Abstract
Although a clear association has been established between IL-33 and inflammatory bowel disease, mechanistic studies to date, primarily using acute murine models of colitis, have yielded contradicting results, demonstrating both pathogenic and protective roles. We used a well-characterized, spontaneous model of inflammatory bowel disease [ie, SAMP1/YitFc (SAMP) mice] to investigate the role of IL-33 during chronic intestinal inflammation. Our results showed marked eosinophil infiltration into the gut mucosa with increased levels of eotaxins and type 2 helper T-cell (Th2) cytokines as disease progressed and became more severe, which could be reversed upon either eosinophil depletion or blockade of IL-33 signaling. Exogenous IL-33 administration recapitulated these effects in ilea of uninflamed (parental) control AKR/J mice. Human data supported these findings, showing colocalization and up-regulation of IL-33 and eosinophils in the colonic mucosa of inflammatory bowel disease patients versus noninflamed controls. Finally, colonization of commensal flora by fecal material transplantation into germ-free SAMP and the presence of the gut microbiome induced IL-33, subsequent eosinophil infiltration, and mounting of Th2 immune responses, leading to exacerbation of chronic intestinal inflammation characteristic of SAMP mice. These data demonstrate a pathogenic role for IL-33–mediated eosinophilia and activation of Th2 immunity in chronic intestinal inflammation that is dependent on the gut microbiome. Targeting IL-33 may represent a novel therapeutic approach to treat patients with inflammatory bowel disease.
IL-33 (alias IL-1F11) is the newest member of the IL-1 family. IL-33 represents a protein with dual function that can act as both a signaling cytokine and an intracellular nuclear factor.1, 2 IL-33 is widely distributed throughout various organ systems, primarily in nonhematopoietic cells, including fibroblasts, adipocytes, smooth muscle cells, endothelial cells, and bronchial and intestinal epithelial cells (IECs), as well as in cells of hematopoietic origin, particularly in restricted populations of professional antigen-presenting cells.1, 3 IL-33 was initially associated with the development of type 2 helper T-cell (Th2) immunity, on the basis of the expression of its cell-bound receptor, ST2L (IL-1R4), on polarized Th2 lymphocytes1 and, more recently, on innate lymphoid cells,4 as well as its ability to potently induce Th2 cytokine production (eg, IL-4, IL-5, and IL-13). In regard to disease pathogenesis, IL-33 is primarily involved in Th2-mediated disorders, such as airway inflammation and allergic reactions5; however, IL-33 has also been described to exacerbate arthritis, widely considered a Th1/Th17-mediated pathology.6, 7
One of the original observations and prominent functions of IL-33 is its ability to activate and induce eosinophil (EOS) infiltration into mucosal organs exposed to the external environment, including the respiratory and gastrointestinal tracts.1 In fact, IL-33 has emerged as an essential mediator in the development of EOS-mediated allergic inflammation and asthma,8 and plays a pivotal role in EOS recruitment and helminth expulsion after parasitic hookworm infection.9 EOS development and recruitment depends on IL-5, which represents the primary factor for EOS maturation and differentiation, but is also important in EOS activation and recruitment.10, 11 Chemokine ligand (CCL) 11 and CCL24 (alias eotaxin-1 and eotaxin-2, respectively) are EOS-specific chemokines that bind to the chemokine receptor, CCR3, expressed on the surface of EOS, and are critical for EOS recruitment.10, 11
A growing body of evidence also supports the importance of EOS in the pathogenesis of inflammatory bowel disease (IBD), a disorder previously attributed to dysregulated and overly aggressive T-effector cell responses. This paradigm has been challenged in recent years by the concept that the primary cause of IBD resides in dysfunction of host innate immunity and aberrant interactions with the gut microbiome, which together shape downstream adaptive immune responses. In regard to EOS, although their numbers are elevated in both ulcerative colitis (UC) and Crohn's disease (CD), two of the main etiopathogenic forms of IBD, most studies report the prevalence of EOS/EOS activity in UC compared with CD.12, 13, 14, 15
In 2010, several groups made the initial observation showing the increased expression and association of IL-33 with UC, and to a lesser extent, CD.3, 16, 17, 18 Others have since confirmed these findings and together have established that in active UC, IL-33 is localized to, and is potently up-regulated in, IECs and less so in infiltrating lamina propria (LP) mononuclear cells belonging to the monocyte/macrophage and B-cell lineages.3, 17 IL-33 is also expressed in activated subepithelial myofibroblasts situated below ulcerative lesions in UC patients,18, 19 suggesting a potential role for IL-33 in ulcer/wound healing, which may be different in UC versus CD. As such, although it is now widely accepted that IL-33 is important in IBD, its precise role has not yet been clearly defined. In fact, mechanistic studies primarily using acute, chemically induced models of colitis have resulted in ambiguous findings, supporting both pathogenic20, 21, 22, 23 and protective24, 25 functions. To date, the IL-33/ST2 axis has not been mechanistically evaluated in a chronic, immunologically mediated model of IBD.
The SAMP1/YitFc (SAMP) mouse strain represents a chronic model of Th1/Th2-mediated ileitis and provides an excellent system to study the initiation and progression of chronic intestinal inflammation.26 Because SAMP were derived from brother-sister mating of wild-type AKR/J (AKR; parental strain), the phenotype occurs spontaneously, as in the human condition, without chemical, genetic, or immunological manipulation. Relevant to the present study, we have previously reported elevated levels of serum and ileal IL-33 in SAMP compared with AKR mice.3
Herein, we used the SAMP model to mechanistically investigate whether IL-33 served any role in the development of chronic intestinal inflammation mediated by EOS. Our results show the following: i) EOS increase with the progression and severity of chronic intestinal inflammation (ie, reversed upon EOS depletion); ii) exogenous IL-33 administration induces EOS infiltration into the gut and a potent mucosal Th2 immune response, whereas IL-33 blockade reduces these effects and diminishes the overall severity of disease; and iii) the commensal flora is essential for the induction of IL-33, subsequent EOS infiltration, and production of Th2 cytokines, which exacerbates ileitis characteristic of SAMP mice. Together, these data demonstrate an important pathogenic role for IL-33 during chronic, spontaneous intestinal inflammation that is dependent on the gut microbiome and downstream Th2 immune responses. As such, targeting the IL-33/ST2 axis for therapeutic purposes may prove to be beneficial for the treatment of patients with IBD.
Materials and Methods
Animals
SAMP and AKR mice were propagated at Case Western Reserve University (CWRU; Cleveland, OH), with SAMP founders provided by S. Matsumoto (Yakult Central Institute for Microbiological Research, Tokyo, Japan).27, 28 Mice were maintained under specific pathogen-free (SPF) conditions, fed standard laboratory chow (Harlan Teklad, Indianapolis, IN), and kept on 12-hour light/dark cycles. Original AKR were purchased from The Jackson Laboratory (Bar Harbor, ME). Germ-free (GF)-SAMP were maintained at Taconic Farms (Albany, NY) and shipped to CWRU in GF vessels. All procedures were approved by the Institutional Animal Care and Use Committee at CWRU and followed the American Association for Laboratory Animal Care guidelines.
Fecal Material Transplantation Experiments
GF-SAMP recipients were maintained at CWRU's Animal Resource Center inside high-efficiency particulate air–filtered pressurized isolators and feeding on a double-irradiated diet (Irradiated Prolab Isopro RMH 3000; Lab Diet, St. Louis, MO) designed for maintenance and breeding of GF mice. On the day of transplantation, fresh fecal samples (single, approximately 100-mg pellet per mouse), collected from a representative cohort of SPF-raised SAMP (n = 6 per sex), were used to prepare donor inoculum. In brief, fecal pellets were homogenized using 200 mg of 1.4-mm-diameter sterile ceramic beads in 6 mL prechilled phosphate-buffered saline (PBS) by gentle agitation for 20 seconds using a vortex mixer. Recipient mice were orally gavaged with 100-μm filtered homogenates (108 to 109 colony-forming units in 300 μL) within 60 minutes of inoculum preparation. Transplanted mice were maintained following strict aseptic techniques, following GF standards for food, water, and nonpalatable bedding on the basis of aspen wood shavings. Prolab Isopro RMH 3000 diet was autoclaved, and a special isolation facility used to prevent mouse-mouse cross-contamination (1 to 2 mice per cage). Mice were sacrificed 6 weeks after fecal material transplantation (FMT), and ilea were harvested for later assays.
Human Endoscopic Biopsy and Surgical Specimens
Mucosal biopsy specimens were obtained during colonoscopy of adult UC patients and uninflamed controls. Diagnosis of UC was established by clinical, macroscopic, and histological criteria. Endoscopies were performed at the Endoscopy Unit of the first Department of Internal Medicine, Propaedeutic, at Laikon Hospital (Athens, Greece) and at the Gastroenterology and Endoscopy Unit of IRCCS Policlinico San Donato, San Donato Milanese (Milano, Italy). In most cases, samples were taken from the same individual, from both normal-appearing mucosa (noninvolved) and areas with evident macroscopic inflammation (involved). Individuals undergoing screening colonoscopy, who did not display endoscopic and histopathological mucosal abnormalities, served as controls. Specimens were maintained at 4°C for 1 to 2 days in RNAlater Solution (Ambion, Life Technologies, Austin, TX) until later total RNA isolation. Full-thickness colonic surgical specimens were obtained from UC patients, as well as noninflammatory controls, who were admitted to Case Medical Center/University Hospitals and underwent therapeutic bowel resection for either inflammatory disease or malignant and other nonmalignant, noninflammatory conditions. Tissues were subsequently processed, as previously described,3 and evaluated for EOS and IL-33 expression. All diagnoses were confirmed by clinical, macroscopic, and histological criteria. All studies were approved by the Ethics Committee of Laikon Hospital and IRCCS Policlinico San Donato, as well as by the Internal Review Board of Case Medical Center.
Tissue Harvest, Histologic Assessment, and EOS Count
Experimental mice were sacrificed, and terminal ilea were removed, opened longitudinally, and rinsed in PBS. Gut specimens were divided in half along the longitudinal plane, with one part placed in RNAlater Solution and maintained at 4°C until later total RNA extraction, and the other submerged in Bouin's solution (LabChem, Inc., Pittsburgh, PA). Bouin's fixed tissues were processed, as previously described,3 sectioned (3 μm thick), stained with hematoxylin and eosin, and histologically evaluated by a trained gastrointestinal pathologist (W.X.) in a blinded manner, using a validated semiquantitative scoring system.3 In addition, EOS counts were performed (W.X.) on major basic protein (MBP)–stained slides with 10 randomly selected areas per section evaluated under 40× high-power field (Olympus superwide eyepiece; 0.53-mm diameter; BX41 Laboratory Microscope; Olympus America, Inc., Melville, NY). Only intact MBP+ cells were counted, with an average number of EOS calculated and reported.
Experimental in Vivo Studies
Four-, 12-, and 20-week-old SAMP and age-matched AKR were used to evaluate baseline inflammation and progression of disease. EOS depletion was performed by i.p. injection (twice per week for 6 weeks) of 20-week-old SAMP with monoclonal antibodies against mouse IL-5 and CCR3 (GS2-19-4 and TRFK-5, respectively; Mayo Clinic Arizona, Scottsdale, AZ), both at 5 mg/kg, administered either alone or in combination. Control mice were treated with an isotype mouse IgG antibody (IR-MS-GF-ED; Innovative Research, Novi, MI), using the same dose and treatment schedule. For IL-33 administration experiments, 4- and 12-week-old SAMP and AKR were injected i.p. (33 μg/kg, daily for 1 week) with either murine recombinant IL-33 (ALX-522-101; Enzo Life Sciences, Farmingdale, NY) or PBS (vehicle controls). IL-33 blockade was achieved using 4- and 14-week-old SAMP treated with a murinzied rat IgG1 antibody against mST2, and controls were administered an isotype mouse IgG1 antibody (i.p., twice per week for 6 weeks; 5 mg/kg; Amgen, Seattle, WA).
Quantitative RT-PCR
Total RNA was isolated from patient endoscopic biopsy specimens and mouse ilea using the RNeasy Mini Kit (Qiagen, Germantown, MD) and reverse transcribed (RNA-to-cDNA kit; Applied Biosystems, Forest City, CA), both according to the manufacturer's instructions. Quantitative RT-PCR was performed, as previously described, using primers for mouse IL-33,3 IL-4, IL-5, IL-13,29 CCL11 (forward, 5′-TGTCTCCCTCCACCATGCA-3′; reverse, 5′-GATCTTCTTACTGGTCATGATAAAGCA-3′), CCL24 (forward, 5′-TGCATCTCCCCATAGATTCTGT-3′; reverse, 5′-ACTCGGTTTTCTGGAATTTTCTTG-3′), and β-actin (forward, 5′-CAGGGTGTGATGGGAATG-3′; reverse, 5′-GTAGAAGGTGTGGTGCCAGAT-3′), and primers for human IL-33,3 IL-5 (PPH00692A-200), CCL11 (PPH0057B-200), CCL24 (PPH01162B-200), CCL26 (PPH0163E-200), and β-actin (330001 PPH00073E-200) (all from Qiagen), and on an Applied Biosystems Step Plus machine (Applied Biosystems). Reaction mixture consisted of 15% volume first-strand synthesis in 20 μL total volume that included Power SYBR Green core reagents (Applied Biosystems) and 500 nmol/L final concentration of primers. Thermal cycling conditions were 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Expression of all target mRNA transcripts was normalized to β-actin, and reported as relative fold difference among groups within an experiment, with control group set arbitrarily at 1.
Cytokine Protein Levels
Cytokine protein levels were measured in cell culture supernatants [mouse Cytokine Screen-IR 16-plex (Quansys Biosciences, Logan, UT) and mouse IL-13 Ready-Set-Go ELISA kit, 88-7137-22 (eBioscience, San Diego, CA)] for EOS depletion and IL-33 administration experiments. Ready-Set-Go ELISA kits (eBioscience) were used for measuring levels of IL-4 (88-7044-22), IL-5 (88-7054-22), and IL-13 (88-7137-22) for IL-33 neutralization experiments.
IHC Data
Immunohistochemical (IHC) staining was performed using a polyclonal goat anti-mouse IL-33 IgG at 1:100 (AF3626; R&D Systems, Minneapolis, MN), a goat anti-human IL-33 IgG at 1:50 (AF3625; R&D Systems), and monoclonal rat anti-mouse MBP IgG (clone MT-14.7) or mouse anti-human EOS protein X IgG antibodies (clone MM25-82.2), both at 1:500 (J.J. Lee, Mayo Clinic, Scottsdale, AZ). Paraffin-embedded tissues were sectioned (3 to 4 μm thick), placed on Superfrost Plus glass slides (Thermo Scientific, Logan, UT), deparaffinized, and incubated in normal serum for non-specific blocking. Samples were blocked for endogenous peroxidase activity using 1.75% H2O2, and antigen retrieval was performed on human samples by microwave exposure (twice for 5 minutes), while submerged in unmasking solution (Vector Labs, Burlingame, CA). After incubation with primary antibodies at 4°C, slides were rinsed in PBS, incubated with appropriate biotinylated secondary antibodies (Vector Labs), rinsed in PBS, and further assayed using Vectastain ABC Kits (Vector Labs). Immunoreactive cells were visualized by addition of a diaminobenzidine substrate (Vector Labs), counterstained with hematoxylin, and mounted using an 80% glycerol mount. All incubations were conducted at room temperature unless otherwise noted. Negative controls were prepared under identical conditions in the absence of primary antibodies.
Flow Cytometry
Bone marrow was harvested from femurs and tibias of 20-week-old SAMP and AKR and placed in RPMI 1640 medium supplemented with 10% fetal bovine serum. Cell suspensions were washed and filtered through 70-μm cell strainers, and erythrocytes were lysed. A total of 106 cells were stained with Zombie NIR dye (Biolegend, San Diego, CA), and with the following antibodies: Brilliant Violet 421–labeled CD11b (M1/70), Alexa Fluor 488–labeled Gr-1 (RB6-8C5; Biolegend, San Diego, CA), and PE-CF594–labeled Siglec-F (E50-2440; Becton Dickinson, San Jose, CA). Samples were analyzed with an LSR II flow cytometer, and data were processed with FacsDiva Software (both from Becton Dickinson).
Image Acquisition
Images were obtained on an Axiophot microscope, captured on an Axiocam, and assembled on an Axiovision Release 4.5 (Carl Zeiss, Inc., Thornwood, NY). Objective lenses used were a Plan-Apochromat 20×/0.60 ∞/0.17 for murine tissues and an EC-Plan-neofluor 10×/0.3 Ph1 ∞/− for human tissues.
Statistical Analysis
Data were analyzed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Selection of appropriate statistical tests was on the basis of variance and underlying distribution of data. Global effects between groups were first assessed using one-way analysis of variance with Bonferroni correction for multiple comparisons; Pearson's r was used for correlation analyses. Differences between individual groups were directly compared using a two-sample unpaired t-test, and results expressed as means ± SEM, with P < 0.05 considered significant.
Results
Marked Increase in EOS Infiltration Correlates with Disease Progression and Severity in SAMP
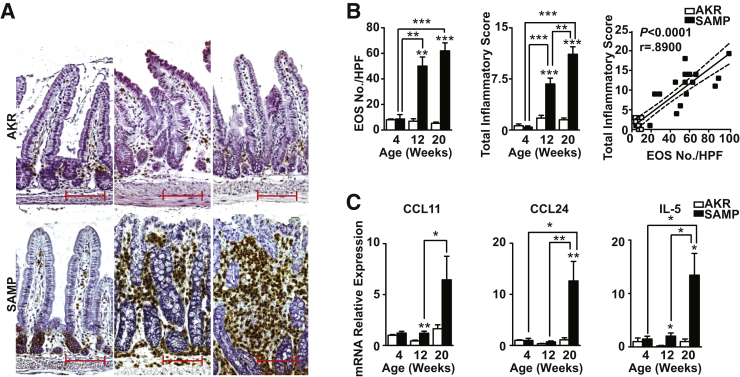
Before the onset of histologically evident inflammation, 4-week-old SAMP ilea displayed sparse, scattered EOS, identified by positive staining for MBP, one of the most abundant proteins specifically produced by EOS,10, 11 which was similar to that observed in age-matched AKR, but with evident accumulation already present within the base of the crypts (Figure 1A). By 12 weeks, frank inflammation was present in SAMP with expansion of the LP and loss of conventional villous architecture. Massive EOS infiltration was seen throughout the LP, with marked accumulation close to the base of the crypts and in the deep submucosa immediately adjacent to the muscularis propria (Figure 1A). This phenotype persisted in 20-week-old SAMP, whereas age-matched AKR showed scant EOS scattered throughout the LP, with no histological evidence of inflammation (Figure 1A). Quantitation of EOS revealed no difference between 4-week-old SAMP and AKR, and both strains showed little to no inflammation (Figure 1B). Conversely, EOS number in SAMP was vastly increased by 12 weeks versus AKR (P < 0.01), with an associated elevation in inflammation (P < 0.001). These trends were sustained in SAMP compared with AKR through 20 weeks (P < 0.001) (Figure 1B). Overall, EOS number positively correlated with inflammation (P < 0.0001) (Figure 1B), and confirmed the association of EOS infiltration and ileitis severity in SAMP mice.
Figure 1.
Eosinophil (EOS) infiltration increases and correlates with severity as disease progresses in SAMP1/YitFc (SAMP) ileitis. A: Representative images of full-thickness ilea from 4- (left column), 12- (middle column), and 20- (right column) week-old mice evaluated for major basic protein–positive EOS show sparse, scattered staining within lamina propria of 4-week-old SAMP, before histological evidence of ileitis, whereas 12- and 20-week-old SAMP with established disease show a marked and progressive increase in EOS infiltration as disease severity escalates. Little to no staining is observed in age-matched AKR/J (AKR; top panels). EOS count, total inflammatory score (TIS), correlation between EOS count and TIS (B), and IL-5, eotaxin-1 (CCL11) and eotaxin-2 (CCL24) in full-thickness ilea from SAMP versus age-matched AKR (C). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus age-matched AKR and as indicated. N = 6 (B and C). Scale bar = 100 μm (A). Original magnification, ×20 + 1.25 (A). CCL, chemokine ligand; HPF, high-power field.
No differences in IL-5, CCL11, or CCL24 were observed in ileal tissues comparing uninflamed, 4-week-old SAMP with age-matched AKR (Figure 1C). However, by 12 weeks, IL-5 and CCL11 showed an 11.6- and 2.7-fold increase, respectively, compared with AKR (both P < 0.01), and further increased at 20 weeks, wherein IL-5, CCL11, and CCL24 were elevated by 13.3-, 3.9-, and 11.4-fold, compared with AKR (P < 0.01, P = 0.068, P < 0.01, respectively). Consistent with these data, a remarkable abundance of EOS in SAMP bone marrow was observed compared with AKR (P < 0.01) (Supplemental Figure S1), suggesting that robust generation of EOS occurs in SAMP, followed by recruitment to the ileum that is likely facilitated, at least in part, by the previously mentioned EOS-associated mediators. Together, these data show that EOS-associated cytokines/chemokines are elevated during SAMP ileitis, and although they may be critical in the initial activation and infiltration of EOS, they may also be important in exacerbating chronic intestinal inflammation and sustaining established disease.
EOS Depletion Potently Decreases Ileal Inflammation in SAMP with Established Disease
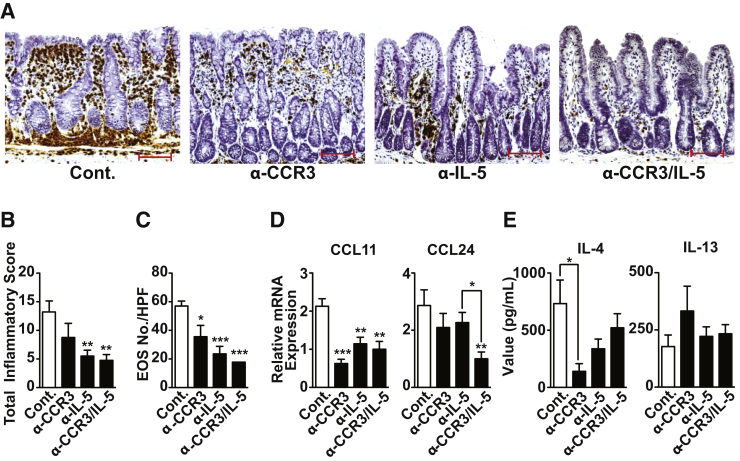
Twenty-week-old SAMP with established disease were treated with anti-CCR3 and anti–IL-5 antibodies, alone and in combination, to observe the effects of EOS depletion. IHC of MBP revealed a significant decrease in EOS infiltration and EOS numbers (Figure 2, A and C) after either anti-CCR3 or anti–IL-5 (P < 0.05 and P < 0.001, respectively), which was even more evident with combination anti-CCR3/IL-5 versus controls (P < 0.001). Moreover, our results showed that anti-CCR3 had a moderate effect at decreasing inflammation, whereas anti–IL-5 and combination anti-CCR3/IL-5 were more effective when compared with IgG-treated controls (both P < 0.01) (Figure 2B). Histologically, anti–IL-5 and anti-CCR3/IL-5 led to restoration of epithelial villous structure and an impressive decrease of inflammation compared with controls (Supplemental Figure S2). Anti-CCR3 and anti–IL-5, either alone or together, also strongly down-regulated ileal CCL11 by 3.4- (P < 0.001), 1.9-, and 2.1-fold (both P < 0.01), respectively, whereas only combination anti-CCR3/IL-5 was effective at decreasing CCL24 (by 2.9-fold) versus controls (P < 0.01) (Figure 2D). Finally, EOS depletion using anti-CCR3 and anti–IL-5 alone and in combination produced a trend toward decreased IL-4 protein levels that was significant only in SAMP treated with anti-CCR3 alone versus controls (P < 0.05) (Figure 2E). Conversely, no differences were observed in IL-13 protein levels after EOS depletion in SAMP compared with controls, regardless of treatment strategy (preventive and therapeutic) (Figure 2E). These findings indicate that EOS alone are not responsible for the induction and elevated levels of IL-13 observed in ileitis-prone SAMP mice; however, EOS likely contribute, at least in part, to increased ileal IL-4 in these mice. Together, these data demonstrate that EOS depletion effectively decreases ileal inflammation, as well as eotaxin and IL-4 expression, in SAMP mice.
Figure 2.
Eosinophil (EOS) depletion markedly reduces severity of SAMP1/YitFc (SAMP) ileitis. A: Representative images of major basic protein–stained ilea show decreased inflammatory cell infiltrates, including EOS, with restoration of villous architecture and striking improvement in overall gut morphology with either anti-CCR3 or anti–IL-5 treatment alone, or in combination, versus IgG-treated controls (Cont.). Histological evaluation of disease severity (B) and EOS number (C) in ilea from EOS-depleted, 20-week-old SAMP. Eotaxin mRNA expression in ilea (D) and type 2 helper T-cell cytokine protein levels from ex vivo T-cell receptor–activated mesenteric lymph node cells from EOS-depleted versus Cont. SAMP (E). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus Cont. and as indicated. N ≥ 4 (B–E). Scale bar = 100 μm (A). Original magnification, ×20 + 1.25 (A).
IL-33 Induces Robust Ileal EOS Infiltration and a Potent Mucosal Th2 Response from GALT
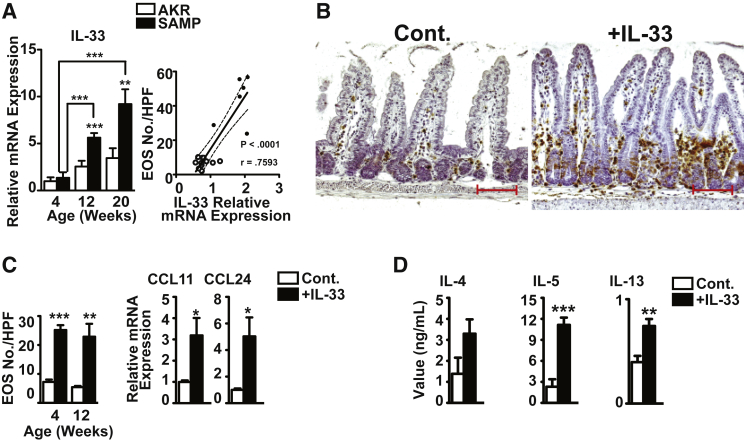
We investigated whether IL-33 had a direct effect on EOS infiltration and function in the gut mucosa that potentially leads to chronic intestinal inflammation. We confirmed that IL-33 was up-regulated in the ilea of 12- and 20-week-old SAMP versus age-matched AKR (P < 0.001 and P < 0.01), and markedly increased with disease progression (4.1- and 6.8-fold versus 4-week-old SAMP, both P < 0.001) (Figure 3A). More important, ileal IL-33 levels positively correlated with disease severity and the number of infiltrating EOS (P < 0.001) (Figure 3A).
Figure 3.
Exogenous administration of recombinant IL-33 (rIL-33) induces ileal eosinophil (EOS) infiltration and a potent type 2 helper T-cell (Th2) immune response in gut-associated lymphoid tissues from normal, uninflamed AKR/J (AKR) mice. A:Il33 in full-thickness ilea from SAMP1/YitFc (SAMP) and age-matched AKR and correlation with EOS number. B: Representative images of full-thickness AKR ilea show abundant infiltration of major basic protein–positive EOS after rIL-33 administration compared with vehicle-treated controls (Cont.). EOS counts and eotaxin mRNA levels in ilea (C) and Th2 cytokine protein levels from ex vivo T-cell receptor–activated mesenteric lymph node cells from IL-33– versus Cont.-treated mice (D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus age-matched AKR or Cont. N ≥ 9 (A, C, and D). Scale bar = 100 μm (B). Original magnification, ×20 + 1.25 (B). CCL, chemokine ligand.
In the absence of background inflammation, exogenous administration of rIL-33 induced robust EOS infiltration into the ilea of 4- and 12-week-old AKR compared with vehicle controls (P < 0.001 and P < 0.01) (Figure 3, B and C), with eotaxin levels higher by 3.2-fold for CCL11 and fivefold for CCL24 in 4-week-old IL-33–treated AKRs versus controls (both P < 0.05) (Figure 3C). IL-33 administration also induced a potent Th2 mucosal immune response in gut-associated lymphoid tissues (GALT) by producing copious amounts of IL-4 (P < 0.05), and particularly of IL-5 (P < 0.001) and IL-13 (P < 0.01), in ex vivo T-cell receptor–activated unfractionated mesenteric lymph node cells from IL-33–treated AKR versus controls (Figure 3D). These data confirm the ability of IL-33 to markedly induce EOS infiltration into the gut mucosa, and also establish that IL-33 potently elicits Th2 cytokine production from GALT in a model of chronic intestinal inflammation.
Blockade of IL-33 Decreases EOS Infiltration, Th2 Cytokine Expression, and Disease Severity in SAMP
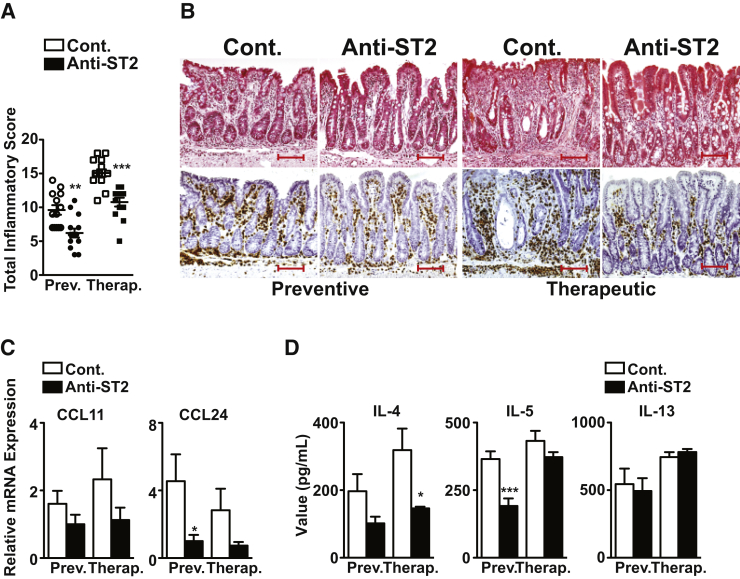
IL-33 bioactivity was blocked by administration of an antibody against the IL-33R (ST2/IL-1R4) to 4- and 20-week-old SAMP for a period of 6 weeks using two different experimental strategies, to prevent disease progression and treat established disease, respectively. Using the preventive protocol, anti-ST2 reduced disease severity by 35% compared with IgG-treated controls (P < 0.01), while treating established disease was also effective, decreasing severity by 29% (P < 0.001) (Figure 4A). Histologically, anti-ST2 decreased inflammatory cell infiltration, improved villous structure and overall gut morphology, and reduced the number of infiltrating EOS (Figure 4B). A decrease was also observed for ileal CCL24 in SAMP treated with anti-ST2 versus controls, particularly using the preventive protocol (P < 0.05), whereas minimal changes were observed for CCL11 (Figure 4C). Similarly, anti-ST2, using either a preventive or therapeutic approach, dampened production of the Th2 cytokines, IL-4 (P < 0.05, therapeutic protocol), IL-5 (P < 0.001, preventive protocol), but not IL-13 (Figure 4D). Together, these results indicate that IL-33 blockade in a chronic model of IBD is effective at decreasing overall gut inflammation, EOS infiltration, and specific GALT-derived Th2 cytokines.
Figure 4.
Blockade of IL-33 signaling decreases chronic intestinal inflammation in SAMP1/YitFc (SAMP) mice. Disease severity (A) and representative images (B) of major basic protein–stained SAMP ilea show marked reduction in inflammation, improvement in villous structure, and overall gut morphology, and decreased infiltrating eosinophil using both preventive and therapeutic strategies after anti-ST2 administration compared with IgG-treated control (Cont.). Ileal eotaxin mRNA expression (C) and type 2 helper T-cell cytokine protein levels (D) from ex vivo T-cell receptor–activated mesenteric lymph node cells. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus Cont. N = 13 to 14 (A); N = 8 (C and D). Scale bar = 100 μm (B). Original magnification, ×20 + 1.25 (B). CCL, chemokine ligand; Prev., preventive; Therap., therapeutic.
EOS Colocalize with IL-33 in the Inflamed Colonic Mucosa of UC Patients
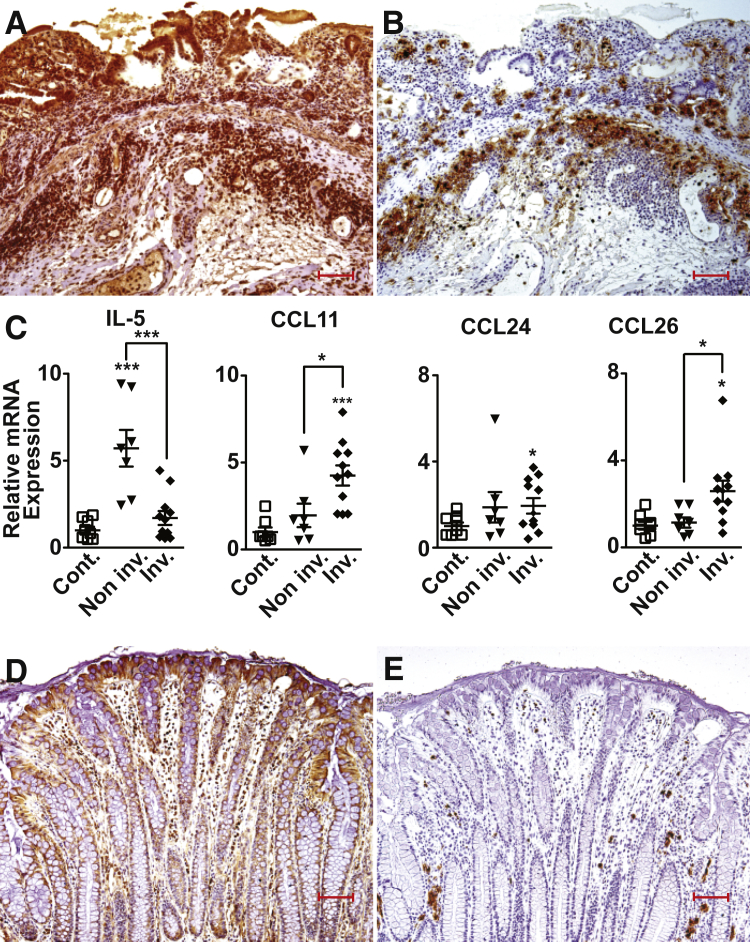
IHC studies revealed abundant IL-33 expression in the colonic mucosa of UC patients that intensified as disease became more severe and ulcerated (Figure 5A). Diffuse IL-33 staining was observed throughout the LP, in IECs, and scattered mononuclear cells within the inflamed gut mucosa, and in submucosal mononuclear cells accumulating in areas marginating the muscularis mucosa. Similar to the staining pattern in SAMP, EOS positive for EOS protein X, specific for detecting human EOS,30 collected primarily at the mucosal/submucosal interface adjacent to the muscularis propria and colocalized, but did not overlap, with cells that were positive for IL-33 (Figure 5B). Conversely, in noninflamed controls, IL-33 staining was largely limited to IEC with scattered staining of LP mononuclear cells, whereas EOS were sparsely present in areas close to the colonic crypts (Figure 5, D and E). Interestingly, IL-5 in UC noninvolved areas expressed a 5.7-fold increase (P < 0.001), but were unchanged in UC involved areas, compared with noninflamed controls (Figure 5C), whereas the eotaxins, CCL11, CCL24, and CCL26, were all elevated by 4.3-, 1.9-, and 2.6-fold, respectively, in severely involved UC colonic biopsy specimens compared with noninflamed controls (P < 0.05 for CCL24 and CCL26 and P < 0.001 for CCL11) (Figure 5C).
Figure 5.
Colonic IL-33, eosinophil (EOS) infiltration, and EOS-associated mediators are elevated in ulcerative colitis (UC) patients. Representative images of surgically resected, full-thickness colonic tissues stained for either IL-33 (A and D) or EOS protein X (B and E) show abundant IL-33 localized within the mucosa and submucosa of ulcerated, inflamed areas of UC patients (A) accompanied by marked accumulation of EOS distributed at the mucosal/submucosal interface in close proximity to IL-33 (B). D and E: Noninflamed areas of control (Cont.) patients show less IL-33, primarily limited to surface epithelial cells, and sparsely scattered EOS. C:IL5 and eotaxin-1 (CCL11), eotaxin-2 (CCL24), and eotaxin-3 (CCL26) in involved and noninvolved colonic mucosal biopsy specimens from UC patients compared with Cont. ∗P < 0.05, ∗∗∗P < 0.001 versus Cont. N ≥ 7 (C). Scale bar = 100 μm (D and E). Original magnification, ×10 + 1.25 (D and E). CCL, chemokine ligand; Inv., involved; Non inv., not involved.
Gut Microbiome Is Essential for Induction of IL-33, EOS Infiltration, and Th2 Immune Responses in the Development of Chronic Intestinal Inflammation
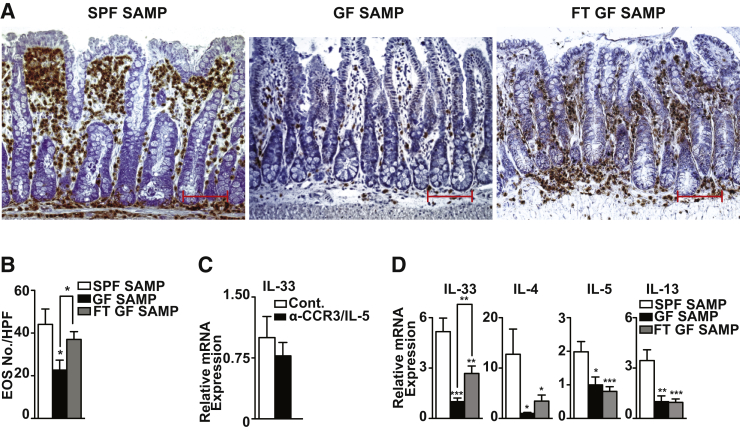
Previous studies showed that, despite being raised under GF conditions, SAMP develop chronic ileitis, but with delayed onset, lower penetrance, and decreased severity versus age-matched SPF-reared SAMP.31 Interestingly, although T-cell receptor–activated mesenteric lymph node cells from GF-SAMP display a robust, intact Th1 immune response, they are unable to mount a vigorous Th2 effector response in the absence of bacterial flora.31 Herein, we showed that in the absence of commensal flora, a significant decrease was observed in the number of infiltrating EOS in ilea of GF- compared with SPF-raised SAMP (P < 0.05) (Figure 6, A and B). After FMT using SPF-SAMP donors into GF-SAMP, EOS numbers increased and returned to values similar to those of SPF-SAMP (Figure 6, A and B). GF-SAMP also displayed a 5.2-fold decrease (P < 0.001) in IL-33 compared with age-matched SPF-SAMP, and after 6 weeks after FMT, showed a 2.7-fold increase in IL-33 versus GF-SAMP (P < 0.01) (Figure 6D). Th2 cytokine mRNA levels in ileal tissues were reduced in GF versus SPF-SAMP by 12.7-, 2-, and 3.5-fold for IL-4, IL-5 (both P < 0.05), and IL-13 (P < 0.01), respectively; however, aside from IL-4, neither IL-5 nor IL-13 increased after FMT into GF-SAMP (Figure 6D), which may require more time after FMT to be generated. More important, induction of IL-33 appeared to occur independently of EOS infiltration because EOS depletion in age-matched SPF-SAMP by combination anti-CCR3/IL-5 treatment did not affect IL-33 versus controls (Figure 6C), suggesting that up-regulation of IL-33 likely occurs before EOS recruitment into the gut.
Figure 6.
Presence of commensal flora is essential for induction of IL-33, eosinophil (EOS) infiltration, and type 2 helper T-cell (Th2) immune responses in SAMP1/YitFc (SAMP) ileitis. Representative images (A) and quantitation of EOS (B) in MBP-stained full-thickness ilea show decreased infiltrating EOS in germ free (GF)-compared with specific pathogen-free (SPF) SAMP and GF-SAMP after fecal material transplantation (FMT). C: Il-33 in SPF-SAMP treated with combination anti-CCR3/IL-5 antibodies versus IgG-treated control (Cont.). D: Il-33 and Th2 cytokine mRNA expression in ilea of SPF- compared to age-matched GF-SAMP and GF-SAMP after fecal transplantation (FT). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus SPF-SAMP and as indicated. N = 7 to 8 (B and C); N ≥ 7 (D). Scale bar = 100 μm (A). Original magnification, ×20 + 1.25 (A).
Discussion
To date, the role of EOS in the pathogenesis of IBD has not been fully elucidated, although studies in animal models indicate that epithelial barrier disruption and damage to gut mucosal surfaces occur after EOS degranulation and release of EOS granule proteins, resulting in acute colitis.10, 11 Other studies have suggested that EOS can act as immunoregulatory cells, stimulating T-cell activation and proliferation, and subsequent release of Th1 and Th2 cytokines,32 thereby perpetuating disease chronicity. Little is known, however, regarding the early events leading to eosinophilia in IBD.
IL-33 in Early EOS Activation and Migration in Chronic Intestinal Inflammation
In fact, the presence of eosinophilia in the intestinal mucosa of SAMP, and its robust infiltration as inflammation and disease become more severe, suggests that EOS also play a pathogenic role in chronic ileal inflammation. This hypothesis was confirmed by depletion of EOS after combination anti-CCR3/IL-5 administration. In addition, anti-CCR3 alone was also shown to dampen gut inflammation and attenuate EOS infiltration in another SAMP-related mouse strain (ie, SAMP1/SkuSlc).33, 34 In the present study, however, although significant diminution in EOS number and gut inflammation was globally achieved, differential effects were observed between either single administration of anti-CCR3 and anti–IL-5 or in combination, comparing locally produced intestinal eotaxins. For example, although anti-CCR3 or anti–IL-5 alone and in combination were effective in down-regulating CCL11, only combination anti-CCR3/IL-5 significantly decreased CCL24, suggesting that EOS depletion may have a direct and differential effect on eotaxin-expressing gut mucosal cells, including epithelial and submucosal endothelial cells, as well as macrophages and, to a lesser extent, T lymphocytes. Alternatively, EOS themselves are also known to be a source of eotaxins,10, 11 wherein CCL11 and CCL24 expression may differentially reflect either the temporal presence of EOS or how these eotaxins can affect EOS chemotaxis during different phases of chronic intestinal inflammation. In fact, after allergen challenge, CCL11 in the human lung is induced early and correlates with the initial phase of EOS recruitment, whereas CCL24 appears to be more important in EOS accumulation.34 Consistent with these findings, a similar time course, wherein CCL11 is expressed earlier than CCL24, is observed during the development of SAMP ileitis.
The observation that increased gut mucosal IL-5, CCL11, and CCL24 either coincided with or occurred later than, but not before, the increase in EOS infiltration and was greatest during chronic, established disease indicates that EOS migration and activation may take place independently of eotaxin and/or IL-5 expression, particularly during the early phase of disease. Another mediator, such as IL-33, could be responsible for early EOS events. Several lines of evidence corroborate this hypothesis, including the following: i) the increasing temporal expression of IL-33 in SAMP, as ileitis develops and correlates with EOS numbers; ii) recombinant IL-33 administration initiates acute EOS infiltration and up-regulation of CCL11 and CCL24 in the gut mucosa of uninflamed AKR; and iii) EOS depletion of chronically inflamed SAMP does not affect gut mucosal levels of IL-33, but blockade of IL-33 potently decreases EOS infiltration. Together, these data indicate that up-regulation of IL-33 occurs independently of, and likely before, EOS activation and migration in chronic intestinal inflammation.
Requirement of the Gut Microbiota for Local IL-33 Expression and the Development of Pathogenic Th2 Immune Responses Leading to IBD
Presence of commensal flora is required for the early induction of IL-33 in SAMP and the downstream activation and infiltration of EOS into the gut mucosa. Because we have previously shown that the primary source of IL-33 in SAMP is the intestinal epithelium, particularly at an early age,3 it is likely that stimulation of IECs via bacteria/bacterial products initiates the cascade of events leading to IL-33–dependent EOS activation and infiltration. Alternatively, the possibility exists that increased bacterial translocation because of the inherent epithelial barrier defect in SAMP mice35 facilitates activation of underlying antigen-presenting cells, another source of IL-33 within the gut mucosa, that can similarly induce EOS activation/infiltration.
A genetic study reporting that specific IL-33, IL1RL1/ST2 polymorphisms confer an increased risk of developing both UC and CD36 further strengthens the link between this important cytokine-receptor pair and IBD. In addition, although increased IL-33 has been primarily associated with UC, a recent study showed that elevated levels of IL-33 specifically occur in pediatric Crohn's patients with complicated fibrostenotic disease.37 Indeed, earlier studies reporting the prevalence of IL-33 in UC compared with CD almost exclusively investigated patients with Crohn's colitis versus ileitis; as such, the possibility exists that IL-33 may play an important role in a specific subpopulation of CD patients who present with fibrosis because elevated IL-33 has also been associated with several other fibrotic diseases.38 Interestingly, SAMP mice also display transmural ileal fibrosis with stenotic lesions that progresses over time.38
Nonetheless, although the association between the IL-33/ST2 axis and human IBD has been firmly established, mechanistic studies using variations of chemically induced colitis models have generated conflicting results. The most commonly used model has been dextran sodium sulfate (DSS)–induced colitis, which represents a T-cell–independent model of epithelial damage and acute inflammation, primarily driven by innate immune responses. DSS given to mice deficient in either IL-33 or ST2 developed less severe colitis than wild-type controls, with decreased granulocyte infiltration, whereas exogenous administration of IL-33 to DSS-treated mice further aggravated acute colitis and triggered the influx of neutrophils into the gut mucosa,20, 21, 23, 25 supporting a pathogenic role for IL-33, at least in an acute inflammatory setting.
Conversely, as opposed to results obtained from IL-33 treatment of acute DSS colitis, Groß et al25 showed that IL-33 administration during repeated cycling of DSS caused a reduction of colitis, suppressed interferon-γ, and decreased bacterial translocation, indicating a protective role for IL-33. Similar results were obtained using the trinitrobenzene sulfonic acid model of colitis,24 another chemically induced model of colonic inflammation described to elicit Th1 immune responses, particularly if performed using a chronic protocol. Although an acute, 4-day challenge of trinitrobenzene sulfonic acid was used in the previously mentioned study, exogenous IL-33 administration was shown to ameliorate colitis.24 Both groups hypothesize that the mechanism for protection rests on IL-33's ability to promote switching from Th1- to Th2-driven immune responses, implying that Th2 skewing results in anti-inflammatory activity. However, although several lines of evidence exist that support the role of Th2 immunity in gut mucosal protection (particularly for parasitic helminth infection), it is now generally accepted that pathogenic Th2-dependent immune processes, primarily driven by IL-5 and IL-13, are also crucial for the development of UC and fibrosis-associated CD. In fact, a recent study attributes IL-33 induction of IL-5 and IL-13, by direct activation of IL-4/IL-4R, to the exacerbation of inflammation in DSS colitic mice.39
IL-33 as a Master Regulator of Gut Mucosal Th2 Immunity
An alternative mechanism for IL-33–induced gut protection has been recently demonstrated by Monticelli et al,40 again using the acute model of DSS colitis, in which either IL-33 treatment or adoptive transfer of group 2 innate lymphoid cells (ILC2s) was found to ameliorate disease severity by up-regulation of the growth factor, amphiregulin, derived from ILC2s. ILC2s represent a subpopulation of newly defined innate immune cells that are prevalent within, but not limited to, mucosal barrier surfaces and that express type 2 cytokines, such as IL-4, IL-5, and IL-13. Relevant to the present study, signaling via IL-33 can also induce EOS migration through the expansion and activation of ILC2s; and in fact, IL-5 derived from ILC2s has been shown to maintain homeostasis and survival of EOS within the gut39 and in other sites, such as lung and adipose tissues.41, 42 In experimental models of colitis, ILC1s and ILC3s, the other predominant ILC subsets, have been implicated in the pathogenesis of IBD; however, aside from findings derived from the previously mentioned study by Monticelli et al,40 the precise role of ILC2s in intestinal inflammation, particularly during chronic disease, is largely unknown.43 Interestingly, IL-13–producing ILCs have been reported to contribute to intestinal fibrosis in Crohn's patients,44 and IL-33–dependent ILC2s have been shown to promote fibrosis in both the liver and lungs,45, 46 suggesting a pathogenic role for these cells during chronic inflammatory conditions. In the SAMP mice, our initial findings indicate an increased frequency and expansion in absolute numbers of gut mucosal ILC2s compared with AKR controls (data not shown), which may be a consequence of elevated local levels of IL-33. It has yet to be determined, however, whether these cells possess pathogenic or protective functions, or both, during the course leading to chronic intestinal inflammation characteristic of these mice, which is an area of ongoing investigation in our laboratory.
In fact, the SAMP model is an established model of Th1/Th2-driven enteritis that, unlike the previously mentioned models of chemically induced colitis, spontaneously develops chronic gut inflammation that recapitulates several features of human IBD.26 In SAMP, Th1 immune responses are predominant early during the induction phase of disease and are sustained throughout the chronic phase, whereas Th2 immune responses are prevalent only later, during the chronic phase when disease is most severe. In agreement with these findings, neutralization of the Th1 cytokines, tumor necrosis factor and interferon-γ, is effective in decreasing inflammation when given during either the early or the late phases of disease,28, 29 whereas blocking the Th2 cytokines, IL-4 and IL-5, has greater efficacy administered when intestinal inflammation is established.29, 47
Because IL-33 is regarded as a possible “master” cytokine in driving Th2 immunity, and both systemic and ileal IL-33 are markedly up-regulated in SAMP,3 we investigated whether blockade of IL-33 could effectively dampen disease in this model of chronic gut inflammation. Our results showed definitive efficacy in down-regulating inflammation in SAMP when using both a preventive and a therapeutic strategy, which is consistent with use of other biological agents (eg, anti-tumor necrosis factor, anti–interferon-γ, and anti–IL-4),26 and anti–IL-5 performed in this and other studies.47 The preventive effects may be attributed to interfering with IL-33's ability to potentiate initial Th1 immune responses and/or to induce early EOS activation, whereas IL-33 blockade during established disease may directly affect the ability to mount pathogenic Th2 immune responses from CD4+ T-effector cells.
In addition, although neutralization of IL-33 was effective in uniformly dampening EOS infiltration into the gut mucosa of SAMP independent of treatment strategy, its prevention of Th2 cytokine expression varied. For example, anti-ST2 was ineffective at modulating IL-13 using both preventive and therapeutic approaches. Similarly, results obtained after EOS depletion showed that IL-4 production was decreased and clearly affected by anti-CCR3 administration, whereas IL-13 levels did not change in any of the EOS depletion treatments tested. It is possible that because EOS depletion was performed in SAMP mice during established disease (ie, at 20 weeks of age), CD4+ T cells, the likely source of IL-13, are unaffected by EOS depletion, and continue to produce copious amounts of IL-13. Consistent with these findings, recent studies have reported the absence for a role of IL-13 in IBD48 and that administration of anrukinzumab, a monoclonal antibody against IL-13, did not show therapeutic efficacy in patients with active UC.49
Th2 cytokine expression also varied depending on whether IL-33 blockade was performed before the onset of inflammation or during established, chronic disease. For instance, anti-ST2 was effective in decreasing IL-5 production when administered early during the development of gut inflammation, but did not alter IL-5 levels after disease was already established. Interestingly, IL-5 in UC patients appeared to be decreased, at least at the mRNA level, in involved versus noninvolved tissues, which may explain the ineffectiveness of anti-ST2 in decreasing IL-5 when disease is established and more severe. These results may also reflect the sum effects of specific ST2-bearing effector cell types with the ability to produce IL-5 that are present or prevalent during early versus late phases of disease development (eg, EOS versus CD4+ Th2 effector cells), or the specific dose (5 mg/kg) and administration regimen that was used in this study. In fact, a higher dose of 10 mg/kg using the same anti-ST2 antibody was shown to down-regulate inflammation in an experimental model of rheumatoid arthritis,7 whereas a 2 mg/kg dose was efficacious in ameliorating acute DSS-induced colitis.23 Interestingly, the effectiveness of the lower dose of anti-ST2 in the latter, particularly in an acute versus chronic model of IBD, may be because of the emerging concept that, similar to other ligands of the IL-1 family, IL-33 possesses dichotomous roles during the course of colitis development, wherein IL-33's established, pathogenic effects must be counterbalanced with its protective functions, including the ability to induce epithelial proliferation, induce mucous production, and promote gut mucosal wound healing,38 and more recently, its ability to promote T-regulatory cell function in the intestine after infection with Helicobacter hepaticus and administration of an IL-10R blocking antibody.50
Conclusion and Future Challenges
As such, total blockade of IL-33 at different stages during the progression of chronic intestinal inflammation may be effective in down-regulating its pathogenic functions, but may also interfere with its protective functions. Further investigation is warranted and currently underway in our laboratory to determine optimal dosage and time course regimens of IL-33 blockade to effectively treat chronic intestinal inflammation, as well as determine whether simultaneous (versus single) blockade of both Th1 and Th2 pathways will improve disease outcome. Therefore, designing therapeutic strategies to target key cytokine pathways, such as the IL-33/ST2 axis, combined with manipulation of the commensal flora, may represent a novel approach for the treatment of IBD.
Footnotes
Supported by NIH grants DK056762, DK091222, and AI102269 (T.T.P.), DK055812 (F.C.), DK097948 and DK042191 (T.T.P./F.C.), and Crohn's and Colitis Foundation of America grant RFA326877 (C.D.S.).
Disclosures: D.E.S. is an employee and stockholder of Amgen Corp.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.11.028.
Supplemental Data
Eosinophil (EOS) frequency is dramatically increased in bone marrow of ileitis-prone SAMP1/YitFc (SAMP) mice with established disease. Bone marrow cells were isolated from 20-week-old SAMP and age-matched AKR/J (AKR) control mice, and analyzed by flow cytometry. Aggregates and dead cells were excluded on the basis of forward scatter (FSC)-A versus FSC-H and live/dead dye staining, respectively, and EOS were gated as CD11b+ side scatter (SSC)hi Gr-1neg Siglec-F+ cells. A: Representative plots displaying 5000 events. B: Percentages of EOS within the live cell gate in SAMP and AKR mice. ∗∗P < 0.01, ∗∗∗P < 0.001 versus age-matched AKR. N = 6 (B).
Eosinophil (EOS) depletion markedly reduces the severity of ileitis in SAMP1/YitFc (SAMP) mice with established disease. A: Histological evaluation of ilea from EOS-depleted 20-week-old SAMP versus IgG control-treated SAMP by analysis of four parameters composing total inflammatory score. B: Representative images of hematoxylin and eosin–stained ileal tissues. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus control (Cont.) and as indicated. N = 8 (A). Scale bar = 100 μm (B). Original magnification, ×20 + 1.25 (B).
References
- 1.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D.M., Bazan J.F., Kastelein R.A. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Carriere V., Roussel L., Ortega N., Lacorre D.A., Americh L., Aguilar L., Bouche G., Girard J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastorelli L., Garg R.R., Hoang S.B., Spina L., Mattioli B., Scarpa M., Fiocchi C., Vecchi M., Pizarro T.T. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K., Bucks C., Kane C.M., Fallon P.G., Pannell R., Jolin H.E., McKenzie A.N. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurowska-Stolarska M., Kewin P., Murphy G., Russo R.C., Stolarski B., Garcia C.C., Komai-Koma M., Pitman N., Li Y., Niedbala W., McKenzie A.N., Teixeira M.M., Liew F.Y., Xu D. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 6.Xu D., Jiang H.R., Kewin P., Li Y., Mu R., Fraser A.R., Pitman N., Kurowska-Stolarska M., McKenzie A.N., McInnes I.B., Liew F.Y. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer G., Talabot-Ayer D., Lamacchia C., Toy D., Seemayer C.A., Viatte S., Finckh A., Smith D.E., Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 8.Stolarski B., Kurowska-Stolarska M., Kewin P., Xu D., Liew F.Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 9.Hung L.Y., Lewkowich I.P., Dawson L.A., Downey J., Yang Y., Smith D.E., Herbert D.R. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci U S A. 2013;110:282–287. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra A., Hogan S.P., Lee J.J., Foster P.S., Rothenberg M.E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan S.P., Rosenberg H.F., Moqbel R., Phipps S., Foster P.S., Lacy P., Kay A.B., Rothenberg M.E. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 12.Lampinen M., Ronnblom A., Amin K., Kristjansson G., Rorsman F., Sangfelt P., Safsten B., Wagner M., Wanders A., Winqvist O., Carlson M. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54:1714–1720. doi: 10.1136/gut.2005.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampinen M., Backman M., Winqvist O., Rorsman F., Ronnblom A., Sangfelt P., Carlson M. Different regulation of eosinophil activity in Crohn's disease compared with ulcerative colitis. J Leukoc Biol. 2008;84:1392–1399. doi: 10.1189/jlb.0807513. [DOI] [PubMed] [Google Scholar]
- 14.Ahrens R., Waddell A., Seidu L., Blanchard C., Carey R., Forbes E., Lampinen M., Wilson T., Cohen E., Stringer K., Ballard E., Munitz A., Xu H., Lee N., Lee J.J., Rothenberg M.E., Denson L., Hogan S.P. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol. 2008;181:7390–7399. doi: 10.4049/jimmunol.181.10.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampinen M., Waddell A., Ahrens R., Carlson M., Hogan S.P. CD14+CD33+ myeloid cell-CCL11-eosinophil signature in ulcerative colitis. J Leukoc Biol. 2013;94:1061–1070. doi: 10.1189/jlb.1212640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidelin J.B., Bjerrum J.T., Coskun M., Widjaya B., Vainer B., Nielsen O.H. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128:80–85. doi: 10.1016/j.imlet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Beltran C.J., Nunez L.E., Diaz-Jimenez D., Farfan N., Candia E., Heine C., Lopez F., Gonzalez M.J., Quera R., Hermoso M.A. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1097–1107. doi: 10.1002/ibd.21175. [DOI] [PubMed] [Google Scholar]
- 18.Kobori A., Yagi Y., Imaeda H., Ban H., Bamba S., Tsujikawa T., Saito Y., Fujiyama Y., Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 19.Sponheim J., Pollheimer J., Olsen T., Balogh J., Hammarstrom C., Loos T., Kasprzycka M., Sorensen D.R., Nilsen H.R., Kuchler A.M., Vatn M.H., Haraldsen G. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177:2804–2815. doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oboki K., Ohno T., Kajiwara N., Arae K., Morita H., Ishii A., Nambu A., Abe T., Kiyonari H., Matsumoto K., Sudo K., Okumura K., Saito H., Nakae S. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imaeda H., Andoh A., Aomatsu T., Uchiyama K., Bamba S., Tsujikawa T., Naito Y., Fujiyama Y. Interleukin-33 suppresses Notch ligand expression and prevents goblet cell depletion in dextran sulfate sodium-induced colitis. Int J Mol Med. 2011;28:573–578. doi: 10.3892/ijmm.2011.718. [DOI] [PubMed] [Google Scholar]
- 22.Rani R., Smulian A.G., Greaves D.R., Hogan S.P., Herbert D.R. TGF-beta limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol. 2011;41:2000–2009. doi: 10.1002/eji.201041135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedhom M.A., Pichery M., Murdoch J.R., Foligne B., Ortega N., Normand S., Mertz K., Sanmugalingam D., Brault L., Grandjean T., Lefrancais E., Fallon P.G., Quesniaux V., Peyrin-Biroulet L., Cathomas G., Junt T., Chamaillard M., Girard J.P., Ryffel B. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62:1714–1723. doi: 10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan L., Chen J., Zhang H., Yang H., Zhu P., Xiong A., Xia Q., Zheng F., Tan Z., Gong F., Fang M. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3(+) regulatory T-cell responses in mice. Mol Med. 2012;18:753–761. doi: 10.2119/molmed.2011.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groß P., Doser K., Falk W., Obermeier F., Hofmann C. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis. 2012;18:1900–1909. doi: 10.1002/ibd.22900. [DOI] [PubMed] [Google Scholar]
- 26.Pizarro T.T., Pastorelli L., Bamias G., Garg R.R., Reuter B.K., Mercado J.R., Chieppa M., Arseneau K.O., Ley K., Cominelli F. SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–2584. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto S., Okabe Y., Setoyama H., Takayama K., Ohtsuka J., Funahashi H., Imaoka A., Okada Y., Umesaki Y. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–78. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosiewicz M.M., Nast C.C., Krishnan A., Rivera-Nieves J., Moskaluk C.A., Matsumoto S., Kozaiwa K., Cominelli F. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bamias G., Martin C., Mishina M., Ross W.G., Rivera-Nieves J., Marini M., Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 30.Protheroe C., Woodruff S.A., de Petris G., Mukkada V., Ochkur S.I., Janarthanan S., Lewis J.C., Pasha S., Lunsford T., Harris L., Sharma V.K., McGarry M.P., Lee N.A., Furuta G.T., Lee J.J. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755.e11. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bamias G., Okazawa A., Rivera-Nieves J., Arseneau K.O., De La Rue S.A., Pizarro T.T., Cominelli F. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–1818. doi: 10.4049/jimmunol.178.3.1809. [DOI] [PubMed] [Google Scholar]
- 32.Woerly G., Roger N., Loiseau S., Dombrowicz D., Capron A., Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med. 1999;190:487–495. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masterson J.C., McNamee E.N., Jedlicka P., Fillon S., Ruybal J., Hosford L., Rivera-Nieves J., Lee J.J., Furuta G.T. CCR3 blockade attenuates eosinophilic ileitis and associated remodeling. Am J Pathol. 2011;179:2302–2314. doi: 10.1016/j.ajpath.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masterson J.C., Capocelli K.E., Hosford L., Biette K., McNamee E.N., de Zoeten E.F., Harris R., Fernando S.D., Jedlicka P., Protheroe C., Lee J.J., Furuta G.T. Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohn's ileitis. Inflamm Bowel Dis. 2015;21:2429–2440. doi: 10.1097/MIB.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takedatsu H., Mitsuyama K., Matsumoto S., Handa K., Suzuki A., Takedatsu H., Funabashi H., Okabe Y., Hara T., Toyonaga A., Sata M. Interleukin-5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur J Immunol. 2004;34:1561–1569. doi: 10.1002/eji.200324680. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann N., Hershey G.K., Foster P.S., Rothenberg M.E. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–242. doi: 10.1067/mai.2003.139. quiz 43. [DOI] [PubMed] [Google Scholar]
- 37.Olson T.S., Reuter B.K., Scott K.G., Morris M.A., Wang X.M., Hancock L.N., Burcin T.L., Cohn S.M., Ernst P.B., Cominelli F., Meddings J.B., Ley K., Pizarro T.T. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera-Nieves J., Bamias G., Vidrich A., Marini M., Pizarro T.T., McDuffie M.J., Moskaluk C.A., Cohn S.M., Cominelli F. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–982. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 39.Nussbaum J.C., Van Dyken S.J., von Moltke J., Cheng L.E., Mohapatra A., Molofsky A.B., Thornton E.E., Krummel M.F., Chawla A., Liang H.E., Locksley R.M. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monticelli L.A., Osborne L.C., Noti M., Tran S.V., Zaiss D.M., Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith S.G., Chen R., Kjarsgaard M., Huang C., Oliveria J.P., O'Byrne P.M., Gauvreau G.M., Boulet L.P., Lemiere C., Martin J., Nair P., Sehmi R. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 42.Hashiguchi M., Kashiwakura Y., Kojima H., Kobayashi A., Kanno Y., Kobata T. IL-33 activates eosinophils of visceral adipose tissue both directly and via innate lymphoid cells. Eur J Immunol. 2015;45:876–885. doi: 10.1002/eji.201444969. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg R., Prescott N., Lord G.M., MacDonald T.T., Powell N. The unusual suspects: innate lymphoid cells as novel therapeutic targets in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:271–283. doi: 10.1038/nrgastro.2015.52. [DOI] [PubMed] [Google Scholar]
- 44.Bailey J.R., Bland P.W., Tarlton J.F., Peters I., Moorghen M., Sylvester P.A., Probert C.S., Whiting C.V. IL-13 promotes collagen accumulation in Crohn's disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS One. 2012;7:e52332. doi: 10.1371/journal.pone.0052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McHedlidze T., Waldner M., Zopf S., Walker J., Rankin A.L., Schuchmann M., Voehringer D., McKenzie A.N., Neurath M.F., Pflanz S., Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li D., Guabiraba R., Besnard A.G., Komai-Koma M., Jabir M.S., Zhang L., Graham G.J., Kurowska-Stolarska M., Liew F.Y., McSharry C., Xu D. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134:1422–1432.e11. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pushparaj P.N., Li D., Komai-Koma M., Guabiraba R., Alexander J., McSharry C., Xu D. Interleukin-33 exacerbates acute colitis via interleukin-4 in mice. Immunology. 2013;140:70–77. doi: 10.1111/imm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biancheri P., Di Sabatino A., Ammoscato F., Facciotti F., Caprioli F., Curciarello R., Hoque S.S., Ghanbari A., Joe-Njoku I., Giuffrida P., Rovedatti L., Geginat J., Corazza G.R., MacDonald T.T. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol. 2014;44:370–385. doi: 10.1002/eji.201343524. [DOI] [PubMed] [Google Scholar]
- 49.Reinisch W., Panes J., Khurana S., Toth G., Hua F., Comer G.M., Hinz M., Page K., O'Toole M., Moorehead T.M., Zhu H., Sun Y., Cataldi F. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut. 2015;64:894–900. doi: 10.1136/gutjnl-2014-308337. [DOI] [PubMed] [Google Scholar]
- 50.Schiering C., Krausgruber T., Chomka A., Frohlich A., Adelmann K., Wohlfert E.A., Pott J., Griseri T., Bollrath J., Hegazy A.N., Harrison O.J., Owens B.M., Lohning M., Belkaid Y., Fallon P.G., Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Eosinophil (EOS) frequency is dramatically increased in bone marrow of ileitis-prone SAMP1/YitFc (SAMP) mice with established disease. Bone marrow cells were isolated from 20-week-old SAMP and age-matched AKR/J (AKR) control mice, and analyzed by flow cytometry. Aggregates and dead cells were excluded on the basis of forward scatter (FSC)-A versus FSC-H and live/dead dye staining, respectively, and EOS were gated as CD11b+ side scatter (SSC)hi Gr-1neg Siglec-F+ cells. A: Representative plots displaying 5000 events. B: Percentages of EOS within the live cell gate in SAMP and AKR mice. ∗∗P < 0.01, ∗∗∗P < 0.001 versus age-matched AKR. N = 6 (B).
Eosinophil (EOS) depletion markedly reduces the severity of ileitis in SAMP1/YitFc (SAMP) mice with established disease. A: Histological evaluation of ilea from EOS-depleted 20-week-old SAMP versus IgG control-treated SAMP by analysis of four parameters composing total inflammatory score. B: Representative images of hematoxylin and eosin–stained ileal tissues. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus control (Cont.) and as indicated. N = 8 (A). Scale bar = 100 μm (B). Original magnification, ×20 + 1.25 (B).