Abstract
Retinoic acid (RA) in association with chemotherapy or with arsenic trioxide (ATO) results in high cure rates of acute promyelocytic leukemia (APL). We show that RA-induced differentiation of human leukemic cell lines and primary blasts dramatically increases their sensitivity to endoplasmic reticulum (ER) stress-inducing drugs at doses that are not toxic in the absence of RA. In addition, we demonstrate that the PERK pathway, triggered in response to ER stress, has a major protective role. Moreover, low amounts of pharmacologically induced ER stress are sufficient to strongly increase ATO toxicity. Indeed, in the presence of ER stress, ATO efficiently induced apoptosis in RA-sensitive and RA-resistant APL cell lines, at doses ineffective in the absence of ER stress. Our findings identify the ER stress-related pathways as potential targets in the search for novel therapeutic strategies in AML.
Introduction
Acute promyelocytic leukemia (APL) is characterized by the chromosomal translocation t(15;17) resulting in the expression of fusion protein PML-RARα,1 which impedes the differentiation program driven by RARα, and arrests the cells at the promyelocytic stage. APL is successfully treated by all-trans retinoic acid (RA) in combination with arsenic trioxide (ATO) or by RA and chemotherapy.2 RA is able to activate RARα-mediated transcription, thereby resuming differentiation,3 and to target PML-RARα for degradation.4 ATO targets the PML moiety of the hybrid protein synergizing with RA in PML-RARα degradation and induces apoptosis of APL blasts via caspase and reactive oxygen species (ROS)-mediated mechanisms.4 Two randomized studies have recently shown the advantage of the RA-ATO combination over conventional RA plus chemotherapy establishing the former approach as the new standard at least in non-high-risk patients.5, 6 Despite showing a considerably improved safety profile, either RA or ATO are not devoid of toxicity, with the most important and potentially life-threatening one being the so-called RA differentiation syndrome.2, 5, 6, 7
RA drives leukemic blasts toward granulocytic differentiation, characterized by the production of secretory granules. Increased secretory protein folding demands in the endoplasmic reticulum (ER) can cause imbalance between the folding capacity and the amount of unfolded client proteins, defined as ER stress. To cope with stress, the ER triggers a series of pathways, emanating from three ER transmembrane receptors, ATF6, IRE1α and PERK, collectively known as the unfolded protein response (UPR). The UPR aims at restoring protein folding homeostasis8 but under conditions of prolonged stress, it activates pro-apoptotic signaling pathways among which the ATF4/CHOP/GADD34 axis has a major role.9, 10 We hypothesized that the RA-induced differentiation of APL cells and the consequent rise in the ER activity render them particularly sensitive to ER stress, shifting the balance of the UPR from pro-survival to pro-apoptotic. Here we show that the APL cell line NB4 and primary human APL cells become sensitive to pharmacologically generated ER stress upon differentiation induction by RA and that such sensitivity mainly involved the PERK pathway. Furthermore, we observed a strong synergistic cytotoxic effect of ATO and the ER stress-inducing drug Tunicamycin (Tm), in both RA-sensitive and RA-resistant APL cell lines.
Materials and methods
Cell lines and primary leukemic blasts cultures and treatments
The drug doses to treat NB4 and NB4-R4 cell lines were as follows: 10 nM RA, 50ng/ml Tm, 17 μM Guanabenz Acetate, 300 nM GSK2606414 (GSK), 200 or 500 nM ATO and 20 mM N-acetyl-cysteine. Bone marrow samples were seeded in Methocult 4035 medium with or without 10 nM RA, 50 ng/ml Tm and 500 nM ATO alone or in combination.
Cell death, cell cycle and cell differentiation
Cell death was evaluated by the propidium iodide exclusion assay. Cell cycle of NB4 and NB4-R4, of APL primary blasts and of healthy bone marrow cells, growth in Methocult media for 11 or 8 days respectively, was analyzed by flow cytometry. Cell differentiation was assessed by morphological analysis of cytospin preparations stained with Wright–Giemsa stain and by flow cytometry after staining with antibody against myeloid markers (CD11b and CD14).
Immunofluorescence analysis
Confocal microscopy was performed on cytospin preparations stained first with primary anti-calreticulin, anti-calnexin, anti-BiP or anti-PML antibodies, then with anti-rabbit Alexa Fluor-488 or anti-mouse Alexa Fluor-555.
Quantitative reverse transcription-PCR
Quantitative RT-PCR was analyzed by the ΔΔCt method using histone 3 as endogenous control for standardization.
Western blotting
Twenty to 40 μg of total protein extract were separated by SDS-polyacrylamide gel electrophoresis, with or without the addition of 50 mM DDT followed or not by boiling 5′ for western blotting in reducing or non-reducing conditions, respectively.
Lentiviral transduction
Lentiviral particles containing the short hairpin RNA targeting CHOP or the non-silencing control sequence were prepared in HEK293 cells using the GIPZ lentiviral short hairpin RNA and the packaging vectors described in De Palma et al.46 The cells successfully transduced were selected by the addition of puromycin in the culture medium for 48 h.
Results
RA-induced granulocytic differentiation sensitizes NB4 cells to ER stress
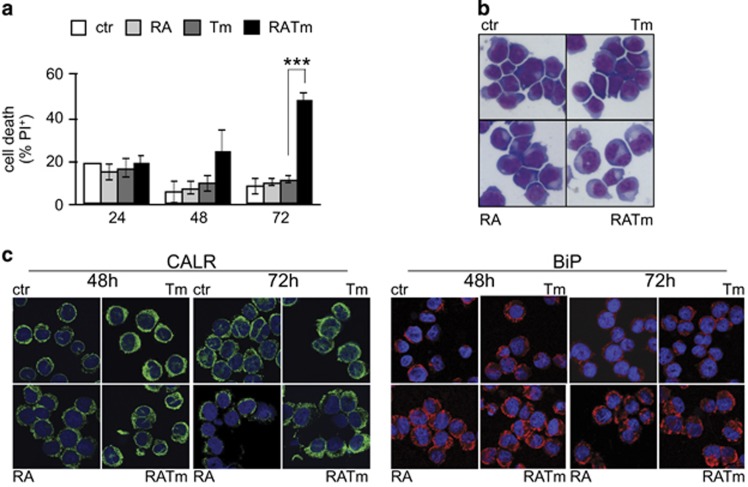
To assess whether RA-driven differentiation would sensitize APL cells to ER stress, we treated the human APL cell line NB4 with RA and the N-glycosylation inhibitor Tm, an ER stress inducer. After 72 h of combined treatment, the cells reached a much lower cellular density than the untreated control (Supplementary Figure S1a). Although the effect on the proliferation rate was negligible in the presence of RA plus Tm (Supplementary Figure S1b), we observed a high percentage of apoptotic cells at 72 h (Figure 1a and Supplementary Figure S1c), using low doses of Tm that alone exhibited only a slight effect on cellular density and none on cell viability. A drug washout experiment suggested that the cells that did not respond to RA plus Tm were able to recover (Supplementary Figure S1d). We obtained similar results generating ER stress with the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor Thapsigargin, ruling out specific effects of Tm (Supplementary Figure S1e). Low concentrations of RA were able to affect NB4 cells resistance to Tm or Tg. Indeed, we used a physiological concentration of RA that was sufficient to induce differentiation (although incomplete), as indicated by the slower proliferation rate and by the increased expression of the granulocytic marker CD11b (Supplementary Figures S1a and f). Morphological analysis confirmed the differentiation of RA-induced cells, evidenced by cytosol enlargement and decreased basophilia with respect to control cells (Figure 1b). We observed that cytosol enlargement was even more evident in the cells treated with RA plus Tm. As ER stress causes ER dilatation, we examined its morphology performing immunofluorescence staining of the ER chaperones Calreticulin and HSPA5/BiP (Figure 1c). As expected during granulocytic differentiation, we observed early expansion of the ER followed by its regression. Cells treated with Tm alone showed swelled ER at 48 h that reverted to normal appearance at 72 h, in accordance with resolution of stress. On the contrary, induction of ER stress in differentiating cells caused further expansion of the ER up to 72 h following treatments. These results show that RA-induced differentiation strongly sensitizes the APL cells NB4 to amounts of ER stress that are easily managed in growth conditions.
Figure 1.
RA-induced differentiation sensitizes NB4 cells to ER stress. (a) NB4 cells treated with 10 nM RA and 50 ng/ml Tm, alone or in combination as indicated, were analyzed for propidium iodide (PI) uptake as an indication of cell death (n=3±s.e.m.; Student’s T-test ***P-value<0.005; full analysis of variance (ANOVA) reported in Supplementary Table 1). (b) Morphological analysis of NB4 treated for 72 h. (c) The same cells were stained with anti-calreticulin (CARL) and anti-GRP78 (BiP) antibodies, and analyzed by confocal microscopy.
RA sensitizes APL primary blasts to ER stress
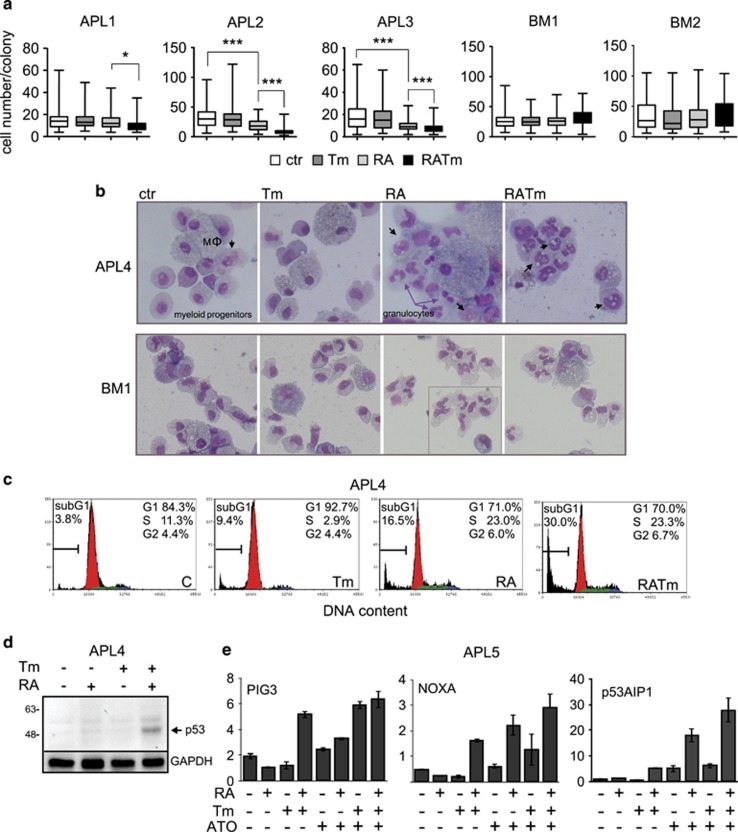
Next, it was important to determine whether freshly isolated differentiating human primary APL blasts showed increased sensitivity to ER stress. Colony-forming unit assays revealed that RA-treated primary cells produced smaller colonies upon induction of ER stress in comparison with cells treated with RA and Tm alone or untreated (Figure 2a and Supplementary Figure S2a), although the total number of colonies was the same (data not shown). Importantly, treatment with RA and Tm in combination showed no toxicity on mononucleated bone marrow cells obtained from healthy donors (Figure 2a and Supplementary Figure S3a), indicating higher sensitivity of APL cells to ER stress. We found no difference in the differentiation pattern (Figure 2b and Supplementary Figure S2c) but a slower proliferation rate (Supplementary Figure S2b) in APL cells treated with RA plus Tm relatively to those treated with RA only. Morphological analysis revealed the presence of numerous vacuoles in most of the APL cells treated with RA and Tm, suggesting apoptosis, but not in healthy cells (Figure 2b and Supplementary Figure S2c). Accordingly, cell cycle analysis demonstrated a higher percentage of apoptotic cells in the APL samples treated with RA and Tm than in those treated with RA alone (Figure 2c and Supplementary Figure S3b). As a further control of toxicity of the combination of RA and Tm on normal cells, peripheral blood mononucleated cells and healthy bone marrow cells were seeded in liquid culture with the addition or not of RA and Tm alone or in combination. After 72 h cell death and the relative percentage of lymphocytes and myeloid cells were evaluated by flow cytometry, finding no differences between control and treated cells (Supplementary Figure S3c and d). Previous studies have demonstrated in both APL murine models and APL patients that RA- or ATO-dependent degradation of PML-RARα and the consequent re-emergence of PML nuclear bodies, activates tumor protein p53, a condition necessary to achieve disease eradication in mouse.11 The p53 pathway is activated in response to multiple stresses, including ER stress.12 Therefore, we analyzed the p53 pathway activation in primary APL samples, expressing wt p53 (Supplementary Table 2), in response to RA and/or ATO and Tm combined treatment. We observed that although there was no activation of the pathway with RA or Tm alone at the low doses used in our experiments, the combination of the two drugs stabilized the p53 protein and increased the expression of the p53 targets NOXA, PIG3 and p53AIP (Figures 2d and e). Altogether, these observations indicate that primary APL blasts, treated ex vivo, are more sensitive to ER stress upon differentiation induced by RA.
Figure 2.
RA sensitizes APL primary blasts to ER stress. (a) APL blasts isolated from three patients’ bone marrow (APL1, APL2 and APL3) were treated in semisolid medium for 8 days. Bone marrow mononucleated cells isolated from two healthy donors (BM1 and BM2) were treated in semisolid medium for 4 days. The box plots report the distribution of the number of cells forming the colonies (APL1 n⩾40, APL2 and APL3 n⩾200 for each treatment; Student’s T-test *P-value<0.05, ***P-value<0.005; BM1 n=200; BM2 n⩾120; analysis of variance (ANOVA) presented in Supplementary table 1). (b) Morphological analysis of patient APL4 cells and of healthy donor BM1 cells isolated from 8 days colonies obtained as in a. The black arrows indicate cell vacuoles, MΦ macrophages, the inset in BM1 RA panel delimits cells from another field. Quantification of the different cell types and of vacuolation is shown in Supplementary Figure 2c. (c) Cell cycle analysis of the same cells to evaluate cell death as subG1 DNA content. (d) Protein extracts of cells isolated from patient APL4, treated in semisolid medium for 4 days, were analyzed by western blot analysis for the expression of p53 protein. GAPDH was used as loading control. (e) Total RNA from cells isolated from patient APL5, treated as in d, with the addition of ATO (see below, Figure 6), for 4 days were analyzed by quantitative reverse transcriptase-PCR (qRT-PCR) for the expression of the p53 targets PIG3, NOXA and p53AIP1.
Increased sensitivity of differentiating NB4 cells to ER stress correlates with the strength of UPR activation
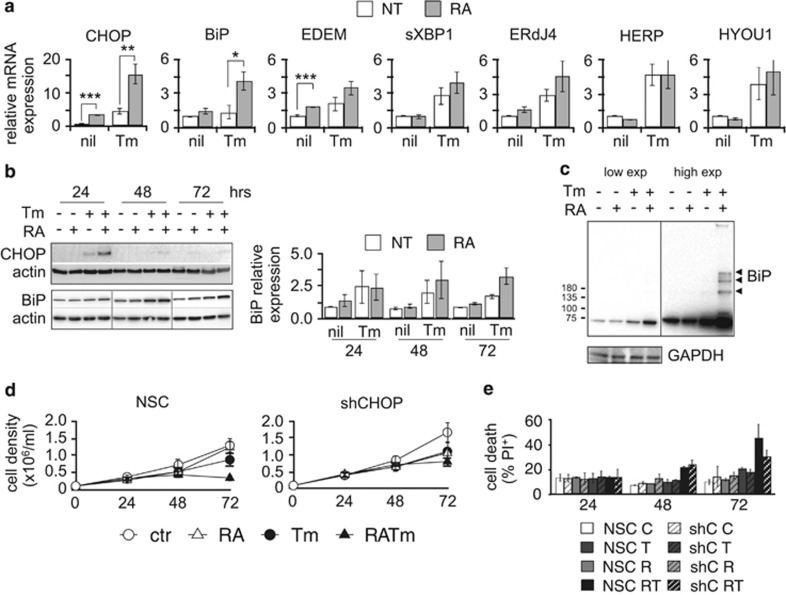
To clarify the molecular mechanisms underlying the inability of differentiating NB4 to cope with low levels of ER stress, we investigated UPR activation. We measured the expression of genes generally upregulated by the UPR. Treatment with Tm induced the expression of most of the UPR markers examined in cells cultured in growth medium but the expression of CHOP and BiP was significantly increased in differentiating cells (Figure 3a). CHOP protein expression peaked 24 h upon treatment decreasing completely at later time points. BiP protein expression increased in a similar manner in cells treated with Tm alone or with Tm and RA up to 48 h, decreasing at 72 h in the cells treated with Tm only. On the contrary, its expression remained higher in cells undergoing combined treatment (Figure 3b). As BiP is a main ER chaperone, binding unfolded proteins to retain them in the ER,13 an increase in ER stress would cause BiP to form more complexes with unfolded client proteins. Indeed, western blot analysis in non-reducing conditions revealed the presence of BiP-containing complexes in the cells treated with RA and Tm (Figure 3c). These observations, together with the swelling pattern of the ER described in Figure 1c, support the conclusion that differentiating NB4 cells are not able to overcome the stress induced by Tm compared with those not stimulated by RA. Moreover, the higher expression of the pro-apoptotic protein CHOP compared with UPR factors mostly involved in the recovery of homeostasis, suggests a shift of the response from pro-survival to pro-apoptotic. To understand the contribution of CHOP in the apoptosis of NB4 cells treated with RA and Tm, we generated NB4 cells stably expressing a short hairpin against CHOP mRNA (NB4-shCHOP) (Supplementary Figure S4a and b). These cells were more resistant to the combined treatment than the non-silencing-control cells (NB4-NSC) (Figures 3d and e). Although we were not able to achieve a strong silencing of CHOP, the results we obtained suggest that it has a role in driving ER stress-related death of differentiating NB4 cells.
Figure 3.
Increased sensitivity of differentiating NB4 cells to ER stress correlates with the strength of UPR activation. (a) Quantitative reverse transcriptase-PCR (qRT-PCR) for the expression of UPR target genes from NB4 samples treated for 24 h (n=4±s.e.m.; Student’s T-test *P-value<0.05, **P-value<0.02, ***P-value<0.005; analysis of variance (ANOVA) presented in Supplementary Table 1). (b) Western blot analysis of total protein extracts from NB4 cells for CHOP and BiP proteins. Actin was used as loading control. The histogram reports the average quantification of BiP protein (n=3±s.e.m.). (c) Western blotting in non-reducing conditions of total protein extracts from NB4 cells treated for 72 h. A shorter exposure is shown on the left, a longer one on the right. The black arrowheads point to BiP complexes. GAPDH was used as loading control. (d) Growth curve and (e) propidium iodide (PI) uptake analysis of NSC and shCHOP NB4 cells (n=2±s.e.m., ANOVA analysis in Supplementary Table 1).
Attenuation of global translation protects differentiating NB4 cells from ER stress-induced death
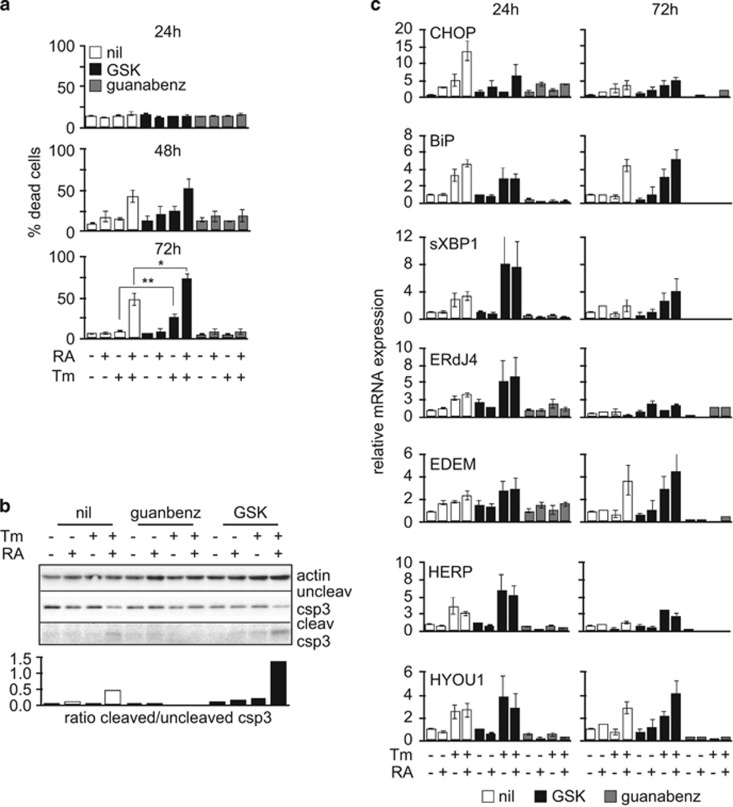
We then wanted to understand the role had by the different UPR pathways in sensitizing differentiating NB4 to ER stress. Thus, we repeated the experiments described above in the presence of specific inhibitors of UPR key signaling proteins. Upon activation, PERK phosphorylates the translation initiation factor eIF2α, thus attenuating global protein synthesis in order to reduce the load of newly synthetized proteins into the ER. Phosphorylation of eIF2α leads to selective translation of the transcription factor ATF4 that regulates the expression of genes involved in protein folding, autophagy, antioxidant response and apoptosis. ATF4 targets CHOP that in turn promotes the expression of PPP1R15A/GADD34, the regulatory subunit of the protein phosphatase PP1c that de-phosphorylates eIF2α restoring translation.14 We inhibited PERK with the GSK2606414 (GSK) compound15 and GADD34 with its inhibitor Guanabenz.16, 17 Inhibition of PERK further increased the sensitivity of NB4 differentiating cells to ER stress, whereas Guanabenz blunted the toxicity of the combination of RA and Tm (Figure 4a and Supplementary Figure S5a). The ratio between cleaved and uncleaved caspase 3 confirmed a higher apoptotic rate in NB4 cells exposed to RA and Tm in the presence of GSK than in those treated with RA and Tm only (Figure 4b). With the exception of the downregulation of CHOP and BiP expression, we noticed a slightly increased activation of the UPR following PERK inhibition. Inhibition of GADD34 significantly reduced the expression of UPR target genes, suggesting reduced ER stress (Figure 4c). Inhibition of IRE1α by the 4 μ8 compound showed no significant effects (Supplementary Figure S5b and c) implying that Ire1α pathway has no major role in this context. Considering the opposite effects of PERK or GADD34 inhibition, we conclude that the attenuation of translation is important for survival of differentiating NB4 cells in the context of ER stress.
Figure 4.
Attenuation of global translation protects differentiating NB4 cells from ER stress-induced death. (a) Propidium iodide (PI) uptake analysis of NB4 cells treated with RA and/or Tm, in the absence (nil) or presence of the PERK inhibitor GSK (300 nM), or of the GADD34 inhibitor Guanabenz (17 μM) (n=4±s.e.m.; Student’s T-test *P-value<0.05, **P-value<0.02; analysis of variance (ANOVA) presented in Supplementary Table 1). (b) Western blot analysis to assess the ratio between cleaved and uncleaved caspase-3. (c) Quantitative reverse transcriptase-PCR (qRT-PCR) for UPR target genes from samples treated as in (a) (n=3±s.e.m. at 24 h; n=2±s.e.m. at 72 h; ANOVA analysis in Supplementary Table 1).
ER stress does not impair RA-driven PML-RARα degradation
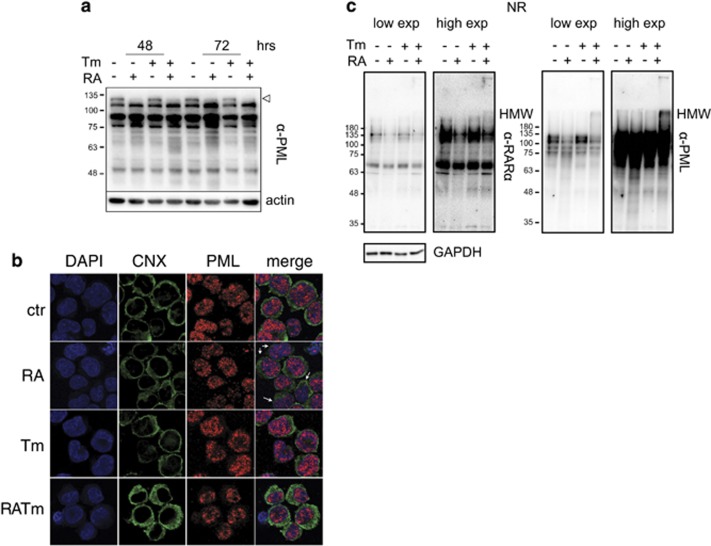
The oncogenic fusion protein PML-RARα modifies the function of the transcription factor RARα and of the tumor suppressor PML that is the central component of the PML nuclear bodies (PML-NBs).18 By heterodimerizing with PML, PML-RARα disrupts the PML-NBs and its degradation, driven by RA, restores PML functions and NB structure. As PML-RARα degradation is dependent both on the ubiquitin–proteasome system and on autophagy, and these cellular processes are highly interconnected with the ER functions, we wanted to assess whether drug-induced ER stress in NB4 cells treated with RA could interfere with PML-RARα degradation and PML nuclear distribution. Cells treated with RA or with RA and Tm similarly degraded PML-RARα (Figure 5a). Analysis of PML nuclear distribution by immunofluorescence revealed a slight difference in the microspeckled pattern between samples treated with RA alone and those treated with RA plus Tm (Figure 5b): some of the cells treated with RA showed less microspeckles, in comparison with control or Tm-treated cells, suggesting initiation of NB formation, whereas the cells undergoing combined treatment showed clustering of PML/PML-RARα at the nucleus periphery. However, when we used the pharmacological dose of 1 μM RA the combination with Tm did not affect PML-NBs generation (Supplementary Figure S6). PML-NB genesis depends on intermolecular disulphide bonds formation and this process is promoted by the presence of ROS;18, 19 thus, both PML and PML-RARα are prone to disulphide bond-dependent aggregation and oxidative stress. As the UPR generates oxidative stress we investigated the level of PML-RARα and PML aggregation in NB4 cells treated with 10 nM RA alone or with Tm by non-reducing western blotting. We found that a fraction of PML and to a lesser extent of PML-RARα, formed high-molecular-weight complexes in differentiating cells in conditions of ER stress (Figure 5c). This finding could explain the different distribution of PML/PML-RARα observed by immunofluorescence. Altogether, these results indicate that ER stress does not affect PML-RARα degradation but suggest that it generates a more oxidative environment.
Figure 5.
ER stress does not impair RA-driven PML-RARα degradation. (a) Western blot analysis of total extracts from NB4 cells with 10 nM RA with or without 50 ng/ml Tm, decorated with an anti-PML antibody. The empty arrowhead points to PML-RARα, the other bands are different PML isoforms. Actin was used as loading control. (b) Confocal microscopy analysis of NB4 cells treated for 72 h as in (a) stained with anti-Calnexin (CNX, green) and anti-PML PGM3 (red) antibodies. The white arrows point to differentiating cells with a reduced amount of microspeckles and an irregularly shaped nucleus indicating differentiation. (c) Western blotting in non-reducing conditions of total protein extracts NB4 cells treated as in a for 72 h decorated with an anti-RARα and, after stripping, with an anti-PML antibody. GAPDH was used as loading control.
ER stress and ATO exhibit synergistic toxicity in RA-sensitive NB4 and in RA-resistant NB4-R4 cells
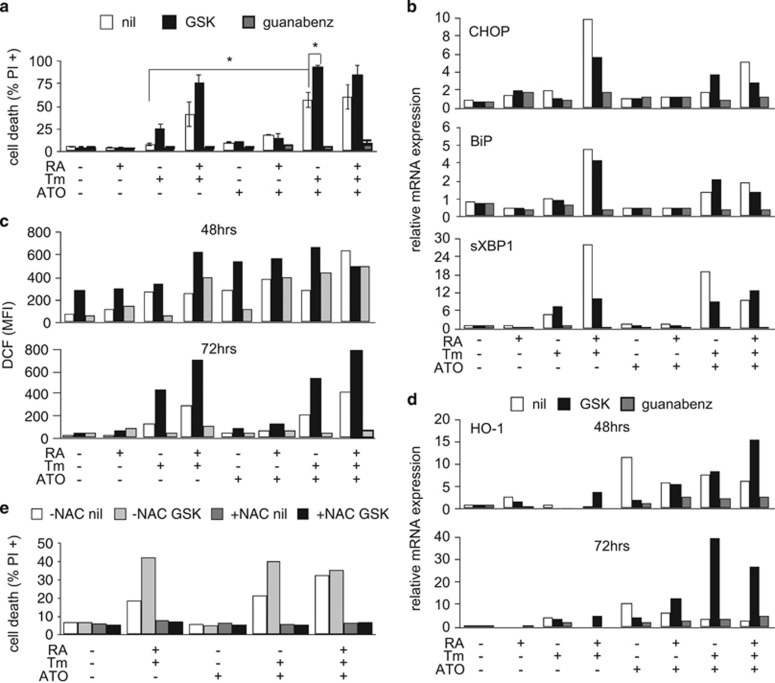
ER stress is closely related to oxidative stress.20 The ATF4/CHOP axis in particular has been shown to induce UPR-related cell death by increasing ROS levels, among other mechanisms.14 As the mechanisms of action of ATO involve generation of ROS,18, 21 we wondered whether ATO could further sensitize cells treated with RA and Tm, possibly by exacerbating oxidative stress. Thus, we treated NB4 cells with RA and/or Tm as described above, in the presence or absence of sub-lethal doses of ATO (200 or 500 nM). ATO and Tm alone did not significantly affect cell viability whereas treatment with both drugs together resulted in apoptotic cell death, independently from the presence or absence of RA (Figure 6a and Supplementary Figures S7a and b). Importantly, the combined treatments resulted not toxic on healthy peripheral blood mononucleated cell and bone marrow mononucleated cells (Supplementary Figure S3). Inhibition of PERK by GSK worsened the toxicity of Tm and ATO whereas inhibition of GADD34 by Guanabenz protected the cells. Not surprisingly, considering the strong link between oxidative and ER stress, ATO amplified the UPR in response to Tm (Figure 6b). Direct measurement of ROS levels showed that Tm and ATO alone, both increased ROS levels at 48 h. Likewise, Tm and ATO with or without RA, and RA and Tm increased ROS levels after 48 h of treatment. Interestingly, although at 72 h the cultures treated with each drug alone recovered from the oxidative stress, those treated with the various combinations did not (Figure 6c). Importantly, perturbation of the PERK pathway by GSK or Guanabenz caused augmented amounts of ROS especially in the cells undergoing combined treatments at 48 h. However, at 72 h, in the presence of GSK the ROS levels remained significantly increased, whereas in the presence of Guanabenz the levels returned comparable to those observed in control cells. Analysis of the expression of heme oxigenase-1 (HO-1) mRNA, a key element in the response to oxidative stress,22 confirmed that inhibition of the PERK pathway leads to an amplified response to Tm in combination with ATO (Figure 6d). To understand the importance of oxidative stress in driving apoptosis in this context, we repeated the same experiments in the presence or absence of the reducing agent N-acetyl cysteine. Strikingly, the presence of N-acetyl cysteine was sufficient to completely counteract the toxic effects of Tm plus ATO and also of Tm plus RA, indicating that generation of oxidative stress is a main driving mechanism leading to apoptosis triggered by ER stress (Figure 6e).
Figure 6.
ER stress and ATO exhibit synergic toxicity in NB4 cells. NB4 cells were treated with 10 nM RA and/or 50 ng/ml Tm and/or 500 nM ATO as indicated, in the absence (nil) or presence of the PERK inhibitor GSK (300 nM), or of the GADD34 inhibitor Guanabenz (17 μM) for the indicated time points. (a) Propidium iodide (PI) uptake analysis after 72 h of treatment (n=2±s.e.m.; Student’s T-test *P-value⩽0.05; analysis of variance (ANOVA) in Supplementary Table 1, see results for Supplementary Figure S6a). (b) Quantitative reverse transcriptase-PCR (qRT-PCR) analysis for the expression of the UPR genes CHOP, BiP and sXBP1 in samples treated for 24 h. (c) Evaluation of ROS levels by flow cytometry following loading of the CM-H2DCFDA oxidative stress indicator. The histogram reports the mean fluorescence values (MFI). (d) qRT-PCR for the expression of the Heme-Oxigenase-1 (HO-1) in the same samples (e) PI uptake analysis of cells treated with the addition of 20 mM N-acetyl -cysteine (NAC) for 72 h.
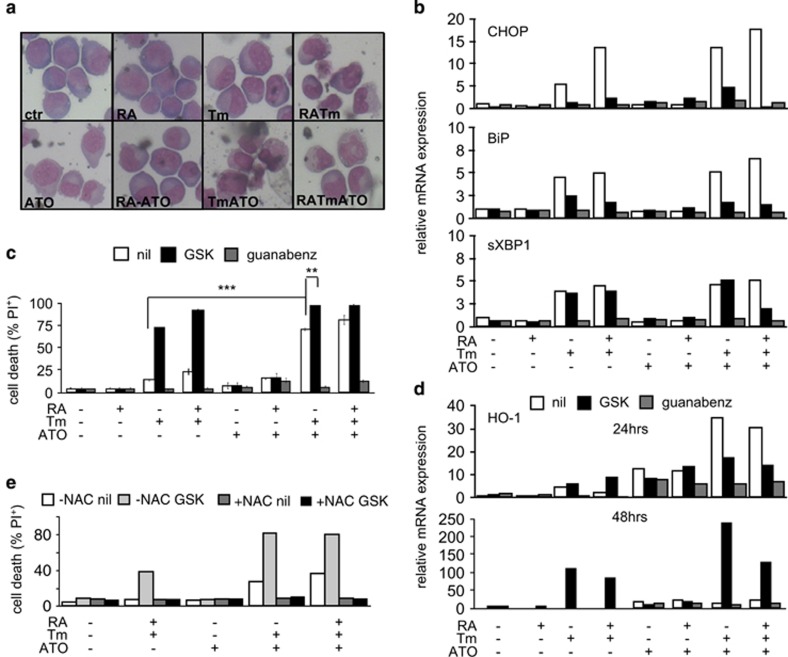
As we did not observe additional toxicity following treatment with the combination of RA with Tm plus ATO, we tested the sensitivity of RA-resistant cells NB4-R4 (R4) to the different treatments. Surprisingly, even though they did not respond to RA alone, as expected and verified by morphological analysis, they nonetheless appeared differentiated in the presence of RA plus Tm (Figure 7a). Indeed the upregulation of the UPR target genes was similar to that observed in NB4 (Figure 7b). A previous study showed that even though this sub-clone has lost regulation of part of the RA-signaling pathway it still retains retinoid induction of some targets.23 Remarkably, R4 cells proved very sensitive to the combination of Tm plus ATO, even when this was used at 200 nM (Figure 7c and Supplementary Figure S7a), and such sensitivity was exacerbated by the inhibition of PERK by GSK, as it was in the case of Tm alone or Tm plus RA. As observed in NB4 cells, inhibition of GADD34 by Guanabenz resulted in total protection from toxicity. Owing to the very high percentage of cell death following treatments, the ROS measurements did not provide clear data (data not shown). However, analysis of the expression of heme oxigenase-1 (Figure 7d) and the striking protection provided by N-acetyl cysteine (Figure 7e) prove that the apoptotic effects of the combinations of RA, Tm and ATO, amplified by GSK, depend on the generation of oxidative stress. Both in NB4 and NB4-R4 we found that in the presence of ER stress there were no differences in PML-RARα and PML degradation induced by ATO at 72 h (Supplementary Figure S7c). In conclusion, these data indicate that the combined effect of low doses of ER stress induced by Tm and of oxidative stress induced by ATO, results in increased toxicity in APL cells, independently from their ability to respond to RA.
Figure 7.
ER stress and ATO exhibit synergic toxicity in RA-resistant NB4-R4 cells. NB4-R4 cells were treated with 10 nM RA and/or 50 ng/ml Tm and/or 500 nM ATO as indicated, in the absence (nil) or presence of the PERK inhibitor GSK (300 nM), or of the GADD34 inhibitor Guanabenz (17 μM) for the indicated time points. (a) Morphological analysis of cells treated for 72 h. (b) Quantitative reverse transcriptase-PCR (qRT-PCR) analysis for the expression of the UPR genes CHOP, BiP and sXBP1 in samples treated for 24 h. (c) Propidium iodide (PI) uptake analysis following 72 h of treatment (n=2±s.e.m.; Student’s T-test **P-value<0.02, ***P-value<0.005; analysis of variance (ANOVA) in Supplementary Table 1, see results for Supplementary Figure S6b). (d) qRT-PCR for the expression of the Heme-Oxigenase-1 (HO-1) in the same samples. (e) PI uptake analysis of cells treated with the addition of 20 mM N-acetyl-cysteine (NAC) for 72 h.
Discussion
The primary aim of the UPR is to restore cellular homeostasis24 but in the event of a prolonged ER stress the response shifts from pro-survival to pro-apoptotic.10 There has been a growing interest in the role of the UPR in cancer25, 26 with the idea of aggravating ER stress to target tumor cells that use the UPR as an adaptive response.27, 28 Importantly, the UPR is activated in about 25% of AML patients,29, 30 identifying a subpopulation where aggravation of ER stress in combination with the existing therapies could be particularly beneficial. How to induce ER stress in vivo is still to be defined. Tm presents general toxicity in vivo31 but low amounts of Tm can be selectively effective on tumor cells in mice.32 Notably, we used a dose of Tm not toxic for the same cells in the absence of RA and 20–100 times lower than the doses commonly used to induce lethal ER stress in cell lines.9, 17, 33 Moreover, drugs targeting different pathways can indirectly exacerbate ER stress and some of these already provided interesting results in AML.34, 35, 36, 37 Another way of aggravating ER stress is to inhibit the pathways having a protective role during the response. We found that a direct inhibition of PERK strongly worsened the toxicity of the combination of RA/ATO and Tm, whereas inhibition of GADD34 was highly protective. Transient inhibition of general translation following PERK activation and eIF2α phosphorylation is a pivotal survival mechanism upon UPR activation9 explaining why the use of Guanabenz reduced ER stress and impeded cell death. Inhibition of PERK and of GADD34 both resulted in decreased CHOP expression but had opposite effects on cell survival, suggesting differing mechanisms of action. In the case of GADD34 inhibition eIF2α phosphorylation is favored and protein synthesis downregulated. This causes a strong reduction of ER and oxidative stress, and promotes survival; the decrease in ER stress shuts down the UPR leading to reduced UPR target gene expression, including CHOP. In contrast, PERK inhibition hinders eIF2α phosphorylation (and CHOP expression that is directly downstream this pathway), leading to sustained protein translation with consequent increase of ER and especially oxidative stress. Our findings are in accordance with those from the laboratories of Randal Kaufman and Anne Bertolotti, who elegantly demonstrated the prominent role of P-eIF2α-mediated inhibition of protein synthesis in survival after ER stress.9, 17
APL is curable with therapies combining RA plus chemotherapy or RA plus ATO. These strategies result in very high rates of long-term remission, however both RA and ATO are associated with some adverse events including differentiation syndrome, which represents a potentially fatal complication.2, 5, 6, 7 Combining RA with ER stress we obtained malignant promyelocytic cell death using a dose of RA hundred times lower than the therapeutic reference range.38 Furthermore, we obtained a strong cytotoxic effect of ATO in combination with Tm using ATO doses 2 to 10 times lower than the therapeutic reference.39 Indeed, we found no toxicity of the combined treatments on bone marrow mononucleated cells from healthy donors (Figures 2a and b, and Supplementary Figure S3). RA- and ATO-mediated PML-RARα degradation, via the proteasome system40, 41 and autophagy,4, 18, 42, 34 is the underlying mechanisms by which APL is cured.4, 41, 43 The possible overload of the proteasome due to ER stress could hamper PML-RARα degradation but this was not the case in our experimental conditions. APL represents a paradigm of targeted therapy but retinoids proved unsuccessful in non-APL AML.44 Importantly, we found that RA sensitized the myeloblastic leukemia cell line HL6045 to ER stress induced by Tm (Supplementary Figure S8), although the molecular mechanisms of action were different from those observed in NB4 cells. Even though these observations need to be investigated further, they suggest the possibility that some types of non-APL cells could also respond to RA in combination with ER stress.
In conclusion, this work explored new strategies that could lead to the development of less toxic and more effective therapies, exploiting the idea of combining different sources of cellular stress to target malignant promyelocytes.
Acknowledgments
This work was supported by A.I.R.C. (StG 4841), FILAS-RU-2014-1020 and ‘Progetti Ateneo’ Sapienza University of Rome to FF, EPIGEN Flagship Project (13/05/R/42) to GB and Italian Ministry of Health (GR-2011-02348567) to GF. We gratefully acknowledge Fabrizio Padula and Stefania De Grossi for technical assistance, the UOC Immunohematology and transfusion medicine of Sapienza University of Rome for providing blood from healthy donors, and Billiana Lozanoska-Ochser for critical reading of the manuscript.
Author contributions
SM, EC, SDP and GF performed experiments and contributed to experimental design. TO, MD and NIN characterized and provided leukemic bone marrow samples. CB and AP characterized and provided healthy bone marrow samples. SM, GB, FLC and FF planned the research strategy and wrote the paper. All authors read and approved the final manuscript.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
The authors declare no conflict of interest.
Supplementary Material
References
- Kakizuka A, Miller Jr WH, Umesono K, Warrell Jr RP, Frankel SR, Murty VV et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 1991; 66: 663–674. [DOI] [PubMed] [Google Scholar]
- Lo‐Coco F, Cicconi L, Breccia M. Current standard treatment of adult acute promyelocytic leukaemia. Br J Haematol 2016; 172: 841–854. [DOI] [PubMed] [Google Scholar]
- Fazi F, Travaglini L, Carotti D, Palitti F, Diverio D, Alcalay M et al. Retinoic acid targets DNA-methyltransferases and histone deacetylases during APL blast differentiation in vitro and in vivo. Oncogene 2005; 24: 1820–1830. [DOI] [PubMed] [Google Scholar]
- de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer 2010; 10: 775–783. [DOI] [PubMed] [Google Scholar]
- Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol 2015; 16: 1295–1305. [DOI] [PubMed] [Google Scholar]
- Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369: 111–121. [DOI] [PubMed] [Google Scholar]
- Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood 2014; 123: 2777–2782. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529. [DOI] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin Y-H, Gildersleeve R, Shan J et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 2013; 15: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 2011; 13: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablain J, Rice K, Soilihi H, De Reynies A, Minucci S, de Thé H. Activation of a promyelocytic leukemia-tumor protein 53 axis underlies acute promyelocytic leukemia cure. Nat Med 2014; 20: 167–174. [DOI] [PubMed] [Google Scholar]
- Bourougaa K, Naski N, Boularan C, Mlynarczyk C, Candeias MM, Marullo S et al. Endoplasmic reticulum stress induces G2 cell-cycle arrest via mRNA translation of the p53 isoform p53/47. Mol Cell 2010; 38: 78–88. [DOI] [PubMed] [Google Scholar]
- Hendershot LM. Immunoglobulin heavy chain and binding protein complexes are dissociated in vivo by light chain addition. J Cell Biol 1990; 111: 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 2013; 15: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 2013; 5: 206ra138. [DOI] [PubMed] [Google Scholar]
- Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 2011; 332: 91–94. [DOI] [PubMed] [Google Scholar]
- Das I, Krzyzosiak A, Schneider K, Wrabetz L, D'Antonio M, Barry N et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 2015; 348: 239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne M, Lallemand-Breitenbach V, Ferhi O, Koken M, Le Bras M, Duffort S et al. PML/RARA oxidation and arsenic binding initiate the antileukemia response of As2O3. Cancer Cell 2010; 18: 88–98. [DOI] [PubMed] [Google Scholar]
- Sahin U, Ferhi O, Jeanne M, Benhenda S, Berthier C, Jollivet F et al. Oxidative stress-induced assembly of PML nuclear bodies controls sumoylation of partner proteins. J Cell Biol 2014; 204: 931–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 2014; 21: 396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata K, Yokoo H, Shimazaki R, Okabe S. Classification of heavy-metal toxicity by human DNA microarray analysis. Environ Sci Technol 2007; 41: 3769–3774. [DOI] [PubMed] [Google Scholar]
- Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 2016; 73: 3221–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Benedetti L, Lamph WW, Nervi C, Miller WH. A retinoid-resistant acute promyelocytic leukemia subclone expresses a dominant negative PML-RARα mutation. Blood 1997; 89: 4282–4289. [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334: 1081–1086. [DOI] [PubMed] [Google Scholar]
- Dufey E, Urra H, Hetz C. ER proteostasis addiction in cancer biology: novel concepts. Semin Cancer Biol 2015; 33: 40–47. [DOI] [PubMed] [Google Scholar]
- Wang J, Yin Y, Hua H, Li M, Luo T, Xu L et al. Blockade of GRP78 sensitizes breast cancer cells to microtubules‐interfering agents that induce the unfolded protein response. J Cell Mol Med 2009; 13: 3888–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica 2012; 2012: 857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer 2004; 4: 966–977. [DOI] [PubMed] [Google Scholar]
- Haefliger S, Klebig C, Schaubitzer K, Schardt J, Timchenko N, Mueller BU et al. Protein disulfide isomerase blocks CEBPA translation and is up-regulated during the unfolded protein response in AML. Blood 2011; 117: 5931–5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt JA, Weber D, Eyholzer M, Mueller BU, Pabst T. Activation of the unfolded protein response is associated with favorable prognosis in acute myeloid leukemia. Clin Cancer Res 2009; 15: 3834–3841. [DOI] [PubMed] [Google Scholar]
- Lee J-S, Zheng Z, Mendez R, Ha S-W, Xie Y, Zhang K, Pharmacologic ER. stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett 2012; 211: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Zhang X, Li H, Huang S, Zhang S, Wang F et al. Tunicamycin enhances the antitumor activity of trastuzumab on breast cancer in vitro and in vivo. Oncotarget 2015; 6: 38912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. IRE1a induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 2012; 16: 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Alex A, Chendamarai E, Balasundaram N, Palani H, David S et al. Rationale and efficacy of proteasome inhibitor combined with arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 2016; 30: 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, van Anken E, Sitia R. Proteostenosis and plasma cell pathophysiology. Curr Opin Cell Biol 2011; 23: 216–222. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Dou QP. Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov Med 2011; 12: 471. [PMC free article] [PubMed] [Google Scholar]
- Ishizawa J, Kojima K, Chachad D, Ruvolo P, Ruvolo V, Jacamo RO et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal 2016; 9: ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muindi JR, Frankel SR, Huselton C, DeGrazia F, Garland WA, Young CW et al. Clinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Cancer Res 1992; 52: 2138–2142. [PubMed] [Google Scholar]
- Firkin F, Roncolato F, Ho WK. Dose‐adjusted arsenic trioxide for acute promyelocytic leukaemia in chronic renal failure. Eur J Haematol 2015; 95: 331–335. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kitamura K, Tanaka K, Omura S, Miyazaki T, Hachiya T et al. Accelerated degradation of PML-retinoic acid receptor α (PML-RARA) oncoprotein by all-trans-retinoic acid in acute promyelocytic leukemia: possible role of the proteasome pathway. Cancer Res 1996; 56: 2945–2948. [PubMed] [Google Scholar]
- Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med 2008; 14: 1333–1342. [DOI] [PubMed] [Google Scholar]
- Isakson P, Bjoras M, Boe SO, Simonsen A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood 2010; 116: 2324–2331. [DOI] [PubMed] [Google Scholar]
- Ablain J, Leiva M, Peres L, Fonsart J, Anthony E, de The H. Uncoupling RARA transcriptional activation and degradation clarifies the bases for APL response to therapies. J Exp Med 2013; 210: 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Redner RL. An ATRActive future for differentiation therapy in AML. Blood Rev 2015; 29: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton WJ, Ahearn M, McCredie KB, Freireich EJ, Stass S, Trujillo J. HL-60 cell line was derived from a patient with FAB-M2 and not FAB-M3. Blood 1988; 71: 242–247. [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Naldini L. In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors. Hum Gene Ther 2003; 14: 1193–1206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.