Abstract
A hallmark of idiopathic pulmonary fibrosis (IPF) is excessive and disordered deposition of extracellular matrix. Although the lung extracellular matrix normally plays an essential role in development and maintenance of lung tissue through reciprocal interactions with resident cells, the disordered matrix in the diseased lung is increasingly recognized as an active and important contributor to IPF pathogenesis. This working group summary from a recently conducted National Heart, Lung, and Blood Institute strategic planning workshop for IPF research highlights recent advances, challenges, and opportunities in the study of matrix biology in IPF. Particular attention is given to the composition and mechanical properties of the matrix in normal and diseased lungs, and the biochemical and biomechanical influences exerted by pathological matrix. Recently developed model systems are also summarized as key tools for advancing our understanding of matrix biology in IPF. Emerging approaches to therapeutically target the matrix in preclinical and clinical settings are discussed, as are important concepts, such as alterations of the matrix with aging and the potential for the resolution of fibrosis. Specific recommendations for future studies in matrix biology of IPF are also proposed.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
The structural and social organization of cells into functional units within tissues/organs of multicellular organisms requires adhesion to their neighbors and to the acellular extracellular matrix (ECM). The matrisome, composed of almost 300 ECM proteins, is one of the most plastic and rapidly evolving compartments of the proteome.1 In addition to providing essential physical scaffolding for cellular constituents, the ECM serves to integrate biochemical and biomechanical cues essential for tissue morphogenesis, differentiation, and homeostasis.2 ECM deposition, remodeling, and resorption are dynamic processes that are precisely orchestrated and controlled during normal development, homeostasis, and tissue repair. Loss of control of these processes may lead to pathological tissue remodeling, as in emphysema or idiopathic pulmonary fibrosis (IPF). A hallmark of IPF is the formation of a stiff, fibrotic ECM that obliterates normal alveolar structures and impairs gas exchange. In November 2012, the National Heart, Lung, and Blood Institute convened a workshop in Bethesda, MD, to outline future directions and priorities for research in IPF. This workshop summary highlights recent advances in our understanding of the active role of the ECM in determining the phenotype and fate of lung cells participating in tissue repair, as well as the challenges and opportunities that will allow studies of the ECM to inform future diagnostic tools and therapeutic approaches in IPF.
The ECM in Lung Development and Aging
A central role for the ECM in cell specification and fate determination during normal lung development is well appreciated.3 Branching morphogenesis, alveolar septation, and terminal differentiation of the various cells of the lung are all dependent on proper ECM signals.4 Maintenance of cell polarity and regulation of surfactant synthesis by type II alveolar epithelium are dependent on the ECM, including its three-dimensional (3D) and topographic cues.5 The concept of an aberrant recapitulation of lung development in IPF has been proposed,6, 7 although mechanisms are not well defined. Understanding the differences in lung ECM composition and organization during development and in postnatal life may provide insights into mechanisms of the aberrant remodeling in IPF.
IPF is a disease of aging,8 yet studies on ECM composition, topography, or stiffness of the aging lung are lacking. In aging tissues, collagen fibers may be prone to inappropriate cross-linking, which leads to tissue stiffening and decreased elasticity.9 Oxidative stress associated with aging may influence this process,10, 11, 12 although the relative contributions of enzymatic and nonenzymatic mechanisms of ECM cross-linking to disease pathogenesis require further study.
ECM Composition and Its Bioactive Components
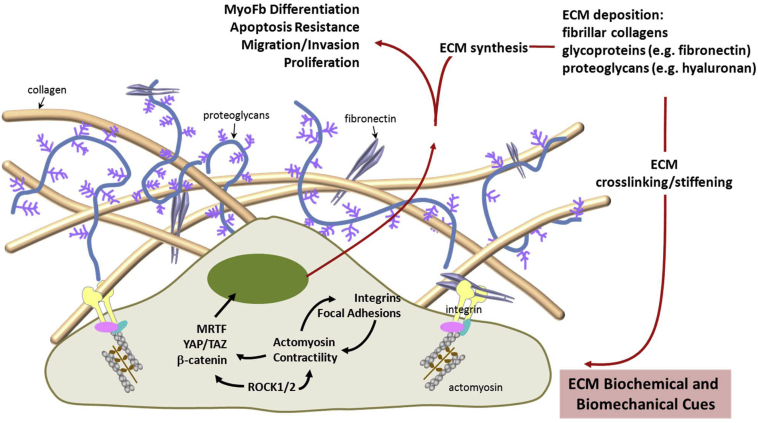
The fibrotic ECM in IPF is composed of a variety of molecules that include proteins, glycoproteins, proteoglycans, and polysaccharides, each of which has distinct biochemical and biomechanical properties (Figure 1). The fibrotic matrix in IPF tissue is rich in fibrillar collagens, including type I and III collagen, proteoglycans, such as hyaluronan, and various glycoproteins, prominent of which is cellular [extra domain (EDA)-containing] fibronectin.13, 14, 15 During physiological repair, fibrillar type I (polymerized) collagen functions to limit fibroblast proliferation through the activation of key phosphatases via engagement of the α2β1 integrin.16, 17, 18 This physiological restraint may be lost in IPF fibroblasts,19, 20 which potentially contributes to their expansion in pathological fibrosis. These studies suggest that cell-autonomous phenotypes and behavior influence cell-matrix interactions.
Figure 1.
Biochemical and biomechanical interactions between fibroblasts and the ECM. Biochemical and biomechanical stimuli derived from the fibrotic ECM initiate intracellular signals through multiple pathways. Integrin, microfilament, and transcription factor–mediated signals are highlighted. These signals promote the fibrogenic phenotype of fibroblasts. Reciprocally, cellular signals can feedback to the ECM and regulate the composition and mechanical properties of the ECM. These bidirectional interactions between fibroblasts and the ECM provide positive feedback loops that sustain/perpetuate progression of lung fibrosis. TAZ, transcriptional coactivator with PDZ-binding motif; YAP, yes-associated protein.
Ultrastructural studies indicate that fibroblastic foci are composed of myofibroblasts within an ECM rich in collagen and EDA-fibronectin.15 EDA-fibronectin has been reported to be required for the myofibroblast differentiation by transforming growth factor-β1 (TGF-β1)21 and cellular motility.22 Although the mice deficient in EDA-fibronectin are protected from bleomycin-induced fibrosis, they succumb early to unremitting inflammation,23 supporting important roles for fibronectin and its spliced variants in the control of inflammation and fibrosis. Periostin is another glycoprotein that is overexpressed in IPF lungs, regulates profibrotic fibroblast phenotypes, and promotes fibrogenesis in experimental models.24
Hyaluronan (HA) is a large-molecular-weight glycosaminoglycan that is synthesized by HA synthases (HAS). It is widely expressed during tissue injury and remodeling. HA and HA fragments accumulate at sites of tissue injury and play pivotal roles in normal wound healing and fibrosis.25 When HAS2 is overexpressed in myofibroblasts, severe fibrosis develops after lung injury in mice; silencing of HAS2 or inhibition of CD44, a cell surface proteoglycan receptor, inhibits IPF fibroblast invasion.26 These data provide evidence that HA-CD44 interactions regulate IPF fibroblast motility and invasion, which may be critical for pathological remodeling in IPF.
Taken together, these studies indicate that specific ECM components, such as type I collagen, EDA-fibronectin, periostin, and hyaluronan, are critical regulators of fibroblast phenotypes and fibrogenesis. A disease-specific, contextual understanding of these ECM signals via integrins and proteoglycan cell surface receptors will uncover therapeutic strategies to halt or slow fibrotic progression.
Biomechanical Signaling by the ECM
In addition to its chemical composition, ECM dimensionality, organization/topography, and mechanical properties influence cellular phenotypes and fates that promote fibrosis. Our current understanding of ECM mechanics in normal and IPF lungs is limited. For example, the long held assumption that lung elasticity can be partitioned into contributions from elastin at low volume and collagen at high volume is likely an oversimplification.27, 28 At the organ scale, lung mechanical properties are typically reported as compliance (or elastance) measured from pressure-volume relationships, and at this level, there is evidence, although not universal, that fibrosis is associated with decreases in lung compliance (increased stiffness). Such measurements that treat the lung as a homogenous continuum do not address the heterogeneity of tissues at the regional, cellular, and molecular levels. Newer technologies, such as atomic force microscopy microindentation, allow for measurements of tissue/cellular stiffness.14, 29 Interestingly, application of atomic force microscopy to isolated lung epithelial cells shows that the cytoplasm of type II cells is stiffer (approximately twofold median difference) than that of type I cells,30 whereas fibroblast stiffness increases with age.31
Within the remodeled fibrotic ECM itself, we have relatively little understanding of how mechanical properties are perturbed, and whether this arises from changes in composition, density, cross-linking, other post-translational modifications, or some combination of these. Most studies of tissue mechanics (in the normal and diseased states), thus far, have focused on matrix stiffness or elasticity. Tissues, however, are viscoelastic and nonlinear, meaning that incremental strain (change in dimension normalized to some initial dimension) changes with time and strain levels as stress is applied; both of these properties may be altered during ECM remodeling.32 Interestingly, fibroblasts change their mode of 3D migration depending on whether matrices exhibit linear or nonlinear elastic properties, demonstrating one important influence of matrix physical properties on fibroblast phenotype.33 Mechanical characterization of the lung ECM requires replication of surface tension effects within the intact lung microstructure and the distending prestress normally present in the thoracic cavity. Intravital microscopy may open opportunities to visualize events within the alveoli and microvasculature of the intact lung.34 In addition, newer applications of noninvasive imaging modalities, such as microfocal X-ray computed tomography,35 magnetic resonance elastography,36 and transient elastography,37 may allow for assessments of tissue biomechanical properties of the lung in vivo. Transient elastography has already been applied to the study of liver disease. The Fibroscan (Echosens, Paris, France) is an ultrasound-based device that uses vibration-controlled transient elastography by which a controlled vibration produces a mechanical shear wave with consistent amplitude and frequency. By using low-frequency ultrasound, the speed and depth of shear wave propagation through the tissue are recorded and represented in graphic form and as Young’s modulus (stiffness).37 Similarly, magnetic resonance elastography uses a magnetic resonance–compatible ultrasound to generate mechanical waves through the liver, which are then measured using a standard 1.5-T magnetic resonance imager with a modified phase-contrast, gradient-echo sequence. The wavelength visible on the readout can then be used to generate elastograms (maps of tissue stiffness) and calculate local Young’s modulus.38
Several groups have shown that fibroblast function and myofibroblast differentiation are tightly linked to the deformability (rigidity and stiffness) of the ECM.29, 39, 40, 41, 42 Intriguingly, several of these studies indicate that the effects of matrix stiffness may be independent of soluble profibrotic factors, such as TGF-β, or may selectively promote the effects of these factors.29, 39, 41 In addition to matrix stiffness, interstitial fluid flow43 and stretch44 may also regulate fibroblast signaling and differentiation.
Both the outside-in transmission of mechanical forces to the cell and the inside-out transmission of traction forces from the cell to the ECM fundamentally involve the cytoskeleton. Force transmission in both directions flows through focal adhesions (FAs), which link the cytoskeleton with the ECM through transmembrane integrin receptors. FA assembly itself is greatly augmented by both forces applied externally to cells and forces generated in cells internally by myosin-driven contraction.45 Both externally applied and internally produced forces transmitted by the cytoskeleton generate biochemical signals by inducing FA protein conformational changes that activate FA kinase.45 Adhesion signaling via FA kinase is essential for TGF-β1–induced myofibroblast differentiation46 and survival,47, 48 as well for the induction of mechanical force-induced hypertrophic scarring of the skin.49
In addition to FA signaling, actin cytoskeletal dynamics are sufficient to activate transcriptional programs that regulate profibrotic genes. The myocardin-related transcription factors (MRTFs), MRTF-A and MRTF-B, have been identified as important links between actin dynamics and gene expression (Figure 1).50 MRTF-A has recently been demonstrated to be required for intrinsic matrix stiffness-induced fibroblast α-smooth muscle actin expression and myofibroblast differentiation.41 Signaling by the Rho GTPase RhoA and its downstream Rho-associated coiled-coil–forming kinases, ROCK1 and ROCK2, regulates effector proteins that modulate the polymerization equilibrium of G- and F-actin, and consequently regulate MRTF nuclear translocation.50 Recent studies demonstrate that inhibition of this mechanosensitive signaling pathway induces myofibroblast apoptosis by the intrinsic mitochondrial pathway and ameliorates experimental pulmonary fibrosis.51 Other mechanosensitive pathways that are relevant to fibrosis include the Wnt/β-catenin pathway52, 53; yes-associated protein and transcriptional coactivator with PDZ-binding motif, downstream effectors of the Hippo pathway54; and ion channels.55 Further studies on therapeutically tenable biomechanical signaling pathways that sustain profibrotic cellular phenotypes will lead to novel, and hopefully more effective, therapies for fibrosis.
ECM Dynamics and Fibrosis Resolution
The process of fibrosis is dynamic and best described as a process of continuous matrix remodeling, which results in net ECM deposition. For example, the collagenous matrix of the rabbit lung turns over by as much as 10% per day under homeostatic conditions,56 and the rapid accumulation of collagen after lung injury is the result of increased rates of collagen synthesis and decreased rates of degradation.57 The mechanisms that lead to an imbalance in ECM synthesis/deposition and degradation in IPF are not well understood.
There is evidence that fibrosis of diverse organ systems, such as the liver, kidney, and heart, are at least partially reversible in experimental animal models and in humans58, 59; similar observations have been made in lung fibrosis.60, 61 Indeed, survivors of acute respiratory distress syndrome, which is associated with variable degrees of fibrosis and restrictive physiological features, demonstrate almost complete restoration of normal lung function, between 3 and 6 months after lung injury.62 For resolution of fibrosis to occur, at a minimum, recruited/activated myofibroblasts must be eliminated and the deposited ECM must be degraded and cleared; in most cases, it appears that both of these processes occur synchronously.63
Although the origin of myofibroblasts has received much attention in recent years,64 mechanisms that control the fate of myofibroblasts are largely unknown. Elimination of myofibroblasts during fibrosis resolution may involve apoptosis,63, 65 dedifferentiation,66, 67 or elimination by immune cells.68 Which of these mechanisms are subverted in IPF is not known. Myofibroblasts acquire an apoptosis-resistant phenotype in IPF, and multiple mechanisms have been proposed69, 70; an understanding of how myofibroblasts evade apoptosis or potentially lose the capacity for dedifferentiation may provide new opportunities for therapeutic intervention.
Although one might surmise that net ECM deposition is due to a relative deficiency in ECM degrading enzymes, evidence suggests far greater complexity.71 Indeed, several studies demonstrate increased collagenase activity and the expression of several other matrix metalloproteinases in IPF.72, 73 The plasminogen activation system also participates in injury repair, and through modulation of proteolytic and nonproteolytic functions, plasminogen activator inhibitor-1 may contribute to fibrosis.74, 75 Macrophage subpopulations participate in this process and may facilitate fibrosis resolution.76 It remains to be determined whether the IPF matrix is intrinsically resistant to proteolytic degradation. Interestingly, mice harboring a cleavage-resistant form of collagen I fail to demonstrate regression of liver fibrosis.77 Thick, acellular scars and those with extensive collagen and elastin cross-linking (via the action of tissue transglutaminases, lysyl oxidases, and other cross-linking enzymes) may be more resistant to degradation and fibrosis resolution.78
Therapeutic Targeting of the Matrix and Matrix Sensing
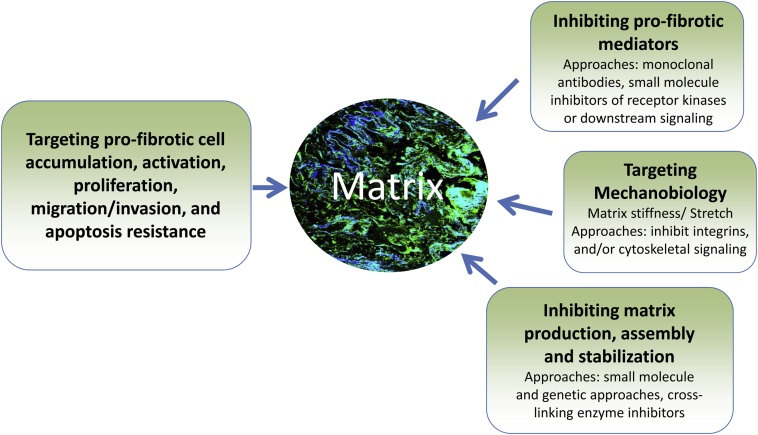
Given the essential role of the ECM in the initiation and progression of fibrosis, therapeutic strategies that target the matrix or its sensing by lung cells may prove beneficial in halting or even reversing fibrosis (Figure 2). Pharmacological agents that inhibit ECM synthesis, deposition, and stabilization or promote its degradation are now being developed; many have shown to be efficacious in preclinical models and a few have reached phase 1 and even phase 2 clinical trials. Potential matrix-directed therapeutic targets include the following: cytokines that activate ECM-producing lung cells79; matrix metalloproteinases73; plasminogen activation74; integrins80; matrix-sensing intracellular proteins51; and matrix cross-linking enzymes, such as tissue transglutaminase-281 and lysyl oxidase.82 Success with therapeutic approaches that target the ECM and matrix sensing will be bolstered by further studies of the IPF matrix and mechanisms that drive aberrant ECM remodeling, as well as the availability of selective pharmacological agents.
Figure 2.
Potential matrix-directed therapeutic strategies for lung fibrosis.
ECM Model Systems
The translation of the accumulating knowledge of matrix biology to the development of new diagnostic biomarkers and therapeutic agents for IPF will be facilitated by improved in vitro, in vivo, and ex vivo model systems. Ideally, these systems must replicate key features of the in vivo IPF lung, in particular its dimensionality, stiffness, and matrix composition. Classically, studies of the ECM use tissue culture plastic dishes coated with a single ECM protein (or mixture of proteins) on which cells are cultured. This approach has been informative for purposes of identifying receptors, elucidating signaling pathways, and understanding the fundamental biological processes. However, in vivo, cells reside in the 3D environment that is neither as planar nor as stiff as a tissue culture plastic, and includes a complex mixture of ECM proteins.
There are now a variety of culture systems available that mimic the mechanical properties and dimensionality of tissues in vivo (Table 1). One widely used system is mechanically tunable polyacrylamide gels with variable concentrations of bis-acrylamide, which imparts known degrees of stiffness to the matrix.29, 39, 41, 83, 84 These matrices can be coated with purified ECM proteins or mixtures to provide a biologically relevant culture substrate with appropriate (normal) or augmented (fibrotic) stiffness. A limitation of this system is that cells can be cultured only in two dimensions. Newer materials are being developed that permit cell growth in 3D, and that enable gradual softening or stiffening. Other such matrix models in or on which cells can be cultured (eg, type I collagen gels) exist. Another approach that may provide some degree of dimensionality and more physiological stiffness is to extract cell-derived matrices from ex vivo fibroblast cultures, followed by reseeding of the cells to be studied.33 These techniques (or combinations thereof) may address difficult-to-answer questions regarding the role of both composition and dimensionality of the ECM in regulating profibrotic cellular phenotypes. Research is urgently needed, however, to develop additional culture systems that not only enable study of mechanical stiffness in a 3D environment but also incorporate other biomechanical factors, including viscosity, flow, stretch, and hydrostatic pressure.
Table 1.
ECM Model Systems
| Variable | Uses | Advantages | Limitations |
|---|---|---|---|
| In vitro | |||
| Tissue culture plastic coated with purified ECM | Identify direct ECM receptor interactions; study signaling pathways and ECM effects on cell behavior | Study the function of a specific ECM molecule or receptor; trace downstream effects from ECM adhesion to biological responses; precise definition of phenotypic responses to ECM molecules | Coating on plastic likely changes ECM conformations; planar surface prevents determining effects in a 3D environment; stiffness of tissue culture plastic is significantly higher than physiological range; unable to assess effects of multiple ECM proteins in a physiological mixture |
| Tissue culture plastic coated with ECM from homogenized tissue | Identify effects of tissue ECM on cell behavior; understanding roles of simultaneous signaling by multiple ECM proteins | Interrogation of ECM adhesion signaling; precise definition of phenotypic responses to mixtures of ECM molecules in physiological ratios; assess the role of receptors using function-blocking strategies | Coating on plastic likely changes ECM conformations and relationships between proteins in the mixture; planar surface prevents determining effects in a 3D environment; stiffness of tissue culture plastic is significantly higher than physiological range |
| Mechanically tunable acrylamide and other hydrogels | Identify effects of ECM on cell behavior under physiological or pathological stiffness | Allows for the study of matrices of varying stiffness, potentially in gradients; depending on the hydrogel system, stiffness may increase or decrease with time in culture; can be coated with single or multiple proteins or protein mixtures; different ECM proteins can be presented in different patterns | Planar surface prevents determining effects in a 3D environment; mixtures of proteins may not interact physiologically after cross-linking to hydrogel; cross-linking may alter ECM protein conformation |
| Type I collagen gels | Study cell behavior as it relates to collagen I; can study cell contractility in 3D | Cells can be implanted within the gel, accounting for dimensionality; studies of proteolysis, cell migration, and other cell behaviors are possible; multiple imaging modalities available for determining effects of live cells on collagen organization | Studies primarily limited to effects from collagen I; unable to control stiffness of the gel |
| Basement membrane extract gels | Studies of cell behavior in 3D | Can be used both in vitro and in vivo; supports growth of mesenchymal, endothelial, and epithelial cells | ECM content and growth factor content vary lot to lot; commercially available preparations largely derived from murine tissue (human tissue preparations now available) |
| Fibroblast- or other cell-derived matrices | Studies of cell behavior in 3D | Fibroblasts or other cells can be extracted, leaving behind a 3D matrix of varying proteins, in a physiological orientation; matrices can be reseeded with cells of choice | Unable to control stiffness of matrix, which may be reflected from the underlying tissue culture plastic substrate; does not account for ECM derived from other cell types; potentially technically difficult |
| Polyelectrolyte multilayers | Identify direct ECM receptor interactions; study signaling pathways and ECM effects on cell behavior | Allows control of physical properties of the culture system; proteins potentially interact in a physiological way; protein mixtures more uniform than with other methods | Potentially technically difficult; may be difficult to control stiffness of the system; does not permit study of cells in 3D |
| In vivo | |||
| Basement membrane extract gels | Studies of cell behavior in 3D | Can be used both in vitro and in vivo; supports growth of mesenchymal, endothelial, and epithelial cells; other matrix proteins and cells can be mixed in | ECM content and growth factor content vary lot to lot; commercially available preparations derived from murine instead of human tissue |
| Ex vivo | |||
| Acellular human (or experimental animal) matrix | Study effects of ECM on cell behavior using human (or experimental animal)–, organ-, and/or disease-specific matrices; can be used to study tissue regeneration | Uses human (or experimental animal) ECM in an organ- or disease-specific manner; can be processed to provide uniform disks of matrix; retains dimensionality and stiffness of the native organ; retains a proportion of normal ECM composition and growth factor inclusion; a single human organ can provide a multitude of disks for study; experimental animal tissue is relatively easy to obtain | Human tissue can be difficult to obtain; complete decellularization must first be confirmed; compositional differences may be present in tissues from donors of different ages; some ECM components may be lost during the decellularization process |
Another model being used to study the effects of ECM on lung cell biology is the acellular human lung ECM. In this system, human lungs from normal donors or patients with IPF are decellularized using a series of detergent, salt, and DNase washes. Subsequently, tissues are sliced on a vibratome to generate individual culture disks that retain both their native stiffness and ECM composition.14 This model system has multiple advantages, including that it incorporates a human matrix and allows investigators to study matrices from the disease of choice, or in relevant animal models; more important, it mimics the matrix organization, mechanics, and dimensionality of the lung microenvironment. Other methods for generating mixtures of matrix proteins (eg, polyelectrolyte multilayers) and studying individual matrix proteins or simple protein mixtures (eg, collagens and proteoglycans) in mechanically tunable systems need to be developed.
Among other aspects of ECM biology in IPF that remain poorly understood are the factors affecting the capacity of ECM to bind growth factors and growth factor–binding proteins (eg, latent TGF-β binding proteins). Although much has been gleaned from basic biochemical investigations with respect to the molecular interactions through which these factors bind ECM, little is known about how stiffness, stretch, and fluid flow (eg, tissue fluid flow, cyclic stretch of lung ECM, hydrostatic pressures, and elastin cross-linking) affect binding capacity of these proteins to the ECM. Thus, development of models that can account for these various features will almost certainly enhance our knowledge and provide more realistic platforms on which to test hypotheses regarding the ECM in IPF and lung fibrogenesis.
Recommendations
On the basis of the working group summary, we recommend further studies to: i) characterize the alterations of the IPF lung ECM in relation to composition, organization, topography, and stiffness; and determine how each of these may influence specific profibrotic cellular phenotypes; ii) determine genetic, epigenetic, age, and environmental factors that may account for the altered IPF lung ECM; iii) identify key ECM proteins and their receptors, which promote and perpetuate fibrotic remodeling (eg, the invasive fibroblast phenotype, aberrant proliferation, and cellular senescence); iv) elucidate the mechanisms that promote matrix stiffening (eg, the role of matrix cross-linking enzymes) and determine how stiffness sensing is transmitted to fibrogenic signals; v) determine the extent to which fibrosis resolution occurs in the lung and strategies that may be used to reverse established fibrosis, including, but not limited to, the induction of myofibroblast apoptosis, dedifferentiation, and immune surveillance; vi) facilitate the development of matrix-directed therapeutics, including proof-of-concept studies in preclinical cell/animal models and early-phase clinical studies in IPF; and vii) develop model systems that simulate the in vivo lung ECM environment for basic studies of lung cell biology and preclinical testing.
Acknowledgments
We thank Dr. Jerry Eu (National Heart, Lung, and Blood Institute) for programmatic development and organization of the workshop.
All authors (except Y.Z.) participated in the on-site National Heart, Lung, and Blood Institute workshop; Y.Z. contributed to manuscript preparation.
Footnotes
A guest editor acted as the editor-in-chief for this manuscript. No person at the University of Alabama at Birmingham was involved in the peer review process or final disposition for this article.
Disclosures: None declared.
References
- 1.Hynes R.O., Naba A. Overview of the matrisome: an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGowan S.E. Extracellular matrix and the regulation of lung development and repair. FASEB J. 1992;6:2895–2904. [PubMed] [Google Scholar]
- 4.Shannon J.M. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166:600–614. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- 5.Shannon J.M., Hyatt B.A. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 6.Selman M., Pardo A., Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardie W.D., Glasser S.W., Hagood J.S. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghu G., Weycker D., Edelsberg J., Bradford W.Z., Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 9.Robins S.P. Biochemistry and functional significance of collagen cross-linking. Biochem Soc Trans. 2007;35:849–852. doi: 10.1042/BST0350849. [DOI] [PubMed] [Google Scholar]
- 10.Kinnula V.L., Fattman C.L., Tan R.J., Oury T.D. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larios J.M., Budhiraja R., Fanburg B.L., Thannickal V.J. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem. 2001;276:17437–17441. doi: 10.1074/jbc.M100426200. [DOI] [PubMed] [Google Scholar]
- 12.Iyer S.S., Ramirez A.M., Ritzenthaler J.D., Torres-Gonzalez E., Roser-Page S., Mora A.L., Brigham K.L., Jones D.P., Roman J., Rojas M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L37–L45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghu G., Striker L.J., Hudson L.D., Striker G.E. Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis. 1985;131:281–289. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 14.Booth A.J., Hadley R., Cornett A.M., Dreffs A.A., Matthes S.A., Tsui J.L., Weiss K., Horowitz J.C., Fiore V.F., Barker T.H., Moore B.B., Martinez F.J., Niklason L.E., White E.S. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn C., 3rd, Boldt J., King T.E., Jr., Crouch E., Vartio T., McDonald J.A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 16.Koyama H., Raines E.W., Bornfeldt K.E., Roberts J.M., Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 17.Ivaska J., Nissinen L., Immonen N., Eriksson J.E., Kahari V.M., Heino J. Integrin alpha 2 beta 1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3 beta. Mol Cell Biol. 2002;22:1352–1359. doi: 10.1128/mcb.22.5.1352-1359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia H., Seeman J., Hong J., Hergert P., Bodem V., Jessurun J., Smith K., Nho R., Kahm J., Gaillard P., Henke C. Low alpha(2)beta(1) integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the beta-catenin pathway. Am J Pathol. 2012;181:222–233. doi: 10.1016/j.ajpath.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia H., Khalil W., Kahm J., Jessurun J., Kleidon J., Henke C.A. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol. 2010;176:2626–2637. doi: 10.2353/ajpath.2010.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia H., Diebold D., Nho R., Perlman D., Kleidon J., Kahm J., Avdulov S., Peterson M., Nerva J., Bitterman P., Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serini G., Bochaton-Piallat M.L., Ropraz P., Geinoz A., Borsi L., Zardi L., Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen A.L., Sackey B.K., Marcinkiewicz C., Boettiger D., Wells R.G. Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts. Gastroenterology. 2012;142:928–937.e3. doi: 10.1053/j.gastro.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muro A.F., Moretti F.A., Moore B.B., Yan M., Atrasz R.G., Wilke C.A., Flaherty K.R., Martinez F.J., Tsui J.L., Sheppard D., Baralle F.E., Toews G.B., White E.S. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik P.K., Bozyk P.D., Bentley J.K., Popova A.P., Birch C.M., Wilke C.A., Fry C.D., White E.S., Sisson T.H., Tayob N., Carnemolla B., Orecchia P., Flaherty K.R., Hershenson M.B., Murray S., Martinez F.J., Moore B.B., COMET Investigators Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teder P., Vandivier R.W., Jiang D., Liang J., Cohn L., Pure E., Henson P.M., Noble P.W. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 26.Li Y., Jiang D., Liang J., Meltzer E.B., Gray A., Miura R., Wogensen L., Yamaguchi Y., Noble P.W. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalcante F.S., Ito S., Brewer K., Sakai H., Alencar A.M., Almeida M.P., Andrade J.S., Jr., Majumdar A., Ingenito E.P., Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol. 2005;98:672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- 28.Fust A., LeBellego F., Iozzo R.V., Roughley P.J., Ludwig M.S. Alterations in lung mechanics in decorin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2005;288:L159–L166. doi: 10.1152/ajplung.00089.2004. [DOI] [PubMed] [Google Scholar]
- 29.Liu F., Mih J.D., Shea B.S., Kho A.T., Sharif A.S., Tager A.M., Tschumperlin D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azeloglu E.U., Bhattacharya J., Costa K.D. Atomic force microscope elastography reveals phenotypic differences in alveolar cell stiffness. J Appl Physiol. 2008;105:652–661. doi: 10.1152/japplphysiol.00958.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulze C., Wetzel F., Kueper T., Malsen A., Muhr G., Jaspers S., Blatt T., Wittern K.P., Wenck H., Kas J.A. Stiffening of human skin fibroblasts with age. Biophys J. 2010;99:2434–2442. doi: 10.1016/j.bpj.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolhnikoff M., Mauad T., Ludwig M.S. Extracellular matrix and oscillatory mechanics of rat lung parenchyma in bleomycin-induced fibrosis. Am J Respir Crit Care Med. 1999;160:1750–1757. doi: 10.1164/ajrccm.160.5.9812040. [DOI] [PubMed] [Google Scholar]
- 33.Petrie R.J., Gavara N., Chadwick R.S., Yamada K.M. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J Cell Biol. 2012;197:439–455. doi: 10.1083/jcb.201201124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuebler W.M., Parthasarathi K., Lindert J., Bhattacharya J. Real-time lung microscopy. J Appl Physiol. 2007;102:1255–1264. doi: 10.1152/japplphysiol.00786.2006. [DOI] [PubMed] [Google Scholar]
- 35.Sera T., Fujioka H., Yokota H., Makinouchi A., Himeno R., Schroter R.C., Tanishita K. Localized compliance of small airways in excised rat lungs using microfocal X-ray computed tomography. J Appl Physiol. 2004;96:1665–1673. doi: 10.1152/japplphysiol.00624.2003. [DOI] [PubMed] [Google Scholar]
- 36.McGee K.P., Hubmayr R.D., Ehman R.L. MR elastography of the lung with hyperpolarized 3He. Magn Reson Med. 2008;59:14–18. doi: 10.1002/mrm.21465. [DOI] [PubMed] [Google Scholar]
- 37.Foucher J., Castera L., Bernard P.H., Adhoute X., Laharie D., Bertet J., Couzigou P., de Ledinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411–412. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Rouviere O., Yin M., Dresner M.A., Rossman P.J., Burgart L.J., Fidler J.L., Ehman R.L. MR elastography of the liver: preliminary results. Radiology. 2006;240:440–448. doi: 10.1148/radiol.2402050606. [DOI] [PubMed] [Google Scholar]
- 39.Li Z., Dranoff J.A., Chan E.P., Uemura M., Sevigny J., Wells R.G. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 40.Balestrini J.L., Chaudhry S., Sarrazy V., Koehler A., Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 41.Huang X., Yang N., Fiore V.F., Barker T.H., Sun Y., Morris S.W., Ding Q., Thannickal V.J., Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wipff P.J., Rifkin D.B., Meister J.J., Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng C.P., Hinz B., Swartz M.A. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci. 2005;118:4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 44.Boudreault F., Tschumperlin D.J. Stretch-induced mitogen-activated protein kinase activation in lung fibroblasts is independent of receptor tyrosine kinases. Am J Respir Cell Mol Biol. 2010;43:64–73. doi: 10.1165/rcmb.2009-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldmann W.H. Mechanotransduction and focal adhesions. Cell Biol Int. 2012;36:649–652. doi: 10.1042/CBI20120184. [DOI] [PubMed] [Google Scholar]
- 46.Thannickal V.J., Lee D.Y., White E.S., Cui Z., Larios J.M., Chacon R., Horowitz J.C., Day R.M., Thomas P.E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 47.Horowitz J.C., Rogers D.S., Sharma V., Vittal R., White E.S., Cui Z., Thannickal V.J. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia H., Nho R.S., Kahm J., Kleidon J., Henke C.A. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 49.Wong V.W., Rustad K.C., Akaishi S., Sorkin M., Glotzbach J.P., Januszyk M., Nelson E.R., Levi K., Paterno J., Vial I.N., Kuang A.A., Longaker M.T., Gurtner G.C. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson E.N., Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y., Huang X., Hecker L., Kurundkar D., Kurundkar A., Liu H., Jin T.H., Desai L., Bernard K., Thannickal V.J. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samuel M.S., Lopez J.I., McGhee E.J., Croft D.R., Strachan D., Timpson P., Munro J., Schroder E., Zhou J., Brunton V.G., Barker N., Clevers H., Sansom O.J., Anderson K.I., Weaver V.M., Olson M.F. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charbonney E., Speight P., Masszi A., Nakano H., Kapus A. Beta-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition. Mol Biol Cell. 2011;22:4472–4485. doi: 10.1091/mbc.E11-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 55.Sukharev S., Sachs F. Molecular force transduction by ion channels: diversity and unifying principles. J Cell Sci. 2012;125:3075–3083. doi: 10.1242/jcs.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laurent G.J. Rates of collagen synthesis in lung, skin and muscle obtained in vivo by a simplified method using [3H]proline. Biochem J. 1982;206:535–544. doi: 10.1042/bj2060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurent G.J., McAnulty R.J. Protein metabolism during bleomycin-induced pulmonary fibrosis in rabbits: in vivo evidence for collagen accumulation because of increased synthesis and decreased degradation of the newly synthesized collagen. Am Rev Respir Dis. 1983;128:82–88. doi: 10.1164/arrd.1983.128.1.82. [DOI] [PubMed] [Google Scholar]
- 58.Duffield J.S., Lupher M., Thannickal V.J., Wynn T.A. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson N.C., Iredale J.P. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci. 2007;112:265–280. doi: 10.1042/CS20060242. [DOI] [PubMed] [Google Scholar]
- 60.Lee C.G., Cho S.J., Kang M.J., Chapoval S.P., Lee P.J., Noble P.W., Yehualaeshet T., Lu B., Flavell R.A., Milbrandt J., Homer R.J., Elias J.A. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawson W.E., Polosukhin V.V., Stathopoulos G.T., Zoia O., Han W., Lane K.B., Li B., Donnelly E.F., Holburn G.E., Lewis K.G., Collins R.D., Hull W.M., Glasser S.W., Whitsett J.A., Blackwell T.S. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol. 2005;167:1267–1277. doi: 10.1016/S0002-9440(10)61214-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McHugh L.G., Milberg J.A., Whitcomb M.E., Schoene R.B., Maunder R.J., Hudson L.D. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:90–94. doi: 10.1164/ajrccm.150.1.8025779. [DOI] [PubMed] [Google Scholar]
- 63.Iredale J.P., Benyon R.C., Pickering J., McCullen M., Northrop M., Pawley S., Hovell C., Arthur M.J. Mechanisms of spontaneous resolution of rat liver fibrosis: hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blackwell T.S., Tager A.M., Borok Z., Moore B.B., Schwartz D.A., Anstrom K.J. Future directions in idiopathic pulmonary fibrosis research: an NHLBI workshop report. Am J Respir Crit Care Med. 2014;189:214–222. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desmouliere A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 66.Kisseleva T., Cong M., Paik Y., Scholten D., Jiang C., Benner C., Iwaisako K., Moore-Morris T., Scott B., Tsukamoto H., Evans S.M., Dillmann W., Glass C.K., Brenner D.A. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hecker L., Jagirdar R., Jin T., Thannickal V.J. Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res. 2011;317:1914–1921. doi: 10.1016/j.yexcr.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thannickal V.J., Horowitz J.C. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kis K., Liu X., Hagood J.S. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardo A., Selman M., Kaminski N. Approaching the degradome in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:1141–1155. doi: 10.1016/j.biocel.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 72.Gadek J.E., Kelman J.A., Fells G., Weinberger S.E., Horwitz A.L., Reynolds H.Y., Fulmer J.D., Crystal R.G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979;301:737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- 73.Dancer R.C., Wood A.M., Thickett D.R. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J. 2011;38:1461–1467. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- 74.Sisson T.H., Simon R.H. The plasminogen activation system in lung disease. Curr Drug Targets. 2007;8:1016–1029. doi: 10.2174/138945007781662319. [DOI] [PubMed] [Google Scholar]
- 75.Horowitz J.C., Rogers D.S., Simon R.H., Sisson T.H., Thannickal V.J. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol. 2008;38:78–87. doi: 10.1165/rcmb.2007-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duffield J.S., Forbes S.J., Constandinou C.M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Issa R., Zhou X., Trim N., Millward-Sadler H., Krane S., Benyon C., Iredale J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47–49. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 78.Issa R., Zhou X., Constandinou C.M., Fallowfield J., Millward-Sadler H., Gaca M.D., Sands E., Suliman I., Trim N., Knorr A., Arthur M.J., Benyon R.C., Iredale J.P. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Sime P.J., O’Reilly K.M. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 80.Horan G.S., Wood S., Ona V., Li D.J., Lukashev M.E., Weinreb P.H., Simon K.J., Hahm K., Allaire N.E., Rinaldi N.J., Goyal J., Feghali-Bostwick C.A., Matteson E.L., O’Hara C., Lafyatis R., Davis G.S., Huang X., Sheppard D., Violette S.M. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 81.Olsen K.C., Sapinoro R.E., Kottmann R.M., Kulkarni A.A., Iismaa S.E., Johnson G.V., Thatcher T.H., Phipps R.P., Sime P.J. Transglutaminase 2 and its role in pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:699–707. doi: 10.1164/rccm.201101-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li S., Yang X., Li W., Li J., Su X., Chen L., Yan G. N-acetylcysteine downregulation of lysyl oxidase activity alleviating bleomycin-induced pulmonary fibrosis in rats. Respiration. 2012;84:509–517. doi: 10.1159/000340041. [DOI] [PubMed] [Google Scholar]
- 83.Georges P.C., Hui J.J., Gombos Z., McCormick M.E., Wang A.Y., Uemura M., Mick R., Janmey P.A., Furth E.E., Wells R.G. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 84.Wells R.G. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39:S158–S161. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]