Abstract
AIM
To evaluate whether fish oil (FO) can protect liver injury induced by intestinal ischemia/reperfusion (I/R) via the AMPK/SIRT-1/autophagy pathway.
METHODS
Ischemia in Wistar rats was induced by superior mesenteric artery occlusion for 60 min and reperfusion for 240 min. One milliliter per day of FO emulsion or normal saline was administered by intraperitoneal injection for 5 consecutive days to each animal. Animals were sacrificed at the end of reperfusion. Blood and tissue samples were collected for analyses. AMPK, SIRT-1, and Beclin-1 expression was determined in lipopolysaccharide (LPS)-stimulated HepG2 cells with or without FO emulsion treatment.
RESULTS
Intestinal I/R induced significant liver morphological changes and increased serum alanine aminotransferase and aspartate aminotransferase levels. Expression of p-AMPK/AMPK, SIRT-1, and autophagy markers was decreased whereas tumor necrosis factor-α (TNF-α) and malonaldehyde (MDA) were increased. FO emulsion blocked the changes of the above indicators effectively. Besides, in LPS-stimulated HepG2 cells, small interfering RNA (siRNA) targeting AMPK impaired the FO induced increase of p-AMPK, SIRT-1, and Beclin-1 and decrease of TNF-α and MDA. SIRT-1 siRNA impaired the increase of SIRT-1 and Beclin-1 and the decrease of TNF-α and MDA.
CONCLUSION
Our study indicates that FO may protect the liver against intestinal I/R induced injury through the AMPK/SIRT-1/autophagy pathway.
Keywords: Fish oil, AMPK/SIRT1/autophagy, Liver injury, Intestinal ischemia/reperfusion
Core tip: Intestinal ischemia/reperfusion (I/R) injury is a remarkable problem in many clinical conditions. Increased evidence indicates AMPK/SIRT-1 pathway linked autophagy exhibits a protective effect in liver diseases. Fish oil (FO) emulsion improves outcomes in patients with parenteral nutrition associated liver injury. We aimed to evaluate whether FO can protect liver injury induced by intestinal I/R via the AMPK/SIRT-1/autophagy pathway. Our results indicate that FO may protect the liver against intestinal I/R induced injury through the AMPK/SIRT-1/autophagy pathway. To our knowledge, we maybe for the first time present that FO attenuated intestinal I/R induced liver injury by inducing autophagy both in vivo and in vitro through the AMPK/SIRT-1 signaling pathway.
INTRODUCTION
Ischemia-reperfusion (I/R) injury of the intestine is a remarkable problem in many clinical conditions. Patients who suffer from conditions including abdominal aortic aneurysm surgery, cardiopulmonary bypass, intestinal transplantation, necrotizing enterocolitis, strangulated hernias, collapse of systemic circulation, and hypovolemic and septic shock have a potential risk for intestinal I/R[1-3]. Intestinal I/R is associated with a high morbidity and mortality[4]. Despite intensive study efforts over the past decades, pharmacologic therapies for liver injury induced by intestinal I/R have remained ineffective and controversial[5]. Patients who underwent persistent intestinal ischemia are often accompanied with the complication of septic shock, which eventually induces multiorgan dysfunction syndrome (MODS) and death[6].
The liver is the first organ which is affected by intestinal I/R because of the washout of toxic substances from the re-perfused intestine[7]. In addition, it is presumably due to the fact that the vasculature of the liver is associated with the intestine[8]. The gut-liver axis with cytokine cascade may play an important role in the liver injury induced by intestinal I/R[9]. The pathophysiology of acute liver injury is unclear; however, the prominent neutrophil infiltration and other related injury models suggest that oxygen radical species and inflammatory cascade crosstalk in this pathogenesis, which eventually results in final liver failure and even MODS [6].
Autophagy is a regulated cellular pathway involved in the turnover of cytoplasmic organelles and proteins through a lysosome-dependent degradation process[10]. Recent studies indicated that autophagy deficiency promoted inflammatory reaction and oxidative stress[11,12], implicating a protective function. In addition, autophagy can be modulated by several pathways, the most crucial one of which is the adenosine 5’-monophosphate-activated protein kinase/sirtuin 1 (AMPK/SIRT-1) pathway[13]. Increasing evidence indicates that AMPK/SIRT-1 pathway linked autophagy plays a protective effect in various diseases including liver injury. Studies also reported that resveratrol, a kind of natural polyphenol, regulates autophagy by activating the AMPK/SIRT-1 signaling pathway in neuroblastoma and endothelial cells[14]. Furthermore, inducing autophagy by activating AMPK/SIRT-1 alleviates oxidized low-density lipoprotein (oxLDL)-induced human umbilical vein endothelial cell injury[15]. However, the role of the AMPK/SIRT-1/autophagy pathway in intestinal I/R-challenged liver injury is still unknown.
Fish oil (FO), containing major ingredients as ω-3 polyunsaturated fatty acids including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), has been widely used as a therapeutic intervention in critical care settings[16]. Parenteral FO improves outcomes in patients with parenteral nutrition associated-liver injury[17]. FO has been shown to be a potent activator of the AMPK/SIRT-1 pathway against lung injury induced by intestinal I/R[16]. Herein, we hypothesized that FO could be an appropriate agent for intestinal I/R-induced liver injury via the AMPK/SIRT-1/autophagy pathway.
MATERIALS AND METHODS
Animals
Male Wistar rats weighing 180-220 g from the Animal Center of Dalian Medical University were used in this study, and maintained under standard laboratory conditions. All rats were fed standard laboratory food and water. Rats were housed in a barrier system at 25 °C with 12 h light-12 h dark cycles and acclimated for 1 wk before experimentation. All procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Dalian Medical University (Permit number: SCXK 2008-0002). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize animal suffering.
Experimental design
Intestinal I/R was induced in rats according to a previously standardized method[16]. Briefly, after a midline laparotomy was performed, the superior mesenteric artery was gently isolated and occluded with an atraumatic microvascular clamp for 60 min and then followed by reperfusion for 240 min. When mesenteric pulsations ceased and the intestine became pale in color, occlusion was confirmed. And reperfusion was confirmed when the pusatile flow returned to the mesenteric artery and its branches. For sham operation, the superior mesenteric artery was only isolated without occlusion.
The rats were randomly divided into three groups (n = 8 each): (1) sham group; (2) I/R group; and (3) FO group. In the FO group, rats were pretreated intraperitoneally with 1 mL/d of FO emulsion (100 mL of FO emulsion contained 2.82 g of EPA and 3.09 g of DHA; Fresenius Kabi, Bad Homburg, Germany) for five consecutive days, and then surgery was performed as that in the I/R group. The same volume of normal saline was administered in the sham group and I/R group.
The dose of FO emulsion pretreatment was selected according to a previous study[16], and a pilot study has been performed. All animals were sacrificed at the end of reperfusion and tissue and blood samples were obtained for further analysis.
Intestinal and liver morphological assessment
Intestinal and liver tissues were fixed in 10% formalin, embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin (H&E) for light microscopy. Scores of liver and intestine pathology were evaluated according to previous studies[7-9].
Measurement of serum aspartate aminotransferase, alanine aminotransferase, tumor necrosis factor-α, and liver malonaldehyde
Serum was obtained from blood samples by centrifugation (1000 g, 10 min, 4 °C) and stored at -80 °C for assessment. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured with an OLYMPUS AU1000 automatic analyzer (Aus Bio Laboratories Co., Ltd., Beijing, China). Serum tumor necrosis factor-α (TNF-α) was quantified using a rat enzyme-linked immunosorbent assay kit (BOSTER Bioengineering Co Ltd, Wuhan, China). Liver tissues were harvested and homogenized immediately on ice in five volumes of normal saline. Homogenates were centrifuged at 1200 g for 10 min. Liver malonaldehyde (MDA) concentration in the supernatant was determined using an assay kit (Nanjing Jiancheng Corp., Nanjing, China), according to the manufacturer’s recommendations. The activity of MDA is expressed in nmol/mg prot.
Cell culture and treatment
HepG2 cells were cultured in Dulbecco’s Modified Eagle’s Medium (Gibco, CA, United States) supplemented with 10% fetal bovine serum. The cells were kept at 37 °C in a humidified atmosphere with 5% CO2, 100 units/mL of penicillin, and 10 μg/mL of streptomycin. Media were collected and centrifuged 48 h after treatment with lipopolysaccharide (LPS; 10 μg/mL, Sigma-Aldrich, United States) in the presence or absence of FO (50 mmol/L EPA/50 mmol/L DHA each mixture) and TNF-α was measured with a specific ELISA kit (Jiancheng, Nanjing, China) according to the manufacturer’s protocol.
The doses of FO and LPS pretreatment were selected according to previous studies[16,18], and a pilot study has been performed.
Western blot analysis of p-AMPK, SIRT-1, LC3 II, Beclin-1, and P62 in liver tissue and HepG2 cells
For detecting p-AMPK, SIRT-1, LC3 II, Beclin-1, and P62 contents in liver tissue, 30 μg of protein was separated by 12% or 15% SDS-PAGE (Bio-Rad, Hercules, United States), and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, United States). The membranes were then incubated with primary anti-p-AMPK (Abcam Ltd., Cambridge, United Kingdom), anti-SIRT-1 (Abcam Ltd., Hong Kong, China), anti-LC3 I, anti-LC3 II, anti-P62, anti-Beclin-1, or anti-β-actin antibody (Santa Cruz Biotechnology, United States) in Tris buffered solution (TBS) containing 5% skimmed milk overnight at 4 °C. After washing three times in TBS with 0.1% Tween 20 (TTBS), the membranes were incubated with biotinylated secondary antibody diluted 1:1000 in PBST containing 5% skimmed milk for 2 h at 37 °C. After extensive washing with TTBS, the membranes were exposed to enhanced chemiluminescence-plus reagents (Beyotime Institute of Biotechnology, Hangzhou, China). Emitted light was documented with a BioSpectrum-410 multispectral imaging system with a Chemi HR camera 410 (UVP, Upland, CA, United States) and analyzed with Gel-Pro Analyzer Version 4.0 (Media Cybernetics, Bethesda, United States).
Quantitative real-time polymerase chain reaction (RT-PCR) analysis of liver AMPK and SIRT-1 mRNA levels
Total RNA was extracted from rat liver using Trizol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. Reverse transcription into cDNA was performed using a TaKaRa RNA polymerase chain reaction (PCR) kit Version 3.0 (TaKaRa, Dalian, China) for PCR analysis; primers are shown as follows: AMPK forward, 5’-G TGGTGTTATCCTGTATGCCCTTCT-3’ and reverse, 5’-CTGTTTAAACCATTCATGCTCTCGT-3’; SIRT-1 forward, 5’-AGCTGGGGTTTCTGTTTCCTGTGG-3’ and reverse, 5’-CGAACATGGCTTGAGGATCTGGGA-3’; β-actin forward, 5’-AGAGGGAAATCGTGCGTGAC-3’ and reverse, 5’-CAATAGTGATGACCTGGCCGT-3’. RT-PCR was performed with the SYBR Premix Ex Taq kit (Takara, Dalian, China) for fluorescence detection during amplification on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). PCR cycling was performed under the following conditions: initial denaturation at 95 °C for 30 s and 40 thermal cycles of 95 °C for 5 s, 60 °C for 34 s, and 72 °C for 30 s.
RNA interference
HepG2 cells were transfected with small interfering RNAs (siRNAs) directed against AMPK α1 and SIRT-1, with scrambled siRNAs used as controls. Both specific and control siRNAs were obtained from GenePharma (Shanghai, China), and transient siRNA transfection was performed as previously described. After siRNA transfection for 48 h, the cells were treated with FO for an additional 6 h. The cells were then collected for the protein and mRNA analyses.
RESULTS
Effect of FO emulsion on intestinal and liver injury induced by intestinal I/R
Histopathological analysis of the liver in the I/R group showed apparent injury compared with the sham group, which was manifested as edema, hemorrhage, and neutrophil infiltration (Figure 1A). However, liver injury was significantly improved by pretreatment with FO emulsion, and the histopathological score was significantly reduced compared with the I/R group (Figure 1A, P < 0.01).
Figure 1.
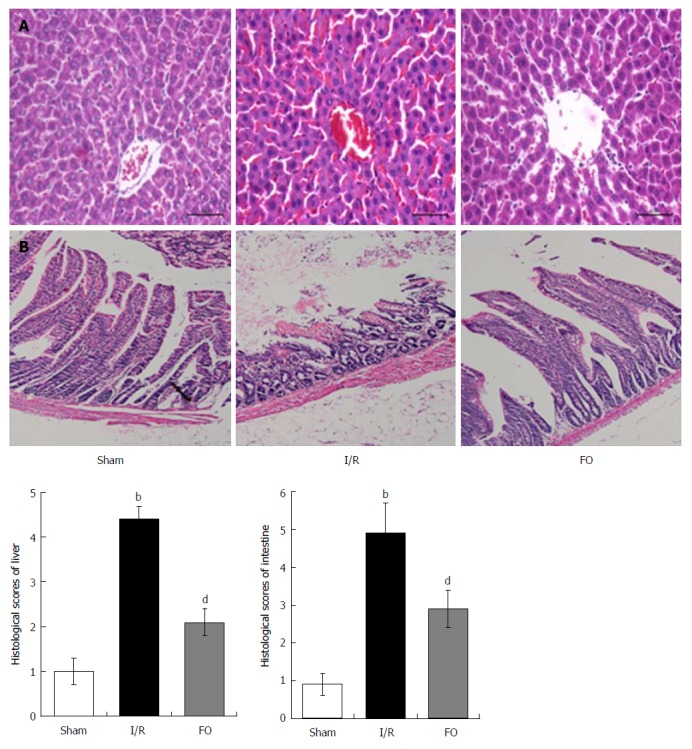
Fish oil emulsion improves I/R induced liver and intestinal injury histopathologically. Pathologic changes of liver (A) and intestinal (B) tissues in different groups (mean ± SD, n = 8). bP < 0.01 vs sham group; dP < 0.01 vs I/R group.
Compared with the sham group, intestinal mucosal injury was also found in the I/R group with apparent hemorrhage and loss of lamina propria villi and glands (Figure 1B). Pretreatment with FO emulsion significantly reduced the intestinal damage and histopathology score (Figure 1B, P < 0.01).
Effect of FO emulsion on serum ALT, AST, TNF-α, and liver MDA levels
In the I/R group, there was a significant increase in serum ALT and AST levels compared with the sham group. However, pretreatment with FO significantly decreased serum levels of both ALT and AST compared with the I/R group (Figure 2A, P < 0.01). This indicated that FO emulsion was effective in improving liver function.
Figure 2.
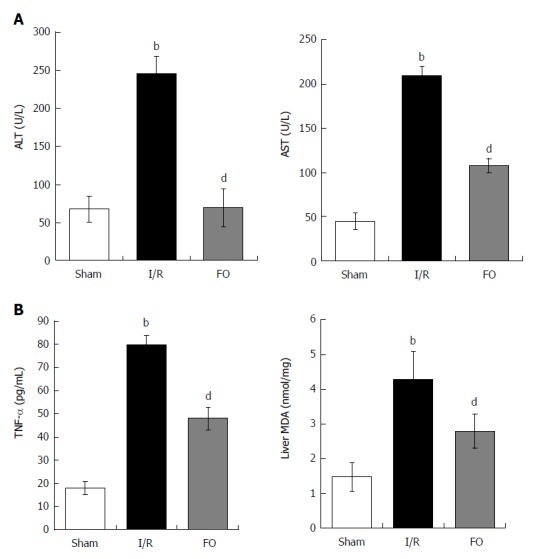
Alanine aminotransferase and aspartate aminotransferase (A) as well as TNF-α and liver MDA levels (B) in different groups (mean ± SD, n = 8). bP < 0.01 vs sham group; dP < 0.01 vs I/R group.
Moreover, serum TNF-α and liver MDA increased significantly after intestinal I/R compared with the sham group (P < 0.01). Furthermore, FO emulsion pretreatment dramatically reduced proinflammatory cytokine levels (Figure 2B, P < 0.01).
Effect of FO emulsion on LC3 II, Beclin-1, and P62 expression in liver tissue after intestinal I/R
Western blot analysis was performed to examine the expression of LC3 II, Beclin-1, and P62 in liver tissue. Compared with the sham group, both LC3 II and Beclin-1 expression was markedly decreased but P62 expression increased in the I/R group. However, pretreatment with FO emulsion markedly increased the expression of both LC3 II and Beclin-1, and decreased the expression of P62 compared with the I/R group (Figure 3, P < 0.01), indicating that FO emulsion activated autophagy in intestinal I/R-induced liver injury.
Figure 3.
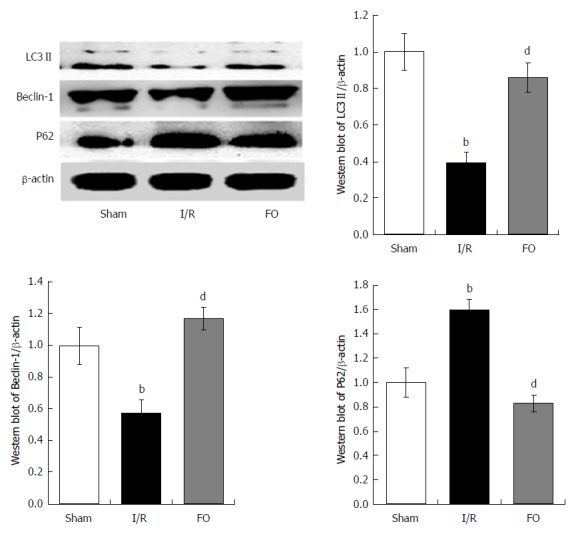
Western blot analysis of liver autophagy (LC3 II, Beclin-1, and P62) levels in different groups (mean ± SD, n = 8). bP < 0.01 vs sham group; dP < 0.01 vs I/R group.
Effect of AMPK/SIRT-1 activation by FO emulsion in liver tissue after intestinal I/R
Western blot and RT-PCR analyses were performed to determine AMPK and SIRT-1 protein and mRNA contents in the liver. After intestinal I/R, the ratio of p-AMPK/AMPK protein and AMPK mRNA in the liver was dramatically decreased compared with the sham group (Figure 4A, P < 0.01). Similarly, both SIRT-1 protein and mRNA expression in the liver were dramatically decreased compared with the sham group (Figure 4B, P < 0.01). Nevertheless, not only the ratio of p-AMPK/AMPK protein and AMPK mRNA, but also the SIRT-1 protein and mRNA expression in the liver were dramatically increased by pretreatment with FO emulsion compared with the I/R group (Figure 4A and B, P < 0.01, P < 0.05).
Figure 4.
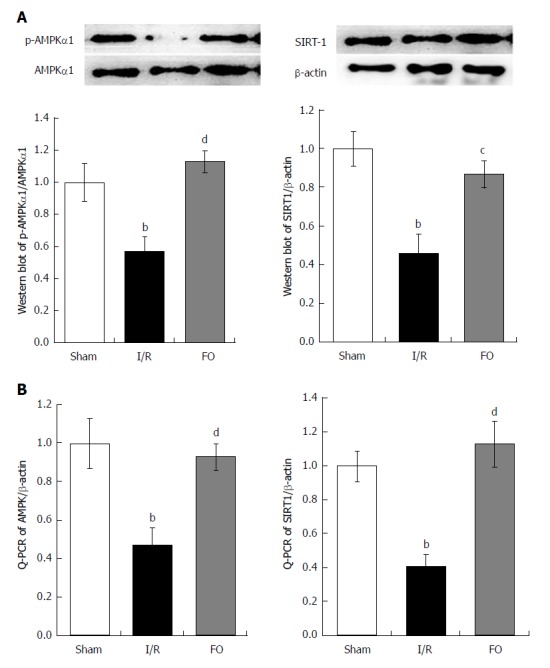
Western blot analysis (A) of liver p-AMPKα1/AMPKα1 and qRT-PCR analysis (B) of liver AMPK expression in different groups (mean ± SD, n = 8). bP < 0.01 vs sham group; cP < 0.05, dP < 0.01 vs I/R group.
FO emulsion ameliorates LPS induced HepG2 cell damage through the AMPK/SIRT-1/autophagy pathway
To investigate the role of the AMPK/SIRT-1/autophagy pathway affected by FO emulsion in intestinal I/R-induced liver injury, HepG2 cells were employed and challenged with LPS to mimic the circumstance in vivo. Western blot analysis was then performed to examine the AMPK, SIRT-1, and Beclin-1 expression in LPS-stimulated HepG2 cells. Further, TNF-α and MDA were determined using experimental kits according to the manufacturer’s protocols. Compared with the con group (control + LPS), the expression of p-AMPK, SIRT-1, and Beclin-1 was significantly increased in the FO group (FO + LPS) (Figure 5A, P < 0.05). This was associated with a significant decrease of TNF-α and MDA levels (Figure 5B, P < 0.01). However, p-AMPK, SIRT-1, and Beclin-1 expression was decreased significantly in the FO + siA (FO + AMPK siRNA + LPS) group compared with the FO group. Moreover, reduced SIRT-1 and Beclin-1 expression was observed in the FO + siS (FO + siRNA of SIRT-1 + LPS) group compared with the FO group (Figure 5A, P < 0.05). This was associated with a significant increase of TNF-α and MDA levels in HepG2 cells, although there was no change of p-AMPK (Figure 5B, P < 0.05). Thus, FO emulsion may ameliorate liver injury induced by intestinal I/R through the AMPK/SIRT-1/autophagy pathway.
Figure 5.
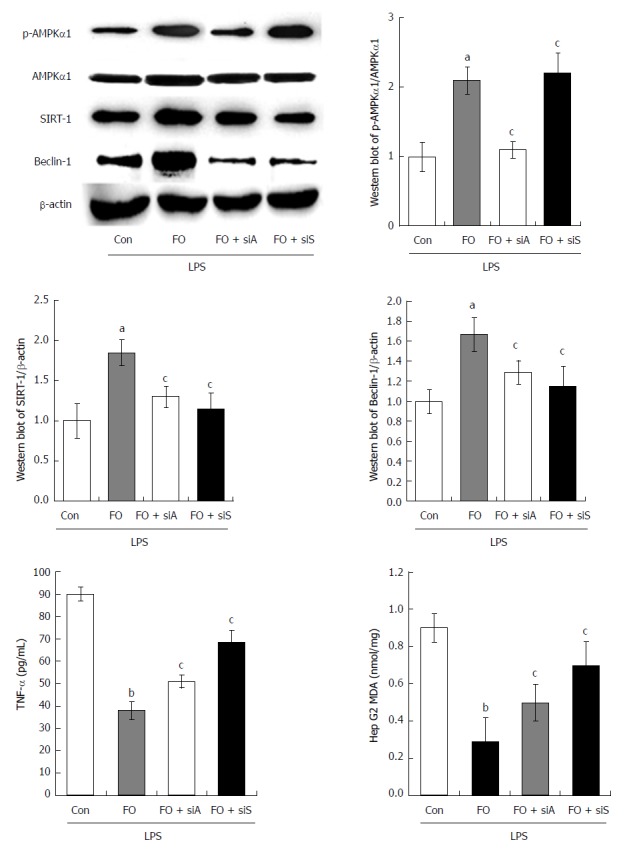
p-AMPK, AMPK, SIRT-1, Beclin-1, TNF-α, and MDA expression in HepG2 cells challenged with LPS, LPS + FO, LPS + FO + siAMPK, or LPS + FO + siSIRT-1 (mean ± SD, n = 3). aP < 0.05, bP < 0.01 vs control; cP < 0.05 vs FO.
DISCUSSION
To our knowledge, we may provide the first evidence of the protective effect of AMPK/SIRT-1/autophagy activated by FO in intestinal I/R induced liver injury. In the present study, we demonstrated that intestinal I/R caused significant liver injury, which was evidenced by: (1) apparent liver tissue edema, hemorrhage, and neutrophil infiltration; (2) liver dysfunction (increased serum AST and ALT levels) associated with increased TNF-α and MDA; (3) decreased protein and mRNA expression of p-AMPK/AMPK and SIRT-1 associated with autophagy (decreased LC3 II and Beclin-1 expression but increased P62 expression); (4) FO emulsion restored the balance of the factors and alleviated liver injury; and (5) in LPS-stimulated HepG2 cells, AMPK siRNA impaired the FO-induced increase of p-AMPK, SIRT-1, and Beclin-1 but the decrease of TNF-α and MDA. SIRT-1 siRNA impaired the FO-induced increase of SIRT-1 and Beclin-1 but the decrease of TNF-α and MDA.
Intestinal I/R injury is the initiator of MODS and the promoter of distant organ injury[6]. Elucidating the series of events which lead to distant organ injury and a lethal consequence may provide clues to the pathogenesis of MODS and may further improve survival in critically ill patients. The liver is the most vulnerable organ even beyond the intestine per se when suffering intestinal I/R[7-9]. Liver malfunction often indicates a poor prognosis in MODS patients[9].
Oxidative stress and inflammatory cascade are two critical pathogeneses in liver injury induced by intestinal I/R. Intestinal I/R can induce leukocyte (including neutrophils and lymphocytes) infiltration into the liver and results in an oxidative stress in sinusoids that contributes to subsequent hepatocellular injury[19]. It has been proposed that activated Kupffer cells and infiltrating neutrophils producing oxygen radicals and chemical inflammatory mediators such as TNF-α and IL-6 are involved in the gut I/R induced neutrophil accumulation in the liver[9]. TNF-α is regarded as the most important proinflammatory cytokine which contributes to both morbidity and mortality in inflammation cascade. Because the biological effects of TNF-α both in the liver and elsewhere, it has been widely used as an indicator of liver injury[9]. Further, MDA as an indicator of lipid peroxidation was determined in the present study[19]. We now demonstrated that serum TNF-α and liver MDA were increased after intestinal I/R. This is consistent with previous studies from our and other laboratories[7-9].
Autophagy is a lysosome-mediated degradative pathway of cellular mechanisms for degrading misfolded proteins[10]. The execution of autophagy involves three critical proteins including LC3 II, P62/SQSTM1, and Beclin 1[20-22]. Autophagy not only plays a crucial role in normal liver but also diseased liver. Moderate autophagy is benefit for cell survival against various stress stimulations. Nevertheless, excessive activation of autophagy results in cell death[23,24]. Autophagy also exhibits dual roles in different disease circumstances and also links to oxidative stress and inflammatory reaction[11,12]. Previous studies indicated that autophagy is a cellular self-protective mechanism against oxLDL-induced injury[13]. In the current study, decreased expression of essential autophagy regulators (decrease of Beclin 1 and LC3 II and increase of P62) was observed in rat liver challenged by intestinal I/R. FO induced this self-protective mechanism, which manifested as an increase of rat liver autophagy in case of intestinal I/R. Similarly, FO also induced this self-protective mechanism in HepG2 cells in the presence of I/R. Our results suggested that FO conferred liver protection and amplified the activation of autophagy during intestinal I/R.
In addition, we identified the role of AMPK and SIRT-1 in FO stimulated autophagy in liver injury induced by intestinal I/R. SIRT-1 belongs to the mammalian family of sirtuins, a highly conserved family of NAD-dependent deacetylases that regulate cellular energy and lifespan in mammals[25]. A study of SIRT-1 knockout mice showed that SIRT-1 is an important regulator of autophagy[26]. SIRT-1 interacts with several essential components of the autophagy machinery and elevates autophagy. We and others have demonstrated that AMPK can enhance the activity of SIRT-1, which is associated with the cellular energy balance in animal models of stroke[26,27]. It has been reported that AMPK phosphorylation regulates SIRT-1 activity[16]. Herein, we found that a decrease of p-AMPK/AMPK and SIRT-1 protein and mRNA expression in rat liver suffering from intestinal I/R. However, FO promoted AMPK phosphorylation and SIRT-1 expression in intestinal I/R-induced liver injury. This is parallel with the autophagy levels but contradictory with TNF-α and MDA expression. This is consistent with a previous study indicating that AMPK decreases after cold I/R injury of intestinal preservation for transplantation and may be because of energy deprivation under ischemic conditions[28,29]. In addition, knockdown of AMPK impaired the FO-induced increase of p-AMPK, SIRT-1, and Beclin-1 but the decrease of TNF-α and MDA induced by in LPS-stimulated HepG2 cells, and knockdown of SIRT-1 impaired the FO-induced increase of SIRT-1 and Beclin-1 but the decrease of TNF-α and MDA. These indicated that FO stimulated autophagy through the AMPK/SIRT-1 signaling pathway in liver injury induced by intestinal I/R.
To our knowledge, we presented here, maybe for the first time, that FO attenuated intestinal I/R induced liver injury by inducing autophagy both in vivo and in vitro through the AMPK/SIRT-1 signaling pathway (Figure 6). Our results provide experimental evidence for future clinical applications of FO or FO-related products to prevent intestinal I/R associated liver disease. This study has some limitations, and further studies should be performed using gene knockout animals and clinical application should be concentrated.
Figure 6.
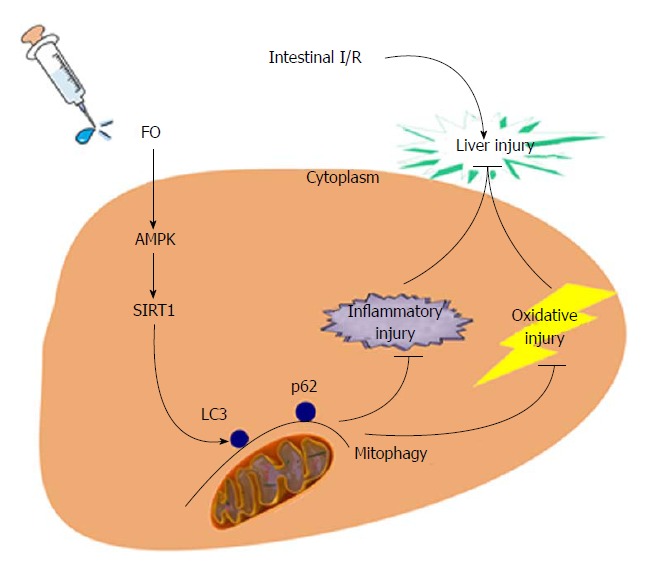
Schematic representation of the novel potential protective mechanism of fish oil in liver injury induced by intestinal I/R through the AMPK/SIRT-1/autophagy pathway.
ARTICLE HIGHLIGHTS
Research background
The liver is the most vulnerable organ after intestinal ischemia/reperfusion (I/R) because the liver and intestine share the anatomical common pathway such as coupled vasculature. The mechanism is obscure and has multiple overlapping pathways. Increased evidence indicates that the AMPK/SIRT-1/autophagy pathway may be involved in this process. Fish oil (FO) may regulate the AMPK/SIRT-1/autophagy pathway to affect liver injury induced by intestinal I/R.
Research motivation
Understanding and regulating AMPK/SIRT-1/autophagy pathway using a safe and effective substance like FO will be an important area of future research.
Research objectives
This study aimed to provide evidence that FO can attenuate intestinal I/R induced liver injury by inducing autophagy through the AMPK/SIRT-1 pathway. This will provide a therapeutic target for future clinical applications of FO to prevent intestinal I/R associated liver disease.
Research methods
This research was performed using Wistar rats challenged by intestinal I/R and HepG2 cells stimulated with LPS to mimic the in vivo pathogenesis. Further, RNA interference has been employed, which laid a foundation for the future gene knockout animal study.
Research results
Intestinal I/R induced apparent liver injury, including histopathological injury and liver dysfunction, and this was associated with increased TNF-α and MDA, and decreased AMPK/SIRT-1/autophagy molecular function. FO emulsion restored the balance of the factors and alleviated liver injury. The similar results were observed in HepG2 cells stimulated with LPS.
Research conclusions
Intestinal I/R induced liver injury is associated with decreased AMPK/SIRT-1/autophagy (LC3 II, Beclin-1, and P62 expression) molecular function. FO emulsion restored the beneficial factors and alleviated liver injury. Similar results were observed in HepG2 cells. We present a novel theory here that FO can prevent intestinal I/R associated liver disease via the AMPK/SIRT-1/autophagy pathway. This may be a potential novel target for patients. We may provide the first evidence of AMPK/SIRT-1/autophagy regulated by FO in liver injury induced by intestinal I/R. Our hypothesis was confirmed using rat models and HepG2 cells treated with LPS and RNA interfere to mimic the conditions in vivo. Thus, FO and FO-related products may have novel clinical applications to prevent intestinal I/R associated liver disease in future.
Research perspectives
This study has some limitations and further studies should be performed using gene knockout animals and clinical application should be concentrated.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by the National Natural Science Foundation of China, No. 81600446; Natural Science Foundation of Liaoning Province, China, No. 201102048; and Natural Science Foundation of Dalian Medical Association, No. WSJ/KJC-01-JL-01.
Institutional review board statement: The study was reviewed and approved by the review board of Dalian Medical University, Liaoning Province, China.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Dalian Medical University (Permit number: SCXK 2008-0002).
Conflict-of-interest statement: There is no conflict of interest in this study.
Data sharing statement: No additional data are available.
Peer-review started: October 30, 2017
First decision: November 15, 2017
Article in press: January 15, 2018
P- Reviewer: Beltowski J, Dai ZJ S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Hui-Rong Jing, Department of General Surgery, Second Affiliated Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China.
Fu-Wen Luo, Department of General Surgery, Second Affiliated Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China.
Xing-Ming Liu, Department of General Surgery, Second Affiliated Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China.
Xiao-Feng Tian, Department of General Surgery, Second Affiliated Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China.
Yun Zhou, Department of Clinical Nutrition, Second Affiliated Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China. zydy2ynutrition@126.com.
References
- 1.Mainous MR, Ertel W, Chaudry IH, Deitch EA. The gut: a cytokine-generating organ in systemic inflammation? Shock. 1995;4:193–199. [PubMed] [Google Scholar]
- 2.Pastores SM, Katz DP, Kvetan V. Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol. 1996;91:1697–1710. [PubMed] [Google Scholar]
- 3.Valentine RJ, Hagino RT, Jackson MR, Kakish HB, Bengtson TD, Clagett GP. Gastrointestinal complications after aortic surgery. J Vasc Surg. 1998;28:404–411; discussion 411-412. doi: 10.1016/s0741-5214(98)70125-9. [DOI] [PubMed] [Google Scholar]
- 4.Schütz A, Eichinger W, Breuer M, Gansera B, Kemkes BM. Acute mesenteric ischemia after open heart surgery. Angiology. 1998;49:267–273. doi: 10.1177/000331979804900404. [DOI] [PubMed] [Google Scholar]
- 5.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 6.Wilmore DW, Smith RJ, O’Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery. 1988;104:917–923. [PubMed] [Google Scholar]
- 7.Towfigh S, Heisler T, Rigberg DA, Hines OJ, Chu J, McFadden DW, Chandler C. Intestinal ischemia and the gut-liver axis: an in vitro model. J Surg Res. 2000;88:160–164. doi: 10.1006/jsre.1999.5767. [DOI] [PubMed] [Google Scholar]
- 8.Jing H, Shen G, Wang G, Zhang F, Li Y, Luo F, Yao J, Tian XF. MG132 alleviates liver injury induced by intestinal ischemia/reperfusion in rats: involvement of the AhR and NFκB pathways. J Surg Res. 2012;176:63–73. doi: 10.1016/j.jss.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan N, Yagmurdur H, Kilinc K, Baltaci B, Tezel S. The protective effects of intravenous anesthetics and verapamil in gut ischemia/reperfusion-induced liver injury. Anesth Analg. 2007;105:1371–1378, table of contents. doi: 10.1213/01.ane.0000284696.99629.3a. [DOI] [PubMed] [Google Scholar]
- 10.Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 11.Swanson MS, Molofsky AB. Autophagy and inflammatory cell death, partners of innate immunity. Autophagy. 2005;1:174–176. doi: 10.4161/auto.1.3.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, Ma W, Peng X, Zhou Y, Mi M. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol Nutr Food Res. 2014;58:1941–1951. doi: 10.1002/mnfr.201400161. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H, Chen Y, Liao L, Wu W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovasc Drugs Ther. 2013;27:189–198. doi: 10.1007/s10557-013-6442-4. [DOI] [PubMed] [Google Scholar]
- 16.Jing H, Yao J, Liu X, Fan H, Zhang F, Li Z, Tian X, Zhou Y. Fish-oil emulsion (omega-3 polyunsaturated fatty acids) attenuates acute lung injury induced by intestinal ischemia-reperfusion through Adenosine 5’-monophosphate-activated protein kinase-sirtuin1 pathway. J Surg Res. 2014;187:252–261. doi: 10.1016/j.jss.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, Zhou J, Duggan C, Gura KM. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA. 2000;97:10144–10149. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Z, Jing H, Yao J, Li Y, Hu X, Shao H, Shen G, Pan J, Luo F, Tian X. The protective effects of curcumin on experimental acute liver lesion induced by intestinal ischemia-reperfusion through inhibiting the pathway of NF-κB in a rat model. Oxid Med Cell Longev. 2014;2014:191624. doi: 10.1155/2014/191624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 21.Bjørkøy G, Lamark T, Johansen T. p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy. 2006;2:138–139. doi: 10.4161/auto.2.2.2405. [DOI] [PubMed] [Google Scholar]
- 22.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 23.Czaja MJ, Ding WX, Donohue TM Jr, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang LM, Wang YJ, Cui M, Luo WJ, Wang XJ, Barber PA, Chen ZY. A dietary polyphenol resveratrol acts to provide neuroprotection in recurrent stroke models by regulating AMPK and SIRT1 signaling, thereby reducing energy requirements during ischemia. Eur J Neurosci. 2013;37:1669–1681. doi: 10.1111/ejn.12162. [DOI] [PubMed] [Google Scholar]
- 28.Salehi P, Walker J, Madsen KL, Sigurdson GT, Strand BL, Christensen BE, Jewell LD, Churchill TA. Relationship between energetic stress and pro-apoptotic/cytoprotective kinase mechanisms in intestinal preservation. Surgery. 2007;141:795–803. doi: 10.1016/j.surg.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Zaouali MA, Boncompagni E, Reiter RJ, Bejaoui M, Freitas I, Pantazi E, Folch-Puy E, Abdennebi HB, Garcia-Gil FA, Roselló-Catafau J. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: a role for melatonin and trimetazidine cocktail. J Pineal Res. 2013;55:65–78. doi: 10.1111/jpi.12051. [DOI] [PubMed] [Google Scholar]


