiseksomphot Endowment Fund to Sintusek P; The Special Task Force for Activating Research in Immune Response in Children with Chronic Liver Diseases and Children after Liver Transplantation, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand to Sintusek P; the Research Chair Grant from the National Science and Technology Development Agency, No. P-15-50004 to Poovorawan Y; and The Center of Excellence in Clinical Virology, Chulalongkorn Unversity and King Chulalongkorn Memorial Hospital, No. GCE 5900930-005 to Poovorawan Y.
Institutional review board statement: The study was reviewed and approved by the Ethics Committee, Faculty of Medicine, Chulalongkorn University (IRB number 614/60).
Informed consent statement: All study participants, or their legal guardian, provided verbal consent prior to study enrolment.
Conflict-of-interest statement: None of the authors has any potential conflict to declare.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at voranush.c@chula.ac.th. Consent was not obtained but the presented data are anonymized and the risk of identification is low.
Peer-review started: December 6, 2017
First decision: December 21, 2017
Article in press: January 20, 2018
P- Reviewer: Hori T, Roohvand F S- Editor: Gong ZM L- Editor:A E- Editor: Ma YJ
Contributor Information
Palittiya Sintusek, Division of Gastroenterology and Hepatology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand.
Nawarat Posuwan, Center of Excellence in Clinical Virology, Chulalongkorn University, Bangkok10330, Thailand.
Piyaporn Wanawongsawad, Excellence Center of Organ Transplantation, King Chulalongkorn Memorial Hospital, Bangkok 10330, Thailand.
Suttiruk Jitraruch, Division of Gastroenterology and Hepatology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand.
Yong Poovorawan, Division of Gastroenterology and Hepatology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand; Center of Excellence in Clinical Virology, Chulalongkorn University, Bangkok10330, Thailand.
Voranush Chongsrisawat, Division of Gastroenterology and Hepatology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand. voranush.c@chula.ac.th.
References
- 1.World Health Organization. 2015. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva. [PubMed] [Google Scholar]
- 2.Poovorawan Y, Sanpavat S, Pongpunglert W, Chumdermpadetsuk S, Sentrakul P, Vandepapelière P, Safary A. Long term efficacy of hepatitis B vaccine in infants born to hepatitis B e antigen-positive mothers. Pediatr Infect Dis J. 1992;11:816–821. doi: 10.1097/00006454-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Stevens CE, Toy PT, Taylor PE, Lee T, Yip HY. Prospects for control of hepatitis B virus infection: implications of childhood vaccination and long-term protection. Pediatrics. 1992;90:170–173. [PubMed] [Google Scholar]
- 4.Poovorawan Y, Theamboonlers A, Hirsch P, Vimolket T, Sinlaparatsamee S, Chaiear K, Siraprapasiri T, Khwanjaipanich S, Owatanapanich S, Chunsuttiwat S. Persistence of antibodies to the surface antigen of the hepatitis B virus (anti-HBs) in children subjected to the Expanded Programme on Immunization (EPI), including hepatitis-B vaccine, in Thailand. Ann Trop Med Parasitol. 2000;94:615–621. doi: 10.1080/00034983.2000.11813584. [DOI] [PubMed] [Google Scholar]
- 5.Bartholdy B, Matthias P. Transcriptional control of B cell development and function. Gene. 2004;327:1–23. doi: 10.1016/j.gene.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Arvilommi H. ELISPOT for detecting antibody-secreting cells in response to infections and vaccination. APMIS. 1996;104:401–410. doi: 10.1111/j.1699-0463.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 7.Diana A, Posfay-Barbe KM, Belli DC, Siegrist CA. Vaccine-induced immunity in children after orthotopic liver transplantation: a 12-yr review of the Swiss national reference center. Pediatr Transplant. 2007;11:31–37. doi: 10.1111/j.1399-3046.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 8.Leung DH, Ton-That M, Economides JM, Healy CM. High prevalence of hepatitis B nonimmunity in vaccinated pediatric liver transplant recipients. Am J Transplant. 2015;15:535–540. doi: 10.1111/ajt.12987. [DOI] [PubMed] [Google Scholar]
- 9.Su WJ, Ho MC, Ni YH, Chen HL, Hu RH, Wu YM, Chang MH, Lee PH. High-titer antibody to hepatitis B surface antigen before liver transplantation can prevent de novo hepatitis B infection. J Pediatr Gastroenterol Nutr. 2009;48:203–208. doi: 10.1097/MPG.0b013e3181819ad4. [DOI] [PubMed] [Google Scholar]
- 10.Lin CC, Chen CL, Concejero A, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B, et al. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant. 2007;7:195–200. doi: 10.1111/j.1600-6143.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 11.Rao W, Xie M, Yang T, Zhang JJ, Gao W, Deng YL, Zheng H, Pan C, Liu YH, Shen ZY. Risk factors for de novo hepatitis B infection in pediatric living donor liver transplantation. World J Gastroenterol. 2014;20:13159–13166. doi: 10.3748/wjg.v20.i36.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danzinger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009;9 Suppl 4:S258–S262. doi: 10.1111/j.1600-6143.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 13.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–318. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 14.Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:311–317. doi: 10.1111/ajt.12122. [DOI] [PubMed] [Google Scholar]
- 15.Uemoto S, Sugiyama K, Marusawa H, Inomata Y, Asonuma K, Egawa H, Kiuchi T, Miyake Y, Tanaka K, Chiba T. Transmission of hepatitis B virus from hepatitis B core antibody-positive donors in living related liver transplants. Transplantation. 1998;65:494–499. doi: 10.1097/00007890-199802270-00007. [DOI] [PubMed] [Google Scholar]
- 16.Angelico M, Nardi A, Marianelli T, Caccamo L, Romagnoli R, Tisone G, Pinna AD, Avolio AW, Fagiuoli S, Burra P, et al. Hepatitis B-core antibody positive donors in liver transplantation and their impact on graft survival: evidence from the Liver Match cohort study. J Hepatol. 2013;58:715–723. doi: 10.1016/j.jhep.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Perrillo R. Hepatitis B virus prevention strategies for antibody to hepatitis B core antigen-positive liver donation: a survey of North American, European, and Asian-Pacific transplant programs. Liver Transpl. 2009;15:223–232. doi: 10.1002/lt.21675. [DOI] [PubMed] [Google Scholar]
- 18.Saab S, Waterman B, Chi AC, Tong MJ. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core antibody-positive donors: a systematic review. Liver Transpl. 2010;16:300–307. doi: 10.1002/lt.21998. [DOI] [PubMed] [Google Scholar]
- 19.Bienzle U, Günther M, Neuhaus R, Vandepapeliere P, Vollmar J, Lun A, Neuhaus P. Immunization with an adjuvant hepatitis B vaccine after liver transplantation for hepatitis B-related disease. Hepatology. 2003;38:811–819. doi: 10.1053/jhep.2003.50396. [DOI] [PubMed] [Google Scholar]
- 20.Lu SC, Jiang T, Lai W, Liu Y, Zhang J, Zeng DB, Li CY, Wang ML, Lin DD, Zhu Y, et al. Reestablishment of active immunity against HBV graft reinfection after liver transplantation for HBV-related end stage liver disease. J Immunol Res. 2014;2014:764234. doi: 10.1155/2014/764234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bialek SR, Bower WA, Novak R, Helgenberger L, Auerbach SB, Williams IT, Bell BP. Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: a 15-year follow-up study. Pediatr Infect Dis J. 2008;27:881–885. doi: 10.1097/INF.0b013e31817702ba. [DOI] [PubMed] [Google Scholar]
- 22.Hammitt LL, Hennessy TW, Fiore AE, Zanis C, Hummel KB, Dunaway E, Bulkow L, McMahon BJ. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine. 2007;25:6958–6964. doi: 10.1016/j.vaccine.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 23.Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561–565. [PubMed] [Google Scholar]
- 24.Duca P, Del Pont JM, D’Agostino D. Successful immune response to a recombinant hepatitis B vaccine in children after liver transplantation. J Pediatr Gastroenterol Nutr. 2001;32:168–170. doi: 10.1097/00005176-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Ni YH, Ho MC, Wu JF, Chen HL, Wu YM, Hu RH, Lee PH, Chang MH. Response to booster hepatitis B vaccines in liver-transplanted children primarily vaccinated in infancy. Transplantation. 2008;86:1531–1535. doi: 10.1097/TP.0b013e318189064c. [DOI] [PubMed] [Google Scholar]
- 26.Bauer T, Günther M, Bienzle U, Neuhaus R, Jilg W. Vaccination against hepatitis B in liver transplant recipients: pilot analysis of cellular immune response shows evidence of HBsAg-specific regulatory T cells. Liver Transpl. 2007;13:434–442. doi: 10.1002/lt.21061. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, Newson J, Karra E, Winstanley A, Alazawi W, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518–523. doi: 10.1038/nm.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleeson MW, Dickson RC. Albumin gains immune boosting credibility. Clin Transl Gastroenterol. 2015;6:e86. doi: 10.1038/ctg.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]