Abstract
AIM
To determine the clinical characteristics of patients undergoing palliative surgery for Krukenberg tumors, including disease presentation, outcomes, and prognostic factors.
METHODS
This was a retrospective clinical study of all patients who underwent palliative surgery for Krukenberg tumors between January 2004 and December 2015. Patient information was obtained from inpatient and outpatient case notes as well as the hospital electronic records. Patients who underwent potentially curative resection, and patients with Krukenberg tumors who did not undergo surgery were also excluded from the study. Palliative surgery was defined as those performed for either alleviation of symptoms or for asymptomatic patients for whom surgical removal of the tumors were deemed necessary following a multidisciplinary consensus. Tumors were diagnosed pre-operatively by computed tomography scans and all had histologic confirmation of the surgical specimens.
RESULTS
Over the study duration, 38 female patients underwent palliative surgery for Krukenberg tumors at our institution. Mean age was 54.2 ± 11.7 years. The colon was the most frequent primary source of metastases (n = 21) followed by the stomach (n = 4). Prophylactic palliative surgery was performed for eight (21.1%) asymptomatic patients. Median post-operative length of stay was 8 d (IQR 6-12 d). Five patients (13.2%) experienced post-operative complications, although high grade morbidity was only seen in one patient (2.6%). Median overall survival from surgery was 17 mo (95%CI: 12.1-21.9) at a median follow-up duration of 12 mo (IQR 8-17 mo). The median survival was shorter for patients who underwent emergency surgery, younger patients, those with a colorectal primary, larger tumors, or synchronous peritoneal or hepatic metastases.
CONCLUSION
Palliative surgery for Krukenberg tumors can be performed safely with acceptable complication rates. Bilateral oophorectomy should be performed to prevent the risk of symptomatic contralateral tumors.
Keywords: Krukenberg tumor, Palliative surgery
Core tip: Krukenberg tumors represent metastases to the ovary and convey a poor prognosis. By reporting our 12-year experience with palliative surgery for Krukenberg tumors and discussing the existing literature, we hope to shed light and establish best practices on the approach to management of the disease. Palliative surgery for patients with Krukenberg tumors can be performed safely in an experienced unit with acceptable complication rates. Where possible, bilateral oophorectomy should be performed to obviate the risk of the contralateral ovary developing symptomatic tumors. Appropriate selection by a multidisciplinary consensus is essential for asymptomatic patients who may benefit from prophylactic surgery.
INTRODUCTION
A Krukenberg tumor is a rare ovarian tumor which has metastasized from a primary site, accounting for 1%-2% of all tumors of the ovary[1]. The stomach was previously described to be the most common primary site, followed by the colon, appendix and breast[1]. Recent literature reveals an increased incidence of tumors originating from the colon[2]. Compared with primary ovarian tumors, the prognosis of patients with Krukenberg tumors is bleak, even with metastatic disease confined to the ovaries.
Various studies have been published over the past decade with regards to the prognostic factors and outcomes of surgery for Krukenberg tumors. Metastasectomy, or surgical removal of one or both involved ovaries, has been found to improve overall survival[3-5]. Cytoreductive surgery incorporating Krukenberg tumor removal has also been found to have a beneficial effect, with a 7% overall 5-year survival[6]. However, extensive cytoreductive surgery may have significant associated morbidity and mortality. To our knowledge, no previous study focuses exclusively on the group of patients for whom surgery is considered palliative.
Here we discuss the existing literature and report our experience with palliative surgery for Krukenberg tumors, including clinical characteristics of patients, disease presentation, surgical outcomes, safety, and prognostic factors.
MATERIALS AND METHODS
Data was collected for all patients who underwent surgery for Krukenberg tumors between January 2004 and December 2015. Patient information was obtained from inpatient and outpatient case notes as well as the hospital electronic records. Protocols were approved by hospital Institutional Review Board and the data was analyzed retrospectively. Patients with ovarian metastases who underwent potentially curative resection were excluded from the study. Patients with Krukenberg tumors who did not undergo surgery were also excluded from the study.
Over this period, surgery for Krukenberg tumors was performed by experienced surgeons from 3 departments within the same institution; General Surgery, Surgical Oncology, as well as Obstetrics and Gynaecology. The diagnosis of metastatic disease was made pre-operatively at a multidisciplinary tumor board meeting in 37 cases and post-operatively in one patient who underwent emergency surgery. Most Krukenberg tumors were diagnosed pre-operatively by computed tomography (CT) scans and all had histologic confirmation based on the pathological evaluation of the surgical specimens.
Palliative surgery in our series was defined as those performed for either alleviation of symptoms caused by the Krukenberg tumor (e.g., abdominal pain, distension or obstruction), or for asymptomatic patients for whom surgical removal of the tumors were deemed necessary following a multidisciplinary consensus.
Statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, United States). Survival was defined as the time from the date of surgery for the Krukenberg tumor to the date of death. Patients in whom the event of death had not occurred at the time of this study were censored. Survival rates were calculated using the Kaplan-Meier method and the differences in median survival times between groups assessed using the log-rank test. Cox proportional hazards model was used to determine the hazard ratios of variables affecting survival. P < 0.05 was taken as significant.
RESULTS
Patient and disease characteristics
Between January 2004 and December 2015, 38 female patients underwent palliative surgery for Krukenberg tumors at our institution. Patient and disease characteristics are summarised in Tables 1 and 2 respectively. Mean patient age was 54.2 ± 11.7 years. Almost 40% of the patients with Krukenberg tumors presented with abdominal pain or had pain as their most significant symptom. Median tumor size was 11.8 cm [Interquartile range (IQR) 7.8–16.0 cm]. Apart from the ovaries, all patients had at least one other site of metastases, mostly commonly the peritoneum or liver. The colon was the most frequent primary source of metastases followed by the stomach. In one patient, the primary tumor was not identified despite extensive investigation. The Krukenberg tumor was diagnosed during the same setting as the primary in just under half of the patients. Amongst the patients who had ovarian metastases detected metachronously, the median duration of diagnosis of the Krukenberg tumor from the primary was 19.5 mo (IQR 9-24 mo).
Table 1.
Patient characteristics n (%)
| Variable | Frequency |
| Age | |
| ≥ 50 | 25 (65.8) |
| < 50 | 13 (34.2) |
| Race | |
| Chinese | 33 (86.8) |
| Malay | 3 (7.9) |
| Others | 2 (5.3) |
| Presenting symptoms | |
| Pain | 15 (39.5) |
| Distension | 9 (23.7) |
| Intestinal obstruction | 3 (7.9) |
| Obstructive symptoms | 3 (7.9) |
| Asymptomatic | 8 (21.1) |
| ASA score at KT surgery | |
| 2 | 30 (78.9) |
| 3 | 8 (21.1) |
SD: Standard deviation; ASA: American Society of Anesthesiologists; KT: Krukenberg tumour.
Table 2.
Disease characteristics n (%)
| Variable | Frequency |
| Site of primary | |
| Stomach | 4 (10.5) |
| Colon | 21 (55.3) |
| Rectum | 1 (2.6) |
| Pancreas | 3 (7.9) |
| Breast | 2 (5.3) |
| Peritoneum | 2 (5.3) |
| Appendix | 1 (2.6) |
| Endometrium | 1 (2.6) |
| Bladder | 1 (2.6) |
| Lung | 1 (2.6) |
| Unknown | 1 (2.6) |
| Previous surgery for primary | |
| Yes | 17 (44.7) |
| No | 21 (55.3) |
| Detection of KT and primary | |
| Synchronous | 18 (47.4) |
| Metachronous | 20 (52.6) |
| KT size | |
| ≥ 12 cm | 19 (50.0) |
| < 12 cm | 19 (50.0) |
| Ovaries involved | |
| Unilateral | 20 (52.6) |
| Bilateral | 18 (47.4) |
| Peritoneal metastases | |
| Yes | 21 (55.3) |
| No | 17 (44.7) |
| Hepatic metastases | |
| Yes | 16 (42.1) |
| No | 22 (57.9) |
KT: Krukenberg tumour; SD: Standard deviation.
Twenty-six patients (68.4%) had undergone chemotherapy prior to Krukenberg tumor surgery and of these, 19 continued post-operative palliative chemotherapy. A total of 27 patients (71.1%) received chemotherapy post-operatively, including eight patients who commenced chemotherapy for the first time after surgery. Four patients (10.5%) had never received chemotherapy.
Surgical characteristics
Surgical procedures performed were; a unilateral or bilateral salphingo-opherectomy alone, or a total hysterectomy in addition to a bilateral salphingo-opherectomy (Table 3). Two other patients underwent additional surgical procedures during the same sitting as the Krukenberg tumor surgery; one patient had a small bowel resection while the other had a right hemicolectomy performed. Prophylactic palliative surgery was performed for eight asymptomatic patients; two were performed for histological confirmation of metastases to guide subsequent treatment, two were for progression of the Krukenberg tumors while on chemotherapy despite adequate extra-ovarian disease control, and four were prophylactic for sizeable tumors which would have likely caused symptoms during the patient’s lifetime. Five patients underwent emergent surgery; two for ruptured Krukenberg tumors, the third for a patient with fever and abdominal pain whose pre-operative CT scan was incorrectly reported as tubo-ovarian abscesses, the fourth for ovarian torsion, and in the last patient for acute intestinal obstruction secondary to compression of the bowel from the Krukenberg tumor.
Table 3.
Surgery characteristics n (%)
| Variable | Frequency |
| Urgency | |
| Elective | 33 (86.8) |
| Emergency | 5 (13.2) |
| Pre-KT surgery chemo | |
| Yes | 26 (68.4) |
| No | 12 (31.6) |
| Surgery | |
| USO | 10 (26.3) |
| BSO | 12 (31.6) |
| THBSO | 16 (42.1) |
| Post-op clavien-dindo score | |
| Grade 0 | 33 (86.8) |
| Grade I | 2 (5.3) |
| Grade II | 2 (5.3) |
| Grade IIIa | 1 (2.6) |
SD: Standard deviation; ASA: American Society of Anesthesiologists; KT: Krukenberg tumour; USO: Unilateral salphingo-opherectomy; BSO: Bilateral salphingo-opherectomy; THBSO: Total hysterectomy and bilateral salphingo-opherectomy.
Peri-operative morbidity
Median post-operative length of stay was 8 d (IQR 6-12 d). The majority of patients had an uneventful recovery period (86.8%). Five patients (13.2%) suffered complications; two Clavien-Dindo grade I, two grade II, and one grade IIIa (Table 3). Grade I complications included a urinary tract infection requiring antibiotics and supraventricular tachycardia which aborted with medication. Both grade II complications were cases of post-operative ileus requiring total parenteral nutrition. In these two patients, peritoneal carcinomatosis was thought to be a likely contributory factor. The grade IIIa complication was a post-operative intraabdominal collection requiring radiological guided drainage. This patient had undergone emergency surgery for a large ruptured Krukenberg tumor.
Survival
The median follow-up duration was 12 mo (IQR 8-17 mo). Two patients were lost to follow-up and one patient returned to her country of origin for continued care shortly after discharge. Twenty patients (52.6%) died during the follow-up period. Median overall survival (OS) from Krukenberg tumor surgery was 17 mo (95%CI: 12.1-21.9) (Figure 1). Based on univariate analysis (Table 4), only emergency surgery was associated with a significantly worse survival outcome. The median survival for patients with a colorectal primary compared to all other origins was 15 mo (95%CI: 10.5-19.5) and 38 mo respectively (95%CI: 5.8-70.2), P = 0.845. The median OS was also shorter for patients younger than 50 years old (13 mo, 95%CI: 8.0-18.0 vs 20 mo, 95%CI: 16.4-23.6), patients with a pre-operative American Society of Anaesthesiologists (ASA) score of 3 vs 2 (8 mo, 95%CI: 4.5-12.1 vs 29 mo, 95%CI: 18.6-40.1), larger tumors ≥ 12 cm (14 mo, 95%CI: 9.4-18.6 vs 20 mo, 95%CI: 14.8-25.2), those with peritoneal metastases (17 mo, 95%CI: 11.3-22.7 vs 20 mo, 95%CI: 9.7-30.3) and those with hepatic metastases (14 mo, 95%CI: 8.4-19.6 vs 17 mo, 95%CI: 9.8-24.2).
Figure 1.
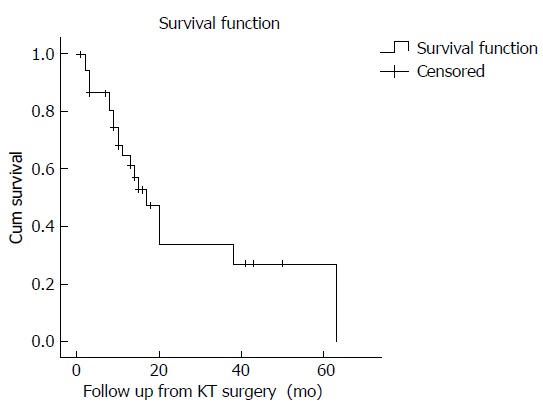
Kaplan-Meier survival analysis of 38 patients who underwent palliative surgery for Krukenberg tumours.
Table 4.
Univariate analyses of variables affecting survival
| Variable | HR (95%CI) | P-value |
| Age < 50 | 2.169 (0.876-5.369) | 0.094 |
| ASA score 3 | 2.830 (0.873-9.171) | 0.083 |
| Colorectal primary | 1.089 (0.424-2.795) | 0.860 |
| Previous surgery for primary | 0.894 (0.359-2.226) | 0.810 |
| Synchronous lesion | 1.240 (0.497-3.096) | 0.645 |
| Size ≥ 12 cm | 1.106 (0.424-2.884) | 0.837 |
| Bilateral disease | 1.473 (0.597-3.636) | 0.400 |
| Peritoneal metastases | 1.337 (0.509-3.511) | 0.555 |
| Hepatic metastases | 1.055 (0.419-2.656) | 0.909 |
| Emergency surgery | 3.382 (1.051-10.89) | 0.027 |
| Pre-op chemotherapy | 1.575 (0.521-4.758) | 0.421 |
| Serum CA 125 > 35 kU/L | 0.750 (0.187-3.004) | 0.684 |
| Serum CEA ≥ 30 μg/L | 0.785 (0.234-2.626)1 | 0.694a |
Among patients with a colorectal primary only. HR: Hazard ratio; CI: Confidence interval; ASA: American Society of Anesthesiologists; CEA: Carcinoembryonic antigen.
Pre-operative serum tumor marker level did not correlate with OS. Serum CA 125 level was performed in 21 patients (55.3%) before Krukenberg tumor surgery, for which median CA 125 level was 42.4 kU/L (IQR 14.1-206.5). Pre-operative serum carcinoembryonic antigen (CEA) testing was done in 30 patients (78.9%), including 20 out of 21 patients who had a colorectal primary. For these 20 patients, the median CEA level was 30.3 μg/L (IQR 10.8-144.0). Using a cut-off value of 35 and 30 for CA 125 and CEA respectively (Table 4), it appeared that the pre-operative values of these tumor markers did not reflect patient prognosis. These values were chosen based on the normal reference range of CA 125 (0-35 kU/L) and the median CEA level amongst patients with a colorectal primary in our study.
DISCUSSION
The eponymous Krukenberg tumor was first described in 1896 by the German doctor Friedrich Krukenberg (1871-1946). Representing advanced metastatic disease, this entity clearly conveys a poor prognosis. Gastric cancer was previously reported to account for the majority of primary tumors responsible[7]. However, recent literature suggests an increased frequency of Krukenberg tumors with a colorectal origin[2]. In our study, more than half of the patients had a colorectal primary compared to only 10% with a gastric primary. This is likely related to the increasing frequency of colorectal cancer locally as well as in other developed countries. In Singapore, the age-standardized incidence rate of colorectal cancer is one of the highest among ethnic Chinese populations in the world[8]. With the longer survival generally seen in patients with metastatic colorectal cancer as compared with that of gastric cancer, the option of pursing palliative surgery in these patients may be seemingly more attractive.
Many aspects of Krukenberg tumors still remain controversial, including the mechanism of metastases or even the pathologic tumor characteristics used for diagnosis. The current criteria used by the World Health Organisation, established in 1973 by Serov and Scully[9], may not adequately reflect the complexity of the tumor considering the multiple different primary sites possible. Treatment generally consists of surgery, chemotherapy or radiotherapy but guidelines concerning treatment of choice and appropriate timing of intervention have yet to be established.
Over the past decade, a number of retrospective studies have attempted to determine the prognostic factors for patients with Krukenberg tumors. Current evidence suggests a survival benefit for patients who undergo metastasectomy, compared to those without surgery. A recent multivariate analysis of 128 patients with Krukenberg tumors (58 colorectal, 41 gastric origin) showed that synchronous tumors, pelvic invasion and ascites were independent factors predicting for a poorer overall survival[10]. Other factors found to negatively influence overall prognosis include R1 or suboptimal resection[3], metastatic disease beyond the ovaries[11], tumors of gastric origin compared to colorectal origin[2], and patients with poorer Karnofsky performance status scores[4]. For gastric cancer, metastasectomy in addition to chemotherapy was also found to improve survival compared to palliative chemotherapy alone[12]. For colorectal cancers, ovarian metastases have been shown to be less responsive to chemotherapy compared to extra-ovarian sites[13]. Surgical resection was therefore recommended for these “metastatic sanctuaries” even in the palliative setting, as they would often progress and result in symptoms while on chemotherapy. In a separate study comparing 83 patients with ovarian metastases from a colorectal primary who underwent an oophorectomy vs 47 historical controls who did not undergo surgery, metastasectomy conferred a significantly longer OS at 20.8 mo vs 10.9 mo[14]. In terms of overall survival, surgery for patients with a colorectal primary may confer a greater advantage over that for a gastric primary in view of less aggressive tumor biology.
In contrast to the usually straightforward oopherectomy, the operative circumstances for patients with Krukenberg tumors may be more challenging. These patients often have undergone previous surgeries with resultant intraabdominal adhesions, or have synchronous pelvic peritoneal disease. Therefore, while the benefit of potentially curative metastasectomy is clear, clinicians may be hesitant to subject patients to palliative surgery, particularly if the patient is asymptomatic or in a debilitated state. In our series, five patients (13.2%) experienced post-operative complications, although high grade morbidity was only seen in one patient (2.6%). Both patients with ileus resumed enteral feeding within two weeks and were discharged well. The 30-d mortality rate was zero and median length of stay was about a week.
Close to half of all patients were found to have bilateral ovarian disease. Of these, the diagnosis of contralateral ovarian involvement was not apparent on pre-operative imaging and only established following surgery and histological examination of the resected specimen in a quarter of the cases (n = 4). Even if not synchronously affected there is a high chance of subsequent contralateral ovary involvement resulting in symptoms. We therefore recommend that bilateral ovarian resection be routine if surgery is to be performed for palliation.
Following our analyses, emergency surgery was the only factor that was found to significantly reduce survival prognosis. Although patients who were less than 50 years old, had a higher ASA score, with tumor size larger than 12 cm, or had hepatic or peritoneal metastases tended to have a shorter median survival, this was not found to be statistically significant. Surprisingly, tumors with a colorectal primary tended to fare worse than tumors of all other origins with a median overall survival of 15 mo vs 38 mo. Meaningful comparisons could not be performed for patients with a gastric primary in view of the small sample size (n = 4) with one patient defaulting follow-up and the other continuing further management overseas. While a single study found that a pre-operative CA 125 level of more than 75 kU/L significantly correlated to poorer survival[15], in our series no association could be found with this value nor the reference range cut-off. Pre- and post-operative trending of CA 125 levels therefore remains of questionable utility. CEA levels do not appear to prognosticate survival in patients with a colorectal primary, but may serve as an adjunct to imaging for reflecting disease response to chemotherapy.
Over a fifth of the patients in our series were asymptomatic at the time of surgery, all of whom had uncomplicated post-surgery recovery periods. Appropriate patient selection for prophylactic surgery is essential, with patient fitness and prognosis along with tumor size, location and anticipated development of symptoms being key considerations. With emergency surgery conferring a significantly worse outcome, the role of prophylactic surgery should certainly be explored further. The possibility of Krukenberg tumors being chemo-resistant “metastatic sanctuaries” was also evident in two asymptomatic patients in our study who had progression of the ovarian tumors despite good systemic control of disease elsewhere.
In conclusion, Palliative surgery for patients with Krukenberg tumors can be performed safely in an experienced unit with acceptable complication rates. The decision to proceed with metastasectomy is influenced by several factors including the presence of symptoms, synchronous disease, and tumor response to chemotherapy, and should be made as part of a multidisciplinary team consensus. Where possible, bilateral oophorectomy should be performed to obviate the significant risk of symptomatic contralateral ovarian involvement. Tumor markers need not be routinely trended peri-operatively. Proper selection is essential for asymptomatic patients who may benefit from prophylactic surgery. Further studies can be done to determine if symptom-free survival can be prolonged or quality of life improved with palliative surgery.
ARTICLE HIGHLIGHTS
Research background
A Krukenberg tumor is a rare ovarian tumor which has metastasized from a primary site, accounting for 1%-2% of all tumors of the ovary, and conveys a poor prognosis even with disease confirmed to the ovaries. A handful of studies have been published over the past decade with regards to the prognostic factors and outcomes of surgery for Krukenberg tumors, but no previous study focuses exclusively on the group of patients for whom surgery is considered palliative.
Research motivation
Many aspects of Krukenberg tumors still remain controversial, and guidelines concerning treatment of choice and appropriate timing of intervention have yet to be established. We report our experience with palliative resection of Krukenberg tumors over the past 12 years and discuss the existing literature, so as to shed light and establish best practices on the approach to management of the disease.
Research objectives
We aimed to determine the clinical characteristics of patients undergoing palliative surgery for Krukenberg tumors, including disease presentation, outcomes, and prognostic factors.
Research methods
This was a retrospective clinical study of all patients who underwent palliative surgery for Krukenberg tumors between January 2004 and December 2015. Patient information was obtained from inpatient and outpatient case notes as well as the hospital electronic records. Patients who underwent potentially curative resection, and patients with Krukenberg tumors who did not undergo surgery were also excluded from the study. Palliative surgery was defined as those performed for either alleviation of symptoms or for asymptomatic patients for whom surgical removal of the tumors were deemed necessary following a multidisciplinary consensus. Tumors were diagnosed pre-operatively by computed tomography scans and all had histologic confirmation of the surgical specimens.
Research results
Over the study duration, 38 female patients underwent palliative surgery for Krukenberg tumors at our institution. Mean age was 54.2 ± 11.7 years. The colon was the most frequent primary source of metastases (n = 21) followed by the stomach (n = 4). Prophylactic palliative surgery was performed for eight (21.1%) asymptomatic patients. Median post-operative length of stay was 8 d (IQR 6-12 d). Five patients (13.2%) experienced post-operative complications, although high grade morbidity was only seen in one patient (2.6%). Median overall survival from surgery was 17 mo (95%CI: 12.1-21.9) at a median follow-up duration of 12 mo (IQR 8-17 mo). The median survival was shorter for patients who underwent emergency surgery, younger patients, those with a colorectal primary, larger tumors, or synchronous peritoneal or hepatic metastases.
Research conclusions
Palliative surgery for patients with Krukenberg tumors can be performed safely in an experienced unit with acceptable complication rates. The decision to proceed with metastasectomy is influenced by several factors including the presence of symptoms, synchronous disease, and tumor response to chemotherapy, and should be made as part of a multidisciplinary team consensus. Where possible, bilateral oophorectomy should be performed to obviate the significant risk of symptomatic contralateral ovarian involvement. Tumor markers need not be routinely trended peri-operatively. Proper selection is essential for asymptomatic patients who may benefit from prophylactic surgery.
Research perspectives
Further studies can be done to determine if symptom-free survival can be prolonged or quality of life improved with palliative surgery.
Footnotes
Institutional review board statement: The study was reviewed and approved by the SingHealth Institutional Review Board.
Informed consent statement: Informed consent was not required for this retrospective study and all details that might disclose the identity of the subjects under study was omitted or anonymized.
Conflict-of-interest statement: All authors declare no conflict-of-interest.
Data sharing statement: Consent for data sharing was not obtained as presented data is anonymized and risk of identification is low.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: September 24, 2017
First decision: November 7, 2017
Article in press: November 25, 2017
P- Reviewer: Hori T, Morris DL S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
Contributor Information
Isaac Seow-En, Department of Colorectal Surgery, Singapore General Hospital, Singapore 169608, Singapore.
Gwen Hwarng, Duke-NUS Medical School, Singapore 169857, Singapore.
Grace Hwei Ching Tan, Department of Surgical Oncology, National Cancer Centre, Singapore 169610, Singapore.
Leonard Ming Li Ho, Department of Colorectal Surgery, Singapore General Hospital, Singapore 169608, Singapore.
Melissa Ching Ching Teo, Department of Surgical Oncology, National Cancer Centre, Singapore 169610, Singapore.
References
- 1.Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med. 2006;130:1725–1730. doi: 10.5858/2006-130-1725-AILAKT. [DOI] [PubMed] [Google Scholar]
- 2.Jeung YJ, Ok HJ, Kim WG, Kim SH, Lee TH. Krukenberg tumors of gastric origin versus colorectal origin. Obstet Gynecol Sci. 2015;58:32–39. doi: 10.5468/ogs.2015.58.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Surgical management and outcome of metachronous Krukenberg tumors from gastric cancer. J Surg Oncol. 2004;87:39–45. doi: 10.1002/jso.20072. [DOI] [PubMed] [Google Scholar]
- 4.Jiang R, Tang J, Cheng X, Zang RY. Surgical treatment for patients with different origins of Krukenberg tumors: outcomes and prognostic factors. Eur J Surg Oncol. 2009;35:92–97. doi: 10.1016/j.ejso.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Lu LC, Shao YY, Hsu CH, Hsu C, Cheng WF, Lin YL, Cheng AL, Yeh KH. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012;32:3397–3401. [PubMed] [Google Scholar]
- 6.Kim WY, Kim TJ, Kim SE, Lee JW, Lee JH, Kim BG, Bae DS. The role of cytoreductive surgery for non-genital tract metastatic tumors to the ovaries. Eur J Obstet Gynecol Reprod Biol. 2010;149:97–101. doi: 10.1016/j.ejogrb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Yada-Hashimoto N, Yamamoto T, Kamiura S, Seino H, Ohira H, Sawai K, Kimura T, Saji F. Metastatic ovarian tumors: a review of 64 cases. Gynecol Oncol. 2003;89:314–317. doi: 10.1016/s0090-8258(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 8.Singapore Cancer Registry report. 2015. Cancer Incidence and Mortality 2003-2012 and Selected Trends 1973-2012 in Singapore. Ministry of Health Singapore. [Google Scholar]
- 9.Serov SF, Scully RE. International Histological Classification of Tumours. Vol. 9. Geneva: WHO; 1973. Histological typing of ovarian tumors; 2015. [Google Scholar]
- 10.Wu F, Zhao X, Mi B, Feng LU, Yuan NA, Lei F, Li M, Zhao X. Clinical characteristics and prognostic analysis of Krukenberg tumor. Mol Clin Oncol. 2015;3:1323–1328. doi: 10.3892/mco.2015.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HK, Heo DS, Bang YJ, Kim NK. Prognostic factors of Krukenberg’s tumor. Gynecol Oncol. 2001;82:105–109. doi: 10.1006/gyno.2001.6210. [DOI] [PubMed] [Google Scholar]
- 12.Cho JH, Lim JY, Choi AR, Choi SM, Kim JW, Choi SH, Cho JY. Comparison of Surgery Plus Chemotherapy and Palliative Chemotherapy Alone for Advanced Gastric Cancer with Krukenberg Tumor. Cancer Res Treat. 2015;47:697–705. doi: 10.4143/crt.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goéré D, Daveau C, Elias D, Boige V, Tomasic G, Bonnet S, Pocard M, Dromain C, Ducreux M, Lasser P, et al. The differential response to chemotherapy of ovarian metastases from colorectal carcinoma. Eur J Surg Oncol. 2008;34:1335–1339. doi: 10.1016/j.ejso.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Lee J, Lim HY, Kang WK, Choi CH, Lee JW, Kim TJ, Kim BG, Bae DS, Cho YB, et al. Survival benefit from ovarian metastatectomy in colorectal cancer patients with ovarian metastasis: a retrospective analysis. Cancer Chemother Pharmacol. 2010;66:229–235. doi: 10.1007/s00280-009-1150-2. [DOI] [PubMed] [Google Scholar]
- 15.Kikkawa F, Shibata K, Ino K, Nomura S, Kajiyama H, Suzuki T, Kawai M, Mizutani S. Preoperative findings in non-gynecologic carcinomas metastasizing to the ovaries. Gynecol Obstet Invest. 2002;54:221–227. doi: 10.1159/000068388. [DOI] [PubMed] [Google Scholar]


