Abstract
The learning disability fragile X syndrome results from the presence of >200 CGG/CCG repeats in exon 1 of the X-linked gene FMR1. Such alleles arise by expansion from maternally transmitted FMR1 premutation alleles, alleles having 55 to 200 repeats. Expansion risk is directly related to maternal repeat number. However, AGG interruptions to the repeat tract are important modifiers of expansion risk. Thus, the ability to identify such interruptions is crucial for the appropriate genetic counseling of females who are premutation carriers. First-generation triplet-primed PCR assays allow these interruptions to be detected. However, because the triplet primer used has multiple binding sites in the repeat tract, interpreting the results is not straightforward and it is not always possible to unambiguously determine the AGG-interruption status in females because of the difficulties associated with the presence of a second X chromosome. Interpretation is further complicated by any repeat size mosaicism that may be present. We have developed second-generation PCR assays that prime specifically at the interruptions. These assays are simpler to interpret and better able to evaluate this important determinant of expansion risk in females even in those with a mixture of premutation allele sizes.
Fragile X syndrome (Online Mendelian Inheritance in Man no. 300624), the most common heritable cause of intellectual disability and the most common monogenic cause of autism results from the presence of >200 CGG repeats in exon 1 of the X-linked gene FMR1 (MIM* 309550). Such alleles, known as full mutation alleles, arise from maternally transmitted premutation (PM) alleles that have 55 to 200 repeats. The longer the PM allele, the greater the risk of expansion. However, many alleles contain one or more periodically spaced AGG interruptions at the 5′ end of the repeat tract that significantly affect expansion risk.1, 2, 3, 4, 5 About 95% of normal FMR1 alleles have one or two such interruptions, most commonly located at the 10th or 11th triplet and the 20th or 21st triplet from the 5′ end of the repeat,6 whereas PM and full mutation alleles are more likely to lack interruptions.7 Normal alleles have approximately 30 repeats and thus have two interruptions at most, with 20 or fewer uninterrupted CGG repeats at the 3′ end of the repeat tract. Such alleles are stable. Unstable alleles have >54 total repeats, with a minimum of 34 uninterrupted repeats at the 3′ end of the repeat tract.1, 3, 6 Alleles with 55 to 59 repeats and no interruptions have a 19-fold higher risk of expansions than similarly sized alleles with two interruptions. Even alleles with 65 to 69 repeats and no interruptions have a 10-fold higher risk of expansion than alleles with two interruptions.3 Furthermore, how much larger the allele becomes on transmission also correlates with AGG status.3 Thus, the ability to accurately determine the number of AGG interruptions is important for the ascertainment of a woman's risk of having a child with fragile X syndrome.
Most current assays for AGG interruptions use a triplet-primed PCR reaction in which the 3′ end of one of the primers contains variable numbers of CCGs or GGCs.8, 9 As such the reactions effectively assay for the absence of priming in the region containing the interruption because the interruption reduces primer binding to that region. For samples with one or more interruptions this results in a PCR profile that consists of a series of peaks and valleys. However, the resultant profiles are not always easy to interpret. This is particularly problematic in females in whom analysis is complicated by the presence of the second X chromosome. This difficulty is exacerbated when the woman is mosaic for different PM allele sizes. An alternative assay we have described previously takes advantage of the fact that AGG interruptions in the CGG-repeat tract generate cleavage sites for the restriction enzyme EciI.9 This assay allows the number of uninterrupted repeats at the 3′ end of the PM repeat tract to readily be determined even in females. However, this assay has some features that we felt would limit its widespread use, including the requirement for additional reaction steps. We have now developed a pair of new assays for AGG interruptions that are able to reliably and accurately determine the number and position of AGG interruptions even in females with large PM alleles.
Materials and Methods
Reagents
All reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. A 2.2 mol/L stock solution of (NH4)2SO4 (catalog number A4418) and a 5 mol/L stock solution of betaine monohydrate (catalog number B2754) were each made up in water and the solutions filter sterilized. Primers were from Life Technologies (Grand Island, NY) and are listed in Table 1. The gBlock used to generate the internal PCR control described below was synthesized by Integrated DNA Technologies (Coralville, IA). Restriction enzymes, Q5 High-Fidelity DNA polymerase, Phusion DNA polymerase, and Gibson Assembly Master Mix were from New England Biolabs (Ipswich, MA). KAPA2G Robust HotStart DNA polymerase was purchased from Kapa Biosystems (Wilmington, MA).
Table 1.
Primers
| Primer | Sequence |
|---|---|
| Gb_FraxC | 5′-CTGGAGCAATTCCGGCGCGCCGCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3′ |
| Gb_TagtR4 | 5′-CTCGCCCTTGCTCACCATGGGAACATCCTTTACAAATGCCTTGTAGAAAGCGCCATTGGAGCCCCGCA-3′ |
| Not_FraxC | 5′-AGTTCAGCGGCCGCGCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3′ |
| Not_FraxR4 | 5′-CAAGTCGCGGCCGCCTTGTAGAAAGCGCCATTGGAGCCCCGCA-3′ |
| Fmr1_CCG5 | 5′-AGCGTCTACTGTCTCGGCACTTGCCCGCCGCCGCCGCCG-3′ |
| Fmr1_CGG5 | 5′-AGCGTCTACTGTCTCGGCACTTGCCGGCGGCGGCGGCGG-3′ |
| A-primer | 5′-AGCGTCTACTGTCTCGGCACTGTCGGCGGCGGA-3′ |
| T-primer | 5′-AGCGTCTACTGTCTCGGCACTTGCCCGCCGCCGCCT-3′ |
| Not_PsdR4 | 5′-CAAGTCGCGGCCGCAGCCGCGAGAAATGCCTCCTGCGCAATGT-3′ |
The sequence shown in bold is not homologous to the FMR1 sequence. The underlined sequence is also not homologous to the FMR1 sequence but is complementary to the sequence introduced into the positive control template.
DNA Templates
gDNAs
The genomic DNAs (gDNAs) used in this study are listed in Table 2. The HT-51A cell line is an induced pluripotent stem cell line derived in our laboratory.9 The SC120A induced pluripotent stem cell line is a subclone of the cell line obtained from Phil Schwartz (Children's Hospital of Orange County, CA). gDNA was prepared from these cell lines using standard procedures. DNA quantification was performed on a Denovix DS-11 spectrophotometer (Denovix, Wilmington, DE). The remaining patient samples were provided by Stephanie Sherman (Emory University, Atlanta, GA). The size of the repeat tract in each cell line was verified by PCR across the repeat tract as previously described.9 In preparation for PCR analysis, 600 ng of the gDNAs were digested overnight at 37°C in a 40-μL reaction mix containing 50 mmol/L Tris-HCl, pH 9.0, 1.75 mmol/L MgCl2, 22 mmol/L (NH4)2SO4, and 20 U HindIII-HF.
Table 2.
AGG Interruption Profiles in PM Carriers
| Sample | Sex | Normal allele | PM allele |
|---|---|---|---|
| SC120A | M | NA | 97† |
| HT51A | F | 9A9A9 | 9A9A78 |
| C0001∗ | F | 19A9 | 10A98 |
| C0075∗ | F | 9A9A9 | 85, 91† |
| C0076 | F | 10A9A10 | 10A9A68 |
| C0138 | F | 9A12A9 | 10A9A84 |
| C0223 | F | 9A20 | 10A9AX‡ |
| C0317 | F | 9A9A9 | 9A116 |
| C0342 | F | 9A9A9 | 133† |
| C0366 | F | 9A9A9 | 10A9A120 |
| C0386∗ | F | 10A9A9 | 10AX§ |
| C0648 | F | 7A9A9 | 11A54 |
| F0655 | F | 9A12A9 | 105; 112† |
With the exception of the samples indicated with an asterisk, the number of interruptions determined in our assays was consistent with previously published data or results obtained in other laboratories using previously published assays (Stephanie Sherman, personal communication, June 3, 2017).9 The samples marked with an asterisk are those in which the results of the A-primed assay differed from the previous assays. In all three cases the previous results had been flagged as being of low confidence or the number of interruptions could not be determined. The results for these three samples were verified by our previously published assay for AGG interruptions9 and by the complete sequencing of clones of the normal allele and the 5′ end of PM alleles.
F, female; M, male; NA, not applicable; PM, premutation.
No interruptions.
Individual mosaic for a number of PM alleles all ≥110 repeats, that is, X ≥ 89.
Individual mosaic for a number of PM alleles all ≥126 repeats, that is, X ≥ 115.
Generation of Molecular Weight Standards
HT51A gDNA was amplified using Q5 High Fidelity DNA polymerase using Gb_FraxC and Gb_TagtR4 primers and 23 cycles of denaturation, annealing, and extension. The resulting PCR products were cloned into an AscI- and NcoI-digested pGL3Basic-derived cloning vector using a Gibson assembly strategy.10 Clones containing the normal and PM alleles were sequence verified and were shown to have the same repeat number and AGG-interruption pattern as the normal and PM alleles in the gDNA from which they were derived, that is, 29 repeats and 98 repeats, respectively, with interruptions on both alleles at the 10th and 20th repeat. These clones have been submitted to Addgene (www.addgene.org; plasmids #99255 and #99256, respectively). With the use of the A-primed reaction a mixture of the two clones generated PCR products corresponding to 10, 20, 78, and 88 repeats. These products can be used to generate a standard curve that allows the repeat number and the position of interruptions to be more accurately determined than is possible using the standard commercially available molecular weight markers. However, it should be noted that because of a variety of intrinsic factors, the error in measurement of the position of the interruptions, particularly in the case of larger alleles, may be one repeat or more. For accurate position information, the A-primed assay should be used in conjunction with the T-primed assay for the analysis of female samples.
Generation of PseudoHT51, a Positive Control Template
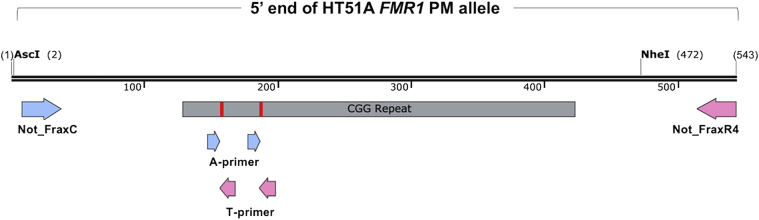
A control template for PCR, PseudoHT51, was generated from the plasmid clone containing the HT51A PM allele described above. A 469-bp region from the FMR1 gene, including 121 bases from the 5′ flank, the repeats, and 54 bases from the 3′-flank, was excised with AscI present in the vector backbone and NheI located just upstream of the Not_FraxR4 binding site (Figure 1). A synthetic gBlock having an NheI recognition site and a BamHI site flanking a binding site for Not_PsdR4, a scrambled version of Not_FraxR4, was digested with NheI and BamHI. The two fragments were cloned back into an empty version of the pGL3-basic–derived vector that had been digested with AscI and BamHI. The construct was verified by sequencing. This construct, like the FMR1 PM allele from which it was derived, has interruptions at the 10th and 20th triplet and 78 uninterrupted repeats at the 3′ end of the repeat tract (Figure 1). Apart from the Not_PsdR4 sequence, the FMR1 sequence of the control template was identical to the original allele. It thus amplified with similar efficiency. The replacement of the Not_FraxR4 sequence with that of Not_PsdR4 allowed it to be specifically amplified even in a mixture with gDNA. This construct has been deposited in Addgene (plasmid #99257).
Figure 1.
Map of the 5′ end of the FMR1 premutation (PM) allele in the HT51 cell line showing the location of the enzymes used for cloning and the primer binding sites used for the various PCR assays described. The positions of the two AGG interruptions present in this allele are indicated by red lines. The primer binding sites of the forward primers used in the new assays are indicated by blue arrows and the reverse primers by pink arrows.
PCR Assays
All of the PCR assays used reaction conditions that were described previously9 with minor modifications.9 Briefly, assays were performed in a final volume of 20 μL containing 75 ng of HindIII-digested gDNA and 0.75U KAPA2G Robust HotStart DNA polymerase in 1X AMP buffer (50 mmol/L Tris-HCl, pH 9.0, 1.75 mmol/L MgCl2, 22 mmol/L (NH4)2SO4, 2.5 mol/L betaine, 2% dimethyl sulfoxide, 0.2 mmol/L each dNTP). This was achieved by mixing 5 μL of the HindIII-digested gDNA solution with 15 μL of a solution containing 50 mmol/L Tris-HCl, pH 9.0, 1.75 mmol/L MgCl2, 22 mmol/L (NH4)2SO4, 3.3 mol/L betaine, 2.67% dimethyl sulfoxide, and 0.27 mmol/L of each dNTP. Care was taken to make sure that the solutions were properly mixed.
Repeat PCR assays for determining the repeat number used the Not_FraxC and Not_FraxR4 primers at a concentration of 0.5 μmol/L each. The PCR cycling conditions were denaturation at 98°C for 3 minutes, followed by 30 cycles of denaturation (98°C for 30 seconds), annealing (59°C for 30 seconds), and extension (72°C for 210 seconds), followed by a single incubation step at 72°C for 10 minutes.
First-generation triplet-primed assays used three primers, Not_FraxC and Not_FraxR4, and a third primer containing either (CGG)5 or (CCG)5 at its 3′ end for the CGG-primed and the CCG-primed assays, respectively. Not_FraxC and Not_Frax4 primers were present at a final concentration of 0.5 μmol/L each. For the CGG-primed assay the Not_FraxR4 primer was HEX-labeled, and the other primers were unlabeled. The repeat-containing primer, Fmr1_CGG5, was used at a final concentration of 0.1 μmol/L. For the CCG-primed assay Not_FraxC was FAM-labeled, and the other primers were unlabeled. The repeat-containing primer, Fmr1_ccg5, was used at a final concentration of 0.025 μmol/L. The PCR cycling conditions were the same as for the repeat PCR assay.
The A-primed assay used a HEX-labeled Not_FraxR4 and the A-primer both at a concentration of 0.5 μmol/L. The A-primer contains the sequence (CGG)3A at its 3′ end and primes specifically at Ts on the template. For reactions containing the positive control template, the NED-labeled Not_PsdR4 primer at a concentration of 0.5 μmol/L was also included along with 0.1 pg of the PseudoHT51 plasmid giving a copy number equivalent to the gDNA. FAM-labeled Not_FraxC primer and the T-primer were used for the T-primed assay both at a concentration of 0.5 μmol/L. The T-primer contains the sequence (CCG)3CCT at its 3′ end and thus primes specifically at As on the template. The optimized PCR cycling conditions for both assays were heating to 98°C for 3 minutes, followed by 30 cycles of incubation at 98°C for 30 seconds, 55°C for 30 seconds, and 72°C for 210 seconds, followed by a single incubation at 72°C for 10 minutes.
The PCR products were subjected to capillary electrophoresis by the DNA sequencing facility at the University of Illinois at Urbana-Champaign (Urbana, IL) using a ABI 3730 genetic analyzer calibrated with a DS-30 matrix standard and MapMarker 1000-ROX (Bioventures, Murfreesboro, TN). The resultant FSA files were displayed using a custom R script (Supplemental Codes S1 and S2). Instructions for use of the R script are provided in Supplemental Appendix S1. Because fragments containing large numbers of CGG repeats migrate aberrantly in capillary electrophoresis, the sizes of the PCR products were determined from a standard curve generated using the repeat-specific molecular weight standards described above and the custom R script.
Results
Development of an A-Primed Assay for AGG Detection
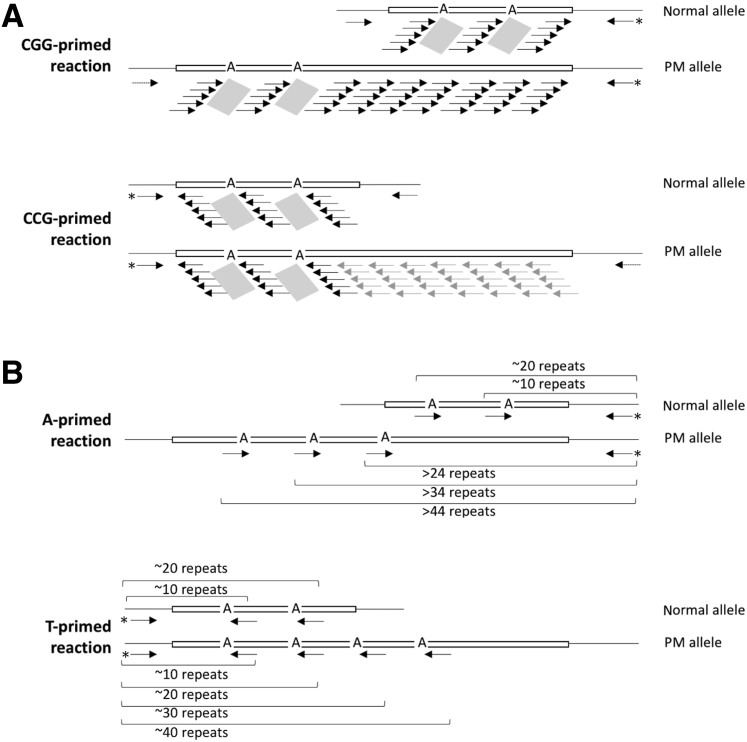
Previously described triplet-primed PCR assays for AGG interruptions in the FMR1 gene typically use a primer that has a small number of anchoring repeats, either CCG or GGC, at its 3′ end.8, 9, 11 The principle of these assays are illustrated in Figure 2A. In these assays the repeat-containing primers can prime at multiple places on the repeat tract but do not prime in the region containing the interruption. When used in conjunction with primers from the flanking region, a PCR profile was generated that allowed the AGG interruptions to be identified from dips in the PCR profile resulting from the failure of the triplet primer to prime. However, in the CGG-primed reaction this dip was not always easy to ascertain on PM alleles (Figure 3). The dips were easier to see in the CCG-primed reaction (Figure 3B). However, in females it was not possible to definitively identify which allele contained the interruptions because the products of amplification of the PM allele and the normal allele overlapped (Figure 2B). This was problematic because female PM carriers are the population for which this analysis is particularly relevant. We rationalized that a clearer result would be obtained by designing primers that directly targeted the AGG interruption with priming being limited to the interruptions themselves (Figure 2B). This would result in a unique peak for each interruption.
Figure 2.
Diagrammatic representation of the principle behind the different triplet-primed assays for AGG interruptions. The principle of the previously published assays (A) and the new generation of assays described here (B).8, 9 The arrows represent the binding sites for the primer used. Asterisks indicate the labeled primer for each reaction, Not_FraxR4 for the CGG-primed reaction and Not_FraxC for the CCG-primed reaction, respectively. The dotted arrow in A illustrates the second flanking primer used in these reactions, Not_FraxC and Not_FraxR4, respectively. Some of the primer binding sites in the CCG-primed assays are shown in gray because smaller PCR products are favored in these reactions; thus, the products resulting from priming at these sites are only a minor fraction of the PCR products produced. The gray parallelograms in A illustrate the location of the region of the repeat tract where priming does not occur and corresponds to the dips seen in the resulting electrophoretograms. PM, permutation.
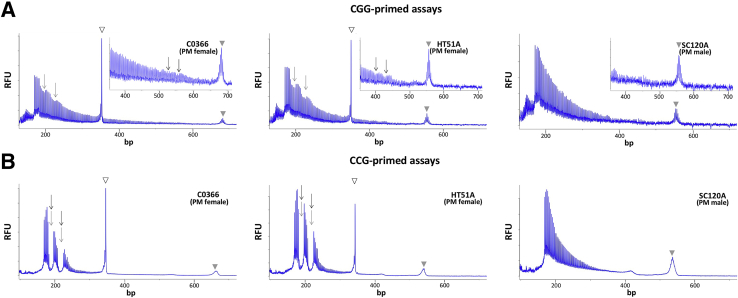
Figure 3.
CGG-primed (A) and CCG-primed (B) PCR assays for C0366, HT51A, and SC120A. PCR amplification of the three genomic templates was performed as described in the Materials and Methods, and the products were resolved by capillary electrophoresis. The insert shown in each electrophoretogram is an enlargement of the 300- to 700-bp region (A). The PCR products corresponding to the full-length normal allele in the permutation (PM) females are indicated by the open arrowhead and the location of full-length PM alleles by the closed arrowhead. The lack of PCR products resulting from failure to prime at interruptions on the PM alleles are shown by the black arrows and those on the normal allele by the gray arrows. RFU, relative fluorescent units.
We therefore designed a primer with a (CGG)3A sequence at the 3′ end and optimized conditions for performing PCR with this primer and the Not_FraxR4 primer. In principle, the output from this reaction would be analogous to that produced by the CGG-primed reaction except that the readout was drastically simplified with individual peaks each corresponding to a T on the template. Normal alleles with approximately 30 repeats and two interruptions would produce two PCR products, with fragment sizes consistent with approximately 10 and approximately 20 repeats (Figure 2B). In the most challenging situation in which a PM allele with 55 repeats and three interruptions was present (9-A-9-A-9-A-25), all of the PCR products from the PM allele would have >24 repeats and thus would migrate at a different location from the products produced from normal alleles with interruptions. PM alleles with 55 repeats but fewer interruptions would produce even larger PCR products, as would larger PM alleles. Gray zone or intermediate alleles with 45 repeats and two interruptions would produce PCR products corresponding to approximately 35 and 25 repeats, respectively. Thus, this assay should be able to distinguish between most combinations of normal and PM or intermediate alleles and should allow the number and location of each interruption to be readily and unambiguously identified.
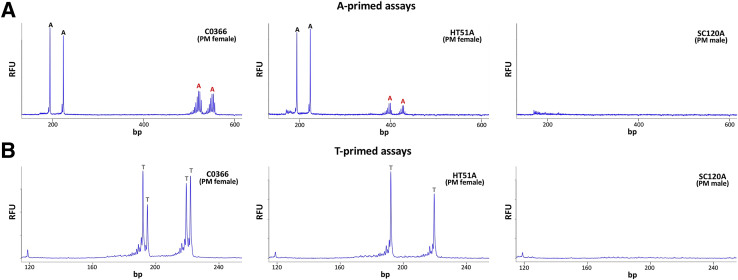
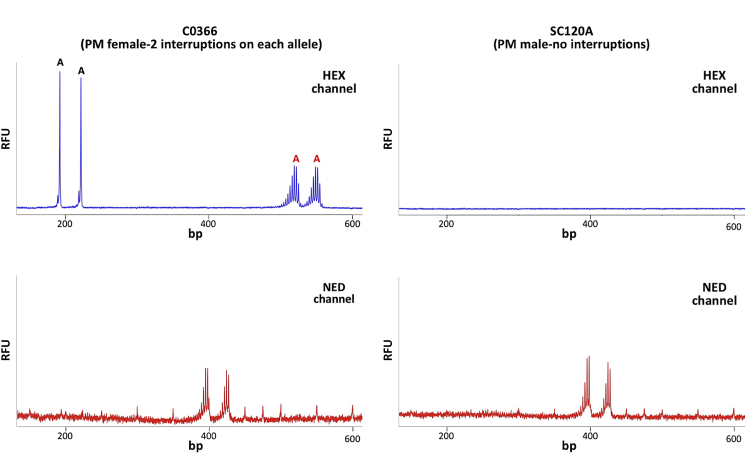
Initially, it was attempted to perform this assay using previously described reaction buffer and Phusion DNA polymerase.9 However, this generated output very similar to the CGG-primed PCR (Figure 3A). It was hypothesized that this was the result of the removal of the terminal A from the primer by Phusion's proof-reading activity. This would allow the primer to prime DNA synthesis from a much larger number of sites, thus swamping priming from the interruption itself. We therefore switched to using KAPA2G Robust HotStart DNA polymerase, a polymerase that lacked proof-reading ability. After modification of the original buffer to optimize the activity of KAPA2G Robust HotStart DNA polymerase, the use of the A-primer resulted in a PCR profile that was easy to interpret unambiguously (Figure 4A), producing four peaks in female samples that had two interruptions in each allele and no peaks in a male sample that did not contain AGG interruptions (SC120A).
Figure 4.
A-primed (A) and T-primed (B) PCR assays for C0366, HT51A, and SC120A. PCR amplification of the three genomic templates was performed as described in Materials and Methods using HEX-labeled Not_FraxR4 and the A-primer for the A-primed reaction and FAM-labeled Not_FraxC and the T-primer for the T-primed reactions. The products were then resolved by capillary electrophoresis. The products arising from priming at AGG interruptions are marked with the letter A (black font for normal alleles, red font for PM alleles). The positions of interruptions in the T-primed reaction are marked with the letter T. RFU, relative fluorescent units.
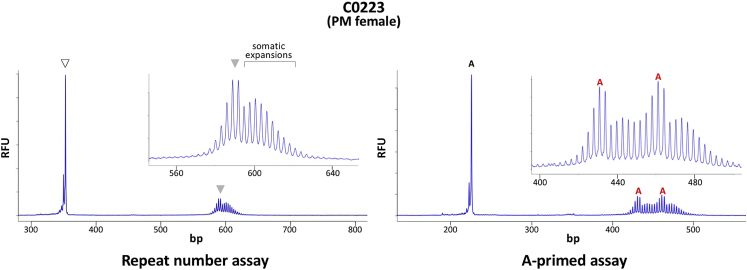
PM females with a heterogeneous mixture of allele sizes were among the most difficult cases to interpret with confidence. This was because the repeat heterogeneity further obscured the dip in the PCR profile diagnostic of interruptions in these assays. However, even in female PM carriers with significant evidence of somatic expansions, the interspersion pattern and AGG repeat number could be unambiguously determined with the A-primed assay (Figure 5).
Figure 5.
Repeat assay and A-primed assays for C0223, a premutation (PM) female with a heterogeneous mixture of allele sizes. PCR amplification of the genomic DNA (gDNA) from C0233 was performed as described in the Materials and Methods using HEX-labeled Not_FraxR4 and Not_FraxC for the repeat number assay and HEX-labeled Not_FraxR4 and the A-primer for the A-primed assay. The products were then resolved by capillary electrophoresis. The inset in each case shows an expanded version of the electrophoretogram. The products corresponding to the full-length alleles are indicated by the open (normal) and closed (PM) arrowheads. The products arising from priming at AGG interruptions are marked with the letter A (black font fornormal alleles, red for PM alleles). The bracket in the repeat number assay indicates the somatic expansions present in this individual. RFU, relative fluorescent units.
The interspersion patterns for an additional nine patient samples are shown in Table 2. In all but three cases, the numbers of AGGs identified were consistent with those identified in other laboratories using different assays (S. Sherman, personal communication, June 3, 2017). In the remaining cases, the original test results were either considered to be of low confidence or no results were obtained. However, our results for these cases were able to be verified using both previously published AGG-interruption assay9 and direct sequencing of individual plasmid clones.
Because large PM or full mutation alleles can be challenging to amplify,8, 9, 11 an internal PCR control template, PseudoHT51, was generated that can be added to samples before the PCR to reduce the likelihood that false negatives will be reported. As described in more detail in Materials and Methods, this template had a CGG-repeat tract with AGG interruptions at the 10th and 20th triplet and 78 uninterrupted CGG-repeats at the 3′ end of the repeat tract and differed only from the genomic sequence by the replacement of the Not_FraxR4 primer-binding site with a nonhomologous sequence with the same G+C content. Inclusion of a third primer, Not_PsdR4, that binds to this nonhomologous region resulted in PCR products that were specific for the control template. This primer was labeled with a fluorescent dye, in this case NED, that has a maximum emission at a wavelength distinct from the dye label on the Not_FraxR4 primer (HEX), allowing them to be distinguished on a suitably calibrated fragment analyzer. The products of PCR on the control template could thus be readily distinguished from the bona fide FMR1 products after capillary electrophoresis because they were detected in different channels (Figure 6). Because the sequence of the control and gDNA templates were identical except for the downstream primer binding regions and because the downstream primer binding regions have the same percentage of G+C, the efficiency of amplification for the control template and PM alleles of up to approximately 100 repeats should be comparable. Thus, the failure to detect a PCR product from the control template would indicate a PCR failure rather than the absence of interruptions.
Figure 6.
A-primed PCR assays for C0366 and SC120A in the presence of the internal PCR control template. PCR amplification of the genomic templates was performed in the presence of an equal molar amount of the internal control fragment as described in Materials and Methods using HEX-labeled Not_FraxR4, NED-labeled Not_PsdR4, and the unlabeled A-primer. The products were then resolved by capillary electrophoresis. The top panels show the reaction products detected in the HEX channel [genomic DNA (gDNA) products] and the bottom panels show the reaction products detected in the NED channel (the internal control products). The products arising from priming at AGG interruptions are marked with the letter A (black font for normal alleles, red for PM alleles). Note that the minor peaks seen in the NED channel are bleed-through/pull-up peaks from the ROX channel (containing the MapMarker 1000-ROX ladder) and thus indicate the positions of the individual fragments in the molecular weight ladder. PM, permutation; RFU, relative fluorescent units.
Development of a T-Primed Assay for AGG Detection
The A-primed assay has the advantage of allowing interruptions in the normal allele to be readily distinguished from interruptions in the PM or full mutation allele in females. However, because large repeat tracts are challenging to amplify, an assay was needed analogous to the CCG-primed assays (Figure 2A), but one that detected the A specifically (Figure 2B). Using a primer with a CCT triplet at the 3′ end and a primer from the upstream region of exon 1 also resulted in a PCR profile that was much easier to interpret than the corresponding CCG-primed assay (Figures 3B and 4B). Because this assay involved the amplification across fewer repeats than in the case of the A-primed assay, the reaction was more robust. Thus, this assay would be particularly useful for the analysis of AGG interruptions in males in whom there is no second X chromosome to complicate data interpretation. However, this assay can also be used to analyze some female samples. For example, the presence of four peaks in the C0366 sample indicated that both alleles have two interruptions and that the position of these interruptions differed between the two alleles (Figure 4B). The presence of only two peaks for the HT51A sample indicated that either both alleles have two interruptions at the same position in repeat tract or that only one allele was interrupted. However, because four peaks were present in the A-primed reaction, the first interpretation must be the correct one. In addition, the data indicated that the normal allele of C0366 had the same interruption pattern as HT51A (9-A-9-A-N) (Figure 4A). To account for the peaks observed, a 10-A-9-A-N interruption pattern could be inferred for the PM allele.
Discussion
Both the A-primed and T-primed assays that are described here result in a greatly simplified readout of the AGG-interruption status compared with the previous generation of triplet-primed PCR assays. This not only makes data interpretation much simpler but also increases the number of samples for which an unambiguous determination of the number and position of AGG interruptions on each allele can be obtained. The downside to these assays is that because they only use one flanking primer they do not provide information about the total number of repeats in the repeat tract. However, for interrupted alleles a reasonable approximation of the total repeat number can easily be deduced from the size of the constituent PCR products in the A-primed reaction. Samples showing no interruptions could then be reflexed to one of our previously described reactions that allow the total number of repeats to be accurately determined.9 An alternative screening algorithm may be more cost-effective for large-scale efforts to identify women at risk of having a child with fragile X syndrome. In this case, it may be better to use PCR-based assays for repeat number coupled with agarose gel electrophoresis to identify women with PM alleles. These women could then be screened using the A-primed assay.
Footnotes
Supported by NIH, National Institute of Diabetes, Digestive, and Kidney Diseases, Intramural program grant DK057808 (K.U.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.jmoldx.2017.06.008.
Supplemental Data
References
- 1.Nolin S.L., Glicksman A., Ersalesi N., Dobkin C., Brown W.T., Cao R., Blatt E., Sah S., Latham G.J., Hadd A.G. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet Med. 2015;17:358–364. doi: 10.1038/gim.2014.106. [DOI] [PubMed] [Google Scholar]
- 2.Latham G.J., Coppinger J., Hadd A.G., Nolin S.L. The role of AGG interruptions in fragile X repeat expansions: a twenty-year perspective. Front Genet. 2014;5:244. doi: 10.3389/fgene.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolin S.L., Sah S., Glicksman A., Sherman S.L., Allen E., Berry-Kravis E., Tassone F., Yrigollen C., Cronister A., Jodah M., Ersalesi N., Dobkin C., Brown W.T., Shroff R., Latham G.J., Hadd A.G. Fragile X AGG analysis provides new risk predictions for 45-69 repeat alleles. Am J Med Genet A. 2013;161A:771–778. doi: 10.1002/ajmg.a.35833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yrigollen C.M., Martorell L., Durbin-Johnson B., Naudo M., Genoves J., Murgia A., Polli R., Zhou L., Barbouth D., Rupchock A., Finucane B., Latham G.J., Hadd A., Berry-Kravis E., Tassone F. AGG interruptions and maternal age affect FMR1 CGG repeat allele stability during transmission. J Neurodev Disord. 2014;6:24. doi: 10.1186/1866-1955-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yrigollen C.M., Durbin-Johnson B., Gane L., Nelson D.L., Hagerman R., Hagerman P.J., Tassone F. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet Med. 2012;14:729–736. doi: 10.1038/gim.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichler E.E., Holden J.J., Popovich B.W., Reiss A.L., Snow K., Thibodeau S.N., Richards C.S., Ward P.A., Nelson D.L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994;8:88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- 7.Falik-Zaccai T.C., Shachak E., Yalon M., Lis Z., Borochowitz Z., Macpherson J.N., Nelson D.L., Eichler E.E. Predisposition to the fragile X syndrome in Jews of Tunisian descent is due to the absence of AGG interruptions on a rare Mediterranean haplotype. Am J Hum Genet. 1997;60:103–112. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Hadd A., Sah S., Filipovic-Sadic S., Krosting J., Sekinger E., Pan R., Hagerman P.J., Stenzel T.T., Tassone F., Latham G.J. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J Mol Diagn. 2010;12:589–600. doi: 10.2353/jmoldx.2010.090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayward B.E., Zhou Y.F., Kumari D., Usdin K. A set of assays for the comprehensive analysis of FMR1 alleles in the Fragile X-related disorders. J Mol Diagn. 2016;18:762–774. doi: 10.1016/j.jmoldx.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson D.G., Smith H.O., Hutchison C.A., III, Venter J.C., Merryman C. Chemical synthesis of the mouse mitochondrial genome. Nat Methods. 2010;7:901–903. doi: 10.1038/nmeth.1515. [DOI] [PubMed] [Google Scholar]
- 11.Tassone F., Pan R., Amiri K., Taylor A.K., Hagerman P.J. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.