Abstract
This Commentary highlights the article by Makarava et al that discusses the formation of synthetic prions and the role of substrate levels in their evolution.
Prion diseases are fatal neurodegenerative disorders that can affect both animals and humans. The prion protein (PrP), which is at the root of these diseases, is found in two different conformational versions: the normal isoform, called PrPC (for cellular PrP), and the infectious isoform, called PrPSc (for scrapie PrP).1 One of the chief characteristics of these diseases is that they can be transmitted from one individual to another, a feature that depends on the ability of PrPSc to template conversion of PrPC into additional molecules of PrPSc. A major effort in the prion field has been to reproduce this molecular transformation in vitro by generating synthetic prions in the test tube with the use of recombinant forms of PrP and demonstrating that these molecules are infectious in animal bioassays. In this issue of the American Journal of Pathology, Makarava et al2 make use of such a system to investigate the formation of synthetic prions and their ability to evolve into pathogenic entities during propagation in vivo.
History of Synthetic Prions
Several different systems have been used to generate infectious prions in a test tube. One, dubbed protein misfolding cyclic amplification (PMCA), is a PCR-like system in which a small amount of infectious PrPSc is used to seed conversion of PrPC substrate in a sample of brain homogenate, with repeated cycles of sonication applied to facilitate the reaction.3 Another method, the one used in the present paper, involves partially denaturing purified, recombinant PrPC with a chaotropic agent such as urea or guanidine. Under appropriate conditions of pH, the protein refolds into β-sheet–rich amyloid fibrils that share some biochemical characteristics of PrPSc but are structurally distinct in terms of the length of the protease-resistant core of the fibril.4, 5 The first report of inducing prion disease in animals with the use of fibrils formed in vitro from recombinant PrP appeared in 2004.6 Interestingly, it was shown that different disease phenotypes (defined by incubation times and patterns of neuropathology) were produced by recombinant mouse PrP fibrils that were prepared under different conditions and that displayed distinct morphologic and biophysical properties.7 This observation was interpreted to mean that different strains of synthetic prions had been generated. The initial transmission studies of these synthetic prions used as hosts transgenic mice that over-expressed PrPC, with the idea that higher levels of substrate would facilitate the conversion process. However, a proportion of these animals spontaneously developed neurologic symptoms without inoculation,8 raising the possible criticism that the injected fibrils had simply accelerated an ongoing disease process, rather than initiating a neurodegenerative syndrome de novo.
In 2010, Baskakov and colleagues9 succeed in producing disease in nongenetically engineered hosts (hamsters) that had been inoculated with recombinant hamster PrP fibrils. Notably, however, clinical symptoms did not appear until the second or subsequent passages. In the first passages, Makarava et al2 detected in the brains of the asymptomatic animals a form of misfolded PrP—atypical PrPres—that was distinct from authentic PrPSc.4, 5 Authentic PrPSc gradually appeared in subsequent passages and eventually became the predominant species, correlating with the development of clinical symptoms. The two forms of PrP could be distinguished biochemically by the characteristic size of the fragments produced after proteolytic digestion. This evolutionary process, in which atypical PrPres was gradually transformed into PrPSc with continued propagation,2 led them to put forward a deformed templating model.4, 5
Theories of Prion Evolution
The concept that prions can evolve or adapt dates to the earliest, classical studies of prion transmission, in which it was observed that the biological properties of different strains could change on repeated passage, particularly during transfer from one host species to another.10 In the modern era, two kinds of molecular explanations have been offered to explain prion evolution. One (the cloud hypothesis11, 12) postulates that even cloned prion strains consist of a spectrum of different PrP conformers, with one of the conformers being predominant. On exposure to an altered set of conditions either in vitro or in vivo (eg, a new host), propagation of a different conformer is favored, and this one becomes predominant. This process was likened to Darwinian evolution (selection of pre-existing mutants). A second proposed mechanism is the deformed templating hypothesis of Baskakov and colleagues.4, 5 In this model, the templating process is imperfect and will sometimes result in generation of altered PrP conformers, a process that can be enhanced by changing propagation conditions. This hypothesis could be said to be Lamarckian as opposed to Darwinian (environmentally induced changes that are subsequently propagated). It was pointed out that these two kinds of explanations are not necessarily mutually exclusive.13
Background to the Current Study
As a substrate for their in vitro, prion-generating system, Makarava et al2 used purified, recombinant, hamster PrPC folded into its native α-helical form, which is not infectious and does not induce a neurologic disease when injected intracerebrally into hamsters. They showed previously that they could produce two different kinds of recombinant hamster PrP fibrils, depending on whether they used 0.5 or 2.0 mol/L guanidine to refold the protein.5 The two kinds of fibrils differed morphologically and biophysically and, importantly, displayed different biological properties when inoculated into recipient hamsters.5 The 0.5 mol/L fibrils eventually produced clinical symptoms of scrapie after secondary passage (but not after primary passage), whereas the 2.0 mol/L fibrils never produced disease even after repeated passage. During the first passage of the 0.5 mol/L fibrils, the brains of inoculated animals accumulated primarily atypical PrPres. During the second passage, the atypical PrPres was replaced by conventional PrPSc, correlating with the appearance of symptoms. Makarava et al2 interpreted their results by postulating a two-step model for the evolution of synthetic prions: first, formation of atypical PrPres from endogenous PrPC in a process catalyzed by the inoculated hamster PrP fibrils; and second, a deformed templating step in which continued propagation of atypical PrPres sometimes resulted in formation of PrPSc molecules, which gradually became the predominant species and caused clinical symptoms.
Description of the Current Study
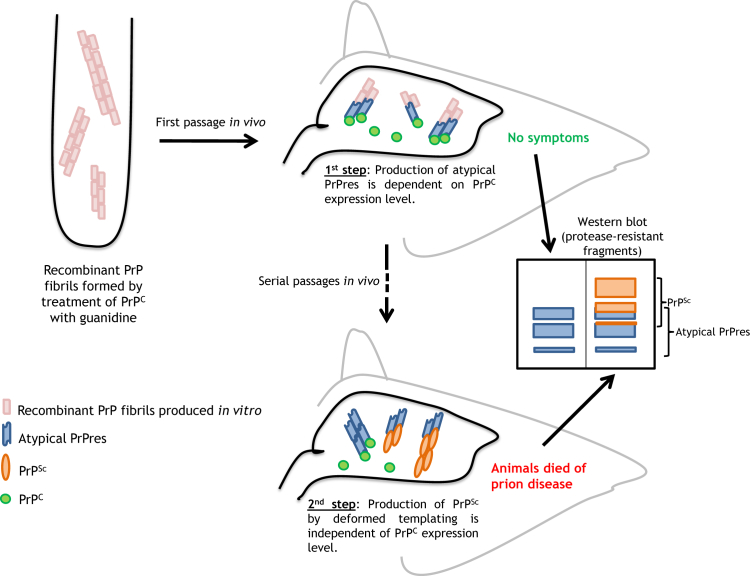
In the discussed study,2 the investigators asked whether increasing the amount of PrPC substrate enhanced the efficiency of each of these two steps (Figure 1). As an inoculum, they used the PrP fibrils formed in 2 mol/L guanidine, which they previously showed did not result in formation of either atypical PrPres, PrPSc, or the appearance of clinical disease after inoculation into hamsters.5 To increase the level of PrPC, they turned to transgenic (Tg7) mice that overexpress hamster PrPC by 3.5-fold in the absence of endogenous mouse PrPC. They found that inoculation of Tg7 mice with 2.0 mol/L fibrils resulted in some animals that accumulated atypical PrPres, indicating that the increased expression level of hamster PrPC in these mice enhanced the formation of atypical PrPres. However, no PrPSc (which could be detected independently on the basis of the sizes of its protease-resistant fragments) was found in animals after the first passage, and the amount of PrPSc that appeared during secondary passage was not higher in Tg7 mice than in hamsters, even though the amount of atypical PrPres was greatly increased. These latter results indicate that the second step, conversion of atypical PrPres to PrPSc, was not enhanced by higher expression of PrPC. Makarava et al2 concluded that the rate of deformed templating does not depend on the expression level of PrPC but is likely to be controlled by other factors, including the nature of the template, the intrinsic rate of conformational errors in templating, and, possibly, the biochemical environment.
Figure 1.
Makarava et al2 test whether overexpression of hamster PrPC in Tg7 transgenic mice enhances the formation of atypical PrPres or PrPSc. After inoculation of Tg7 mice with recombinant hamster PrP fibrils, production of atypical PrPres increases, but PrPSc does not appear and the mice remain healthy. During subsequent passages, PrPSc gradually replaces atypical PrPres, correlating with the development of clinical symptoms and neuropathology. This latter process is not enhanced by increased levels of hamster PrPC substrate. The schematic shows Western blot analysis of protease-resistant fragments of PrP from the brains of the mice, which allows distinction of atypical PrPres from PrPSc. PrP, prion protein; PrPC, cellular prion protein; PrPres, alternative self-replicating state of prion protein; PrPSc, prion protein scrapie isoform; Tg, transgenic.
In a second set of experiments, Makarava et al2 asked whether atypical PrPres and PrPSc compete with each other during the propagation process, as might be expected because they presumably use the same PrPC substrate. They found that such competition is, in fact, observed during propagation with the use of a PMCA system but, surprisingly, not after inoculation into hamsters. They offer several explanations for this discrepancy between the in vitro and in vivo results, including differences between atypical PrPres and PrPSc in clearance rates, neurotropism, cellular replication sites and cofactors, or ability to convert sialylated PrPC substrate.
Remaining Questions
The study of Makarava et al2 raises several intriguing questions. First, what are the factors that control deformed templating? The results presented in the study make it clear that the amount of PrPC substrate is not rate-limiting for this step. In some ways, this is surprising, because it is well known that mice with supraphysiologic levels of PrPC have shorter incubation times after prion inoculation.14 Moreover, the accumulation of atypical PrPres is significantly accelerated by increased expression of PrPC. Why the difference? One possibility is that the switch from atypical PrPres to PrPSc via deformed templating is stochastic, depending, eg, on small, random fluctuations in the conformation of the PrPC template. Another possible explanation could be a role for cofactors such as lipid molecules or RNA, which could favor generation of one product versus the other by binding to critical regions of PrPC. In fact, there is ample evidence for this idea. In a particularly clear example, Supattapone and colleauges15 have produced two autocatalytic recombinant PrP conformers derived from the same original PrPC template, which differ by >105-fold in specific infectivity, depending on whether a lipid cofactor was included in the in vitro amplification reaction. One could hypothesize that, in the discussed study,2 the original PrP fibrils (which were produced without cofactors) gradually produce PrPSc when propagated in vivo in the presence of cofactor molecules such as RNA and lipids. A third potential mechanism to explain deformed templating concerns the nature of the PrPC substrate molecule, in particular the kinds of post-translational modifications present on the protein. It is possible that atypical PrPres and PrPSc preferentially convert PrPC molecules with distinct arrays of post-translational modifications, and that the transition from atypical PrPres to PrPSc involves a rare switch in substrate selection during the conversion process. In fact, recent work from the Baskakov laboratory has highlighted the potential importance of the sialic acid residues on the N-linked glycans of PrPC as a factor in conversion efficiency.16
Another important question raised by the present work is the relation between PrP conformation and pathogenic potential. In particular, why is PrPSc pathogenic, whereas atypical PrPres is not, even when it accumulates to high levels, as in Tg7 mice? There is considerable evidence that neurotoxicity and infectivity are distinct properties, and that only some misfolded conformers of PrP possess both.17, 18 To address this question further, it will be necessary to develop experimental systems that can directly assay prion toxicity.
Finally, this study raises the question of the structural relation between PrPSc, atypical PrPres, and other misfolded forms of PrP that were described, including naturally occurring forms found in human brain and those generated in vitro. The structural differences among these forms have been probed by proteolytic digestion and in some cases by biophysical techniques.15, 19, 20, 21 Importantly, however, a high-resolution atomic structure for infectious PrPSc has yet to be determined.
There is little doubt that the answers to these questions will help move the field forward and shed light on the myriad ways that PrP can misfold, propagate its conformation, and ultimately cause disease.
Footnotes
Supported by NIH grants R01 NS065244 and R01 NS040975 (D.A.H.).
Disclosures: None declared.
See related article on page 1006
References
- 1.Prusiner S.B. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makarava N., Savtchenko R., Alexeeva I., Rohwer R.G., Baskakov I.V. New molecular insight into mechanism of evolution of mammalian synthetic prions. Am J Pathol. 2016;186:1006–1014. doi: 10.1016/j.ajpath.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castilla J., Saa P., Hetz C., Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease. PLoS Pathog. 2011;7:e1002419. doi: 10.1371/journal.ppat.1002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Ostapchenko V.G., Budka H., Rohwer R.G., Baskakov I.V. A new mechanism for transmissible prion diseases. J Neurosci. 2012;32:7345–7355. doi: 10.1523/JNEUROSCI.6351-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legname G., Baskakov I.V., Nguyen H.O., Riesner D., Cohen F.E., DeArmond S.J., Prusiner S.B. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 7.Colby D.W., Giles K., Legname G., Wille H., Baskakov I.V., DeArmond S.J., Prusiner S.B. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci U S A. 2009;106:20417–20422. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colby D.W., Wain R., Baskakov I.V., Legname G., Palmer C.G., Nguyen H.O., Lemus A., Cohen F.E., DeArmond S.J., Prusiner S.B. Protease-sensitive synthetic prions. PLoS Pathog. 2010;6:e1000736. doi: 10.1371/journal.ppat.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makarava N., Kovacs G.G., Bocharova O., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce M.E., Dickinson A.G. Biological evidence that scrapie agent has an independent genome. J Gen Virol. 1987;68:79–89. doi: 10.1099/0022-1317-68-1-79. [DOI] [PubMed] [Google Scholar]
- 11.Collinge J. Prion strain mutation and selection. Science. 2010;328:1111–1112. doi: 10.1126/science.1190815. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Browning S., Mahal S.P., Oelschlegel A.M., Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327:869–872. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makarava N., Baskakov I.V. The evolution of transmissible prions: the role of deformed templating. PLoS Pathog. 2013;9:e1003759. doi: 10.1371/journal.ppat.1003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer M., Rülicke T., Raeber A., Sailer A., Moser M., Oesch B., Brandner S., Aguzzi A., Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 15.Noble G.P., Wang D.W., Walsh D.J., Barone J.R., Miller M.B., Nishina K.A., Li S., Supattapone S. A structural and functional comparison between infectious and non-infectious autocatalytic recombinant PrP conformers. PLoS Pathog. 2015;11:e1005017. doi: 10.1371/journal.ppat.1005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katorcha E., Makarava N., Savtchenko R., Baskakov I.V. Sialylation of the prion protein glycans controls prion replication rate and glycoform ratio. Sci Rep. 2015;5:16912. doi: 10.1038/srep16912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandberg M.K., Al-Doujaily H., Sharps B., De Oliveira M.W., Schmidt C., Richard-Londt A., Lyall S., Linehan J.M., Brandner S., Wadsworth J.D., Clarke A.R., Collinge J. Prion neuropathology follows the accumulation of alternate prion protein isoforms after infective titre has peaked. Nat Commun. 2014;5:4347. doi: 10.1038/ncomms5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiesa R., Piccardo P., Quaglio E., Drisaldi B., Si-Hoe S.L., Takao M., Ghetti B., Harris D.A. Molecular distinction between pathogenic and infectious properties of the prion protein. J Virol. 2003;77:7611–7622. doi: 10.1128/JVI.77.13.7611-7622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groveman B.R., Dolan M.A., Taubner L.M., Kraus A., Wickner R.B., Caughey B. Parallel in-register intermolecular beta-sheet architectures for prion-seeded prion protein (PrP) amyloids. J Biol Chem. 2014;289:24129–24142. doi: 10.1074/jbc.M114.578344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobb N.J., Sonnichsen F.D., McHaourab H., Surewicz W.K. Molecular architecture of human prion protein amyloid: a parallel, in-register beta-structure. Proc Natl Acad Sci U S A. 2007;104:18946–18951. doi: 10.1073/pnas.0706522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Requena J.R., Wille H. The structure of the infectious prion protein: experimental data and molecular models. Prion. 2014;8:60–66. doi: 10.4161/pri.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]