Abstract
Species within the scleractinian genus Pocillopora Lamarck 1816 exhibit extreme phenotypic plasticity, making identification based on morphology difficult. However, the mitochondrial open reading frame (mtORF) marker provides a useful genetic tool for identification of most species in this genus, with a notable exception of P. eydouxi and P. meandrina. Based on recent genomic work, we present a quick and simple, gel-based restriction fragment length polymorphism (RFLP) method for the identification of all six Pocillopora species occurring in Hawai‘i by amplifying either the mtORF region, a newly discovered histone region, or both, and then using the restriction enzymes targeting diagnostic sequences we unambiguously identify each species. Using this approach, we documented frequent misidentification of Pocillopora species based on colony morphology. We found that P. acuta colonies are frequently mistakenly identified as P. damicornis in Kāne‘ohe Bay, O‘ahu. We also found that P. meandrina likely has a northern range limit in the Northwest Hawaiian Islands, above which P. ligulata was regularly mistaken for P. meandrina.
Keywords: Species distribution, Scleractinia, Phenotypic polymorphism, Kāne’ohe Bay, Northwest Hawaiian Islands
Introduction
Species in the scleractinian genus Pocillopora Lamarck 1816 are known to exhibit extreme phenotypic plasticity (Pinzón et al., 2013; Marti-Puig et al., 2014; Paz-García et al., 2015a; Paz-García et al., 2015b; Gélin et al., 2017), making identification in the field difficult, particularly when colonies are small. For example, individuals displaying the classic P. meandrina Dana 1846 morphology in the Society Islands were recently targeted for a population genetic study; however, genetic sequencing of the widely used mitochondrial open reading frame (mtORF) marker (Flot et al., 2008) revealed the presence of six different genetic lineages (Edmunds et al., 2016).
While the mtORF marker (Flot et al., 2008) has been used to delineate up to 16 putative species of Pocillopora (Gélin et al., 2017), this marker does not delineate all species. For example, P. meandrina and P. eydouxi Milne Edwards 1860 share an identical sequence at this locus (mtORF type 1 (Pinzón et al., 2013)) even though genomic data have recently revealed that these lineages are distinct, and valid species (Johnston et al., 2017). Additionally, Schmidt-Roach et al. (2014a) found that P. eydouxi colonies have a styloid columella in Eastern Australia, whereas P. meandrina colonies present mostly convex, oval columellas.
Based on recent genomic work (Johnston et al., 2017), we hypothesized that we could develop a molecular test to delineate Hawaiian Pocillopora species. Here, we present a fast, simple, and inexpensive assay based on restriction fragment length polymorphism (RFLP) of PCR amplicons that unambiguously differentiates all six Pocillopora species found in Hawai‘i. We hypothesized that we could use this molecular assay to show that many species are currently misidentified with traditional gross colony morphology-based approaches using two examples. First, we examined the distribution of P. meandrina and P. ligulata Dana 1846 across the Hawaiian Archipelago, as we hypothesized that the latter, less commonly documented species might often be mistaken as the former. Second, we described the distribution of P. acuta Lamarck 1816 and P. damicornis (Linnaeus 1758) in Kāne‘ohe Bay, O‘ahu. Based on recent work by Schmidt-Roach et al. (2013), we hypothesized that the majority of the colonies previously referred to as P. damicornis in Kāne‘ohe Bay (e.g., Mayfield et al., 2010; Gorospe & Karl, 2013; Putnam & Gates, 2015) were actually P. acuta.
Materials and Methods
Restriction length polymorphism assays
To differentiate individuals of mtORF type 1 (i.e., Pocillopora eydouxi and P. meandrina; 978 bp) from all other species of Pocillopora (species names following Schmidt-Roach et al. (2014a) and mtORF types following Pinzón et al. (2013)), we aligned 50 mtORF sequences taken from Flot et al. (2008), Pinzón et al. (2013) and Johnston et al. (2017), spanning a wide geographic range, using Geneious Alignment in GENEIOUS 6.1.8. In this alignment, we identified a SNP (adenine; 676 bp) fixed for all individuals of mtORF type 1. We then amplified the mtORF region using the FatP6.1 (5′-TTTGGGSATTCGTTTAGCAG-3′) and RORF (5′-SCCAATATGTTAAACASCATGTCA-3′) primers of Flot et al. (2008), and digested the PCR product with the SacI restriction enzyme (Table 1). PCR mixes contained 7.5 μL of BioMix (Bioline Ltd., London, UK), 0.195 μL of each forward and reverse primer (10 μM), 0.14 μL of BSA, 1.5 μL of template DNA (5–50 ng; extracted from coral biopsies using the Omega Bio-Tek EZ 96 Tissue DNA extraction kit; Omega Bio-Tek, Norcross, GA, USA), and 5.47 μL of deionized water to 15 μL final volume. Each PCR followed the cycling protocol of Flot et al. (2008), with a denaturation step of 60 s at 94 °C, followed by 40 cycles of 30 s at 94 °C, 30 s at 53 °C, and 75 s at 72 °C; thermocycling was followed by an incubation at 72 °C for 5 min. PCR products were then digested with 0.5 μL of SacI restriction enzyme (New England BioLabs, Ipswich, MA, USA) and 0.5 μL of the 10X NEBuffer 1.1 for 1 hr at 37 °C, followed by heat inactivation at 65 °C for 20 min (Table 1). Three microliters of the digested products were run on a 2% TAE-agarose gel for 1.5 hr at 70 V.
Table 1. Summary of amplicon, restriction enzyme, restriction site, and respective fragment sizes (bp, base pairs) differentiating Hawaiian Pocillopora species.
| Species | Amplicon | Restriction enzyme site | Fragment sizes (bp) | Amplicon | Restriction enzyme site | Fragment sizes (bp) | Cuts other species? Y/N (Fragment sizes bp) |
|---|---|---|---|---|---|---|---|
| P. eydouxi | (1) mtORF | SacI GAGCT’C C’TCGAG | 298, 680 | (2) PocHistone | XhoI C’TCGAG GAGCT’C | 287, 382 | N |
| P. meandrina | (1) mtORF | SacI GAGCT’C C’TCGAG | 298, 680 | (2) PocHistone | XhoI C’TCGAG GAGCT’C | 669 | N |
| P. verrucosa | (1) mtORF | AciI C’CGC GGC’G | 209, 338, 431 | Y, all species (430, 548) | |||
| P. ligulata | (1) mtORF | AlwNI CAGNNN’CTG GTC’NNNCAG | 467, 511 | Y, mtORF 11 (467, 511) | |||
| P. acuta | (1) mtORF | NlaIV GGN’NCC CCN’NGG | 30, 171, 315, 462 | (2) mtORF | Tsp45I ‘GTSAC CASTG’ | 978 | Y, NlaIV: mtORF 1, 2, 6, 8 (201, 315, 462) Y, NlaIV: mtORF 3i (194, 784) Y, NlaIV: mtORF 3 (516, 462) |
| P. damicornis | (1) mtORF | NlaIV GGN’NCC CCN’NGG | 30, 171, 315, 462 | (2) mtORF | Tsp45I ‘GTSAC CASTG’ | 530, 448 | Y, NlaIV: mtORF 1, 2, 6, 8 (201, 315, 462) Y, NlaIV: mtORF 3i (194, 784) Y, NlaIV: mtORF 3 (516, 462) Y, Tsp451: mtORF 3f (204, 774) |
To then differentiate P. eydouxi from P. meandrina, we aligned 27 total sequences of P. damicornis, P. acuta, P. verrucosa, P. meandrina, P. eydouxi, Pocillopora sp. B, P. ligulata, and mtORF type 11 using Geneious Alignment in GENEIOUS 6.1.8 for the histone 3 region (669 bp) discovered in Johnston et al. (2017). The first half of the gene was identified as an open reading frame of unknown function and contains the SNP (thymine; 279 bp) fixed for P. eydouxi, which falls in the second position of the amino acid leucine; all other Pocillopora examined in this study for this region have guanine at this position, resulting in the amino acid arginine. The latter half of the region (337–659 bp) mapped to partial histone 3 genes from cnidarians Plesiastrea versipora (accession number: HQ203519; Huang et al., 2011) and Nematostella vectensis (accession number: XM_001633243; Putnam et al., 2007). DNA from individuals of mtORF type 1 (P. meandrina and P. eydouxi) were amplified using novel primers for this histone 3 region (PocHistoneF: 5′-ATTCAGTCTCACTCACTCACTCAC-3′ and PocHistoneR: 5′-TATCTTCGAACAGACCCACCAAAT-3′; accession numbers: MG587096–MG587097) (Table 1). PCR mixes were prepared as described above, and the following thermocycling protocol was used: denaturation for 60 s at 94 °C, followed by 40 cycles of 30 s at 94 °C, 30 s at 53 °C, and 60 s at 72 °C, with a final elongation step of 5 min at 72 °C. PCR products were digested with 0.5 μL of XhoI restriction enzyme (New England BioLabs, Ipswich, MA, USA) and 0.5 μL of 1X CutSmart® buffer for 1 hr at 37 °C, followed by heat inactivation at 65 °C for 20 min (Table 1). Three microliters of the digested products were run on a 2% TAE-agarose gel for 1.5 h at 70 V.
Pocillopora verrucosa (Ellis and Solander 1786) (mtORF types 3a, 3b, 3c, 3d, 3e, 3f, 3g, 3h, 3i, 3j; Pinzón et al., 2013) was differentiated from all other Pocillopora species using the mtORF alignment described above, in which P. verrucosa was found to have two fixed SNPs (cytosine and guanine; 210–11 bp), and all Pocillopora species share this same restriction site at 547 bp. The mtORF region (Flot et al., 2008) was amplified and digested using 0.5 μL of the AciI restriction enzyme (New England BioLabs, Ipswich, MA, USA) and 0.5 μL of 1X CutSmart® buffer for 1 hr at 37 °C, followed by heat inactivation at 65 °C for 20 min (Table 1). Three microliters of the digested products were run on a 2% TAE gel for 1.5 h at 70 V.
Pocillopora ligulata was differentiated from all other Pocillopora species using the mtORF alignment described above. In the mtORF alignment, P. ligulata and mtORF type 11 (a haplotype which thus far has only been found in the Society Islands (Forsman et al., 2013; Gélin et al., 2017)) were both found to have a fixed SNP (cytosine; 462 bp). The mtORF region (Flot et al., 2008) was amplified and digested using 0.5 μL of the AlwNI restriction enzyme (New England BioLabs, Ipswich, MA, USA) and 0.5 μL of 1X CutSmart® buffer for 1 hr at 37 °C, followed by heat inactivation at 80 °C for 20 min (Table 1). Three microliters of the digested products were run on a 2% TAE-agarose gel for 1.5 hrs at 70 V.
Pocillopora damicornis and P. acuta (mtORF type 4 and 5, respectively) were differentiated from all other species of Pocillopora using the mtORF alignment described above, in which both species were found to share a restriction site (GGN’NCC; 314, 344, and 515 bp) at three locations, while P. meandrina, P. eydouxi, P. ligulata, and mtORF type 8 share this same restriction site at two locations (314 and 515 bp). The mtORF region (Flot et al., 2008) was amplified and the PCR product digested with 0.5 μL of the NlaIV restriction enzyme (New England BioLabs, Ipswich, MA, USA) and 0.5 μL of 1X CutSmart® buffer for 1 hr at 37 °C, followed by heat inactivation at 65 °C for 20 min (Table 1). Three microliters of the digested products were run on a 2% TAE-agarose gel for 1.5 hrs at 70 V.
In the mtORF alignment described above, P. damicornis was found to have a fixed SNP (cytosine; 534 bp), while all other Pocillopora have adenine in this position. Thomas et al. (2016) used this same SNP to differentiate P. damicornis from all other Pocillopora in Western Australia in their fluorescence-based quantitative real-time PCR (qPCR) assay. To differentiate P. damicornis from P. acuta, DNA from P. damicornis and P. acuta were amplified again with the primers of Flot et al. (2008) and digested with 0.5 μL of the Tsp45I restriction enzyme (New England BioLabs, Ipswich, MA, USA) and 0.5 μL of 1X CutSmart® buffer for 1 hr at 65 °C (Table 1). Three microliters of the digested products were run on a 2% TAE-agarose gel for 1.5 hrs at 70 V.
Pocillopora distribution patterns in Hawai‘i
Pocillopora samples (<10 cm) were collected from colonies displaying P. meandrina morphology (with a subset of colonies verified by J Maragos or P Jokiel) across the Hawaiian Islands haphazardly at depths of 2–20 m on either NOAA research cruises or on SCUBA from shore dives between 2005 and 2016 (Hawai‘i Island, 104; Mau‘i, 59; Lana‘i, 15; Moloka‘i, 10; O‘ahu, 189; Kaua‘i, 16; Ni‘ihau, 26; Nihoa, 32; Kānemiloha‘i (French Frigate Shoals), 44; Pūhāhonu (Gardner Pinnacles), 28; Nalukākala (Maro Reef), 10; Kauō (Laysan Island), 35; Holoikauaua (Pearl and Hermes Atoll), 47; Pihemanu (Midway Atoll), 52; Moku Pāpapa (Kure Atoll), 24). All tissue samples were stored in either salt-saturated DMSO (dimethyl sulfoxide) buffer (Gaither et al., 2011) or >95% ethanol until DNA was extracted. Genomic DNA was extracted from tissues using the Omega E-Z 96 Tissue DNA Kit (Omega Bio-Tek, Norcross, GA, USA). PCRs were prepared as described above and PCR products were first digested with the SacI restriction enzyme. Individuals of mtORF type 1, i.e., either P. eydouxi or P. meandrina, were then amplified using the PocHistone marker and digested with the XhoI restriction enzyme to differentiate P. eydouxi from P. meandrina. Those samples that were neither P. meandrina nor P. eydouxi were then digested with the AlwNI and restriction enzyme to determine if they were P. ligulata. And finally, samples that were not identified as P. meandrina, P. eydouxi, or P. ligulata were digested with the AciI restriction enzyme to determine if they were P. verrucosa.
Twenty-five colonies, varying in length from 5–30 cm, and displaying the full range of P. acuta/P. damicornis morphology (Schmidt-Roach et al., 2014a), were sampled from a 100 m2 area in July 2017 from Kāne‘ohe Bay, O‘ahu (21.456449, −157.794413), at a depth of 4 m, under HIMB special activity permit 2018-3, to determine the relative abundance of these species in the Bay. All tissue samples were stored in salt-saturated DMSO, DNA was extracted as described above, PCRs were prepared as described above, and PCR products were first digested with the NlaIV restriction enzyme to ensure that all samples were either P. acuta or P. damicornis. New PCRs were carried out and the amplicons were digested with the Tsp45I restriction enzyme to differentiate between the two species.
Results
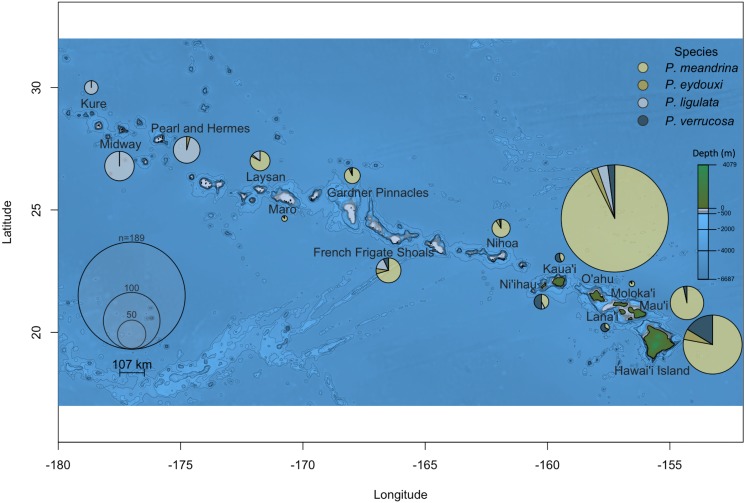
Of the 691 samples displaying classical P. meandrina morphology collected across the Hawaiian Islands, one-third (222 samples) were not P. meandrina. Two striking and previously unknown patterns of Pocillopora distribution stood out. The first was in the Main Hawaiian Islands. Despite the low number of samples collected from Lana‘i, Kaua‘i, and Ni‘ihau, more were P. verrucosa than any other Pocillopora species (10/15 on Lana‘i, 9/16 on Kaua‘i, and 13/26 on Ni‘ihau; Fig. 1). The second surprising pattern was discovered in the Northwest Hawaiian Islands, where none of the 123 presumed P. meandrina samples collected from the three most northerly islands (Pearl and Hermes, Midway, and Kure) were correctly identified; nearly all were P. ligulata, though two collected from Pearl and Hermes were P. eydouxi (Fig. 1).
Figure 1. Pocillopora species composition across the Hawaiian Islands for samples collected from colonies demonstrating P. meandrina morphology.
The size of the pie chart is proportional to the number of individuals sampled per island. Pocillopora species are represented by different colors, specifically: P. meandrina, light yellow; P. eydouxi, dark yellow; P. ligulata, light blue; and P. verrucosa, dark blue.
Previously, all fine branched Pocillopora species in Hawai‘i were identified as P. damicornis, but the presence of P. acuta was also recently confirmed (Schmidt-Roach et al., 2014b; Johnston et al., 2017). This discovery prompted us to look at whether both species were present in Hawai‘i, and if so, what the relative frequency of each in Kāne‘ohe Bay was, the most studied location in the Hawaiian Islands. Of the 25 samples collected from a 100 m2 area in Kāne‘ohe Bay, O‘ahu, 24 were P. acuta and one was P. damicornis, indicating that both species are present, but at very different relative abundances, at least from the wave-exposed, barrier reef site from which these samples were collected.
Discussion
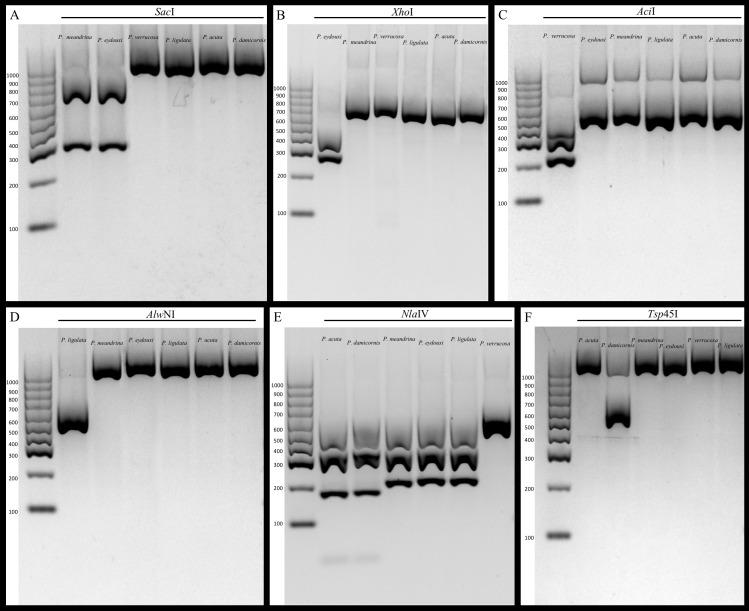
We demonstrate that our assay works to identify all the currently known species of Pocillopora in the Hawaiian Archipelago, and expect that it will be equally useful for other locations throughout the Pacific. However, additional testing by labs in other locations will be needed to confirm reliability among different locations, and some regional modifications are undoubtedly required. For example, the same restriction enzyme used for P. ligulata (AlwNI) in Hawai‘i can be used for haplotype 11 (Forsman et al., 2013) in French Polynesia because P. ligulata does not occur there, but shares the same identifying cut site as haplotype 11 from Moorea (Edmunds et al., 2016). Likewise, Thomas et al. (2016) developed a fluorescence-based quantitative real-time PCR assay that distinguishes P. damicornis from all other Pocillopora species in Western Australia. Our RFLP assay relies on the same SNP to distinguish P. damicornis from P. acuta indicating that replicability is likely inherent in our assay. In contrast, Torda et al. (2013) previously published a RFLP assay that distinguishes P. damicornis and P. acuta from all other Pocillopora in Australia using the putative control region. Despite a concerted effort, these primers failed to amplify our samples, however; this may be due to the fact that we did not purchase the proprietary Qiagen Multiplex PCR Kit used by Torda et al. (2013) in their protocol, or it may be due to regional differences in the sequences selected to differentiate the species that limit the utility of primers. Whatever the reason, our gel-based RFLP approach provides a cost-effective assay without proprietary reagents that quickly allows discrimination amongst Pocillopora species using simple PCR amplification followed by digestion with widely available restriction enzymes (Fig. 2). The sequencing of 691 samples at $3.50 in both directions for two genes would cost approximately $10,000, whereas the cost of all enzymes and reagents used herein was only $500; this represents a cost savings of nearly 95%.
Figure 2. Gel images of amplicons digested with restriction enzymes.
(A) mtORF amplification and digestion with SacI restriction enzyme differentiates mtORF type 1 (P. meandrina and P. eydouxi) from all other Pocillopora. (B) PocHistone amplification and digestion with XhoI restriction enzyme differentiates P. eydouxi from all other Pocillopora. (C) mtORF amplification and digestion with the AciI restriction enzyme differentiates P. verrucosa from all other Pocillopora. (D) mtORF amplification and digestion with the AlwNI restriction enzyme differentiates P. ligulata from all other Pocillopora. (E) mtORF amplification and digestion with the NlaIV restriction enzyme differentiates P. acuta and P. damicornis from all other Pocillopora. (F) mtORF amplification and digestion with the Tsp45I restriction enzyme differentiates P. damicornis from all other Pocillopora.
The occurrence of P. ligulata, and complete absence of P. meandrina, from the three most northern Northwest Hawaiian Islands is striking. Very little is known about P. ligulata. This species is described as having branches with flattened ends and truncated tips that irregularly radiate; the verrucae are widely spaced and irregular (Veron & Stafford-Smith, 2000; Fig. 3). In contrast, P. meandrina has flattened branches that centrally radiate, with neat and uniform verrucae (Veron & Stafford-Smith, 2000; Fig. 3). Genetic surveys to date document P. ligulata only in the Hawaiian Islands (Flot et al., 2008; Forsman et al., 2013; Marti-Puig et al., 2014; Gélin et al., 2017), and its distribution, ecology, and reproductive biology is entirely unknown. Pocillopora meandrina is a widespread species with a distribution spanning from the Eastern Pacific to the east coast of Africa (Flot et al., 2008; Forsman et al., 2013; Schmidt-Roach et al., 2014b; Paz-García et al., 2015a; Gélin et al., 2017). It is hermaphroditic and its reproductive behavior has been characterized in Hawai‘i (Cox, Krupp & Jokiel, 1998) and Australia (Schmidt-Roach et al., 2012), though little is known about its ecology. The Northwest Hawaiian Islands are subtropical and contain the northernmost coral atolls in the world. Colonies displaying the typical P. meandrina morphology from the three most northern atolls (Pearl and Hermes, Midway, and Kure) turned out not to include any individuals of this species. Instead, collections from the three northernmost atolls were dominated by P. ligulata. Although little is known about the ecology of either P. ligulata or P. meandrina, based on these data we hypothesize that P. meandrina may have a northern range limit to the south of Pearl and Hermes, while P. ligulata may be better adapted to the northern edges of the subtropics. Future studies that sample corals across a range of habitat types (e.g., depths, reef types, temperatures, etc.) may reveal that these species are significantly associated with different habitat types (as shown by Mayfield et al., 2015; Mayfield, Chen & Dempsey, 2017a; Mayfield, Chen & Dempsey, 2017b), but we lacked the environmental data from which individual colonies were sampled to perform an equivalent analysis here.
Figure 3. Images of Pocillopora ligulata colonies, (A)–(E); P. meandrina colonies, (F)–(J); and P. eydouxi colonies, (K)–(O) from O‘ahu, Hawai‘i.
The extreme phenotypic plasticity exhibited by P. damicornis and P. acuta has obscured the understanding of the distribution, ecology, and reproductive biology of these two species until very recently (Schmidt-Roach et al., 2013; Schmidt-Roach et al., 2014b). They are often found in the same environments (albeit with differing abundance by depth and exposure (Mayfield et al., 2015; Mayfield, Chen & Dempsey, 2017a; Mayfield, Chen & Dempsey, 2017b)), typically more sheltered locations such as lagoons and back reefs, but can also be found in more exposed environments (Schmidt-Roach et al., 2013; Schmidt-Roach et al., 2014a). These species generally share a sympatric distribution from Hawai‘i to the Indian Ocean, however P. acuta extends its range into the Arabian Gulf (Pinzón et al., 2013). Most studies to date have unknowingly lumped the two species, but both P. damicornis and P. acuta are hermaphroditic and colonies have been documented to both brood and spawn (Richmond & Jokiel, 1984; Jokiel, Ito & Liu, 1985; Ward, 1995; Permatav & Kinzie, 2000; Fan et al., 2002; Combosch & Vollmer, 2013; Massé et al., 2013; Schmidt-Roach et al., 2014b).
Although only P. damicornis was previously known from Hawai‘i, there are two well-characterized ecomorphs, types Y and B, previously documented from Kāne‘ohe Bay. Type B is darker brown, with fine branch tips that are white in color, and releases small planulae on the full moon, whereas Y is stouter, more yellow in color with even pigmentation, and produces larger planulae released on the new moon (Jokiel, Ito & Liu, 1985). Both types were historically common and co-occurred on the reef flats of Kāne‘ohe Bay (Richmond & Jokiel, 1984), although there is nearly continuous variation from one extreme to the other, making identification of intermediate morphologies extremely difficult (PL Jokiel & RH Richmond, pers. comm., 2013). Further, type Y was essentially wiped out during the freshwater kill event in 1988 (Jokiel et al., 1993; Bahr, Jokiel & Toonen, 2015) and has not recovered in the bay since that time (P Jokiel, pers. comm., 2013). Based on the descriptions of the Y and B types, it is not clear if they correspond to P. damicornis and P. acuta, or rather stout vs fine branch morphology that both species appear able to exhibit. Regardless, the frequent misidentification of these species is almost certain to have obscured differences in habitat preference and reproduction likely to exist between them (e.g., Mayfield et al., 2015; Mayfield, Chen & Dempsey, 2017a; Mayfield, Chen & Dempsey, 2017b). The assay developed here will be useful for answering fundamental questions regarding reproductive isolation and habitat differentiation of these two species that are very recently diverged (less than a million years) and frequently lumped in previous studies (Thomas et al., 2014; Johnston et al., 2017). Insofar as our site is representative of wave dominated, barrier reef ecosystems in Kāne‘ohe Bay, our findings indicate that P. acuta is currently far more prevalent than P. damicornis (Fig. 4). Now that it is possible to positively identify the closely related species in this genus using our genetic assay, it will be interesting to determine their habitat preferences and distribution in Hawai‘i, and whether the relative abundance of the species changes over time or space.
Figure 4. Images of the 25 colonies of Pocillopora acuta and P. damicornis collected from Kāne‘ohe Bay, O‘ahu.
Colony L is P. damicornis, all other colonies are P. acuta.
Conclusions
Here, we present an assay that allows rapid and unambiguous identification of all six species of Pocillopora present in Hawai‘i, which we hope will work anywhere these species are found. We present two cases where samples identified morphologically were misidentified to highlight the utility of this approach. Taxonomic confusion can impact a wide range of studies and the ability to rapidly and cost-effectively distinguish among species of Pocillopora will benefit future studies of population structure, ecology, biodiversity, evolution and conservation in this challenging genus.
Supplemental Information
mtORF haplotype number after Pinzón et al. (2013) indicated by either PZ# or Z#.
Acknowledgments
We dedicate this paper to Paul Jokiel, who first introduced us to the Y & B types and encouraged us to see if we could tell them apart genetically. We thank Xavier Pochon, two anonymous referees, and especially Anderson Mayfield for helpful feedback and suggestions that greatly improved the manuscript. We also thank Jean Kenyon, Iliana Baums, Erik Franklin, Greg Concepcion, Jennifer Salerno, Michael Stat, Derek Skillings, Matt Iacchei, Frederique Kandel, Scott Godwin, Chelsie Counsell, ToBo lab, the staff of Papahānaumokuākea and the crew of the R/V Hi‘ialakai for their assistance with collecting Pocillopora meandrina over the years. We would also like to thank Evan Barba for help preparing Fig. 1. This is HIMB contribution number 1712 and SOEST 10304.
Funding Statement
Erika C. Johnston was funded by a National Science Foundation Graduate Research Fellowship, and this work was funded jointly by NSF-OA#1416889 and a Seaver Foundation award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Robert J. Toonen Is an Academic Editor for PeerJ.
Author Contributions
Erika C. Johnston conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Zac H. Forsman conceived and designed the experiments, wrote the paper, reviewed drafts of the paper.
Robert J. Toonen conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Collection of coral tissue samples was approved by the State of Hawai’i, Department of Land and Natural Resources, Division of Aquatic Resources Special Activity Permit 2018-3.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The accession numbers for the histone 3 region of P. eydouxi and P. meandrina are MG587096–MG587097. Sequence data can also be found in the Supplemental Information.
Data Availability
The following information was supplied regarding data availability:
The raw data and code are provided in Supplemental Files.
References
- Bahr, Jokiel & Toonen (2015).Bahr KD, Jokiel PL, Toonen RJ. The unnatural history of Kane‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ. 2015;3:e950. doi: 10.7717/peerj.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combosch & Vollmer (2013).Combosch DJ, Vollmer SV. Mixed asexual and sexual reproduction in the Indo-Pacific reef coral Pocillopora damicornis. Ecology and Evolution. 2013;3(10):3379–3387. doi: 10.1002/ece3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, Krupp & Jokiel (1998).Cox EF, Krupp DA, Jokiel PL. Cox EF, Krupp DA, Jokiel PL, editors. Reproduction in Reef Corals—results of the 1997 Edwin W. Pauley Summer Program in Marine Biology. Honolulu, University of HawaiiTechnical report No. 42. 1998:22–24.
- Edmunds et al. (2016).Edmunds PJ, Leichter JJ, Johnston EC, Tong EJ, Toonen RJ. Ecological and genetic variation in reef-building corals on four Society Islands. Limnology and Oceanography. 2016;61(2):543–557. doi: 10.1002/lno.10231. [DOI] [Google Scholar]
- Fan et al. (2002).Fan TY, Li JJ, Ie SX, Fang LS. Lunar periodicity of larval release by pocilloporid corals in Southern Taiwan. Zoological Studies. 2002;41(3):288–293. [Google Scholar]
- Flot et al. (2008).Flot JF, Magalon H, Cruaud C, Couloux A, Tillier S. Patterns of genetic structure among Hawaiian corals of the genus Pocillopora yield clusters of individuals that are compatible with morphology. Comptes Rendus—Biologies. 2008;331(3):239–247. doi: 10.1016/j.crvi.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Forsman et al. (2013).Forsman ZH, Johnston EC, Brooks AJ, Adam TC, Toonen RJ. Genetic evidence for regional isolation of Pocillopora corals from Moorea. Oceanography. 2013;26(3):153–155. doi: 10.5670/oceanog.2013.58. [DOI] [Google Scholar]
- Gaither et al. (2011).Gaither MR, Szabó Z, Crepeau MW, Bird CE, Toonen RJ. Preservation of corals in salt-saturated DMSO buffer is superior to ethanol for PCR experiments. Coral Reefs. 2011;30(2):329–333. doi: 10.1007/s00338-010-0687-1. [DOI] [Google Scholar]
- Gélin et al. (2017).Gélin P, Postaire B, Fauvelot C, Magalon H. Reevaluating species number, distribution and endemism of the coral genus Pocillopora Lamarck, 1816 using species delimitation methods and microsatellites. Molecular Phylogenetics and Evolution. 2017;109:430–446. doi: 10.1016/j.ympev.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Gorospe & Karl (2013).Gorospe KD, Karl SA. Genetic relatedness does not retain spatial pattern across multiple spatial scales: dispersal and colonization in the coral, Pocillopora damicornis. Molecular Ecology. 2013;22(14):3721–3736. doi: 10.1111/mec.12335. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2011).Huang D, Licuanan WY, Baird AH, Fukami H. Cleaning up the “Bigmessidae”: molecular phylogeny of scleractinian corals from Faviidae, Merulinidae, Pectiniidae and Trachyphylliidae. BMC Evolutionary Biology. 2011;11:37. doi: 10.1186/1471-2148-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston et al. (2017).Johnston EC, Forsman ZH, Flot JF, Schmidt-Roach S, Pinzón H, Knapp ISS, Toonen RJ. A genomic glance through the fog of plasticity and diversification in Pocillopora. Scientific Reports. 2017;7(1):5991. doi: 10.1038/s41598-017-06085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiel et al. (1993).Jokiel PL, Hunter C, Taguchi S, Watarai L. Ecological impact of a fresh-water “reef kill” in Kaneohe Bay, Oahu, Hawaii. Coral Reefs. 1993;12:177–184. doi: 10.1007/BF00334477. [DOI] [Google Scholar]
- Jokiel, Ito & Liu (1985).Jokiel PL, Ito RY, Liu PM. Night irradiance and synchronization of lunar release of planula larvae in the reef coral Pocillopora damicornis. Marine Biology. 1985;88:167–174. doi: 10.1007/BF00397164. [DOI] [Google Scholar]
- Marti-Puig et al. (2014).Marti-Puig P, Forsman ZH, Haverkort-yeh RD, Knapp ISS, Maragos JE, Toonen RJ. Extreme phenotypic polymorphism in the coral genus Pocillopora; micro-morphology corresponds to mitochondrial groups, while colony morphology does not. Bulletin of Marine Science. 2014;90(1):1–22. doi: 10.5343/bms.2012.1080. [DOI] [Google Scholar]
- Massé et al. (2013).Massé LM, Séré MG, Smit AJ, Schleyer MH. Sexual reproduction in Pocillopora damicornis at high latitude off South Africa. Western Indian Ocean Journal of Marine Science. 2013;11(1):55–65. [Google Scholar]
- Mayfield et al. (2015).Mayfield AB, Bruckner AW, Chen C, Chen C. A survey of pocilloporid corals and their endosymbiotic dinoflagellate communities in the Austral and Cook Islands of the South Pacific. Platax. 2015;12:1–17. [Google Scholar]
- Mayfield, Chen & Dempsey (2017a).Mayfield AB, Chen C, Dempsey AC. Biomarker profiling in reef corals of Tonga’s Ha’apai and Vava’u archipelagos. PLOS ONE. 2017a;12(11):e0185857. doi: 10.1371/journal.pone.0185857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield, Chen & Dempsey (2017b).Mayfield AB, Chen C, Dempsey AC. Identifying corals displaying aberrant behavior in Fiji’s Lau Archipelago. PLOS ONE. 2017b;12(5):e0177267. doi: 10.1371/journal.pone.0177267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield et al. (2010).Mayfield AB, Hsiao YY, Fan TY, Chen CS, Gates RD. Evaluating the temporal stability of stress-activated protein kinase and cytoskeleton gene expression in the Pacific reef corals Pocillopora damicornis and Seriatopora hystrix. Journal of Experimental Marine Biology and Ecology. 2010;395(1–2):215–222. doi: 10.1016/j.jembe.2010.09.007. [DOI] [Google Scholar]
- Paz-García et al. (2015a).Paz-García DA, Aldana-Moreno A, Cabral-Tena RA, García-De-León FJ, Hellberg ME, Balart EF. Morphological variation and different branch modularity across contrasting flow conditions in dominant Pocillopora reef-building corals. Oecologia. 2015a;178:207–218. doi: 10.1007/s00442-014-3199-9. [DOI] [PubMed] [Google Scholar]
- Paz-García et al. (2015b).Paz-García DA, Hellberg ME, García-de León FJ, Balart EF. Switch between morphospecies of Pocillopora corals. The American Naturalist. 2015b;186(3):434–440. doi: 10.1086/682363. [DOI] [PubMed] [Google Scholar]
- Permatav & Kinzie (2000).Permatav WD, Kinzie RA. Histological studies on the origin of planulae of the coral Pocillopora damicornis. Marine Ecology Progress Series. 2000;200:191–200. doi: 10.3354/meps200191. [DOI] [Google Scholar]
- Pinzón et al. (2013).Pinzón JH, Sampayo E, Cox E, Chauka LJ, Chen CA, Voolstra CR, LaJeunesse TC. Blind to morphology: genetics identifies several widespread ecologically common species and few endemics among Indo-Pacific cauliflower corals (Pocillopora, Scleractinia) Journal of Biogeography. 2013;40(8):1595–1608. doi: 10.1111/jbi.12110. [DOI] [Google Scholar]
- Putnam & Gates (2015).Putnam HM, Gates RD. Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. Journal of Experimental Biology. 2015;218(15):2365–2372. doi: 10.1242/jeb.123018. [DOI] [PubMed] [Google Scholar]
- Putnam et al. (2007).Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–95. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Richmond & Jokiel (1984).Richmond RH, Jokiel PL. Lunar periodicity in larva release in the reef coral Pocillopora damicornis at Enewetak and Hawaii. Bulletin of Marine Science. 1984;34(2):280–287. [Google Scholar]
- Schmidt-Roach et al. (2014b).Schmidt-Roach S, Johnston E, Fontana S, Jury CP, Forsman Z. Daytime spawning of Pocillopora species in Kaneohe Bay, Hawai‘i. Galaxea, Journal of Coral Reef Studies. 2014b;16:11–12. doi: 10.3755/galaxea.16.11. [DOI] [Google Scholar]
- Schmidt-Roach et al. (2013).Schmidt-Roach S, Lundgren P, Miller KJ, Gerlach G, Noreen AME, Andreakis N. Assessing hidden species diversity in the coral Pocillopora damicornis from Eastern Australia. Coral Reefs. 2013;32(1):161–172. doi: 10.1007/s00338-012-0959-z. [DOI] [Google Scholar]
- Schmidt-Roach et al. (2014a).Schmidt-Roach S, Miller KJ, Lundgren P, Andreakis N. With eyes wide open: a revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zoological Journal of the Linnean Society. 2014a;170(1):1–33. doi: 10.1111/zoj.12092. [DOI] [Google Scholar]
- Schmidt-Roach et al. (2012).Schmidt-Roach S, Miller KJ, Woolsey E, Gerlach G, Baird AH. Broadcast spawning by Pocillopora species on the great barrier reef. PLOS ONE. 2012;7(12):e50847. doi: 10.1371/journal.pone.0050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas et al. (2014).Thomas L, Kendrick GA, Stat M, Travaille K, Shedrawi G, Kennington WJ. Population genetic structure of the Pocillopora damicornis morphospecies along Ningaloo Reef, Western Australia. Marine Ecology Progress Series. 2014;513:111–119. doi: 10.3354/meps10893. [DOI] [Google Scholar]
- Thomas et al. (2016).Thomas L, Stat M, Evans RD, Kennington WJ. A fluorescence-based quantitative real-time PCR assay for accurate Pocillopora damicornis species identification. Coral Reefs. 2016;35(3):895–899. doi: 10.1007/s00338-016-1430-3. [DOI] [Google Scholar]
- Torda et al. (2013).Torda G, Schmidt-Roach S, Peplow LM, Lundgren P, Van Oppen MJH. A rapid genetic assay for the identification of the most common Pocillopora damicornis genetic lineages on the Great Barrier Reef. PLOS ONE. 2013;8(3):e58447. doi: 10.1371/journal.pone.0058447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron & Stafford-Smith (2000).Veron JEN, Stafford-Smith M. Corals of the world. 6th edition Australian Institute of Marine Science; Townsville: 2000. [Google Scholar]
- Ward (1995).Ward S. Two patterns of energy allocation for growth, reproduction and lipid storage in the scleractinian coral Pocillopora damicornis. Coral Reefs. 1995;14(2):87–90. doi: 10.1007/BF00303428. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
mtORF haplotype number after Pinzón et al. (2013) indicated by either PZ# or Z#.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data and code are provided in Supplemental Files.