Abstract
Background
Sepsis is a common cause of death in intensive care units worldwide. Due to the high complexity of this immunological syndrome development of novel therapeutic strategies is urgent. Promising drug targets or biomarkers may depict aquaporins (AQPs) as they regulate crucial key mechanisms of sepsis.
Main body
Here we report on base of the current literature that several AQPs are involved in different physiological processes of sepsis. In immune system mainly AQPs 3, 5 and 9 seem to be important, as they regulate the migration of different immune cells. Several studies showed that AQP3 is essential for T cell function and macrophage migration and that AQP5 and AQP9 regulate neutrophil cell migration and impact sepsis survival. Additionally, to the function in immune system AQPs 1 and 5 play a role in sepsis induced lung injury and their downregulation after inflammatory stimuli impair lung injury. By contrast, AQP4 expression is up-regulated during brain inflammation and aggravates brain edema in sepsis. In kidney AQP2 expression is downregulated during sepsis and can cause renal failure. Some studies also suggest a role of AQP1 in cardiac function.
Conclusion
In conclusion, AQPs are involved in many physiological dysfunctions in sepsis and their expressions are differently regulated. Additional research on the regulatory mechanisms of aquaporins may identify potential therapeutic targets.
Keywords: Aquaporin, AQP; Expression; Immune cells; Migration; Brain; Kidney; Liver; Lung; Heart; LPS, sepsis
Background
Sepsis is one of the most common complications in Intensive Care Units in Germany and the United States [1, 2], and mortality remains unrestrainable high due to the extreme complexity of this immunological syndrome. Predictive biomarkers which characterize this immunological syndrome properly are still missing; hence no individual therapy adapted on the immune status of the unique patient can be conducted. Aquaporins might be convenient biomarkers because they play an important role in inflammation and especially in sepsis as revealed by experimental and association studies [3–6].
Aquaporins (AQPs) are a group of to date 13 identified membrane proteins, which are essential for the regulation of water and salt in- and out flux of the cell. In addition, some AQPs facilitate the passive transport of glycerol and other small solutes such as urea and carbon dioxide through the cell membrane [7]. The water-selective AQPs are involved in many biological functions, including transepithelial fluid transport, cell migration, brain edema and neuroexcitation [7], whereas the aquaglyceroporins participate in cell proliferation, adipocyte metabolism and epidermal water retention. With this study we want to elucidate the possible role of AQPs in pathomechanisms of sepsis on base of the current literature.
Approach of literature research and methodology
A literature search was undertaken using various online sources of English journal articles including ScienceDirect, PubMed and Web of Science. The keywords “aquaporin AND sepsis”, “aquaporins AND sepsis” and “AQP(xy) AND sepsis” were used to search all relevant articles dealing with the role of aquaporins in sepsis. In total 51 studies were found. 10 articles were excluded because they either did not deal with sepsis or with aquaporins. One article was excluded because it was in Russian. The workflow of literature research can be found in Fig. 1. Due to the relative low number of articles dealing with real bacterial sepsis models, endotoxemia models using LPS injection were included in the analysis.
Fig. 1.
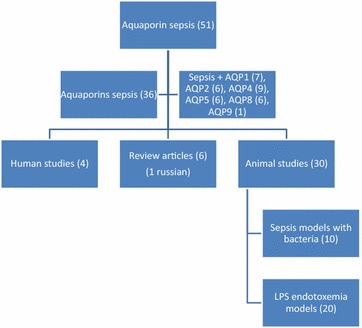
Workflow of literature research
Aquaporin expression during inflammation
To completely understand the role of AQPs in sepsis, it is important to know how their expression is altered during inflammation. It was demonstrated that in leucocytes of septic patients AQP3 expression is reduced 2.5 [8] fold and that simultaneously AQP1 expression is increased twofold [8]. In line with this our group showed that AQP1 expression is increased in the monocytic cell line THP-1 after lipopolysaccharide (LPS) administration, but AQP5 mRNA expression is reduced [9]. AQP6 expression in contrast might play a role in viral infections as it is decreased after viral infection and in turn can reduce the infectivity of Hazara virus [10]. Furthermore, AQP8 is reduced in hepatocytes after LPS administration [11]. In addition, patients with systemic inflammatory response syndrome (SIRS) show increased AQP9 expression in neutrophils compared to healthy controls [12]. Moreover, Gram-negative bacteria as P. aeruginosa induce increased expression, distribution and re-organization of AQP9 in macrophages with is accompanied by changes in macrophage size and morphology. This in turn affects motility, migration and phagocytosis [13].
Aquaporins in cell migration of immune cells
The importance of aquaporins in cell migration has been demonstrated several times before [7, 14–17]. The proposed mechanism by which AQPs enhance cell migration is that they facilitate water influx at the cell’s leading edge. This causes membrane expansion and formation of a concentration gradient of actin polymers which is followed by actin repolymerization to stabilize the membrane protrusion and lamellipodia formation [17]. As immune cell migration is an essential mechanism in sepsis, AQPs might depict key players in this process. Considerable AQPs for immune cell migration are AQP1, AQP3, AQP5, AQP7 and AQP9 as they are expressed in activated B and T lymphocytes (AQP1, 3, 5) [15] as well as immature dendritic cells (AQP3, 5, 7) [15] and neutrophils (AQP9) [15, 18, 19] (Fig. 2f).
Fig. 2.
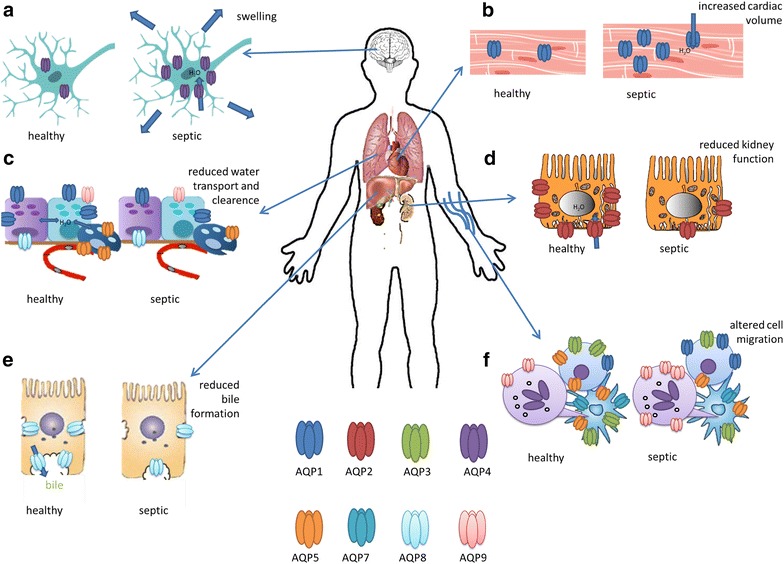
Purposed roles and expressions of aquaporins in sepsis: a AQP4 is expressed in brain and increased in sepsis, b AQP1 expression is increased in cardiac cells in sepsis, c AQP1, AQP8, AQP9 are expressed in bronchiolar epithelial cells and AQP5 can be found in alveolar epithelial cells; their expressions are reduced in sepsis, d AQP2 appears in the apical and subapical part of collecting duct principal cells and is reduced in sepsis, e AQP8 is reduced in hepatocytes in sepsis, f AQP1 and AQP9 expressions are increased in neutrophils and lymphocytes in sepsis, whereas the expression of AQP3, AQP5 and AQP7 is reduced in lymphocytes and dendritic cells
AQP5 seems to be of special interest, because in the past our group demonstrated that the C-allele of the functional AQP5 A(-1364)C promoter polymorphism (rs3759129) is associated with increased survival in severe sepsis [3] but decreased AQP5 expression [20]. Recently we showed that Aqp5-knockout (KO) mice show increased survival compared to wildtype mice after LPS induced endotoxemia. Furthermore, AQP5 overexpression caused increased migration of the T-lymphocytic cell line Jurkat. In addition, neutrophil granulocytes from C-allele carriers showed decreased migration compared to A-allele carriers. Therefore we concluded that the AQP5 genotype and AQP5 protein expression seem to alter neutrophil cell migration and may influence survival in sepsis by altering neutrophil cell migration. Hence AQP5 might be a key protein in inflammation and depict a novel target for developing sepsis therapeutics [21].
Similar to our study Zhu et al. analyzed the effects of Aqp3 expression in a sepsis mouse model. They found that mouse resident peritoneal macrophages (mRPMs) express the aquaglyceroporin Aqp3 and to a low extent Aqp7 and Aqp9 in a plasma membrane pattern [22]. In contrast to our study, Aqp3-KO mice show significantly greater mortality than wildtype mice in a model of bacterial peritonitis. In addition, Aqp3-KO is accompanied by reduced migration of macrophages [22]. Besides to macrophage function, AQP3 seems also to be crucial for T-cell migration. It is suggested that AQP3-mediated H2O2 uptake is required for chemokine-dependent T-cell migration and a sufficient immune response [23].
AQP4 plays a role in the development of regulatory T-cells in the thymus. Aqp4-KO mice show decreased levels of CD4+ CD25+ regulatory T-cells. The decreased amount of regulatory T-cells causes increased microglial inflammatory response in a mouse model of Parkinson with Aqp4-KO mice [24].
Similar to the role of AQP5 and AQP3, AQP9 seems to be responsible for neutrophil migration, as Aqp9-KO mice show reduced neutrophil migration to fMLP [25]. In addition, Aqp7-KO mice have reduced migration of cutaneous dendritic cells. Beside its role in cell migration AQP7 seems to be responsible for antigen uptake as it could be demonstrated that Aqp7-deficient DCs showed a decreased cellular uptake of low-molecular-mass compounds and high-molecular-mass substances [19].
Role of aquaporins in the inflammasome
The inflammasome is an important key modulator of the immune response and affects the immune response by the release of proinflammatory cytokines. It can be found in macrophages and neutrophil granulocytes and can recognize pathogens like bacteria. The inflammasome inter alia consists of NLR family pyrin domain containing 3 (NLRP3) which is up-regulated in sepsis [26]. Activation of NLRP3 inflammasome causes interleukin 1 beta (IL-1β) release. The IL-1β release depends on the pH of the cell and its regulation is caused by water influx mediated by aquaporins. AQP-mediated water movement in macrophages therefore appears as the common element unifying the variety of NLRP3 inflammasome activators [27].
Aquaporins in sepsis induced brain inflammation
One devastating complication of sepsis is septic encephalopathy (SE) [28]. In this context, aquaporins might play an important role, as SE is associated with vasogenic brain edema [29, 30]. The inflammation of the brain occurring in SE is mediated by neutrophil infiltration and causes Aqp4 upregulation which aggravates brain edema [31, 32] (Fig. 2a). Upregulation of Aqp4 in brain after LPS exposure can be attenuated by dexamethasone and this mechanism is mainly regulated by tumornecrosis factor alpha (TNF-α) [33]. However the use of corticosteroids like dexamethasone in sepsis is still discussed and its usage is only recommended under certain conditions [34].
In addition, AQP4 expression is upregulated in astrocytes during sepsis induced delirium (SID) and exosomes carrying AQP4 proteins from astrocytes to the peripheral blood may be utilized as biomarker for SID [35].
Aquaporins in kidney injury
Another common complication in sepsis is acute kidney injury (AKI), former called acute renal failure (ARF), which is frequently associated with polyuria and urine concentration defects and it increases the mortality rate in sepsis [36]. A cecal ligation and puncture (CLP) mouse model for sepsis showed that Aqp2 expression is downregulated through NF-κB pathway and may therefore cause acute renal failure during sepsis [37] (Fig. 2d). Pretreatment of rats with continuous erythropoietin receptor activator (CERA) preserves Aqp2 expression in rat kidneys and protects against sepsis induced AKI [38].
The downregulation of Aqp2 in sepsis models is confirmed by animal models using LPS induced endotoxemia after short time exposure (6 h) [39–42], whereas after long time exposure (18 h) Aqp2 expression is increased in kidney [43]. Another study shows that Aqp2 is downregulated after LPS administration in an LPS sepsis model in rats [44] and that pretreatment but not post-treatment with propofol prevents Aqp2 downregulation and protects renal function during endotoxemia and that this effect may be mediated by regulation of Intercellular Adhesion Molecule 1 (ICAM-1), TNF-α and mediators of apoptosis [44]. Another possibility for Aqp2 preservation after LPS exposure is treatment with α-lipoic acid [45].
Aquaporins in liver dysfunctions during sepsis
Liver has numerous functions in sepsis and is itself a target for sepsis induced injury [46]. For example septic shock and its toxins can cause hypoxic hepatitis, cholestasis due to altered bile metabolism or hepatocellular and acute liver injury [46]. In cholestasis AQP8 might play a role as it is downregulated after LPS stimulation in hepatocytes via TNF-α [11, 47]. The reduced AQP8 expression in turn causes reduced water permeability of hepatocytes, which can result in reduced bile formation and aggravates cholestasis [48, 49] (Fig. 2e). Beside, AQP8 can modulate hepatocellular mitochondria function by modifying water transport [50]. A loss of mitochondria function in turn can cause kidney injury due to loss of cellular energy [51]. In an endotoxemia rat model hepatic mitochondrial Aqp8 expression is reduced [52]. Regulation of Aqp8 in endotoxemia and septic models by substances like tetramethylpyrazine or ethyl pyruvate could stabilize the mitochondria membrane potential, protect hepatocellular mitochondria from damage and might therefore be a therapeutic option in sepsis [51, 53].
Aquaporins in cardiac dysfunction
40–50% of patients with prolonged septic shock develop cardiac dysfunction [54] and newer studies indicate that cardiac dysfunction can occur in all stages of sepsis [55]. The underlying molecular mechanisms are not fully understood yet, but a notable cause is mitochondrial dysfunction which contributes to cardiac dysfunction by causing myocardial energy depletion [56]. Here AQP1 might be important because Aqp1 knockout causes cardiac hypertrophy in mice [57] (Fig. 2b). Another animal study tested the hypothesis if Aqp1 may play a role in cardiac dysfunction during sepsis. They found that Aqp1 expression is increased after LPS exposure in cardiac tissue and that this might influence cardiac function [58].
Aquaporins in acute lung injury
Another common complication in sepsis is acute lung injury that can cause acute respiratory distress syndrome (ARDS), which is associated with increased risk of in-hospital mortality [59]. In lung mainly the aquaporins AQP1 and 5, 8 and to a lower extent AQP9 are expressed [60]. Here, Aqp1 is expressed in all vascular endothelial cells, Aqp5 in the alveolar type I cells and Aqp8 and Aqp9 can be found in the bronchial epithelial cells in lung [61] (Fig. 2c). In 2016 in a small group of septic patients suffering from diffuse alveolar damage is was demonstrated that they have increased expression of AQP3 and AQP5 in the alveolar septum compared to healthy controls [62]. Recently it was demonstrated that Aqp5 expression is decreased after sepsis induction with cecal ligation puncture (CLP) in the lung of rats [63, 64]. This effect can be attenuated by emodin [65] and is regulated by the microRNAs miR-96 and miR-330 [66]. In line with this Aqp1 expression is decreased after LPS exposure in rat lungs [6, 67]. As a therapeutic option it was demonstrated that hydrogen rich saline and parenteral vitamin C can be protective in sepsis related lung injury and that it can attenuate the LPS induced reduction of Aqp1 and Aqp5 expression [5, 68]. In addition, Aqp1 and Aqp5 expression in lung is reduced in lung after an inflammatory pancreatitis models, whereas Aqp8 and Aqp9 expression remains unaffected [61]. Here the traditional Chinese prescription Dai-Huang-Fu-Zi-Tang can upregulate Aqp1 and 5 and attenuate inflammation [61].
Conclusion
The regulatory mechanisms of aquaporins by LPS after endotoxemia and in sepsis seem to be tissue and aquaporin specific, as it can be seen in Table 1 and Fig. 2. As an example and it was demonstrated that AQP8 is downregulated in hepatic cells after LPS administration, though TNF-α pathway [11], while AQP9 expression remains unaffected [33, 69].
Table 1.
Overview of AQP regulation during inflammation (↑ upregulation, ↓ downregulation, ? unknown regulation, = unaffected)
| Aquaporin | Tissue | Regulation during inflammation | References |
|---|---|---|---|
| AQP1 | Immune cells | ↑ In leukocytes and cell lines (THP-1) | [8, 9] |
| Heart | ↑ In cardiac cells | [58] | |
| Lung | ↓ In lung tissue after LPS | [5, 6] | |
| AQP2 | Kidney | ↓ In renal tissue after LPS | [37] |
| AQP3 | Immune cells | ↓ In leukocytes of septic patients | [8] |
| AQP4 | Brain | ↑ In brain and anterior pituitary gland | [31, 75] |
| AQP5 | Lung | ↓ In lung tissue after LPS | [65] |
| Immune cells | ↓ In THP-1 cells after LPS | [9] | |
| AQP7 | Immune cells | ? Mouse resident peritoneal macrophages | [76] |
| AQP8 | Liver | ↓ In hepatic cells | [11] |
| Lung | = In bronchial epithelial cells | [61] | |
| AQP9 | Immune cells | ↑ In neutrophils of SIRS patients | [12] |
| Immune cells | ? Mouse resident peritoneal macrophages | [76] | |
| Lung | = In bronchial epithelial cells | [61] |
In summary, AQPs protein expressions seem to alter differential pathological mechanisms in sepsis and might be key proteins in inflammation. As a limitation of this review it has to be mentioned that several results were concluded from animal studies and that they potentially might to be fully adopted to human physiology. Elucidating the differential regulatory mechanisms of AQP expression in human studies might be helpful for developing novel sepsis therapeutics.
Authors’ contributions
KR analyzed and interpreted the current literature and designed and wrote the manuscript. MA designed the workflow and discussed the topics of the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
All authors agree with the publication in Cell & Bioscience.
Ethics approval and consent to participate
Not applicable.
Funding
We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AQP
aquaporin
- CLP
cecal ligation and puncture
- KO
knockout
- LPS
lipopolysaccharide
- SE
septic encephalopathy
- TNF
tumor necrosis factor
- WT
wildtype
Contributor Information
Katharina Rump, Phone: (+49) (234) 32-29242, Email: katharina.k.rump@rub.de.
Michael Adamzik, Email: michael.adamzik@kk-bochum.de.
References
- 1.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schorr CA, Zanotti S, Dellinger RP. Severe sepsis and septic shock: management and performance improvement. Virulence. 2014;5(1):190–199. doi: 10.4161/viru.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamzik M, Frey UH, Mohlenkamp S, Scherag A, Waydhas C, Marggraf G, Dammann M, Steinmann J, Siffert W, Peters J. Aquaporin 5 gene promoter—1364A/C polymorphism associated with 30-day survival in severe sepsis. Anesthesiology. 2011;114(1528–1175; 0003–3022; 4):912–17. [DOI] [PubMed]
- 4.Liu L, Xie C. Effects of downregulation of aquaporin1 by peptidoglycan and lipopolysaccharide via MAPK pathways in MeT-5A cells. Lung. 2011;189(1432–1750; 0341–2040; 4):331–40. [DOI] [PubMed]
- 5.Tao B, Liu L, Wang N, Wang W, Jiang J, Zhang J. Effects of hydrogen-rich saline on aquaporin 1, 5 in septic rat lungs. J Surg Res. 2016;202(2):291–298. doi: 10.1016/j.jss.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Liu LD, Wu XY, Tao BD, Wang N, Zhang J. Protective effect and mechanism of hydrogen treatment on lung epithelial barrier dysfunction in rats with sepsis. Genet Mol Res. 2016;15(1). 10.4238/gmr.15016050. [DOI] [PubMed]
- 7.Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13:259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassiliou AG, Maniatis NA, Orfanos SE, Mastora Z, Jahaj E, Paparountas T, Armaganidis A, Roussos C, Aidinis V, Kotanidou A. Induced expression and functional effects of aquaporin-1 in human leukocytes in sepsis. Crit Care. 2013;17(5):R199. doi: 10.1186/cc12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rump K, Brendt P, Frey UH, Schafer ST, Siffert W, Peters J, Adamzik M. Aquaporin 1 and 5 expression evoked by the ss-2 adrenoreceptor agonist terbutaline and LPS in mice and in the human monocytic cell line THP-1 is differentially regulated. Shock. 2013;40:430–436. doi: 10.1097/SHK.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 10.Molinas A, Mirazimi A, Holm A, Loitto VM, Magnusson K, Vikström E. Protective role of host aquaporin 6 against Hazara virus, a model for Crimean–Congo hemorrhagic fever virus infection. FEMS Microbiol Lett. 2016;363(8). 10.1093/femsle/fnw058. [DOI] [PubMed]
- 11.Lehmann GL, Carreras FI, Soria LR, Gradilone SA, Marinelli RA. LPS induces the TNF-alpha-mediated downregulation of rat liver aquaporin-8: role in sepsis-associated cholestasis. Am J Physiol Gastrointest Liver Physiol. 2008;294(2):567. doi: 10.1152/ajpgi.00232.2007. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima A, Ogura H, Koh T, Shimazu T, Sugimoto H. Enhanced expression of aquaporin 9 in activated polymorphonuclear leukocytes in patients with systemic inflammatory response syndrome. Shock. 2014;42(4):322–326. doi: 10.1097/SHK.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 13.Holm A, Karlsson T, Vikström E. Pseudomonas aeruginosa lasI/rhlI quorum sensing genes promote phagocytosis and aquaporin 9 redistribution to the leading and trailing regions in macrophages. Front Microbiol. 2015;6:915. doi: 10.3389/fmicb.2015.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YH, Zhou XY, Wang HM, Xu H, Chen J, Lv NH. Aquaporin 5 promotes the proliferation and migration of human gastric carcinoma cells. Tumour Biol. 2013;34(3):1743–1751. doi: 10.1007/s13277-013-0712-4. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson T, Glogauer M, Ellen RP, Loitto VM, Magnusson KE, Magalhaes MA. Aquaporin 9 phosphorylation mediates membrane localization and neutrophil polarization. J Leukoc Biol. 2011;90(5):963–973. doi: 10.1189/jlb.0910540. [DOI] [PubMed] [Google Scholar]
- 16.Jung HJ, Park JY, Jeon HS, Kwon TH. Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLoS ONE. 2011;6(12):e28492. doi: 10.1371/journal.pone.0028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456(0031–6768; 0031–6768; 4):693–700. [DOI] [PMC free article] [PubMed]
- 18.Moon C, Rousseau R, Soria JC, Hoque MO, Lee J, Jang SJ, Trink B, Sidransky D, Mao L. Aquaporin expression in human lymphocytes and dendritic cells. Am J Hematol. 2004;75(0361–8609; 0361–8609; 3):128–33. [DOI] [PubMed]
- 19.Hara-Chikuma M, Sugiyama Y, Kabashima K, Sohara E, Uchida S, Sasaki S, Inoue S, Miyachi Y. Involvement of aquaporin-7 in the cutaneous primary immune response through modulation of antigen uptake and migration in dendritic cells. FASEB J. 2012;26(1):211–218. doi: 10.1096/fj.11-186627. [DOI] [PubMed] [Google Scholar]
- 20.Adamzik M, Frey UH, Bitzer K, Jakob H, Baba HA, Schmieder RE, Schneider MP, Heusch G, Peters J, Siffert W. A novel-1364A/C aquaporin 5 gene promoter polymorphism influences the responses to salt loading of the renin–angiotensin–aldosterone system and of blood pressure in young healthy men. Basic Res Cardiol. 2008;103(1435–1803; 0300–8428; 6):598–610. [DOI] [PubMed]
- 21.Rump K, Unterberg M, Bergmann L, Bankfalvi A, Menon A, Schäfer S, Scherag A, Bazzi Z, Siffert W, Peters J, Adamzik M. AQP5-1364A/C polymorphism and the AQP5 expression influence sepsis survival and immune cell migration: a prospective laboratory and patient study. J Transl Med. 2016;14(1):321. doi: 10.1186/s12967-016-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu N, Feng X, He C, Gao H, Yang L, Ma Q, Guo L, Qiao Y, Yang H, Ma T. Defective macrophage function in aquaporin-3 deficiency. FASEB J. 2011;25(12):4233–4239. doi: 10.1096/fj.11-182808. [DOI] [PubMed] [Google Scholar]
- 23.Hara-Chikuma M, Chikuma S, Sugiyama Y, Kabashima K, Verkman AS, Inoue S, Miyachi Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J Exp Med. 2012;209(10):1743–1752. doi: 10.1084/jem.20112398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi Y, Fan Y, He L, Liu W, Wen X, Zhou S, Wang X, Zhang C, Kong H, Sonoda L, Tripathi P, Li CJ, Yu MS, Su C, Hu G. Novel role of aquaporin-4 in CD4+ CD25+ T regulatory cell development and severity of Parkinson’s disease. Aging Cell. 2011;10(3):368–382. doi: 10.1111/j.1474-9726.2011.00677.x. [DOI] [PubMed] [Google Scholar]
- 25.Moniaga CS, Watanabe S, Honda T, Nielsen S, Hara-Chikuma M. Aquaporin-9-expressing neutrophils are required for the establishment of contact hypersensitivity. Sci Rep. 2015;5:15319. doi: 10.1038/srep15319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esquerdo KF, Sharma NK, Brunialti MKC, Baggio-Zappia GL, Assunção M, Azevedo LCP, Bafi AT, Salomao R. Inflammasome gene profile is modulated in septic patients, with a greater magnitude in non-survivors. Clin Exp Immunol. 2017;189(2):232–240. doi: 10.1111/cei.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabolli V, Wallemme L, Lo Re S, Uwambayinema F, Palmai-Pallag M, Thomassen L, Tyteca D, Octave JN, Marbaix E, Lison D, Devuyst O, Huaux F. Critical role of aquaporins in IL-1beta-mediated inflammation. J Biol Chem. 2014;289:13937–13947. doi: 10.1074/jbc.M113.534594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauber SC, Eiffert H, Brück W, Nau R. Septic encephalopathy and septic encephalitis. Expert Rev Anti Infect Ther. 2017;15(2):121–132. doi: 10.1080/14787210.2017.1265448. [DOI] [PubMed] [Google Scholar]
- 29.Tong D, Zhou Y, Wang G, Chen X, Yang T. Early prediction and outcome of septic encephalopathy in acute stroke patients with nosocomial coma. J Clin Med Res. 2015;7(7):534–539. doi: 10.14740/jocmr2176w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies DC. Blood–brain barrier breakdown in septic encephalopathy and brain tumours. J Anat. 2002;200(6):639–646. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52(3):447–456. doi: 10.1016/j.neuint.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rama Rao KV, Jayakumar AR, Norenberg MD. Brain edema in acute liver failure: mechanisms and concepts. Metab Brain Dis. 2014;29(4):927–936. doi: 10.1007/s11011-014-9502-y. [DOI] [PubMed] [Google Scholar]
- 33.Du Y, Meng Y, Lv X, Guo L, Wang X, Su Z, Li L, Li N, Zhao S, Zhao L, Zhao X. Dexamethasone attenuates LPS-induced changes in expression of urea transporter and aquaporin proteins, ameliorating brain endotoxemia in mice. Int J Clin Exp Pathol. 2014;7(12):8443–8452. [PMC free article] [PubMed] [Google Scholar]
- 34.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent J, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 35.Sfera A, Price AI, Gradini R, Cummings M, Osorio C. Proteomic and epigenomic markers of sepsis-induced delirium (SID) Front Mol Biosci. 2015;2:59. doi: 10.3389/fmolb.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 37.Höcherl K, Schmidt C, Kurt B, Bucher M. Inhibition of NF-kappaB ameliorates sepsis-induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am J Physiol Renal Physiol. 2010;298(1):196. doi: 10.1152/ajprenal.90607.2008. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues CE, Sanches TR, Volpini RA, Shimizu MHM, Kuriki PS, Camara NOS, Seguro AC, Andrade L. Effects of continuous erythropoietin receptor activator in sepsis-induced acute kidney injury and multi-organ dysfunction. PLoS ONE. 2012;7(1):e29893. doi: 10.1371/journal.pone.0029893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olesen ETB, de Seigneux S, Wang G, Lütken SC, Frøkiaer J, Kwon T, Nielsen S. Rapid and segmental specific dysregulation of AQP2, S256-pAQP2 and renal sodium transporters in rats with LPS-induced endotoxaemia. Nephrol Dial Transplant. 2009;24(8):2338–2349. doi: 10.1093/ndt/gfp011. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Li C, Summer SN, Falk S, Wang W, Ljubanovic D, Schrier RW. Role of AQP1 in endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294(6):1473. doi: 10.1152/ajprenal.00036.2008. [DOI] [PubMed] [Google Scholar]
- 41.Versteilen AMG, Heemskerk AEJ, Groeneveld ABJ, van Wijhe M, van Lambalgen AA, Tangelder G. Mechanisms of the urinary concentration defect and effect of desmopressin during endotoxemia in rats. Shock. 2008;29(2):217–222. doi: 10.1097/shk.0b013e3180ca9e53. [DOI] [PubMed] [Google Scholar]
- 42.Grinevich V, Knepper MA, Verbalis J, Reyes I, Aguilera G. Acute endotoxemia in rats induces down-regulation of V2 vasopressin receptors and aquaporin-2 content in the kidney medulla. Kidney Int. 2004;65(1):54–62. doi: 10.1111/j.1523-1755.2004.00378.x. [DOI] [PubMed] [Google Scholar]
- 43.Chagnon F, Vaidya VS, Plante GE, Bonventre JV, Bernard A, Guindi C, Lesur O. Modulation of aquaporin-2/vasopressin2 receptor kidney expression and tubular injury after endotoxin (lipopolysaccharide) challenge. Crit Care Med. 2008;36(11):3054–3061. doi: 10.1097/CCM.0b013e318186a938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui W, Tian A, Bai T. Protective effects of propofol on endotoxemia-induced acute kidney injury in rats. Clin Exp Pharmacol Physiol. 2011;38(11):747–754. doi: 10.1111/j.1440-1681.2011.05584.x. [DOI] [PubMed] [Google Scholar]
- 45.Suh SH, Lee KE, Kim IJ, Kim O, Kim CS, Choi JS, Choi H, Bae EH, Ma SK, Lee JU, Kim SW. Alpha-lipoic acid attenuates lipopolysaccharide-induced kidney injury. Clin Exp Nephrol. 2015;19(1):82–91. doi: 10.1007/s10157-014-0960-7. [DOI] [PubMed] [Google Scholar]
- 46.Strnad P, Tacke F, Koch A, Trautwein C. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14(1):55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 47.Bhogal HK, Sanyal AJ. The molecular pathogenesis of cholestasis in sepsis. Front Biosci (Elite Ed) 2013;5:87–96. doi: 10.2741/E598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marinelli RA, Lehmann GL, Soria LR, Marchissio MJ. Hepatocyte aquaporins in bile formation and cholestasis. Front Biosci (Landmark Ed) 2011;16:2642–2652. doi: 10.2741/3877. [DOI] [PubMed] [Google Scholar]
- 49.Lehmann G, Larocca M, Soria L, Marinelli R. Aquaporins: their role in cholestatic liver disease. World J Gastroenterol. 2008;14(46):7059–7067. doi: 10.3748/wjg.14.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X, Shi Z, Hu J, Yuan B, Huang H, Fang H, Yin X, Nie N, Sheng X. Identification of differentially expressed genes associated with burn sepsis using microarray. Int J Mol Med. 2015;36(6):1623–1629. doi: 10.3892/ijmm.2015.2374. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Zhang L, Tao X, Wei L, Liu B, Huang L, Chen Y. Tetramethylpyrazine upregulates the aquaporin 8 expression of hepatocellular mitochondria in septic rats. J Surg Res. 2013;185(1):286–293. doi: 10.1016/j.jss.2013.05.106. [DOI] [PubMed] [Google Scholar]
- 52.Soria LR, Marrone J, Molinas SM, Lehmann GL, Calamita G, Marinelli RA. Lipopolysaccharide impairs hepatocyte ureagenesis from ammonia: involvement of mitochondrial aquaporin-8. FEBS Lett. 2014;588(9):1686–1691. doi: 10.1016/j.febslet.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Z, Li X, Lin Z, Chen J, Guan X, Chen M. Ethyl pyruvate reduces hepatic mitochondrial swelling and dysfunction in a rat model of sepsis. Int J Clin Exp Pathol. 2015;8(7):7774–7785. [PMC free article] [PubMed] [Google Scholar]
- 54.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35(6):1599–1608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 55.Flynn A, Chokkalingam Mani B, Mather PJ. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart Fail Rev. 2010;15(6):605–611. doi: 10.1007/s10741-010-9176-4. [DOI] [PubMed] [Google Scholar]
- 56.Cimolai MC, Alvarez S, Bode C, Bugger H. Mitochondrial mechanisms in septic cardiomyopathy. Int J Mol Sci. 2015;16(8):17763–17778. doi: 10.3390/ijms160817763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montiel V, Leon Gomez E, Bouzin C, Esfahani H, Romero Perez M, Lobysheva I, Devuyst O, Dessy C, Balligand JL. Genetic deletion of aquaporin-1 results in microcardia and low blood pressure in mouse with intact nitric oxide-dependent relaxation, but enhanced prostanoids-dependent relaxation. Pflugers Arch. 2014;466(2):237–251. doi: 10.1007/s00424-013-1325-x. [DOI] [PubMed] [Google Scholar]
- 58.Madonna R, Jiang J, Geng YJ. Attenuated expression of gelsolin in association with induction of Aquaporin-1 and nitric oxide synthase in dysfunctional hearts of aging mice exposed to endotoxin. Int J Immunopathol Pharmacol. 2012;25(0394–6320; 0394–6320; 4):911–22. [DOI] [PubMed]
- 59.Kim W, Hong S. Sepsis and acute respiratory distress syndrome: recent update. Tuberc Respir Dis (Seoul) 2016;79(2):53–57. doi: 10.4046/trd.2016.79.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong-Min F, Chun-Rong H, Rui Z, Li-Na S, Ya-Jun W, Li L. CGRP 8-37 enhances lipopolysaccharide-induced acute lung injury and regulating aquaporin 1 and 5 expressions in rats. J Physiol Biochem. 2016;73(3):381–386. doi: 10.1007/s13105-017-0563-3. [DOI] [PubMed] [Google Scholar]
- 61.Kang X, Lu X, Zhan L, Liang Z, Guo W, Ma Q, Wang Y, Song J, Feng J, Wang C, Bai L, Song Y, Liu G. Dai-Huang-Fu-Zi-Tang alleviates pulmonary and intestinal injury with severe acute pancreatitis via regulating aquaporins in rats. BMC Complement Altern Med. 2017;17(1):288. doi: 10.1186/s12906-017-1789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pires-Neto RC, Del Carlo Bernardi F, Alves de Araujo P, Mauad T, Dolhnikoff M. The expression of water and ion channels in diffuse alveolar damage is not dependent on DAD etiology. PLoS ONE. 2016;11(11):e0166184. doi: 10.1371/journal.pone.0166184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chinnaiyan AM, Huber-Lang M, Kumar-Sinha C, Barrette TR, Shankar-Sinha S, Sarma VJ, Padgaonkar VA, Ward PA. Molecular signatures of sepsis: multiorgan gene expression profiles of systemic inflammation. Am J Pathol. 2001;159(4):1199–1209. doi: 10.1016/S0002-9440(10)62505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bromberg Z, Raj N, Goloubinoff P, Deutschman CS, Weiss YG. Enhanced expression of 70-kilodalton heat shock protein limits cell division in a sepsis-induced model of acute respiratory distress syndrome. Crit Care Med. 2008;36(1):246–255. doi: 10.1097/01.CCM.0000295473.56522.EF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y, Sun L, Liu S, Song J, Cheng J, Liu J. Effect of emodin on aquaporin 5 expression in rats with sepsis-induced acute lung injury. J Tradit Chin Med. 2015;35(6):679–684. doi: 10.1016/S0254-6272(15)30159-X. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Chen M, Zhang Y, Peng P, Li J, Xin X. miR-96 and miR-330 overexpressed and targeted AQP5 in lipopolysaccharide-induced rat lung damage of disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2014;25(7):731–737. doi: 10.1097/MBC.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 67.Ma T, Liu Z. Functions of aquaporin 1 and α-epithelial Na+ channel in rat acute lung injury induced by acute ischemic kidney injury. Int Urol Nephrol. 2013;45(4):1187–1196. doi: 10.1007/s11255-012-0355-1. [DOI] [PubMed] [Google Scholar]
- 68.Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012;303(1):20. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- 69.Lehmann GL, Marinelli RA. Peritoneal sepsis downregulates liver expression of aquaporin-8: a water channel involved in bile secretion. Liver Int. 2009;29(2):317–318. doi: 10.1111/j.1478-3231.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Thorling CA, Liang X, Bridle KR, Grice JE, Zhu Y, Crawford DHG, Xu ZP, Liu X, Roberts MS. Diagnostic imaging and therapeutic application of nanoparticles targeting the liver. J Mate Chem B. 2015;3(6):939–958. doi: 10.1039/C4TB01611D. [DOI] [PubMed] [Google Scholar]
- 71.Eymael J, Smeets B. Origin and fate of the regenerating cells of the kidney. Eur J Pharmacol. 2016;790:62–73. doi: 10.1016/j.ejphar.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 72.Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273(5 Pt 1):1549. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 73.Nielsen S, Frøkiaer J, Marples D, Kwon T, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82(1):205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 74.Kovach TK, Dighe AS, Lobo PI, Cui Q. Interactions between MSCs and immune cells: implications for bone healing. J Immunol Res. 2015;2015:752510. doi: 10.1155/2015/752510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuwahara-Otani S, Maeda S, Tanaka K, Hayakawa T, Seki M. Systemic administration of lipopolysaccharide increases the expression of aquaporin-4 in the rat anterior pituitary gland. J Vet Med Sci. 2013;75(8):1081–1084. doi: 10.1292/jvms.13-0083. [DOI] [PubMed] [Google Scholar]
- 76.Zhu N, Feng X, He C, Gao H, Yang L, Ma Q, Guo L, Qiao Y, Yang H, Ma T. Defective macrophage function in aquaporin-3 deficiency. FASEB J. 2011;25(1530–6860; 0892–6638; 12):4233–39. [DOI] [PubMed]
- 77.Anonymous: animal primary tissues—OpenStax CNX. http://cnx.org/contents/f8b7e159-1112-46eabe19-5f492747a7b5@4/Animal-Primary-Tissues.
- 78.Anonymous: Brain cells|The Brain Tumour Charity. https://www.thebraintumourcharity.org/understanding-brain-tumours/symptoms-and-information/braincells/.
- 79.Fliesler N. Reversing lung disease in mice by coaxing production of healthy cells. https://vector.childrenshospital.org/2014/01/reversing-lung-disease-in-mice-by-coaxing-production-ofhealthy-cells/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


