Abstract
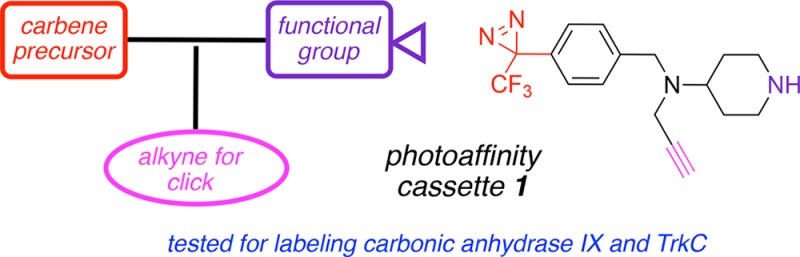
A small molecule 1 was designed to contain an alkyne, a trifluoromethyl phenyldiazirine, and a free piperidine-NH for facile conjugation to protein binding ligands. This “cassette” 1 was synthesized via a relatively direct route involving only routine steps. In this proof-of-concept study, putative ligands for carbonic anhydrase IX and for TrkC were conjugated to 1. Photoaffinity labeling was performed using purified extracellular regions of both these protein-receptors, and using cells that express these receptors (isolation via a pull-down procedure), labeling of the protein was observed in all four experiments.
Keywords: Photoaffinity labeling, click chemistry, carbonic anhydrase IX, TrkC, targeting
Photoaffinity labeling (PAL) is a valuable tool in target identification.1 Preferred embodiments of this method includes photolabile groups that decompose to carbenes or nitrenes, which undergo insertion reactions at reactive centers of proteins drawn into the environment by an attached ligand (Figure 1a). Difficulties with PAL tend to be encountered in detecting the proteins targeted by the reactive species; this usually requires labeling with a fluor or another entity to make the conjugates conspicuous.2 However, fluors incorporated before the photodecomposition step can impact binding of the targeting ligand to the protein. Indeed, even without fluors, addition of PAL-groups to targeting ligands can impact how they bind proteins.3 For these reasons, it is often advantageous to “click”4,5 on a fluor after photodecomposition so that fluor–protein interactions have no impact on the coupling step, but after click the covalent conjugates can be readily detected. This Letter describes a simple, conveniently accessible, “clickable cassette” 1 (Figure 1b) that may be readily conjugated to targeting ligands, and includes an alkyne for click-labeling with azides. Two applications of cassette 1 are also presented, i.e. labeling of carbonic anhydrase IX (CAIX) and labeling of tropomyosin kinase receptor C (TrkC).
Figure 1.
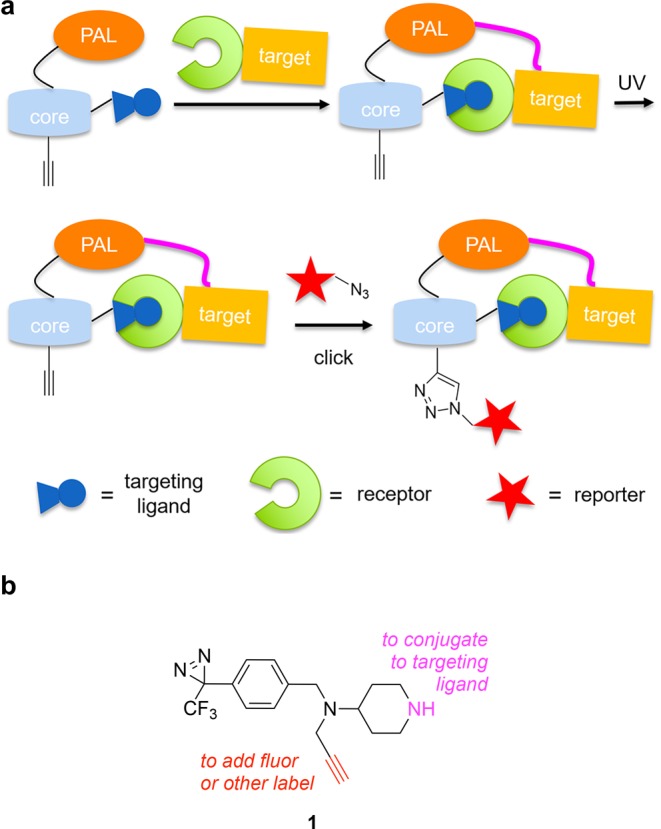
(a) Typical PAL strategy. (b) Cassette featured in this work.
Several criteria were prioritized in the design of PAL-cassette 1, specifically: (i) ease of synthesis; (ii) a trifluoromethyl phenyldiazirine because that substituent is often preferred for PAL;6 (iii) a highly nucleophilic secondary amine for coupling to small-molecule ligands; (iv) an alkyne for click-capture; and (v) small structure overall to minimize nonspecific binding of the cassette fragment to proteins. The presence of a tertiary amine in this system also means that it could be protonated to increase water solubilities of ligand-conjugates.
Scheme 1a describes the synthesis of cassette 1 from the known diazirine precursor A.7 Nucleophilic displacement with the amine 2 (prepared via a reductive amination of propargyl amine with the corresponding ketone, see the Supporting Information) gave the Boc-protected cassette 3; the deprotected cassette 1 was obtained through treatment with acid.
Scheme 1. Synthesis of (a) Cassette 1 and (b) 5, comprising 1 Conjugated with a CAIX Ligand.
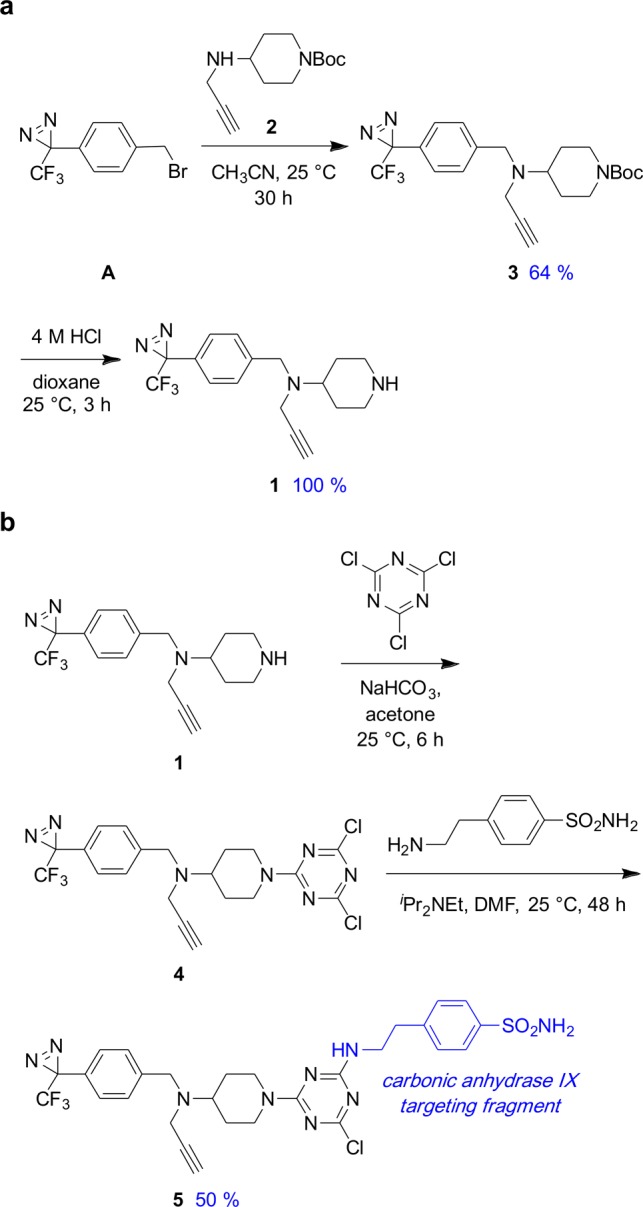
As a test application of 1, this material was coupled with cyanuric chloride to give the pivotal intermediate 4 (Scheme 1b). A second SNAr displacement on the triazine enabled incorporation of a carbonic anhydrase IX ligand in the conjugate 5.
Carbonic anhydrase (CA) IX is a transmembrane protein, one of a family of enzymes that mediate reversible hydration of CO2.8 This enzyme tends to be overexpressed in hypoxic tumors but not significantly expressed in most normal tissues.9 Small molecules that putatively bind CAIX are interesting in tumor targeting.10−12
In a photoaffinity labeling experiment, the conjugate 5 with recombinant CAIX in pH 7.4 HEPES buffer was illuminated at 365 nm (light-emitting diode) for 30 min. Fifty-fold excesses of azide-fluor-488 (compared to ligand 5), copper (2+) salts, and a reducing agent (tris(2-carboxyethyl)phosphine, TCEP) were added and the reaction mixture was held at 25 °C for 3–4 h in the dark to allow the click cycloaddition to proceed. Constituents of the sample were separated on an SDS-PAGE gel; after fluorescence imaging, a band at 55 kDa was observed corresponding to labeled CAIX. Staining all proteins on the same gel with a Coomassie blue dye revealed the protein is present in all lanes, but comparison with the in-gel fluorescent image showed that 50-fold excess of the conjugate without a diazirine6 blocked the photoaffinity labeling (lanes 2 and 4). When the sample was not illuminated (lane 5), no photoaffinity labeling occurred.
The next set of experiments were undertaken to confirm that the cassette could be used on cell lysates, rather than purified recombinant protein, and that the conjugate could be observed via biotin-labeling and a pull-down procedure (Figure 2b). “Triple negative” breast cancer cells, MDA-MB-231, were used since these are known to express CAIX.13 Thus, the overall procedure was photoaffinity labeling, click with biotin azide, pull-down on streptavidin coated beads, and then SDS-PAGE gel and Western blot. This sequence revealed the presence of CAIX (lane 1), but not when excess blocking ligand 6 was present (lane 2) or when no photoaffinity label at all was used (lane 3). Lane 4 is a positive control showing that CAIX was present in the cell lysate before pull-down.
Figure 2.
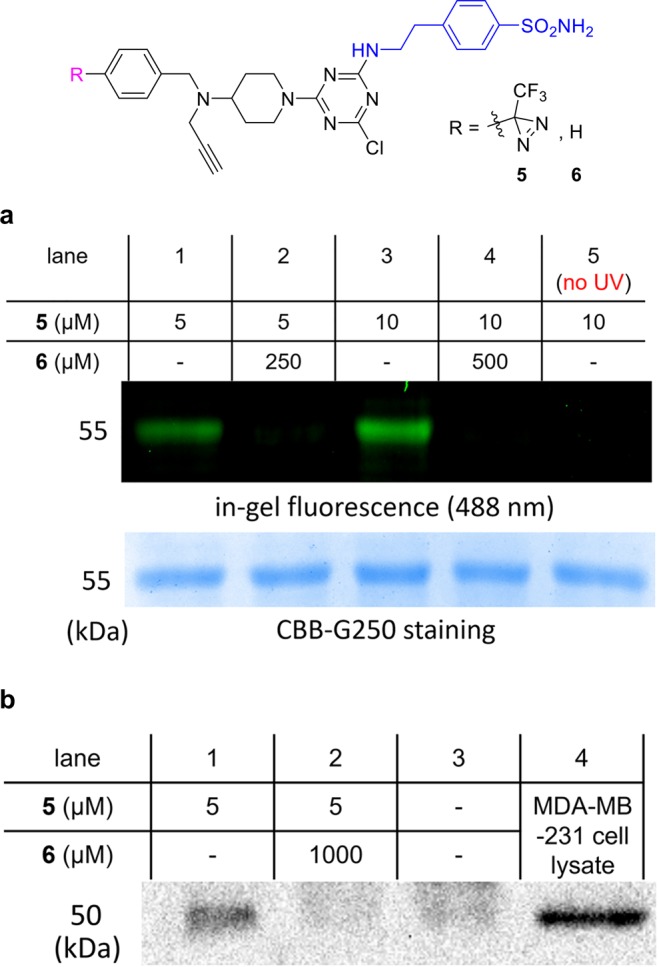
(a) Experiments with isolated protein. Photoaffinity labeling of recombinant CAIX after 30 min illumination with excess ligand 5 and click with azide-fluor-488; the top gel (black background) shows the fluorescence at 488 nm, and the one below depicts protein staining with Coomassie Brilliant Blue (CBB) G250. Competition with 6, which lacks a diazirine, suppresses labeling with 5 (lanes 2 and 4). (b) Experiments with MDA-MB-231 cell lysates. MDA-MB-231 cell lysate was treated with PAL probe 5 under 365 nm illumination for 30 min and then clicked with biotin-azide. NeutrAvidin agarose was used to pull down the biotinylated proteins, and the material captured was run on a SDS-PAGE gel. Lane 1 shows staining of CAIX on the Western blot image with a CAIX Ab, lane 2 shows sample pretreated with blocking ligand 6, lane 3 shows no ligands, and lane 4 depicts the lysate without PAL.
Several studies from our laboratories have featured a bivalent system with dipeptide fragments (blue in structures 7 and 8) that putatively bind the cell surface receptor TrkC.14−19 Thus, conjugates 7 and 8 were formed to test this hypothesis via photoaffinity labeling.
Figure 3a shows in-gel fluorescence after a photoaffinity labeling, click, and SDS-PAGE sequence for solubilized TrkC receptor and the conjugate 7. A band at around 100 kDa was observed (lanes 1 and 3) but not when 80× of the blocking ligand 8 was added (lanes 2 and 4), or if the illumination step was excluded (lane 5). Figure 3b shows a Western blot after illumination of 7 with NIH3T3 cells stably transfected with TrkC, “click” with biotin azide, capture onto NeutrAvidin agarose beads, and then SDS-PAGE. Two TrkC-Ab bands were observed (whereas the parent NIH3T3 cells give none, see the Supporting Information); these are at similar, but not the same, molecular mass as the solubilized extracellular domain of the TrkC receptor in Figure 3a.
Figure 3.
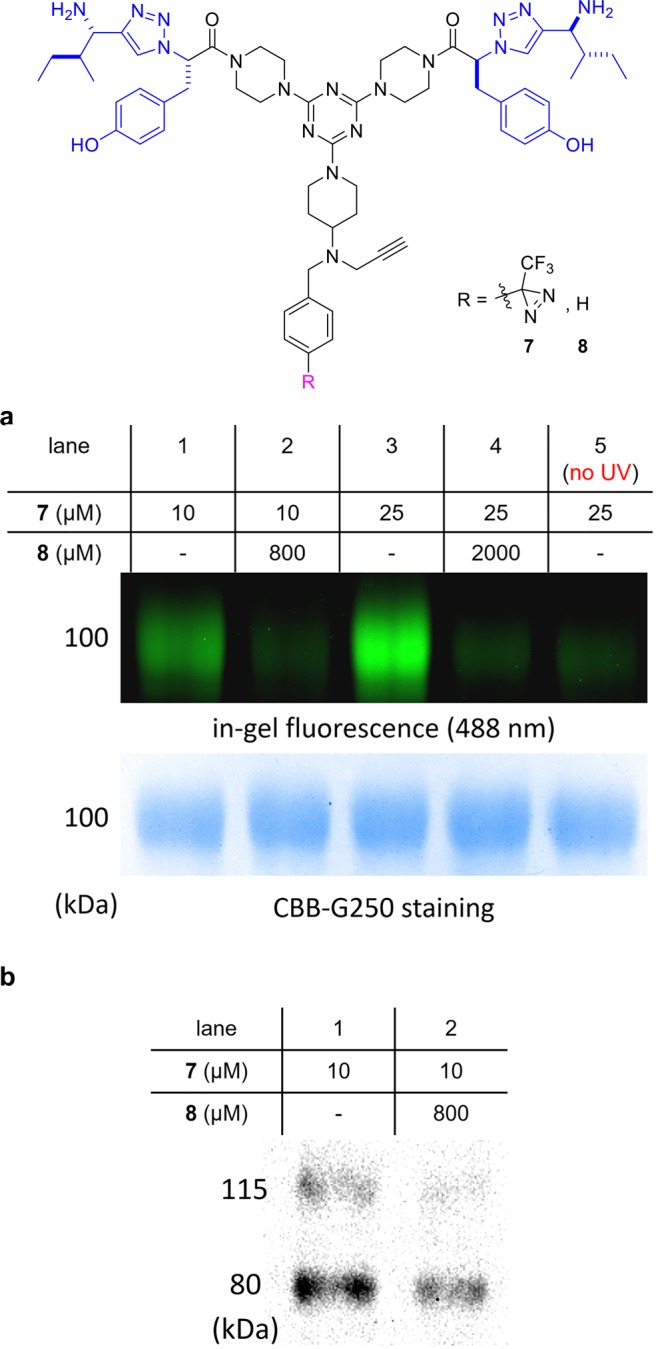
(a) Experiments with solubilized TrkC extracellular domain. Solubilized TrkC extracellular domain was incubated with 7 for 1 h, illuminated for 30 min at 365 nm, clicked with azide-fluor-488, and then subjected to SDS-PAGE. Top gel: fluorescence at 488 nm. Bottom gel: protein staining with CBB G250. Competition with 8, which lacks a diazirine, suppresses labeling with 7 (lanes 2 and 4). (b) Experiments with NIH3T3 TrkC+ cell lysates. Cell lysate was treated with PAL probe 7 under 365 nm illumination for 30 min and then clicked with biotin azide. NeutrAvidin agarose was used to pull down the biotinylated proteins, and the material captured was run on a SDS-PAGE gel. Lane 1 shows staining of TrkC on the Western blot image with anti-TrkC mAb, and lane 2 shows sample pretreated with blocking ligand 8.
Other PAL ligands functionalized with alkynes have been reported prior to our work,20,21 but the synthesis of cassette 1 appears to be more convenient than most and provides another type of system. This is because, unlike some other probes, synthesis of cassette 1 does not require reactive organometallics, except in the first step wherein the starting material was made on scale. Cassette 1 has a secondary amine that can be easily coupled to activated carboxylic acids; this feature makes the probe more portable. Both the small-molecule conjugates prepared in this work labeled their anticipated targets with sufficient efficiencies for observation in SDS-PAGE experiments.
Acknowledgments
We acknowledge DoD BCRP Breakthrough Award (BC141561), CPRIT (RP150559 and RP170144), and The Robert A. Welch Foundation (A-1121). The NMR instrumentation at Texas A&M University was supported by a grant from the National Science Foundation (DBI-9970232) and the Texas A&M University System.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00516.
Synthetic procedures for compounds 1–8, procedures for photoaffinity labeling, full gel images (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sumranjit J.; Chung S. J. Recent advances in target characterization and identification by photoaffinity probes. Molecules 2013, 18, 10425–10451. 10.3390/molecules180910425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinsky D. J. Tandem photoaffinity labeling-bioorthogonal conjugation in medicinal chemistry. Bioorg. Med. Chem. 2012, 20, 6237–6247. 10.1016/j.bmc.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Park J.; Koh M.; Koo J. Y.; Lee S.; Park S. B. Investigation of Specific Binding Proteins to Photoaffinity Linkers for Efficient Deconvolution of Target Protein. ACS Chem. Biol. 2016, 11, 44–52. 10.1021/acschembio.5b00671. [DOI] [PubMed] [Google Scholar]
- Meldal M.; Tornoe C. W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Finn M. G.; Sharpless K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. . [DOI] [PubMed] [Google Scholar]
- Dubinsky L.; Krom B. P.; Meijler M. M. Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 2012, 20, 554–570. 10.1016/j.bmc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- Stromgaard K.; Saito D. R.; Shindou H.; Ishii S.; Shimizu T.; Nakanishi K. Ginkgolide Derivatives for Photolabeling Studies: Preparation and Pharmacological Evaluation. J. Med. Chem. 2002, 45, 4038–4046. 10.1021/jm020147w. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy V. M.; Kaufman G. K.; Urbach A. R.; Gitlin I.; Gudiksen K. L.; Weibel D. B.; Whitesides G. M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein-Ligand Binding. Chem. Rev. 2008, 108, 946–1051. 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P.; Vaughan-Jones R. D.; Harris A. L. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007, 26, 299–310. 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- Thiry A.; Dogne J.-M.; Masereel B.; Supuran C. T. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol. Sci. 2006, 27, 566–573. 10.1016/j.tips.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Krall N.; Pretto F.; Neri D. A bivalent small molecule-drug conjugate directed against carbonic anhydrase IX can elicit complete tumour regression in mice. Chem. Sci. 2014, 5, 3640–3644. 10.1039/C4SC00685B. [DOI] [Google Scholar]
- Krall N.; Pretto F.; Decurtins W.; Bernardes G. J. L.; Supuran C. T.; Neri D. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew. Chem., Int. Ed. 2014, 53, 4231–4235. 10.1002/anie.201310709. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wang H.; Oosterwijk E.; Tu C.; Shiverick K. T.; Silverman D. N.; Frost S. C. Expression and Activity of Carbonic Anhydrase IX Is Associated With Metabolic Dysfunction in MDA-MB-231 Breast Cancer Cells. Cancer Invest. 2009, 27, 613–623. 10.1080/07357900802653464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.; Brahimi F.; Angell Y.; Li Y.-C.; Moscowicz J.; Saragovi H. U.; Burgess K. Bivalent Peptidomimetic Ligands of TrkC are Biased Agonists, Selectively Induce Neuritogenesis, or Potentiate Neurotrophin-3 Trophic Signals. ACS Chem. Biol. 2009, 4, 769–781. 10.1021/cb9001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E.; Kamkaew A.; Burgess K. Small Molecule Ligands for Active Targeting of TrkC-expressing Tumor Cells. ACS Med. Chem. Lett. 2012, 3, 1008–1012. 10.1021/ml300227d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkaew A.; Burgess K. Double-targeting Using a TrkC-Ligand Conjugated to Dipyrrometheneboron Difluoride (BODIPY) Based Photodynamic Therapy (PDT) Agent. J. Med. Chem. 2013, 56, 7608–7614. 10.1021/jm4012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahimi F.; Ko E.; Malakhov A.; Burgess K.; Saragovi H. U. Combinatorial assembly of small molecules into bivalent antagonists of TrkC or TrkA receptors. PLoS One 2014, 9, e89617/89611–e89617/89612. 10.1371/journal.pone.0089617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kue S. C.; Anyanee A.; Lee H. B.; Chung L. Y.; Kiew L. V.; Burgess K. Targeted PDT Agent Eradicates TrkC Expressing Tumors Via Photodynamic Therapy (PDT). Mol. Pharmaceutics 2015, 12, 212–222. 10.1021/mp5005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkaew A.; Li F.; Li Z.; Burgess K. An agent for optical imaging of TrkC-expressing, breast cancer. MedChemComm 2017, 8, 1946–1952. 10.1039/C7MD00328E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. S.; Young R. N. Design and synthesis of an all-in-one 3-(1,1-difluoroprop-2-ynyl)-3H-diazirin-3-yl functional group for photo-affinity labeling. Bioorg. Med. Chem. 2009, 17, 5388–5395. 10.1016/j.bmc.2009.06.048. [DOI] [PubMed] [Google Scholar]
- Li Z.; Hao P.; Li L.; Tan C. Y. J.; Cheng X.; Chen G. Y. J.; Sze S. K.; Shen H.-M.; Yao S. Q. Design and Synthesis of Minimalist Terminal Alkyne-Containing Diazirine Photo-Crosslinkers and Their Incorporation into Kinase Inhibitors for Cell- and Tissue-Based Proteome Profiling. Angew. Chem., Int. Ed. 2013, 52, 8551–8556. 10.1002/anie.201300683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


