Abstract
Pineal gland has and antistressogenic role. Its main hormone, melatonin, has radio protective effect on endocrine and other dynamic tissues. In our previous study, we have shown that pinealectomy changes the behavior of suprarenal gland in totally irradiated rats. The aim of this study is to evaluate the effect of exogenous melatonin on suprarenal gland of rats with or without pineal gland. Four months after pinealectomy (experimental group) or shampine-alectomy (control group), adult Wistar male rats were daily treated with 0,2 mg of melatonin intraperitoneally, during two weeks. Thereafter, all animals were totally irradiated with 8 Gy of Gamma rays produced from Cobalt 60. Animals who survived were sacrificed on the 17th post irradiation day. Qualitative and quantitative characteristics of the suprarenal gland were studied using histological methods. The results show that exogenous melatonin had protective role on suprarenal gland in totally irradiated rats and that those effects were more pronounced in the presence of pineal gland.
Keywords: melatonin, suprarenal gland, pinealectomy, total irradiation
INTRODUCTION
Pineal gland is part of the diencephalon’s epithalamus. It mainly produces neurohormone melatonin which is an integral part of the neuroendocrine system. Production of melatonin significantly increases during the dark period of a day-night cycle. That is how melatonin sends signals of daily and seasonal changes to the body. Melatonin acts in interaction with a family of receptors that are present in brain structures and peripheral tissues. That explains why melatonin has influence on many organic systems. Number of studies on biological action of melatonin is constantly increasing. New studies show that, except for its role in the coordination of body’s circadian watch, melatonin also has antioxidant, antineoplastic, antigonadotropic, immunostimulative, neuro protective, radio protective and many other roles (1). The significance of pineal gland in irradiation stress as well as an increase in the melatonin synthesis has been described in early 1970s in the last century (2, 3). Radio protective acting of the pineal gland and melatonin in totally irradiated rats with a sub-lethal doze of Gamma rays have been proved in the parenchyma of thyroid gland (4) and testicles (5). Effects of pinealectomy (removal of the pineal gland) on suprarenal gland of rats have been described as progressive changes (6), which has been confirmed by Mornjaković et al. (7) in irradiation stress circumstances. Sewerynek et al. (8) believe that pinealectomy significantly increases the mitotic activity in the suprarenal gland and that melatonin inhibits proliferation of cells. Morphologic signs of an increased activity of suprarenal gland were found by Guillot et al. (9) in all layers of the cortex and in medulla, when pineal gland was locally irradiated with laser. Authors believe that their results are similar to those that can be found after pinealectomy and that, once again, it proves that pineal gland, mediated by the light impulses, controls the suprarenal gland. In the available literature, there are not data that point out possible influences of the application of exogenous melatonin on the morphofunctional status of suprarenal gland. Therefore, by conducting our experiment and on the base of qualitative and quantitative histological analysis, we wanted to evaluate possible influence of exogenous melatonin on the suprarenal gland in the model of total irradiation with a high dose of Gamma rays and its eventual dependence on the presence of pineal gland.
MATERIAL AND METHODS
In this study, we have used sexually mature mail Wi-star rats in which, in July, ablation of pineal gland (experimental group, N = 19) has been performed in total anesthesia (Nembutal, 5mg/100g of body weight), i.e. shampinealectomy to the phase of cutting the sagittal sinus (control group, N= 16). After a 4-month adaptation period, animals were injected intraperitoneally with a solution of 0,2mg of melatonin dissolved in 0,5 ccm of hidroalcohol solvent once a day. After a treatment which lasted 14 days, during the light phase of day and in the afternoon, all animals were acutely and totally irradiated with one dose of 8 Gy of Gamma rays (the source was Cobalt 60, focus-skin distance was 60 cm, the strength of the source was 7,4x1013 Bq, the speed of the dose was 1 Gy/min., ionizing chamber 35x35cm). Those rats that survived until 17th post irradiation day (in the experimental group N = 13 and in the control group N = 14 animals) have been sacrificed in the ethereal anesthesia. Suprarenal glands that were talken out by dissection, together with the accompanying periadrenal tissue, were embedded in paraffin using the usual histological procedures and were cut in serial slices to a depth of 6 μιη and stained by hemalauneosin and Azan methods. Qualitative and quantitative analysis of suprarenal gland in each animal was performed using a light binocular microscope. Quantitative analysis was focused on the nuclei of corticocytes and medullocytes of the suprarenal gland. Karyometric measuring (100 nuclei in each of the three zones and 100 nuclei in the medulla for each animal) was performed by ocular micrometer. Absolute nuclei volumes (μιη3) were calculated by the Tonutti equation for rotational ellipsoids (10). Results were evaluated by using statistical methods. The significance of the difference between the results from studied groups was determined by using Student t-test.
RESULTS
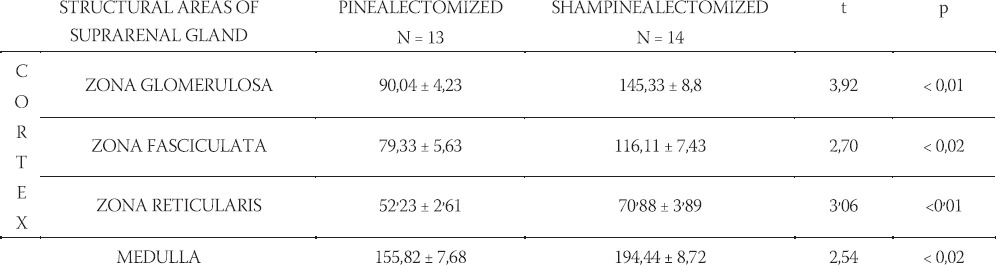
By using a qualitative histological analysis of suprarenal glands of pinealectomized and melatonin treated and totally irradiated rats, a pronounced wide zona glomerulosa (G) with hypertrophic cells, beside the accompanying periadrenal tissue (P), was noticed. In zona fasciculata (F), which has better marked vascular tissue paths, dark corticocytes dominate, as well as in zona reticularis (R), in which hyperemia is strong too. In all cortical zones relative small number degenerated cells was noticed. In medulla (M), together with hyperemia, there are hypertrophic medullocytes and a small number of degenerated cells (Figure 1). In shampinealectomized and melatonin treated totally irradiated rats there is a gross periadrenal tissue (P) with cortex prolapses in which dark cells dominate. In zona glomerulosa (G), there is hyperplasia and sporadic hypertrophy; zona fasciculata (F) looks uneven with sporadic disintegrated cells; in zona re-ticularis (R) there is hyperplasia with small cortico-cytes. In all parts of cortex, hyperemia of blood vessels is pronounced. Medulla (M) is extremely hyperemic (Figure 2). Data that relate to the volume of nuclei of certain cortex zones and medulla of suprarenal glands in studied animals are shown in Table 1. Based on those results, it is obvious that there are statistically significant differences between the compared groups.
FIGURE 1.
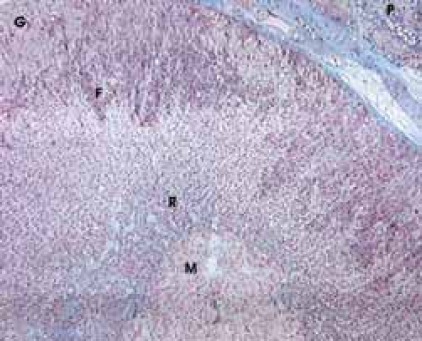
Pinealectomized and melatonin treated irradiated rats. Azan, x 100
FIGURE 2.
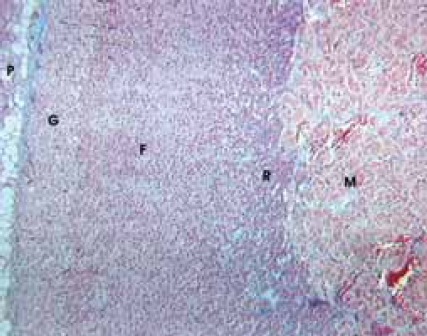
Shampinealectomized and melatonin treated irradiated rats. Azan, x 60.
TABLE 1.
Karyometric analysis (x±Sx) of cortical zones and medulla of suprarenal glands in pinealectomized, shampinealectomized and melatonin treated and irradiated rats
DISCUSSION
Histological qualitative analysis in melatonin treated and irradiated rats shows a surprising preservation of organ structures in all irradiated animals, having in mind the dose that was applied. Healing of radio lesions at 17th post irradiation day is obvious in both studied groups. Morphologic indicators of reactive changes of the progressive type are expressed in the preserved structures of corticocytes and medullocytes, occasional hypertrophy and hyperplasia and pronounced hyperemia of all structures in the region of suprarenal gland. Those results correlate with results from Théret et al. (11) that relate to a significant radio resistance of ACTH cells of adenohypophisis of totally irradiated Wistar rats, as well as to our previous study on qualitative histological analysis of rat’s suprarenal gland’s reactivity in irradiation stress (7). Still, our quantitative results undoubtedly point a significant difference in the size of glandular cells’ nuclei among studied groups. In those studied structural areas of suprarenal gland, nuclei of glandular cells are significantly bigger in the group of shampinealectomized animals. Increased volume of nuclei, which is a morphological indicator of an increased functional activity, Sanchez del Campo (12) finds in the cortex and zona reticularis, while Guillot et al. (9), in their experiment, find it in all layers of cortex and medulla of the suprarenal gland and was mostly pronounced in medulla and zona fasciculata. Comparing our previous studies (7) with results from this study, it is evident that the presence of pineal gland together with exogenous melatonin, modulates the reactivity of suprarenal gland in a positive direction. Besides, reactive cell changes were found such as hyperplasia and hypertrophy, including statistically significant bigger nuclei in all structural areas, with hyperemia which is especially pronounced in the medulla. We will presume that, under those conditions and to a certain extent, effects of endogenous and exogenous melatonin are suming up. The base for such presumption we found in recently published paper by Torres-Farfan et al. from 2003 (13), which deals with the existence of specific receptors for melatonin in the suprarenal gland.
CONCLUSION
-Suprarenal gland has significant potentials of reactivity to stress in the model of total irradiation.
-Exogenous melatonin has protective effect on suprarenal gland, which is potentiated by the presence of pineal gland and is expressed by progressive reactive changes.
REFERENCES
- 1.Šuško I, Mornjaković Z, Aličelebić S, Ćosović E, Beganović A. Retínalníi pinealni melatoninod cirkadijalnog signala do terapijske upotrebe. Med. Arh. 2004;58(1):61–64. [PubMed] [Google Scholar]
- 2.Ellis C, Barfuss DW, Kampen K. Modifications of rat testicular response to X-irradiation by various lighting regimes. 1. The pineal gland and melatonin synthesis as a possible mediator of the response. Life Sci. 1970;9(I):1011. doi: 10.1016/0024-3205(70)90124-4. [DOI] [PubMed] [Google Scholar]
- 3.Hasan SS, Singh PM, Pandey SN, Prasad GC. Trial of a new compound as a chemical radioprotector and its effect on melatonin. J. Nucl. Med. All Sci. 1980;24(3-4):135–137. [PubMed] [Google Scholar]
- 4.Kundurović Z, Šćepović M, Mornjaković Z. Histological characteristics of the thyroid gland in pinealectomized irradiated rats. Veterinaria. 1991;40(1-2):143–150. [Google Scholar]
- 5.Mornjaković Z, Šćepović M, Kundurović Z. Morphometric analysis of the testis in pinealectomized and irradiated rats. Folia Anatomica Yugoslavica. 1989-1990;20(1):47–51. [Google Scholar]
- 6.Devečerski DV. Histofiziološke odlike nadbubrežne žlezde nakon epifizektomije. Disertacija za doktorat medicinskih nauka. Forum, Novi Sad. 1975 [Google Scholar]
- 7.Mornjaković Z, Aličelebić S, Šuško I, Beganović A, Ćosović E, Arslanagić R. Udio pinealne žlijezde u histofiziološkim promjenama nadbubrežne žlijezde u totalno ozračenih pacova. Veterinaria. 2003;52(1-4):61–74. [Google Scholar]
- 8.Sewerynek E, Lewinski A, Choczaj V, Szkudlinski M, Szymc-zykiewicz P. Melatonin-induced suppression of the pinealecto-my-stimulated rat adrenal cortex mitotic activity. Cytobios. 1991;65(261):115–122. [PubMed] [Google Scholar]
- 9.Guillot MD, Guillot A, Martiinez-Soritano F, Smith-Agreda V. Morphological changes of the adrenal gland following soft-laser irradiation. Anat. Anz. 1988;167(4):286–296. [PubMed] [Google Scholar]
- 10.Tonutti E, Bahner F, Muchke E. Die Veränderungen der nebennierenrinde der maus nach hypophysektomic und nach ACTH bechandlung, quantitativ betrachtet am verhalten der zellkern. Endocrinologie. 1954;31:266–284. [PubMed] [Google Scholar]
- 11.Théret CG, Lavedan J, Lalégerie P, Comlan G. Modifications des ultrastructures de l’adénohypophyse de rongeurs apris totale on segmentane an radiocobalt. CRSR. 1971;165(7-8):1546–1549. [PubMed] [Google Scholar]
- 12.Sanchez del Campo FJ. Técnica quirúrgica experimental para realizar la pinealectomia en la rata. An Anat Granada. 1971:1–87196. [Google Scholar]
- 13.Torres-Farfan C, et al. Mt1 Melatonin receptor in the primate adrenal gland: inhibit of adrenocorticotropin-stimulated cortisol production by melatonin. J Clin Endocrinol Metab. 2003;88(1):450–458. doi: 10.1210/jc.2002-021048. [DOI] [PubMed] [Google Scholar]


