Abstract
Purpose
The aim of this study was to report on results of uveal melanoma treatment with ruthenium-106 (106Ru) brachytherapy with long-term follow-up, in terms of local tumor control, eye retention rate, radiation retinopathy, and patients’ survival.
Material and methods
Medical records of patients treated with ruthenium plaque due to uveal melanoma at the Department of Ophthalmology, Poznan University of Medical Sciences, Poland, between 1994 and 2014 were retrospectively reviewed.
Results
We identified 126 patients: 53 men, 73 women, mean age 60.04 years (range, 21-89). The largest basal diameter ranged from 4.04 mm to 18.9 mm (median, 10.67 mm), tumor height was 1.9 mm to 7.42 mm (median, 4.8 mm). Median scleral radiation dose was 570 Gy (range, 235-1,500 Gy), median apical dose 100 Gy (range, 60-129 Gy). Median follow-up was 66.5 months (range, 2-261 months). We noted a total of 19 (15%) recurrences. The actuarial rate of recurrence was 9.5% at 3 years, and 13.5% at 5 years postoperatively. Nine (7%) eye globes were lost, median time to enucleation was 5 years. The eye retention rate at 5 years was 92.7% and 81% at 10 years. Forty-three (34%) patients died before the end of the study, 24 (19%) of them due to metastatic disease. Metastatic death was related to: tumor size and TNM stage at presentation (p = 0.002 vs. p = 0.0006, respectively) but not to age, gender, and plaque dosimetry.
Conclusions
106Ru brachytherapy is an effective, globe sparing treatment that provides good tumor control and a high rate of survival. However, some ocular complications tend to appear late post-treatment, and therefore long-term follow-up is advised.
Keywords: brachytherapy, eye, neoplasm, treatment, uveal melanoma
Purpose
Ruthenium-106 (106Ru) is used mainly in Europe for the treatment of ocular tumors up to 6 mm in thickness. It was introduced to ocular oncology in 1970s by Lommatsch [1]. 106Ru is a beta ray emitter particularly suitable to treat small, circumscribed lesions. Its half-life is approximately one year. The radiation is delivered by the means of different episcleral applicators (plaques) coated with silver, which are manufactured in various shapes and diameters. Other radionuclides traditionally used for ophthalmic brachytherapy include iodine-125 (125I) and palladium-103 (103Pd) [1]. 125I was attenuated for the ophthalmic use with specially designed COMS plaques in the United States. In 1991, the use of iodine plaques was also a subject of a randomized study comparing the use of the plaques versus surgical removal of the globe with uveal melanoma (COMS study) [2]. It showed no difference in terms of 5-year survival between both arms of the study, and proved the safety of ophthalmic radiation treatment [2,3]. However, the I-125 is gamma emitter and more likely to cause radiation side effects than Ru-106, if same-sized tumors are treated [4].
The aim of this study was to report the long-term results of uveal melanoma 106Ru brachytherapy in terms of local tumor control, eye retention rate, radiation retinopathy, and patients’ survival, based on one center experience.
Material and methods
Consecutive patients that underwent ruthenium brachytherapy for choroidal melanoma with or without ciliary body involvement at the Department of Ophthalmology, University of Medical Sciences, Poznan, Poland, between 1994 and 2014 were included in this study. Four patients with no recorded follow-up visit were excluded from the analysis. Other exclusion criteria comprised iris involvement, which is known to have better prognosis, or if the plaque was applied for other type of tumor (i.e., vascular tumor). Large tumor size (thickness over 6 mm, longest basal diameter > 18 mm) tumor location within 1.0 mm from the optic disk or fovea were considered relative contraindications, and possible adverse effects of ruthenium brachytherapy were explained to the patients before they gave informed consent for the treatment [5,6]. Patients presenting with ciliochoroidal melanomas not eligible for ruthenium brachytherapy, because of tumor thickness and/or its location, were offered other types of treatment [5]. The end of follow-up was defined as the last recorded date of follow-up visit. The survival data were checked with the local and national cancer registry.
The diagnosis of uveal melanoma was made after detailed clinical examination, B-scan ultrasound, and in some patients, fluorescein and/or indocyanine green angiography. The patients were screened for distant metastases at the time of diagnosis with liver ultrasound, chest X-rays, and routine blood tests. All procedures were carried out with the use of 20 mm CCB ruthenium plaque. The treatment protocol aimed to deliver the approximate dose of 100 Gy to the tumor apex with the scleral dose not exceeding 1,000 Gy. With large tumors, higher scleral doses were accepted [5,6,7]. Medical physicist and radiation oncologist were involved in the planning procedure. The surgeries were performed under local or general anesthesia according to the tumor location. Postoperatively, patients were seen 4 weeks after the surgery, 3-6 months, and then on annual basis, if no serious side effects were noted.
Main outcome measures were: metastasis free survival, local recurrence, secondary enucleation, and radiation retinopathy. The local recurrence was defined as the increase of tumor thickness in at least two consecutive ultrasound follow-up measurements, or the increase of basal diameter comparing to preoperative photo at any time following the surgery. The appearance of radiation retinopathy was defined as the presence of retinal hemorrhages and/or lipid exudates with macular edema, or as the appearance of retinal hemorrhages in more than two quadrants or lipid exudates without macular edema.
For statistical analysis, a Statistica for Windows v.12, Serial No. JPZP611B316611AR-B, (StatSoft Inc. Tulsa, OK, USA) was used. For the assessment of differences in nominal variables, the χ2 test was used; for categorical variables, the Mann-Whitney U test was applied. In order to evaluate survival probability according to stage and risk for secondary enucleation, the Kaplan-Meier model was used with log rank test for comparing both groups. Risk factors for metastatic death according to initial tumor characteristics were assessed in Cox univariate analysis. Coefficient p lower than 0.005 was considered statistically significant.
Results
We identified 126 patients: 53 men, 73 women, mean age 60.5 years (range, 21-89). Men were older than women at the time of treatment (p = 0.047). The largest basal diameter of the treated melanomas ranged from 4.04 mm to 18.9 mm (median, 10.67 mm), tumor height ranged from 1.9 mm to 7.42 mm (median, 4.8 mm). The median follow-up was 66.5 months (range, 2-261 months). According to TNM staging, 28 tumors were T1 (22%), 89 – T2 (71%), 8 – T3 (6%), and 1 – T4 (1%). Among 126 treated uveal melanomas, 22 were stage I (18%), 90 – stage IIA (71%), 13 – stage IIB (10%), and 1 – stage IIIA (1%). One patient presented with extrascleral extension of a predominantly ciliary body lesion; 7 years post-treatment, the globe is preserved with the best corrected visual acuity of 0.5. The patients’ characteristics is presented in Table 1.
Table 1.
Patients’ characteristics
| Factor | n | % |
|---|---|---|
| Age | ||
| 20-30 | 4 | 3 |
| 31-40 | 9 | 7 |
| 41-50 | 12 | 10 |
| 51-60 | 35 | 28 |
| 61-70 | 36 | 28 |
| > 70 | 30 | 24 |
| Median | 61 | |
| Gender | ||
| Male | 53 | 42 |
| Female | 73 | 58 |
| Laterality | ||
| Left | 53 | 42 |
| Right | 73 | 58 |
| Tumor location | ||
| Anterior to equator | 50 | 40 |
| Posterior to equator | 76 | 60 |
| Tumor size | ||
| T1 | 28 | 22 |
| T2 | 89 | 71 |
| T3 | 8 | 6 |
| T4 | 1 | 1 |
| Tumor stage | ||
| I | 22 | 17 |
| IIA | 90 | 72 |
| IIB | 13 | 10 |
| IIIA | 1 | 1 |
| Tumor height | ||
| < 3 mm | 13 | 10 |
| 3-5 mm | 56 | 45 |
| > 5 mm | 57 | 45 |
| Largest basal diameter | ||
| < 10 mm | 38 | 30 |
| 10-15 mm | 84 | 67 |
| > 15 mm | 4 | 3 |
Median scleral radiation dose was 570 Gy (range, 235-1,500 Gy), and median apical dose 100 Gy (range, 60-129 Gy). The radiation treatment was delivered in the mean time of 4.2 days (range, 1-10 days). In 47 cases, additional transpupillary thermotherapy was applied. Only one patient (0.8%) complained of postoperative diplopia, which persisted longer than 5 weeks post-treatment. It was successfully managed with prisms. There were 21 patients with tumors thicker than 6 mm in the studied group: 9 men, 12 women, mean age was 57 years (range, 21-85). Median tumor thickness was 6.59 mm (range, 6.12-7.42 mm), median basal diameter 12.49 mm (range, 8.64-18.9 mm). Local tumor control was achieved in 18 (86%) cases. We noted 3 recurrences that were managed with enucleation. Twelve (57%) patients died before the end of the study, 7 (33%) out of metastatic disease.
Local tumor control
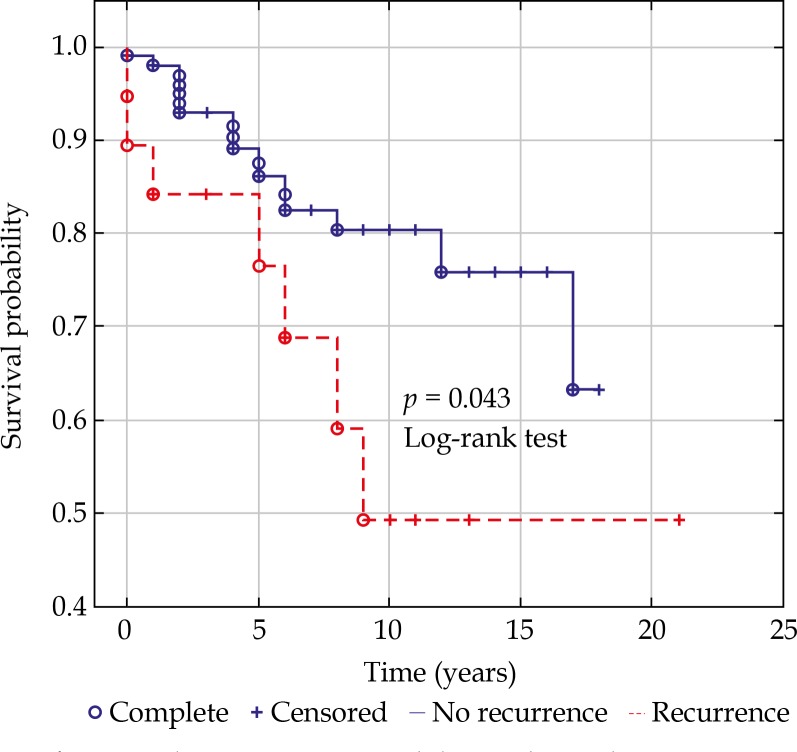
The local tumor control in our series was 89.6% at 3 years, 83.3% at 5 years, and 80% at 10 years post-treatment. We noted a total of 19 (15%) recurrences, median time to recurrence was 24 months. The actuarial rate of recurrence was 9.5% at 3 years, and 13.5% at 5 years postoperatively. The risk of recurrence was associated with: largest basal diameter of the tumor at presentation (p = 0.0281) and the location of the tumor margin within 3 mm to the macula (p = 0.049), but not with tumor thickness (p = 0.897), scleral (p = 0.758), and apex radiation dose (p = 0.266), or the proximity to the optic disk (p = 0.289). Four (21%) recurrences were managed by second ruthenium plaque insertion, 10 (53%) by transpupillary thermotherapy, 4 (21%) by enucleation, and 1 (5%) by endoresection. The local recurrence was associated with poorer survival (Figure 1).
Fig. 1.
Kaplan-Meier curve with log-rank test demonstrating worse prognosis for the patients with a local recurrence
Enucleation rate
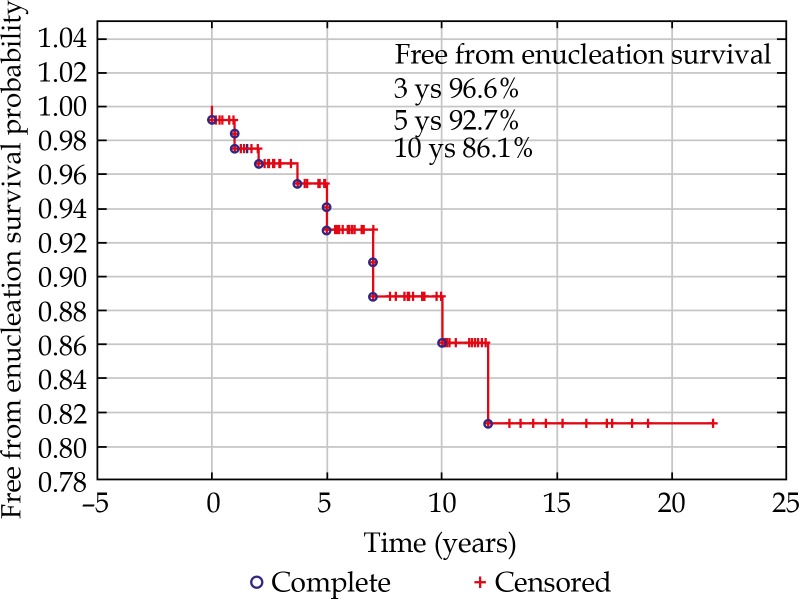
Nine (7%) eyes globes were lost, 5 due to local tumor recurrence, and 4 due to secondary glaucoma. Mean time to enucleation was 5 years. The eye retention rate was 96.6% at 3 years postoperatively, 92.7% at 5 years, and 86.1% at 10 years (Figure 2).
Fig. 2.
The eye retention rate at 3, 5, and 10 years postoperatively
Patients’ survival
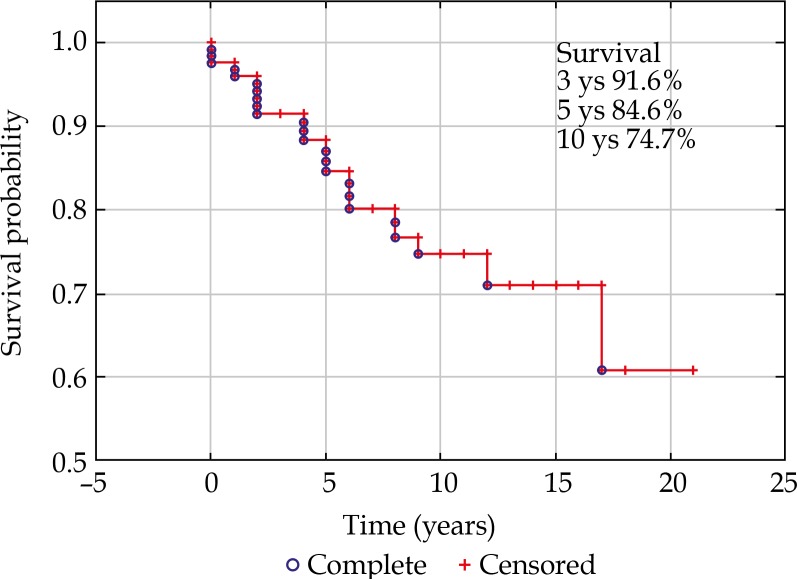
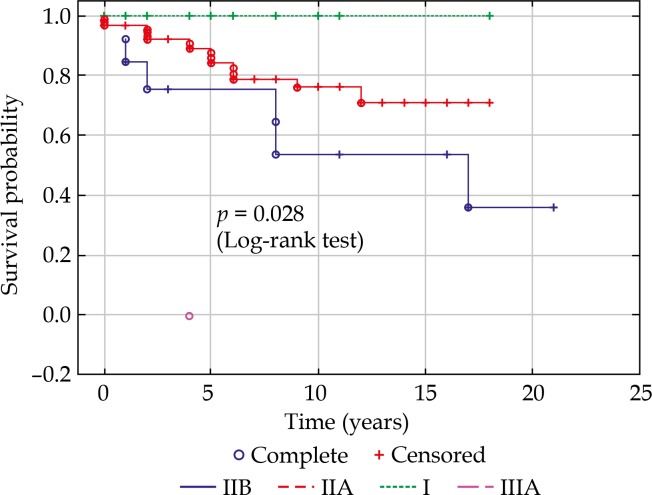
Forty-three (34%) patients died before the end of the study, out of which 24 (19%) due to metastatic disease. Metastatic death was related to: tumor size (TNM) at presentation (p = 0.002), TNM stage (p = 0.0006), but not to age (p = 0.689), largest basal diameter (p = 0.085), or tumor thickness (p = 0.079) alone, or plaque dosimetry: scleral dose (p = 0.181) and apex dose (p = 0.715). Three-year survival in our group was 91.6%, 5-year cancer-free survival reached 84.6% (Figure 3). With regards to stages at 5 years, it was 100% for stage I, 82% for IIA, and 75% for IIB (Figure 4).
Fig. 3.
Disease-free survival for the whole studied group
Fig. 4.
Survival probability with regards to TNM stages at presentation
Radiation retinopathy
Fifty (40%) patients were diagnosed with radiation retinopathy and/or lipid exudation without macular edema during the period of observation (Table 2). Median time to the appearance of first symptoms of radiation damage was 3.5 years. The radiation retinopathy was related to the location of the tumor margin within 3 mm from the fovea (p = 0.047), but not to the largest basal diameter of the irradiated lesion, plaque dosimetry, or time of irradiation (plaque activity). Seventy-two (57%) patients had a useful vision (0.1 Snellen or more) at the last recorded follow-up visit, 36 (29%) patients maintained the vision of 0.5 or more.
Table 2.
Radiation side effects
| Side effects | n |
|---|---|
| Maculopathy | 50 |
| Lipid exudation | 14 |
| Cataract | 7 |
| Vitreous hemorrhage | 9 |
| Neovascular glaucoma | 6 |
Discussion
This study reports the single center experience with the use of episcleral brachytherapy with ruthenium plaques for uveal melanoma during 20 years. The main strength of this study is well-documented long follow-up period, with almost all the follow-up examinations being performed in the same center. The local tumor control in our series was 83.3% at 5 years. This is comparable with what has already been reported by other authors – local tumor control varied from 81.7% [8] to 97% [4], and confirms that ruthenium brachytherapy represents a good treatment option for small and medium-sized melanomas.
Our recurrence rate was 13.5% at 5 years post-treatment, which is acceptable and similar to that reported by other authors: 2% [9], 11.2% [7], 17.3% [10], and 21.2% [11]. The local recurrence was associated with poorer survival, which has already been reported in the literature [12]. The risk of local recurrence in our series was related to largest basal tumor diameter and the posterior location of the tumor. This was also observed by other authors [9,11] and made us review our treatment approach, proposing disinsertion of extraocular muscles to facilitate the plaque positioning under general anesthesia.
Interestingly, the risk of local recurrence was not associated with prescribed doses during the brachytherapy or the time of irradiation. This was also observed by other authors [9,11] who emphasized the necessity of exact plaque positioning. While there are some reports [6] showing that the apical dose might be lower than 100 Gy (70-80 Gy) or higher [7], we considered 100 Gy dose to the tumor apex as safe in terms of local tumor control and radiation side effects. The enucleation rate was 6.3% after 10 years, which is lower to that reported by other authors: 18% [8] and 19.2% [11]. Interestingly, we observed no scleral necrosis despite high scleral doses used in some cases. The main side effect of irradiation was radiation retinopathy that in our series was associated with tumor location within 3 mm from the fovea. The mean time to enucleation was 5 years, showing that most of the postoperative complications tend to appear late post-treatment, and the studies showing shorter follow-ups may underestimate the actuarial rate of side effects.
In the literature there are small series of bigger tumors being irradiated with ruthenium applicators [6,7, 13,14,15], i.e. thicker than 6 mm. Some authors report irradiating tumors thicker than 8 mm [14,15]. In our cohort, there were 21 tumors thicker than 6 mm treated with ruthenium plaque and additional TTT with acceptable clinical outcome.
The survival rate in our group was 89.2% after 5 years, which is as expected with regards to the small or medium size of treated melanomas. The reported overall survival rates for ruthenium brachytherapy vary from 82% [11] and 83.3%[8] to 92% [10]. If divided to stages, the prognosis is more accurate, and proves accuracy and applicability of TNM 8th edition [1,14]. The radiation retinopathy in our series tended to appear slightly later than reported by other authors [1]. We observed some tumors started to present lipid exudation as late as 5 years post-treatment, and this was only possible to document due to long follow-up period of this study.
Conclusions
106Ru brachytherapy provides a good tumor control and relatively high 5-year survival and is an adequate treatment option for uveal melanomas up to 6 mm of height. Nevertheless, some ocular complications tend to appear late post-treatment and therefore long-term followup is advised.
Acknowledgements
The authors acknowledge Stanisław Paradowski, BSc, for his help and support with statistical analysis.
Disclosure
Authors report no conflict of interest.
References
- 1.Seregard S, Damato BE. Uveal malignant melanoma: Management options for brachytherapy. In: Damato B, Singh AD, editors. Clinical Ophthalmic Oncology. Berlin Heidelberg: Springer-Verlag; 2014. pp. 181–196. [Google Scholar]
- 2.Diener-West M, Earle JD, Fine SL, et al. Collaborative Ocular Melanoma Study Group The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969–982. doi: 10.1001/archopht.119.7.969. [DOI] [PubMed] [Google Scholar]
- 3.Rao YJ, Sein J, Badiyan S, et al. Patterns of care and survival outcomes after treatment for uveal melanoma in the post-coms era (2004-2013): a surveillance, epidemiology, and end results analysis. J Contemp Brachytherapy. 2017;9:453–465. doi: 10.5114/jcb.2017.70986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takiar V, Voong KR, Gombos DS, et al. A choice of radionuclide: Comparative outcomes and toxicity of ruthenium-106 and iodine-125 in the definitive treatment of uveal melanoma. Pract Radiat Oncol. 2015;5:e169–176. doi: 10.1016/j.prro.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 5.American Brachytherapy Society – Ophthalmic Oncology Task Force The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13:1–14. doi: 10.1016/j.brachy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Tarmann L, Wackernagel W, Avian A, et al. Ruthenium-106 plaque brachytherapy for uveal melanoma. Br J Ophthalmol. 2015;99:1644–1649. doi: 10.1136/bjophthalmol-2015-306666. [DOI] [PubMed] [Google Scholar]
- 7.Marinkovic M, Horeweg N, Fiocco M, et al. Ruthenium-106 brachytherapy for choroidal melanoma without transpupillary thermotherapy: similar efficacy with improved visual outcome. Eur J Cancer. 2016;68:106–113. doi: 10.1016/j.ejca.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Bergman L, Nilsson B, Lundell G, et al. Ruthenium brachytherapy for uveal melanoma, 1979-2003, survival and functional outcomes in the Swedish population. Ophthalmology. 2005;112:834–840. doi: 10.1016/j.ophtha.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Damato B, Patel I, Campbell IR, et al. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol. 2005;63:385–391. doi: 10.1016/j.ijrobp.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Perri P, Fiorica F, D’Angelo S, et al. Ruthenium-106 eye plaque brachytherapy in the conservative treatment of uveal melanoma: a mono-institiutional experience. Eur Rev Med Pharmacol Sci. 2012;16:1919–1924. [PubMed] [Google Scholar]
- 11.Rouberol F, Roy P, Kodjikian L, et al. Survival, anatomy, and functional long-term results in choroidal and ciliary body melanoma after ruthenium brachytherapy (15 years’ experience with beta-rays) Ophthalmology. 2004;137:893–900. doi: 10.1016/j.ajo.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Ophthalmic Oncology Task Force Local Recurrence Significantly Increases the Risk of Metastatic Uveal Melanoma. Ophthalmology. 2016;123:86–91. doi: 10.1016/j.ophtha.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Fili M, Lundell G, Lundell M, et al. High dose rate and low dose rate ruthenium brachytherapy for uveal melanoma. No association with ocular outcome. Br J Ophthalmol. 2014;98:1349–1354. doi: 10.1136/bjophthalmol-2014-305055. [DOI] [PubMed] [Google Scholar]
- 14.Naseripour M, Jaberi R, Sedaghat A, et al. Ruthenium-106 brachytherapy for thick uveal melanoma: reappraisal of apex and base dose radiation and dose rate. J Contemp Brachytherapy. 2016;8:66–73. doi: 10.5114/jcb.2016.57818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiserman N, Kaiserman I, Hendler K, et al. Ruthenium-106 plaque brachytherapy for thick posterior uveal melanomas. Br J Ophthalmol. 2009;93:1167–1171. doi: 10.1136/bjo.2009.157701. [DOI] [PubMed] [Google Scholar]