Dear Editor
We read the article by Jackson et al.[1] with great interest. The authors concluded that the threshold for causing a brain lesion in a rat model using transcranial direct current stimulation (tDCS) is well below the level previously reported by Liebetanz et al.[2] Using a computational modeling approach, they also indicated that current density at the level of the brain is a better predictor of brain damage than current density at the level of electrode or electrode-skin interface. While we admire their scientific rigor and the use of computational modeling, we are concerned that the units of tDCS dose they chose has affected the relevance of the conclusions in the paper. Our analysis of their data suggests that Liebetanz et al.[2] still offers an estimate of tDCS dose that may result in brain injury that is conservative by an order of magnitude.[1]
First, there is a need to clarify the tDCS stimulation parameters for a single session[3]:
The primary parameters of tDCS dose are:
-
-
Current (mA)
-
-
Duration (minutes)
-
-
Electrode/pad size (cm2)
The derived parameters of tDCS dose are:
-
-
Charge (C) = (Current (mA) ÷ 1000) × (Duration (minutes) × 60)
-
-
Current density (A/m2) = (Current (mA) ÷ 1000) ÷ (Pad size (cm2) ÷ 10,000)
-
-
Charge density (kC/m2) = (Charge (C) ÷ 1000) ÷ (Pad size (cm2) ÷ 10,000)
Second, Jackson et al.[1] claim that anodal stimulation with a current density of 20.0 A/m2 can cause brain lesion, which is “well below” the current density of 142.9 A/m2 reported by Liebetanz et al.[2] However, Liebetanz et al.[2] also showed that “for current densities between 142.9 and 285.7 A/m2, lesion size increased linearly with charge density,” we believe that the charge density would be the most relevant variable for determining a lesion threshold. As shown above, current density is an instantaneous measure that does not consider or involve the duration of stimulation. In other words, current density does not change whether the tDCS is applied for 1 minute or 60 minutes, only offering a “snapshot” in time without the duration factored in. Computational models of tDCS-generated electric fields (EF) also offer a similar snapshot that does not account for duration. When the duration of stimulation is constant between various stimulation scenarios, then current density and charge density (which involves duration of stimulation) are interchangeable and the computational models prove very useful. We argue that any tDCS dose parameter that does not take into account the duration of stimulation is not a good candidate to measure lesion threshold, as it can be compared to the intravenous drip of a medicine of a given concentration at a given rate without defining the duration of the drip (and therefore without determination of total amount of medicine delivered inside the body). Elegant work by McCreery et al.[4] almost 3 decades ago also emphasize the involvement of stimulation duration by comparing how various stimulation parameters like charge density and charge per phase factor in the neural injury. When making a comparison to Liebetanz et al.[2], Jackson et al. do not account for the duration of stimulation. We believe this limits the generalizability of their conclusion, as shown in Table 1.
Table 1.
Comparison of tDCS dose parameters
| Publication | Current (mA) |
Pad Size (mm2) |
Duration (min) |
Current Density (A/m2) |
Charge (C) |
Charge Density (kC/m2) |
|---|---|---|---|---|---|---|
| Jackson et al. 2017 | 0.50 | 25.00 | 60.00 | 20.00 | 1.80 | 72.00 |
| Liebetanz et al. 2009 | 0.50 | 3.50 | 10.00 | 142.90 | 0.30 | 85.74 |
| Liebetanz et al. 2009 lesion threshold | 0.31 | 3.50 | 10.00 | 87.33 | 0.18 | 52.40 |
| Typical human tDCS study | 1.00 | 3500.00 | 20.00 | 0.29 | 1.20 | 0.34 |
| Chhatbar et al. 2017 | 4.00 | 3500.00 | 30.00 | 1.14 | 7.20 | 2.06 |
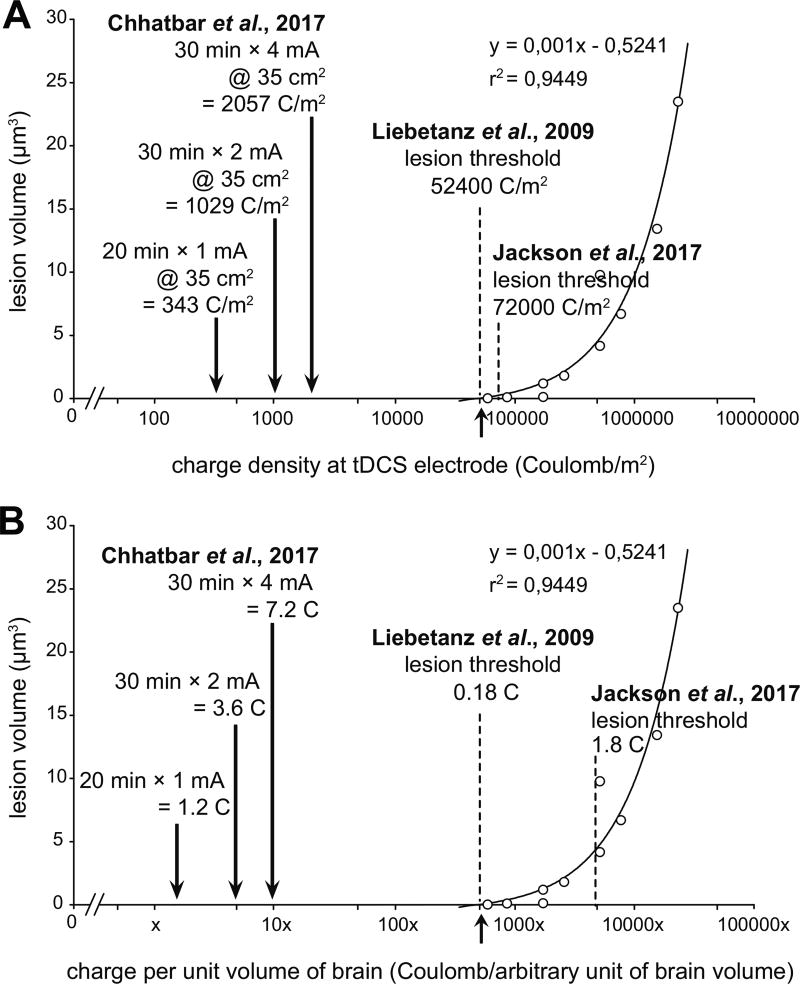
Although Jackson et al.[1] use 0.5 mA current with a 25 mm2 electrode to achieve a current density of 20.0 A/m2, they use a longer duration of stimulation lasting 60 minutes which is 6-times the 10-minute stimulation duration in Liebetanz et al.[2] with a current density of 142.9 A/m2 (0.5 mA current with 3.5 mm2 electrode). Therefore, while current density was very different, the charge density was comparable between the two studies (72.0 kC/m2 for Jackson et al.[1] and 85.7 kC/m2 for Liebetanz et al.[2], respectively). Note that a charge density of 72.0 kC/m2 (see Table 2 of Jackson et al.[1] and Table 1 here) exceeds the lesion threshold of 52.4 kC/m2 derived by Liebetanz et al.[2] using the extrapolation method (see Fig. 4 of Liebetanz et al.[2] and Fig. 1A here).
Figure 1. Comparison of brain lesion threshold between Liebetanz et al. 2009 and Jackson et al. 2017 along with conventional human tDCS dose with the highest dose with evidence of safety on humans.
(A) Charge density plot shows that lesion threshold of Jackson et al. 2017 (72.0 kC/m2) is higher than Liebetanz et al. 2009 (52.4 kC/m2), with much smaller charge densities for typical human studies. (B) Charge delivery per unit volume of the brain (arbitrary, assuming 2000× human to rat brain volume ratio) show almost an order of magnitude higher lesion threshold by Jackson et al. 2017 (1.80 C) when compared with Liebetanz et al. 2009 (0.18 C). Given the much bigger size of the human brain, the total charge delivered to the human brain is much smaller when compared with the rat brain. Note the logarithmic scale on the azimuth in both the plots.
Third, the lesion threshold determined by Jackson et al may have also been influenced by the methodology as well. The cranium diffuses current as it reaches the brain because of its low electrical conductivity. Because a removal of periosteum will decrease the thickness of cranium, the experimental set-up of Jackson et al.[1] may incur a more focused stimulation with a higher current density at the level of the cortex, making it more likely to induce brain injury when compared with Liebetanz et al.[2] where the cranial thickness is not compromised.
Fourth, by generalizing a statement by Jackson et al.[1], we concur that tDCS dose at the level of the cortex is more relevant than dose at the level of the scalp since the skull diffuses direct current due to its low conductivity. If we assume a complete diffusion of current in the brain over time (i.e., total charge), the dose levels determined by Jackson et al.[1] to cause brain lesion (1.80 kC) are an order of magnitude higher than the dose levels established by Liebetanz et al.[2] (0.18 kC) as presented in Table 1 and Fig. 1B.
Finally, Jackson et al.[1] state that “clinical” tDCS typically uses a current density of 2 A/m2. This is about an order of magnitude higher current density than the ones used in human clinical trials, which commonly use 1 mA of current over 35 cm2 pads for 20 minutes (current density: 0.29 A/m2; charge: 1.2 C; and charge density: 0.34 kC/m2, see Fig. 1). Even the recent safety and tolerability study of single session 30-minutes of 4 mA tDCS in stroke patients[5] offered 1.14 A/m2 current density (charge: 7.2 C; charge density: 2.06 kC/m2, see Fig. 1). Note that although higher absolute charges delivered in human studies, the relative charge is much less as the volume of the human brain is ~2000 times greater than the rat brain (~1200 cm3 vs. ~600 mm3)[6, 7].
We have mathematically demonstrated that when charge density is used to represent tCDS dose, the safety limits established by Liebetanz et al.[2], were substantially exceeded in the study of Jackson et al.[1].,By expressing tDCS dose levels as current density instead of charge density, we believe that Jackson et al.[1] reached an incorrect conclusion regarding safety limits for the animal brain.
Acknowledgments
This project is supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM109040) and National Center of Neuromodulation for Rehabilitation (P2CHD086844). PYC acknowledges fellowship grant support from American Heart Association (15SFDRN24480016); PYC and WF acknowledge grant support from America Heart Association (14SDG1829003); SAK acknowledges grant support from the Rehabilitation Research and Development Service of the VA, I01RX001935).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson MP, Truong D, Brownlow ML, Wagner JA, McKinley RA, Bikson M, et al. Safety parameter considerations of anodal transcranial Direct Current Stimulation in rats. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009;120:1161–7. doi: 10.1016/j.clinph.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Chhatbar PY, Ramakrishnan V, Kautz S, George MS, Adams RJ, Feng W. Transcranial Direct Current Stimulation Post-Stroke Upper Extremity Motor Recovery Studies Exhibit a Dose-Response Relationship. Brain Stimul. 2016;9:16–26. doi: 10.1016/j.brs.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;37:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- 5.Chhatbar PY, Chen R, Deardorff R, Dellenbach B, Kautz SA, George MS, et al. Safety and tolerability of transcranial direct current stimulation to stroke patients - A phase I current escalation study. Brain Stimul. 2017;10:553–9. doi: 10.1016/j.brs.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin B, Aslan H, Unal B, Canan S, Bilgic S, Kaplan S, et al. Brain volumes of the lamb, rat and bird do not show hemispheric assymetry: a stereological study. Image Analysis & Stereology. 2001;20:9–13. [Google Scholar]