Abstract
Since our proposal of a dualistic model of epithelial ovarian carcinogenesis more than a decade ago, a large number of molecular and histopathologic studies were published that have provided important insights into the origin and molecular pathogenesis of this disease. This has required that the original model be revised and expanded to incorporate these findings. The new model divides type I tumors into three groups: i) endometriosis-related tumors that include endometrioid, clear cell, and seromucinous carcinomas; ii) low-grade serous carcinomas; and iii) mucinous carcinomas and malignant Brenner tumors. As in the previous model, type II tumors are composed, for the most part, of high-grade serous carcinomas that can be further subdivided into morphologic and molecular subtypes. Type I tumors develop from benign extraovarian lesions that implant on the ovary and which can subsequently undergo malignant transformation, whereas many type II carcinomas develop from intraepithelial carcinomas in the fallopian tube and, as a result, disseminate as carcinomas that involve the ovary and extraovarian sites, which probably accounts for their clinically aggressive behavior. The new molecular genetic data, especially those derived from next-generation sequencing, further underline the heterogeneity of ovarian cancer and identify actionable mutations. The dualistic model highlights these differences between type I and type II tumors which, it can be argued, describe entirely different groups of diseases.
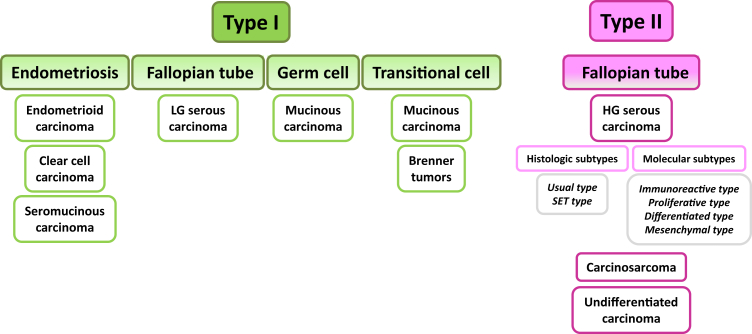
More than a decade ago we proposed a dualistic model of epithelial ovarian carcinogenesis (type I and type II tumors) in an attempt to unravel the complex molecular genetic pathways involved in pathogenesis of primary ovarian carcinomas and to correlate these pathways with the histopathologic classification.1 In the ensuing years, a large number of molecular and histopathologic studies were published that have provided important insights into the origin and development of these tumors. We are now at crossroads where pathogenesis according to morphology and molecular findings intersect. At present, our understanding of carcinogenesis and its role in tumor classification is based largely on morphology, but the importance of molecular classification is becoming increasingly apparent. The new model takes into account the current histopathologic classification and integrates it with the emerging molecular genetic findings to provide a bridge to the future (Figure 1).
Figure 1.
Expanded dualistic model of ovarian carcinogenesis. Ovarian carcinomas derive from endometrial tissue, fallopian tube tissue, germ cells, and transitional epithelium. Type I carcinomas comprise endometrioid, clear cell, LG serous, and mucinous carcinomas. Seromucinous carcinomas and malignant Brenner tumors are rare. It was recently proposed that seromucinous neoplasms be designated mixed Müllerian tumors. Type II carcinomas are largely composed of HG serous carcinoma, carcinosarcoma, and undifferentiated carcinoma. Transitional cell indicates metaplastic transitional epithelium at the tuboperitoneal junction. HG, high-grade; LG, low-grade; SET, solid pseudoendometrioid transitional.
Clinical Features
The salient clinicopathologic and molecular differences between type I and type II tumors are shown in Table 1. Type I carcinomas usually present as large, unilateral, cystic neoplasms. With the exception of clear cell carcinomas, which are not graded but are considered high grade, type I tumors are low grade; they therefore, not surprisingly, tend to behave in an indolent fashion. When confined to the ovary they have an excellent prognosis, but advanced stage tumors have a poor outcome. Type I tumors account for only 10% of the deaths from ovarian cancer. Comprehensive staging of type I tumors is routinely performed, but, in the absence of overt extraovarian disease, the likelihood of detecting occult tumor is remote. One study of >100 women with unilateral mucinous carcinomas reported that staging failed to detect occult disease in any of these patients.2
Table 1.
Clinicopathologic and Molecular Features of Type I and Type II Ovarian Carcinomas
| Features | Type I | Type II |
|---|---|---|
| Stage | Frequently early stage | Almost always advanced stage |
| Tumor grade | Low grade∗† | High grade |
| Proliferative activity | Generally low | Always high |
| Ascites | Rare | Common |
| Response to chemotherapy | Fair | Good (but recur later) |
| Early detection | Possible | Challenging |
| Progression | Slow and indolent | Rapid and aggressive |
| Overall clinical outcome | Good | Poor |
| Risk factors | Endometriosis | Lifetime ovulation cycles; BRCA germline mutations |
| Origin | See Morphologic and Molecular Features of Precursor Lesions | Mostly tubal |
| Precursors | Atypical proliferative (borderline) tumors | Mostly STICs |
| Chromosomal instability | Low | High |
| TP53 mutation | Infrequent | Almost always |
| Homologous recombination repair | Rarely defective | Frequently defective |
| Actionable mutations | Can be present | Rare |
BRCA, breast cancer; STIC, serous tubal intraepithelial carcinoma.
Clear cell carcinoma is not graded, but many consider the tumor as high-grade.
Occasional progression to high grade can be observed.
Type II tumors present in advanced stage in >75% of cases. They are invariably high grade, develop rapidly, and are highly aggressive. The volume of tumor in the ovaries (typically both are involved) is substantially less than that of the type I tumors. However, the volume of extraovarian disease is generally much greater, often with massive disease in the omentum and mesentery, than in the type I tumors. Ascites frequently accompanies the type II tumors but is infrequent with type I tumors. Aggressive surgery and chemotherapy have lengthened progression free survival and, to a very modest extent, of overall survival, but ultimately most patients with type II tumors succumb. These neoplasms account for 90% of the deaths from ovarian cancer.
Morphologic and Molecular Features
In 2014, the World Health Organization updated the histopathologic classification of ovarian tumors.3 The morphologic features of these neoplasms are well illustrated in the World Health Organization book and in textbooks of gynecologic pathology and are therefore only briefly described in this Review. Type I tumors are composed of low-grade serous, endometrioid, clear cell, mucinous carcinomas and malignant Brenner tumors. Type II tumors include high-grade serous carcinoma (HGSC), carcinosarcoma, and undifferentiated carcinoma. In the updated model, seromucinous carcinoma was added to the type I group. It was recently proposed that a more appropriate designation for seromucinous tumors, which more accurately reflects the morphologic, immunohistochemical, and molecular features, is mixed Müllerian tumors.4 These comprise cystadenomas, atypical proliferative (borderline) tumors, and carcinomas. In the type II group, HGSCs were divided into two morphologic subsets: the usual type and a so-called SET variant (solid, pseudoendometrioid, transitional). HGSCs have also been subdivided on the basis of gene expression cluster analysis into four molecular subtypes termed immunoreactive, differentiated, proliferative, and mesenchymal, but a correlation with morphology has not yet been described.
The main molecular genetic feature that separates type I from type II tumors is the relative genetic stability (as reflected by global DNA copy number changes) of type I tumors compared with the marked chromosomal instability of type II tumors. In addition, ubiquitous TP53 mutations characterize type II tumors, in contrast to their infrequency in type I tumors. The other important differences are the frequent somatic mutations involved in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α (PIK3CA)/phosphatase and tensin homolog (PTEN), catenin β1, Kirsten rat sarcoma viral oncogene homolog (KRAS)/B-Raf proto-oncogene, serine/threonine kinase (BRAF)/mitogen-activated protein (MAP) extracellular signal-related kinase (ERK), and AT-rich interaction domain 1A (ARID1A) chromatin remodeling pathways in type I carcinomas, whereas frequent abnormalities in homologous recombination repair, retinoblastoma protein, cyclin E1, forkhead box M1 (FOXM1), and Notch3 pathways occur in type II carcinomas (Figure 2). Although ovarian carcinomas with mixed histologic features (more than one histotype in a given tumor) can be observed, they are rare, with an estimation of <1% with the use of current diagnostic criteria and immunohistochemical staining.5
Figure 2.
The revised dualistic model in the pathogenesis of ovarian epithelial cancer. Type I carcinomas comprise low-grade serous, clear cell, endometrioid, and mucinous carcinomas. Seromucinous carcinomas and malignant Brenner tumors are rare and not shown. Type II carcinomas are largely composed of high-grade serous carcinomas. Carcinosarcoma and undifferentiated carcinoma are relatively uncommon and not illustrated. The areas in individual histotypes reflect their relative prevalence. The inner circle indicates the likely cell of origin of the different type I and type II neoplasms. The origin of mucinous carcinomas is not well established and is discussed in the text. The molecular pathway alterations that characterize each tumor subtype are summarized in the square boxes. Some of the pathway abnormalities are shared by some tumor types and they are shown in two-color fill in boxes. ARID1A, AT-rich interaction domain 1A; BRAF, B-Raf proto-oncogene, serine/threonine kinase; CCNE1, cyclin E1; ERRB2, estrogen-related receptor β2; HR DDR, homologous recombination-mediated DNA damage repair; KRAS, Kirsten rat sarcoma viral oncogene homolog; MEK, mitogen-activated protein (MAP) extracellular signal-related kinase (ERK) kinase; MMR, DNA mismatch repair; NF1, nuclear factor 1; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; Rb, retinoblastoma protein.
Type I Carcinomas
LGSCs
These tumors may be noninvasive LGSC (niLGSC) or invasive (LGSC). The niLGSC is distinguished from an atypical proliferative serous tumor (APST) according to architecture and more importantly on nuclear features. In contrast to APSTs, niLGSCs have nuclear atypia that is identical to invasive LGSC.3 Parenthetically, the 2014 World Health Organization Classification regards the terms serous borderline tumor and atypical proliferative serous tumor as synonymous and serous borderline tumor, micropapillary variant as synonymous with niLGSC.3 We prefer APST and niLGSC and use these terms in this Review.
Unlike the other noninvasive tumors, niLGSCs are often bilateral and may be associated with extraovarian disease (implants) in up to 30% of cases.6 The mechanisms underlying the development of peritoneal implants (noninvasive and invasive) have bedeviled investigators for many years. Recently, it was shown that both types of implants have identical BRAF or KRAS mutation to the ovarian tumors, indicating that they are metastases.7 On the basis of this and other findings, the 2014 World Health Organization Classification considers invasive implants to be metastatic LGSCs.3
After surgery, approximately 10% of these tumors subsequently recur as carcinoma, almost always as low grade.8, 9 Progression to HGSC occurs rarely.10 Studies have reported a better outcome for women whose tumors contain BRAF mutations than for women with KRAS mutations or wild-type BRAF and KRAS.11, 12, 13 Immunohistochemical studies have shown that BRAF mutations correlate with the presence of cells with abundant eosinophilic cytoplasm in the primary tumor.14 These eosinophilic cells are also found in foci of microinvasion, peritoneal implants, and lymph nodes. Furthermore, immunohistochemical studies have shown decreased expression of estrogen receptor (ER), progesterone receptor (PR), Wilms tumor 1 (WT1), and Ki-67 and increased expression of p16 in the eosinophilic cells.15 These findings and their morphologic similarity to cells in culture that are undergoing senescence suggest that the eosinophilic cells are senescent, which is consistent with the excellent outcome for women whose tumors display microinvasion and/or lymph node involvement characterized by the presence of these cells. A distinctive lesion in the fallopian tube designated papillary tubal hyperplasia is associated with APSTs.16
Endometrioid and Clear Cell Carcinomas
Most endometrioid carcinomas are well differentiated, but occasionally moderately and poorly differentiated carcinomas are observed. The frequent finding of well-differentiated areas in the moderate and poorly differentiated neoplasms suggests that the latter de-differentiated from low-grade carcinomas. In contrast to the other type I tumors, clear cell carcinomas are not graded. They are generally regarded as high grade, unlike the other type I tumors. Occasionally, both endometrioid and clear cell carcinoma components coexist in an ovarian tumor.
Somatic inactivating mutations of ARID1A characterize both tumor types and activating mutations of the catenin β1 gene (CTNNB1 encodes β-catenin) occur in roughly 15% to 40% of ovarian endometrioid carcinomas, and mutation of this gene is associated with squamous differentiation, low tumor grade, and favorable outcome.17, 18 In addition, inactivating mutations of PTEN were reported in 15% to 20% of endometrioid carcinomas and in almost 10% of clear cell carcinomas.19 Activating mutations of PIK3CA occur in 20% of endometrioid and clear carcinomas.20, 21 These genes are rarely mutated in other types of ovarian cancer. Inactivating mutations of PTEN and activating mutations of PIK3CA can lead to activation of the phosphatidylinositol 3-kinase signaling pathway. Less than 7% of endometrioid carcinomas have activating mutations of KRAS and BRAF.17, 22 Microsatellite instability has also been reported in up to 20% of endometrioid carcinomas and is usually associated with loss of human mutL homolog 1 (hMLH1), human mutS homolog 2 (hMSH2), mutS homolog 6 (MSH6), and peroxide-sensitive mutant 2 (PSM2) expression. The similar molecular genetic profiles of endometrioid and clear cell carcinomas highlight their close relation and origin from endometriosis (see Morphologic and Molecular Features of Precursor Lesions). However, the morphology and behavior of endometrioid and clear cell carcinomas are different, so it is not surprising that, eg, canonical Wnt signaling pathway defects and microsatellite instability have not been observed with significant frequency in clear cell tumors, unlike endometrioid tumors.23 Studies that used genetically engineered mouse models found that deletion of ARID1A, mimicking its somatic inactivation, is insufficient to drive ovarian tumor formation; however, codeletion of ARID1A and PTEN results in ovarian endometrioid carcinoma,24 whereas codeletion of ARID1A and PIK3CA leads to formation of clear cell-like ovarian neoplasms in mice.25 In addition to these molecular genetic alterations, a recent genome-wide methylation study suggested that clear cell carcinomas have a unique methylation profile compared with the other histologic subtypes.26 Pathway analyses indicate that there is an increase in promoter methylation for multiple genes in the ERα pathway and loss of promoter methylation for multiple genes in the hepatocyte nuclear factor 1 (HNF1) pathway, thus explaining the characteristic immunohistochemical findings in clear cell carcinomas.
TP53 mutations were described in high-grade endometrioid carcinoma with expression profiles similar to those of HGSC, but these tumors may have been misclassified, as suggested by more recent studies reporting a subset of HGSCs that display a pseudoendometrioid pattern27 (see Type II Tumors, HGSC).
Seromucinous Carcinomas (Mixed Müllerian Carcinomas)
Most seromucinous carcinomas are noninvasive. They are generally papillary and resemble niLGSCs but, in fact, are composed of a mixture of epithelial cell types, including endometrioid and squamous cells and endocervical-type mucinous cells. Furthermore, the immunoprofile is characterized by frequent expression of ER (100%), PR (67%), cancer antigen 125 (CA125; 92%), infrequent expression of WT1 (8%), and lack of expression of cytokeratin 20 (CK20) and caudal type homeobox 2 (CDX2), an immunostaining pattern consistent with a Müllerian immunophenotype. Loss of ARID1A expression was reported in more than one-third of cases,28 which is similar to the frequency in endometrioid and clear cell tumors, providing compelling evidence to include them in the group of endometriosis-related neoplasms. Note that mutation of the ARID1A gene or loss of its protein expression has not been found in ovarian neoplasms other than seromucinous, clear cell, and endometrioid carcinomas. Thus, the morphologic, immunohistochemical, molecular genetic data, and frequent association with endometriosis are not consistent with a tumor that is exclusively serous and mucinous; therefore, the term seromucinous is inaccurate and misleading. It was therefore proposed that this group of tumors be designated mixed Müllerian tumors, which can be subcategorized as mixed Müllerian cystadenomas, mixed Müllerian atypical proliferative (borderline) tumors, and mixed Müllerian carcinomas.4
Mucinous Carcinomas
Most mucinous carcinomas are well differentiated; moderate and poorly differentiated tumors are relatively uncommon. Typically, mucinous carcinomas are quite heterogeneous, containing areas of cystadenoma and atypical proliferative tumor intimately admixed with areas of carcinoma. A recent study that used next-generation sequencing found that KRAS-activating mutation is the most common single molecular genetic alteration in mucinous carcinomas, occurring in 65% of cases.29 Interestingly, mutations in KRAS, BRAF, and/or ERRB2 amplification are present in >90% of mucinous carcinomas, indicating frequent RAS/MEK pathway activation in this neoplasm. Another study identified mutations in a novel gene, RNF43.30 By combining the discovery and validation sets, 6 of 29 mucinous carcinomas (21%) were found to harbor the inactivating mutations of RNF43, a zinc finger-dependent E3 ubiquitin protein ligase, suggesting that RNF43 inactivation may characterize a proportion of mucinous cancers.30 In contrast to other type I ovarian carcinomas, TP53 mutation is frequent in mucinous carcinomas, being present in approximately one-half of cases.29, 30 Interestingly, in contrast to serous, endometrioid, and clear cell tumors, mucinous tumors do not express ER or PR, which is consistent with our proposal that they are non–Müllerian-derived tumors. However, paired box 8 (PAX8), a Müllerian marker, is expressed in approximately 50% of mucinous tumors. We have postulated that the nongerm cell mucinous tumors develop from Brenner tumors, which are in turn derived from nests of transitional epithelium at or near the tuboperitoneal junction. Accordingly, a possible explanation for expression of PAX8 by a substantial number of mucinous tumors may be that in the region of the tuboperitoneal junction, where Müllerian-derived tubal epithelium is in close contact with the mesothelium of the tubal serosa and ovarian surface epithelium, overlapping PAX8 expression may occur in both Müllerian and non-Müllerian epithelium.31, 32
Brenner Tumors
Brenner tumors are composed of nests of transitional-type epithelium surrounded by a fibromatous stroma. Typically, the nests of transitional epithelium have a central cystic cavity lined by mucinous epithelium. Most Brenner tumors are benign, less commonly atypical proliferative, and rarely malignant, and consequently a comprehensive molecular analysis of malignant Brenner tumors has not been performed. There have only been a few immunohistochemical and molecular genetic studies of benign and atypical proliferative Brenner tumors. p16 immunostaining was shown to be positive in the epithelial component of 12 of 13 benign Brenner tumors (92%) but completely negative in 7 atypical proliferative Brenner tumors. Fluorescence in situ hybridization identified homozygous deletion of CDKN2A, the gene encoding p16, in the epithelial component of all atypical proliferative tumors, but it was retained in all benign tumors. Two PIK3CA mutations were found in the stromal component in 2 of 20 benign Brenner tumors (5%) but not in the epithelial component. However, one KRAS mutation and two PIK3CA mutations were detected in the epithelial component of two atypical proliferative tumors (29%).33 These findings suggest that loss of CDKN2A may play a role in progression of benign to atypical proliferative Brenner tumors.
Type II Carcinomas
HGSC
Recent morphologic studies correlated with germline breast cancer gene (BRCA) mutations studies have provided evidence that HGSC is more heterogeneous than previously thought.27, 34 The usual type of HGSC is composed of solid masses of cells with slit-like spaces and papillary, glandular, and cribriform patterns often accompanied by necrosis. Another type is composed of solid masses of cells that simulate endometrioid and transitional cell carcinomas. These latter carcinomas, however, have an identical immunoprofile to HGSC, including p53, PTEN, WT1 reactivity and a similar frequency of TP53 mutations, supporting their classification as HGSC.35 To distinguish these tumors from the usual HGSC, it was proposed that they be classified as the SET variant.27 Compared with the usual type of HGSC, the SET tumors have a greater number of tumor-infiltrating lymphocytes and a higher mitotic index.
Correlating the two types of HGSC with germline BRCA1 mutations reveals substantial differences. Compared with the usual HGSC, the SET tumors were substantially more frequently associated with BRCA1 mutations but less frequently associated with serous tubal intraepithelial carcinomas (STICs; see Morphologic and Molecular Features of Precursor Lesions). It was also reported that SET tumors occurred in younger women than the usual-type HGSC and had a better clinical outcome.34 The latter observation may be due to greater chemosensitivity of these neoplasms (deficiency in homologous recombination repair), because it was previously reported that ovarian transitional cell carcinomas are more chemosensitive than are HGSCs and have better survival.36 The Cancer Genome Atlas project analyzed genome-wide sequence mutation, mRNA and protein expression, miRNA expression, promoter methylation, and DNA copy number in a large number of HGSCs and reported that they were characterized by TP53 mutations in almost all tumors.37 Although the number of somatic mutations per case in HGSC is similar to most solid malignant neoplasms, interestingly, unlike the latter, somatic mutations in mutated genes other than TP53 occurred in no >5% of the ovarian HGSCs. Substantial focal DNA copy number aberrations and promoter methylation events that involved 168 genes were also found. Amplification of CCNE1 was another frequent finding.37 Analyses delineated four ovarian cancer transcriptional subtypes, three miRNA subtypes, four promoter methylation subtypes, and a transcriptional signature associated with survival duration. In addition, NOTCH3 and FOXM1 signaling were found to be involved in HGSC pathophysiology.37 More recently, the results of The Cancer Genome Atlas (TCGA) study were largely verified in another genome-wide report.38
Among these various molecular findings, those that are most characteristic of HGSC are widespread DNA copy number or structural aberrations and TP53 mutation. TCGA project reported that >96% of HGSCs have TP53 mutations; however, a study by five gynecologic pathologists who reviewed the negative TP53 cases from TCGA study found that all of the negative tumors except for one were histologically misclassified. The one exception contained a homozygous deletion of the gene, indicating that all properly classified HGSCs have a TP53 abnormality, which is almost always a mutation.39 In addition to widespread copy number alterations, which reflect the history of genomic instability, and ubiquitous TP53 mutations, other common threads in HGSCs include CCNE1 amplification, germline and somatic mutation of BRCA1/2, and other aberrations in pathways that regulate homologous recombination DNA damage repair pathways.38 HGSCs showing BRCA1/2 deficiency are characterized by more extensive DNA copy number alterations, and they usually do not harbor CCNE1 amplification and vice versa.38
A gene expression analysis of >300 HGSCs identified four molecular subtypes40 that were subsequently validated in TCGA study and termed immunoreactive, differentiated, proliferative, and mesenchymal on the basis of gene expression in the clusters (Figure 1).37 These molecular subtypes were associated with distinct clinical outcomes.41, 42 In one study, it was shown that survival differed significantly between the subtypes and was best for the immunoreactive subtype, a finding consistent with the histopathologic observation that HGSCs with large numbers of tumor-infiltrating lymphocytes are associated with a better outcome. Another study reported that BRCA1 disruptions were associated with the immunoreactive subtype.43 It is postulated that these subtypes may reflect distinct patterns of oncogene activation. These studies suggest that high-grade serous carcinogenesis is initiated by disruption of DNA repair, followed by chromosomal instability, copy number change, and segregation into molecular subtypes.
Undifferentiated Carcinoma
These are uncommon tumors that show none of the characteristic features of HGSC or high-grade endometrioid carcinoma. At present, it is not clear whether these are distinct tumors or variants of poorly differentiated HGSCs or high-grade endometrioid carcinomas. They assume a gene expression pattern close to mesenchymal rather than epithelial cells. We are not aware of any molecular studies specifically evaluating undifferentiated carcinomas.
Carcinosarcoma (Malignant Mixed Müllerian Tumor)
Carcinosarcoma is a biphasic tumor composed of carcinoma and sarcoma. The two components are distinct but typically intermixed. Several studies have shown that the epithelial and mesenchymal components are clonal; therefore, most investigators are of the opinion that these are high-grade carcinomas that have undergone sarcomatoid differentiation.44 Carcinosarcomas share several molecular genetic abnormalities with HGSC, including TP53 mutations and CDKN2A overexpression.45
Primary Peritoneal Carcinoma
These tumors almost always resemble HGSC, and most investigators regard them as HGSCs in which little or no tumor involves the ovary. We are not aware of molecular studies specifically evaluating these tumors.
In summary, data are now emerging to indicate that type II tumors are more heterogeneous than previously suspected, although the differences appear to be more subtle than those in the type I tumors.
Functional Aspects of the Molecular Genetic Aberrations in Different Histotypes
The individual molecular genetic alterations that are present in different histotypes of ovarian carcinoma were described in the sections above. These changes can be summarized as abnormalities that affect distinct molecular cancer pathways (Figure 1). In general, type I neoplasms are characterized by activation in the ERRB2/KRAS/BRAF/MEK pathway, PI3K/AKT pathway, and Wnt pathway, and inactivation in the PTEN pathway, ARID1A-related chromatin remodeling, and mismatch repair mechanism, either because of sequence mutations and/or gene amplification/deletion. In contrast, type II neoplasms are mainly characterized by inactivation of the p53-related pathways, and activation in the cyclin E1, Notch3, and FOXM1 pathways. In addition, at least one-third of type II cancers exhibit deficiency in homologous recombination-mediated DNA damage repair. Tumor cells with deficiency in homologous recombination DNA damage repair tend to use error-prone non-homologous end joining (including alternative non-homologous end joining) to repair DNA, leading to exaggerated structure variations in their genomes. Similarly, inactivation of the p53 pathway and activation of the cyclin E1 pathway also indirectly contribute to chromosomal instability that is the cardinal molecular feature of the type II ovarian cancers.
Morphologic and Molecular Features of Precursor Lesions
One of the main features that led us to parse epithelial ovarian cancers into type I and type II groups was the relation of the different histologic subtypes to precursor lesions. Specifically, type I carcinomas were found to develop from well-established benign precursor lesions, notably borderline or atypical proliferative tumors, simulating the adenoma/carcinoma sequence of colorectal carcinoma. In contrast, type II tumors were thought to develop de novo from the ovarian surface epithelium, because no apparent precursor lesion had been identified. In the past few years, studies have shown that many type II carcinomas develop from an intraepithelial carcinoma in the fallopian tube, generally located in the fimbria and designated as STIC.
Type I
LGSC
LGSCs (Table 2) evolve from APSTs in a step-wise fashion and are characterized by sequence mutations in the KRAS, BRAF, and ERBB2 oncogenes, which result in constitutive activation of the MAP kinase signal transduction pathway.46, 47, 48 Studies have implicated a hyperplastic lesion in the fallopian tube, designated papillary tubal hyperplasia as the precursor of APSTs.16 The MAP kinase pathway plays a critical role in the transmission of growth signals into the nucleus and ultimately contributes to neoplastic transformation. KRAS mutations at codons 12 and 13 occur in one-third of APSTs and LGSCs, and BRAF mutations at codon 600 occur in another one- third of APSTs but less commonly in LGSCs.46, 49 Mutations of ERBB2 occur in <5% of these tumors; neuroblastoma RAS (NRAS) viral oncogene homolog gene mutations are also detected in a small percentage of LGSCs.49, 50 Mutations in KRAS, BRAF, and ERBB2 are mutually exclusive and consequently are detected in approximately two-thirds of APSTs and LGSCs. They appear to occur early in the development of these tumors, as evidenced by the finding of KRAS and BRAF mutations in the benign cystadenoma epithelium adjacent to APSTs.51 Pure serous cystadenomas do not harbor these mutations, supporting the interpretation that these mutations are critical in initiating the LGSC pathway. In addition, mutation of NRAS may also be involved in progression in a small number of cases.50
Table 2.
Potential Precursors of Type I and Type II Tumors
| Tumor | Precursor site of origin | Potential precursor lesion | Immediate precursor |
|---|---|---|---|
| Invasive LGSC | Fallopian tube | Papillary tubal hyperplasia | Atypical proliferative tumor/noninvasive LGSC |
| Endometrioid | Endometrium | Endometriosis | Atypical proliferative tumor |
| Clear cell | Endometrium | Endometriosis | Atypical proliferative tumor/noninvasive |
| Seromucinous | Endometrium | Endometriosis | Atypical proliferative tumor/noninvasive |
| Mucinous | Tuboperitoneal junction | Transitional epithelium | Atypical proliferative tumor/intraepithelial carcinoma |
| Mucinous | Ovarian | Mature teratoma | Mucinous epithelium |
| Brenner | Tuboperitoneal junction | Transitional epithelium | Transitional epithelium |
| HGSC | Fallopian tube | STIC | STIC |
| Undifferentiated carcinoma | ? Fallopian tube | ? STIC | ? STIC |
| Carcinosarcoma | Fallopian tube | STIC | STIC |
| Primary peritoneal carcinoma | Fallopian tube | STIC | STIC |
?, possible; HGSC, high-grade serous carcinoma; LGSC, low-grade serous carcinoma; STIC, serous tubal intraepithelial carcinoma.
It is difficult to reconcile the disparate role of BRAF mutation in the pathogenesis of these tumors, because, on the one hand, it leads to senescence, and, on the other hand, it is involved in tumor initiation and occasionally progression to LGSC. We postulate that BRAF mutation, such as KRAS mutation, is required for tumor initiation, because mutations in both genes have multiple tumor-promoting effects, including up-regulation of glucose transporter-1, an essential surface protein that results in an increase in glucose metabolism required for tumor transformation.52 Once an APST develops, the epithelial cells may initiate a mechanism to restrain tumor progression as occurs in many benign neoplasms. This may explain why BRAF-mutated advanced-stage LGSCs are much less common than are BRAF-mutated advanced stage APSTs.12, 13, 53 Despite this restraining mechanism, a few tumors do progress, possibly as a result of hemizygous ch1p36 loss or homozygous or hemizygous deletions of ch9p21 and perhaps others, because these losses are much more common in LGSC than in APSTs.47 The ch1p36 region contains several candidate tumor suppressor genes, including miR-34a, which is required for activation of the DNA damage response and is the direct p53 target that mediates tumor suppressor functions.54, 55 Likewise, the ch9p21 region corresponding to the CDKN2A/B locus encodes three well-known tumor suppressor proteins, p14 (Arf), p16, and p15, that inhibit cyclin-dependent kinase. Thus, deletions or silencing of miR-34a and CDKN2A/B loci may abrogate the checkpoint on mutations and permit some APSTs to progress to LGSCs. In this regard, it was reported that p16 expression levels are significantly decreased in LGSCs compared with in APSTs.56
Endometrioid and Clear Cell Carcinoma
These tumors may be pure or admixed with their corresponding atypical proliferative tumors, suggesting that like LGSCs they develop from atypical proliferative tumors. In addition, both the carcinomas and atypical proliferative tumors are frequently associated with endometriosis. This association was described for several decades and has now been supported by molecular genetic studies. Inactivating mutation of ARID1A, a tumor suppressor gene involved in switch/sucrose non-fermentable (SWI/SNF) chromatin remodeling, was detected in up to 50% of clear cell carcinomas and 30% of endometrioid carcinomas.21, 57, 58 Moreover, mutation and loss of expression of this gene was found in the endometriotic epithelium in endometriomas immediately adjacent to endometrioid and clear cell carcinomas but not in the epithelium in the endometriotic cysts more distant from the carcinoma.57, 58 Somatic mutations of PTEN were also found in endometrioid and clear cell carcinomas and in endometriotic cysts, lending further support to endometriotic cysts as precursors of these neoplasms.59
Seromucinous Carcinoma (Mixed Müllerian Carcinoma)
Evidence is persuasive that these tumors are derived from endometriosis, because approximately one-third are associated with endometriosis, which is similar to the frequency found in endometrioid and clear cell carcinomas.60 In addition, seromucinous tumors are cytokeratin (CK)7+, CK20−, CDX2−, WT1− and usually express ER and PR, which closely resembles the immunoprofiles of endometrioid and clear cell tumors. In contrast, intestinal-type mucinous tumors do not express ER and PR.61 Thus, three tumors, endometrioid, clear cell, and seromucinous carcinomas, all appear to be derived from endometriosis, and we refer to them as endometriosis-related neoplasms.
Mucinous Carcinoma and Malignant Brenner Tumor
Mucinous carcinomas typically display considerable heterogeneity, with foci of mucinous cystadenoma admixed with atypical proliferative tumor and obvious carcinoma. Laser capture microdissection studies have shown the identical KRAS mutation in all three components (adenoma, atypical proliferative, and carcinoma), supporting their clonal relation and thereby providing strong evidence that mucinous cystadenomas are the precursor lesions62, 63; the origin of mucinous cystadenomas, however, is not clear. A minority were found to develop from mucinous epithelium in teratomas, but most mucinous cystadenomas are not associated with teratomas. It was proposed that these nongerm cell mucinous tumors are derived from Brenner tumors.64
Mucinous tumors are typically large, whereas Brenner tumors tend to be smaller. Accordingly, it was suggested that expansion of the mucinous epithelium in the Brenner tumor leads to the development of a cystadenoma, which then compresses and obliterates the Brenner tumor.64 This hypothesis was supported by a recent study showing that, in combined Brenner and mucinous tumors, the Brenner and mucinous components are clonally related.65 It was argued that Brenner tumors arise from nests of transitional epithelium, so-called Walthard cell nests, which can be found adjacent to the fallopian tubes and ovaries at a site termed the tuboperitoneal junction.6 Meticulous sectioning of this region has found a relatively high frequency of nests of transitional metaplasia, which for all practical purposes are Walthard cell nests. Because they are small, they are easily missed in routine sampling.6 Accordingly, evidence is good that some mucinous neoplasms arise from teratomas and that others develop from Brenner tumors. This does not exclude the possibility that some mucinous tumors arise from other sources, but, at present, no data indicate what those sources might be.
Type II
HGSC
One of the main advances in our understanding of the pathogenesis of ovarian cancer that occurred in the past few years was the recognition that many HGSCs may develop from a precursor lesion in the fallopian tube, designated STIC (Table 2). This finding was first described in women at high risk of developing ovarian cancer or who had BRCA germline mutations, when they underwent risk-reducing salpingo-oophorectomy (RRSO).66, 67, 68, 69 Interestingly, similar lesions were not detected in the ovary. This was surprising, because it had been assumed that the precursor of ovarian carcinoma would be in the ovary, although multiple studies in the past three decades failed to find a convincing ovarian precursor lesion. The detection of the lesion in the fallopian tube was due to the introduction of a new method of sampling the fallopian tube in which the entire fallopian tube, with particular attention to the fimbria, was sectioned, the so called sectioning and extensively examining the fimbria technique.70 Subsequently, STICs were detected in 50% to 60% of women with sporadic HGSC when the fallopian tubes were completely processed with the sectioning and extensively examining the fimbria protocol.71, 72 It has been argued that STIC may be a metastasis rather than a precursor of HGSC, but accumulating evidence supports STIC as a precursor lesion for at least many ovarian HGSCs. Briefly, STICs are detected in the absence of an ovarian carcinoma in women at high risk who are undergoing RRSO as noted earlier. More recently, incidental STICs were reported in women undergoing hysterectomy and bilateral salpingo-oophorectomy for nonprophylactic reasons, who were not known to have BRCA mutations in both selected73, 74 and unselected series.75, 76, 77 Other data that support STIC as a precursor are that, in women with a concomitant HGSC in the ovary and a STIC, the identical TP53 mutation is found in both lesions.78 In addition, STICs, compared with the concomitant ovarian tumor, have shorter telomeres,79 and shortened telomeres are one of the earliest molecular changes in carcinogenesis. Finally, in molecularly engineered mouse models, inactivation of BRCA, TP53, and PTEN leads to the development of STICs and ovarian HGSC. When salpingectomy is performed at an early age, no cancers develop, whereas neither oophorectomy nor hysterectomy prevents the development of cancer.80 A recent epidemiologic study showed that in women who had prior salpingectomy, the risk of developing HGSC was significantly decreased compared with that of women with intact fallopian tubes, further supporting the tubal origin of HGSC.81, 82
The diagnostic criteria of STIC are based on morphologic features and immunostaining profiles, which are summarized in a previous report.83 Because STIC is a carcinoma confined to the epithelium, one would expect there to be lesions that are precursors of it; indeed, cytologic atypia in tubal epithelium that falls short of STIC was described and termed serous tubal intraepithelial lesions or tubal intraepithelial lesions in transition. In addition, stretches of normal tubal epithelium that express p53 and termed p53 signatures were reported,84 but the significance, if any, of serous tubal intraepithelial lesions and p53 signatures and their relation to STIC have yet to be established.
On the basis of the clinicopathologic differences of the usual HGSC and the SET variant and their different relations to STICs and germline BRCA1 mutations, some investigators have proposed that HGSCs may develop along two different pathways,34, 85 with the usual HGSC developing from a STIC and the SET variant developing either from STIC, another tubal precursor, or from elsewhere.38 Indeed, a case of a SET HGSC associated with a STIC showing the same morphology suggests that there may be different types of STICs.34 This is an intriguing possibility and one that requires further investigation. The likelihood that other sites, such as the ovarian surface epithelium or ovarian cortical inclusion cysts, are sources of some HGSCs cannot be entirely excluded, but at this time only limited data support this view.
Carcinosarcoma (Malignant Mixed Müllerian Tumor)
Only a few small studies focused on their association with STICs were reported, and these have shown that STICs are a precursor.6, 86
Primary Peritoneal Carcinoma
Studies have shown they are frequently associated with STICs.6, 77
Implications for Diagnosis and Treatment Based on Molecular Features
Current management of ovarian cancer is radical surgery and chemotherapy, which are directed at established cancers, not at the mechanisms by which cells become neoplastic. These modalities are costly, the benefits are limited to lengthening the interval to relapse, and they have not substantially improved overall survival in >50 years. Cure remains elusive; therefore, new approaches that are based on understanding the molecular pathogenesis of ovarian cancer are needed. These approaches should focus on strategies aimed at prevention and screening, because they have proved effective for other diseases. For example, infectious diseases are prevented by vaccines, cardiovascular disease is greatly mitigated by changes in lifestyle (diet and exercise), and cervical cancer is mitigated by both prevention (vaccines) and screening. These methods are cheaper, easier to administer, and more effective than treatment of established disease.
Screening
Over the past two decades, clinical screening trials, primarily using transvaginal ultrasonography and serum cancer antigen 125 concentrations, have not shown a survival benefit.87, 88 Their goal was to detect stage I disease (confined to the ovary) because studies have shown 90% survival for women with stage I disease compared with 30% survival for advanced stage disease.89 Survival differences, however, are not due to stage distribution, but to the biology of type I compared with type II tumors. Type II tumors account for 90% of the deaths from ovarian cancer and are almost nonexistent in International Federation of Gynecology and Obstetrics stage I.89 As discussed, the earliest precursor lesion of these neoplasms is STIC, a minute, microscopic intraepithelial carcinoma in the fallopian tube. Accordingly, the earliest events in the development of HGSC occur in the fallopian tube, and therefore, at inception, HGSC is already International Federation of Gynecology and Obstetrics stage II. Moreover, the microscopic size of the STIC is below the level of detection of current screening methods. Conversely, the tumors that present in stage I are type I neoplasms, which account for 10% of deaths from ovarian cancer.89 They present as large cystic masses that can be detected manually on pelvic examination and by transvaginal ultrasound screening. To make a substantial impact on reducing rates of mortality, the focus must be on type II tumors, and the aim of screening should be directed at detecting a tumor early in its evolution with the use of highly sensitive biomarkers. A step in this direction was recently made with the development of a highly sensitive whole exome sequencing method with the use of a panel of genes commonly mutated in ovarian carcinomas. The test was performed on liquid cervical cytology specimens collected in the operating room at the time patients underwent resection of their ovarian neoplasms. The same mutation was detected in the liquid cytology specimen as in the tumor in 41% of 22 early- and late-stage ovarian carcinomas.90 Although the findings are encouraging, there were only a small number of patients. Clearly, additional large-scale clinical trials must be undertaken to determine whether the test will be useful in screening. The key question to be answered is whether this, or any other biomarker assay, can detect STICs or low-volume disease.
It is important to emphasize that, unlike the precursors of the type I tumors which are benign neoplasms, STICs share many of the morphologic and molecular features of HGSC; thus, once they have acquired the ability to spread, they disseminate as carcinoma. Although STIC is confined to the epithelium, the cells tend to be discohesive; therefore, dissemination can occur in the absence of invasion.91 At present, the transit time for a STIC to disseminate to other sites is not known. In an ultrasonographic study in which investigators attempted to detect early-stage epithelial ovarian cancer in asymptomatic high-risk women, it was found that for all of the cancers detected women had normal ultrasound and physical examinations 6 to 12 months before the cancer diagnosis.92 Although BRCA1 and BRCA2 mutation carriers with incidental invasive carcinoma at RRSO are reported to have a relatively high rate of tumor recurrence despite predominantly early-stage and small-volume disease, STICs rarely recur as carcinoma and may not require adjuvant chemotherapy.93 However, a more recent study of women with germline BRCA1/2 mutations with STICs discovered at RRSO reported recurrences 4 years later, suggesting a longer transit time from STICs to recurrent HGSC.94
Prevention
Approximately 10% of women with ovarian cancer have a genetic predisposition on the basis of germline BRCA mutations, and these women have an increased risk of developing HGSC. Prophylactic bilateral salpingo-oophorectomy substantially reduces their risk of ovarian cancer. Removal of the ovaries at earlier ages, however, can have detrimental effects, as shown by the Nurses' Health Study of 30,000 women which compared hysterectomy and bilateral salpingo-oophorectomy with hysterectomy with ovarian conservation. This study found no overall survival benefit from bilateral oophorectomy at any age. In fact, a significant increase was found in the rate mortality from coronary heart disease, lung cancer, colorectal cancer, and from all causes.95 Accordingly, the recent studies that identified the potential precursor of HGSCs in the fallopian tube have led to the adoption of bilateral salpingectomy with ovarian conservation, thus preserving fertility and hormone function. For high-risk women, the time at which salpingectomy should be performed has not been well established. Incidental STICs discovered in both women at high risk and in women undergoing surgery for benign disease found that STICs were infrequently associated with invasion or disseminated disease younger than 50 years of age.73, 74 It therefore appears that prophylactic surgery can be safely delayed for these women until the age of 45 years. The appropriate management of women who were found to have STIC in the absence of tumor elsewhere is controversial. Although the number of these cases is small, thus far, recurrences are rare,93, 96 suggesting that perhaps not all STICs progress to HGSC. Some investigators have therefore proposed that women diagnosed with STIC after RRSO may not require adjuvant chemotherapy, but more cases should be analyzed to reach a conclusion.93
Women who have taken oral contraceptive pills and women with increased parity have a substantially reduced risk of developing ovarian cancer, whereas women with germline BRCA mutations are at a substantially increased risk, but for the general population no other definitive risk factors were identified. As a result, it has not been possible to target a population of women who need to be screened. For many years, it was recommended that bilateral salpingo-oophorectomy be performed for women older than 40 years at the time of hysterectomy for benign disease, in an effort to reduce the future development of ovarian cancer. For these women, bilateral salpingectomy only is now being advocated.82 Similarly, it was recommended that salpingectomy rather than simply tubal ligation be performed for women desirous of a relatively permanent form of contraception. For the general population of women who are not known to be at high risk, other approaches besides bilateral salpingectomy should be explored. For example, as noted above, studies have shown about a 50% reduction in the risk of ovarian cancer with the use of oral contraceptive pills.97 The mechanism by which this occurs is thought to be a reduction in the number of lifetime ovulatory cycles, because persistent ovulation has been proposed for many years to be an important event in the development of ovarian cancer. In addition, other drugs, such as statins,98 which have been associated with a reduced risk of ovarian cancer, should be evaluated, because these drugs have relatively minimal side effects and are widely available. Statins were also recently reported in a preclinical mouse model to reduce the formation of STIC in mice.99
Treatment
Type I tumors are mostly stage IA (confined to one ovary) and almost always low grade (except clear cell carcinoma). Removal of the affected ovary is generally curative. In contrast, metastatic type I tumors are, for the most part, chemoresistant, because of either slow proliferative activity or intrinsic mechanisms; however, because they are relatively genetically stable and contain activating mutations in genes that are actionable, targeted therapy such as kinase inhibitors may be beneficial. Moreover, those type I carcinomas that exhibit mismatch repair deficiency may benefit from immune checkpoint inhibitors because those tumor cells produce numerous neo-antigens and are thus associated with abundant tumor-infiltrating lymphocytes due to extensive mutation load.100 Those tumor-infiltrating cytotoxic T cells may potentially attack and kill tumor cells once the immune checkpoint is relieved by inhibitors. More recently, preclinical studies have found that type I tumors harboring ARID1A mutations are more sensitive to the treatment with the use of EZH2 inhibitor101 and polyADP ribose polymerase (PARP) inhibitor.102
Although type II tumors are initially chemosensitive, as a result of their highly proliferative activity and defects in DNA repair capacity, chemoresistance almost always emerges. Recent genome-wide studies have elucidated the mechanisms underlying chemoresistance, and these may potentially lead to new therapeutic strategies that target these pathways.38, 103 It should be noted that these new approaches may improve the progression-free survival, they are less likely to affect overall survival, underscoring the importance of early detection.
Tumors with BRCA abnormalities belong to a group of neoplasms that show deficiencies in homologous recombination, which is one of the main mechanisms that repair double-stranded DNA breaks. This is particularly important, because tumors with deficiencies of homologous recombination repair appear sensitive to PARP inhibitor therapy. As previously noted, the subset of women with HGSCs called the SET variant may have a more favorable prognosis than women with the usual type of HGSC, because these tumors are more sensitive to chemotherapy, particularly PARP therapy.104 The mechanism for this increase in chemosensitivity is thought to result from deficiencies in homologous recombination repair. This defect may occur not only in association with germline mutant BRCA1 but also in tumors that contain somatic BRCA mutations and epigenetic silencing of BRCA expression, and those showing BRCA genomic signatures or scarring (collectively, so-called BRCAness).38, 100 The effectiveness of PARP inhibitor therapy in which BRCA inactivation occurs as a result of promoter methylation is likely through synthetic lethality but needs to be further explored. This has important implications because TCGA study found that one-half of all HGSCs have abnormalities in the homologous recombination repair pathway.37 In addition to the therapeutic implications, recognition of the SET variant can be used to direct genetic testing to this group of individuals, as opposed to testing all women with HGSCs.27
Summary
In the expanded dualistic model, type I carcinomas have now been divided into three subtypes as follows: i) endometriosis-related (endometrioid, clear cell, and seromucinous or mixed müllerian neoplasms), ii) tubal-related (low-grade serous tumors), and iii) germ cell or transitional cell-related (mucinous and Brenner tumors). The first two groups are derived from Müllerian tissues, whereas some gastrointestinal-type mucinous tumors develop from mature teratomas in the ovary and others from an outgrowth of mucinous epithelium in Brenner tumors. The latter may develop from transitional epithelium (transitional metaplasia; i.e., Walthard cell nests) at the tuboperitoneal junction. The precursor lesions of the type I carcinomas are benign, ie, endometriosis, papillary tubal hyperplasia, and transitional epithelium, which implant on the ovary and then progress in a stepwise fashion to adenomas and atypical proliferative tumors before undergoing malignant transformation. In contrast, many type II carcinomas, at their inception, are established carcinomas (STIC) arising in the fimbria and capable of implanting on the ovary and other sites in the pelvic and abdominal cavities. This difference in the nature of the precursor lesions may explain why type I carcinomas remain confined to the ovary for long periods and have an indolent course, whereas type II carcinomas spread rapidly and are highly aggressive at their onset. Many type II carcinomas appear to be derived from tubal type epithelium and are therefore of Müllerian origin. Preliminary morphologic data suggest that they can be divided into two subsets as follows: the usual and SET variants. The usual type is more frequently associated with STICs, whereas the SET variant is more often associated with BRCA mutations. In addition, gene expression analysis has identified four molecular subtypes of HGSCs, termed immunoreactive, differentiated, proliferative, and mesenchymal.
In summary, recent molecular studies underline the heterogeneity of ovarian cancer, both for type I and type II tumors, demonstrating that ovarian cancer is a family of related but distinct tumors with substantial differences in molecular features, clinicopathologic characteristics, and behavior. The dramatic differences between the type I and type II tumors, in fact, argue that they describe two completely different groups of diseases. An appreciation of the different biology of these tumors should lead to a more informed approach toward prevention, diagnosis, and treatment, thereby reducing the burden of this devastating disease.
Acknowledgment
We thank Emily Gerry for editorial assistance.
Footnotes
Supported by the US Department of Defense grant OCRP-OC-100517, the Richard W. TeLinde Research Program, Johns Hopkins University, and the Roseman Ovarian Cancer Foundation (R.J.K. and I.M.S.); and NIH grant CA165807 (I.M.S.).
Disclosures: None declared.
References
- 1.Shih IeM., Kurman R.J. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmeler K.M., Tao X., Frumovitz M., Deavers M.T., Sun C.C., Sood A.K., Brown J., Gershenson D.M., Ramirez P.T. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol. 2010;116:269–273. doi: 10.1097/AOG.0b013e3181e7961d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurman R.J., Carcangiu M.L., Herrington S., Young R.H. ed 4. IARC; Lyon, France: 2014. WHO classification of tumours of female reproductive organs. [Google Scholar]
- 4.Kurman R.J., Shih IeM. Seromucinous tumors of the ovary. What's in a name? Int J Gynecol Pathol. 2016;35:78–81. doi: 10.1097/PGP.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie R., Talhouk A., Eshragh S., Lau S., Cheung D., Chow C., Le N., Cook L.S., Wilkinson N., McDermott J., Singh N., Kommoss F., Pfisterer J., Huntsman D.G., Kobel M., Kommoss S., Gilks C.B., Anglesio M.S. Morphologic and molecular characteristics of mixed epithelial ovarian cancers. Am J Surg Pathol. 2015;39:1548–1557. doi: 10.1097/PAS.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidman J.D., Cho K., Ronnett B.M., Kurman R.J. Surface epithelial tumors of the ovary. In: Kurman R.J., Ellenson L.H., Ronnett B.M., editors. Blaustein's Pathology of the Female Genital Tract. ed 6. Springer Verlag; New York: 2011. pp. 679–784. [Google Scholar]
- 7.Ardighieri L., Zeppernick F., Hannibal C.G., Vang R., Cope L., Junge J., Kjaer S.K., Kurman R.J. Shih IeM: Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol. 2014;232:16–22. doi: 10.1002/path.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longacre T.A., McKenney J.K., Tazelaar H.D., Kempson R.L., Hendrickson M.R. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29:707–723. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 9.Shvartsman H.S., Sun C.C., Bodurka D.C., Mahajan V., Crispens M., Lu K.H., Deavers M.T., Malpica A., Silva E.G., Gershenson D.M. Comparison of the clinical behavior of newly diagnosed stages II-IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma. Gynecol Oncol. 2007;105:625–662. doi: 10.1016/j.ygyno.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Dehari R., Kurman R.J., Logani S., Shih IeM. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007;31:1007–1012. doi: 10.1097/PAS.0b013e31802cbbe9. [DOI] [PubMed] [Google Scholar]
- 11.Tsang Y.T., Deavers M.T., Sun C.C., Kwan S.Y., Kuo E., Malpica A., Mok S.C., Gershenson D.M., Wong K.K. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J Pathol. 2013;231:449–456. doi: 10.1002/path.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grisham R.N., Iyer G., Garg K., DeLair D., Hyman D.M., Zhou Q., Iasonos A., Berger M.F., Dao F., Spriggs D.R., Levine D.A., Aghajanian C., Solit D.B. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119:548–554. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong K.K., Tsang Y.T., Deavers M.T., Mok S.C., Zu Z., Sun C., Malpica A., Wolf J.K., Lu K.H., Gershenson D.M. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–1617. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeppernick F., Ardighieri L., Hannibal C.G., Vang R., Junge J., Kjaer S.K., Zhang R., Kurman R.J., Shih IeM. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am J Surg Pathol. 2014;38:1603–1611. doi: 10.1097/PAS.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maniar K.P., Wang Y., Visvanathan K., Shih IeM., Kurman R.J. Evaluation of microinvasion and lymph node involvement in ovarian serous borderline/atypical proliferative serous tumors: a morphologic and immunohistochemical analysis of 37 cases. Am J Surg Pathol. 2014;38:743–755. doi: 10.1097/PAS.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurman R.J., Vang R., Junge J., Hannibal C.G., Kjaer S.K., Shih IeM. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–1614. doi: 10.1097/PAS.0b013e318229449f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu R., Hendrix-Lucas N., Kuick R., Zhai Y., Schwartz D.R., Akyol A., Hanash S., Misek D.E., Katabuchi H., Williams B.O., Fearon E.R., Cho K.R. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Saegusa M., Hashimura M., Yoshida T., Okayasu I. beta- Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001;84:209–217. doi: 10.1054/bjoc.2000.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catasus L., Bussaglia E., Rodrguez I., Gallardo A., Pons C., Irving J.A., Prat J. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360–1368. doi: 10.1016/j.humpath.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama K., Nakayama N., Kurman R.J., Cope L., Pohl G., Samuels Y., Velculescu V.E., Wang T.L., Shih IeM. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther. 2006;5:779–785. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- 21.Jones S., Wang T.L., Shih IeM., Mao T.L., Nakayama K., Roden R., Glas R., Slamon D., Diaz L.A., Jr., Vogelstein B., Kinzler K.W., Velculescu V.E., Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayr D., Hirschmann A., Lohrs U., Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–887. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Willner J., Wurz K., Allison K.H., Galic V., Garcia R.L., Goff B.A., Swisher E.M. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–613. doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Guan B., Rahmanto Y.S., Wu R.C., Wang Y., Wang Z., Wang T.L., Shih IeM. Roles of deletion of Arid1a, a tumor suppressor, in mouse ovarian tumorigenesis. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler R.L., Damrauer J.S., Raab J.R., Schisler J.C., Wilkerson M.D., Didion J.P., Starmer J., Serber D., Yee D., Xiong J., Darr D.B., Pardo-Manuel de Villena F., Kim W.Y., Magnuson T. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6:6118. doi: 10.1038/ncomms7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi K., Huang Z., Matsumura N., Mandai M., Okamoto T., Baba T., Konishi I., Berchuck A., Murphy S.K. Epigenetic determinants of ovarian clear cell carcinoma biology. Int J Cancer. 2014;135:585–597. doi: 10.1002/ijc.28701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soslow R.A., Han G., Park K.J., Garg K., Olvera N., Spriggs D.R., Kauff N.D., Levine D.A. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25:625–636. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 28.Wu C.H., Mao T.L., Vang R., Ayhan A., Wang T.L., Kurman R.J., Shih IeM. Endocervical-type mucinous borderline tumors are related to endometrioid tumors based on mutation and loss of expression of ARID1A. Int J Gynecol Pathol. 2012;31:297–303. doi: 10.1097/PGP.0b013e31823f8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackenzie R., Kommoss S., Winterhoff B.J., Kipp B.R., Garcia J.J., Voss J., Halling K., Karnezis A., Senz J., Yang W., Prigge E.S., Reuschenbach M., Doeberitz M.V., Gilks B.C., Huntsman D.G., Bakkum-Gamez J., McAlpine J.N., Anglesio M.S. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer. 2015;15:415. doi: 10.1186/s12885-015-1421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryland G.L., Hunter S.M., Doyle M.A., Rowley S.M., Christie M., Allan P.E., Bowtell D.D., Australian Ovarian Cancer Study Group. Gorringe K.L., Campbell I.G. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J Pathol. 2013;229:469–476. doi: 10.1002/path.4134. [DOI] [PubMed] [Google Scholar]
- 31.Auersperg N. The origin of ovarian carcinomas: a unifying hypothesis. Int J Gynecol Pathol. 2011;30:12–21. doi: 10.1097/PGP.0b013e3181f45f3e. [DOI] [PubMed] [Google Scholar]
- 32.Adler E., Mhawech-Fauceglia P., Gayther S.A., Lawrenson K. PAX8 expression in ovarian surface epithelial cells. Hum Pathol. 2015;46:948–956. doi: 10.1016/j.humpath.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhn E., Ayhan A., Bahadirli-Talbott A., Zhao C., Shih IeM. Molecular characterization of undifferentiated carcinoma associated with endometrioid carcinoma. Am J Surg Pathol. 2014;38:660–665. doi: 10.1097/PAS.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 34.Howitt B.E., Hanamornroongruang S., Lin D.I., Conner J.E., Schulte S., Horowitz N., Crum C.P., Meserve E.E. Evidence for a dualistic model of high-grade serous carcinoma: BRCA mutation status, histology, and tubal intraepithelial carcinoma. Am J Surg Pathol. 2015;39:287–293. doi: 10.1097/PAS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 35.Kobel M., Kalloger S.E., Lee S., Duggan M.A., Kelemen L.E., Prentice L., Kalli K.R., Fridley B.L., Visscher D.W., Keeney G.L., Vierkant R.A., Cunningham J.M., Chow C., Ness R.B., Moysich K., Edwards R., Modugno F., Bunker C., Wozniak E.L., Benjamin E., Gayther S.A., Gentry-Maharaj A., Menon U., Gilks C.B., Huntsman D.G., Ramus S.J., Goode E.L., Ovarian Tumor Tissue Analysis Consortium Biomarker-based ovarian carcinoma typing: a histologic investigation in the ovarian tumor tissue analysis consortium. Cancer Epidemiol Biomarkers Prev. 2013;22:1677–1686. doi: 10.1158/1055-9965.EPI-13-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva E.G., Robey-Cafferty S.S., Smith T.L., Gershenson D.M. Ovarian carcinomas with transitional cell carcinoma pattern. Am J Clin Pathol. 1990;93:457–465. doi: 10.1093/ajcp/93.4.457. [DOI] [PubMed] [Google Scholar]
- 37.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patch A.M., Christie E.L., Etemadmoghadam D., Garsed D.W., George J., Fereday S. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 39.Vang R., Levine D.A., Soslow R.A., Zaloudek C., Shih IeM., Kurman R.J. Molecular alterations of TP53 are a defining feature of ovarian high-grade serous carcinoma: a rereview of cases lacking tp53 mutations in the Cancer Genome Atlas Ovarian Study. Int J Gynecol Pathol. 2016;35:48–55. doi: 10.1097/PGP.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tothill R.W., Tinker A.V., George J., Brown R., Fox S.B., Lade S., Johnson D.S., Trivett M.K., Etemadmoghadam D., Locandro B., Traficante N., Fereday S., Hung J.A., Chiew Y.E., Haviv I., Australian Ovarian Cancer Study Group. Gertig D., DeFazio A., Bowtell D.D. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 41.Helland A., Anglesio M.S., George J., Cowin P.A., Johnstone C.N., House C.M., Sheppard K.E., Etemadmoghadam D., Melnyk N., Rustgi A.K., Phillips W.A., Johnsen H., Holm R., Kristensen G.B., Birrer M.J., Australian Ovarian Cancer Study Group. Pearson R.B., Borresen-Dale A.L., Huntsman D.G., deFazio A., Creighton C.J., Smyth G.K., Bowtell D.D. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS One. 2011;6:e18064. doi: 10.1371/journal.pone.0018064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konecny G.E., Wang C., Hamidi H., Winterhoff B., Kalli K.R., Dering J., Ginther C., Chen H.W., Dowdy S., Cliby W., Gostout B., Podratz K.C., Keeney G., Wang H.J., Hartmann L.C., Slamon D.J., Goode E.L. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George J., Alsop K., Etemadmoghadam D., Hondow H., Mikeska T., Dobrovic A., deFazio A., Australian Ovarian Cancer Study Group. Smyth G.K., Levine D.A., Mitchell G., Bowtell D.D. Nonequivalent gene expression and copy number alterations in high-grade serous ovarian cancers with BRCA1 and BRCA2 mutations. Clin Cancer Res. 2013;19:3474–3484. doi: 10.1158/1078-0432.CCR-13-0066. [DOI] [PubMed] [Google Scholar]
- 44.Jin Z., Ogata S., Tamura G., Katayama Y., Fukase M., Yajima M., Motoyama T. Carcinosarcomas (malignant mullerian mixed tumors) of the uterus and ovary: a genetic study with special reference to histogenesis. Int J Gynecol Pathol. 2003;22:368–373. doi: 10.1097/01.pgp.0000092134.88121.56. [DOI] [PubMed] [Google Scholar]
- 45.Abeln E.C., Smit V.T., Wessels J.W., de Leeuw W.J., Cornelisse C.J., Fleuren G.J. Molecular genetic evidence for the conversion hypothesis of the origin of malignant mixed mullerian tumours. J Pathol. 1997;183:424–431. doi: 10.1002/(SICI)1096-9896(199712)183:4<424::AID-PATH949>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Singer G., Oldt R., 3rd, Cohen Y., Wang B.G., Sidransky D., Kurman R.J., Shih IeM. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 47.Kuo K.T., Guan B., Feng Y., Mao T.L., Chen X., Jinawath N., Wang Y., Kurman R.J., Shih I.M., Wang T.L. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009;69:4036–4042. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pohl G., Ho C.L., Kurman R.J., Bristow R., Wang T.L., Shih IeM. Inactivation of the mitogen-activated protein kinase pathway as a potential target-based therapy in ovarian serous tumors with KRAS or BRAF mutations. Cancer Res. 2005;65:1994–2000. doi: 10.1158/0008-5472.CAN-04-3625. [DOI] [PubMed] [Google Scholar]
- 49.Jones S., Wang T.L., Kurman R.J., Nakayama K., Velculescu V.E., Vogelstein B., Kinzler K.W., Papadopoulos N., Shih IeM. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012;226:413–420. doi: 10.1002/path.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emmanuel C., Chiew Y.E., George J., Etemadmoghadam D., Anglesio M.S., Sharma R., Russell P., Kennedy C., Fereday S., Hung J., Galletta L., Hogg R., Wain G.V., Brand A., Balleine R., MacConaill L., Palescandolo E., Hunter S.M., Campbell I., Dobrovic A., Wong S.Q., Do H., Clarke C.L., Harnett P.R., Bowtell D.D., deFazio A., Australian Ovarian Cancer Study (AOCS) Genomic classification of serous ovarian cancer with adjacent borderline differentiates RAS pathway and TP53-mutant tumors and identifies NRAS as an oncogenic driver. Clin Cancer Res. 2014;20:6618–6630. doi: 10.1158/1078-0432.CCR-14-1292. [DOI] [PubMed] [Google Scholar]
- 51.Ho C.L., Kurman R.J., Dehari R., Wang T.-L., Shih IeM. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004;64:6915–6918. doi: 10.1158/0008-5472.CAN-04-2067. [DOI] [PubMed] [Google Scholar]
- 52.Sheu J.J., Guan B., Tsai F.J., Hsiao E.Y., Chen C.M., Seruca R., Wang T.L., Shih IeM. Mutant BRAF induces DNA strand breaks, activates DNA damage response pathway, and up-regulates glucose transporter-1 in nontransformed epithelial cells. Am J Pathol. 2012;180:1179–1188. doi: 10.1016/j.ajpath.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ardighieri L., Zeppernick F., Hannibal C.G., Vang R., Cope L., Junge J., Kjaer S.K., Kurman R.J., Shih IeM. Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol. 2014;232:16–22. doi: 10.1002/path.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dankort D., Filenova E., Collado M., Serrano M., Jones K., McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato M., Paranjape T., Muller R.U., Nallur S., Gillespie E., Keane K., Esquela-Kerscher A., Weidhaas J.B., Slack F.J. The mir-34 microRNA is required for the DNA damage response in vivo in C. elegans and in vitro in human breast cancer cells. Oncogene. 2009;28:2419–2424. doi: 10.1038/onc.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlosshauer P.W., Deligdisch L., Penault-Llorca F., Fatemi D., Qiao R., Yao S., Pearl M., Yang Z., Sheng T., Dong J. Loss of p16INK4A expression in low-grade ovarian serous carcinomas. Int J Gynecol Pathol. 2011;30:22–29. doi: 10.1097/PGP.0b013e3181ed89b3. [DOI] [PubMed] [Google Scholar]
- 57.Wiegand K.C., Shah S.P., Al-Agha O.M., Zhao Y., Tse K., Zeng T. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayhan A., Mao T.L., Seckin T., Wu C.H., Guan B., Ogawa H., Futagami M., Mizukami H., Yokoyama Y., Kurman R.J., Shih IeM. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gynecol Cancer. 2012;22:1310–1315. doi: 10.1097/IGC.0b013e31826b5dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato N., Tsunoda H., Nishida M., Morishita Y., Takimoto Y., Kubo T., Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–7056. [PubMed] [Google Scholar]
- 60.Shappell H.W., Riopel M.A., Smith Sehdev A.E., Ronnett B.M., Kurman R.J. Diagnostic criteria and behavior of ovarian seromucinous (endocervical-type mucinous and mixed cell-type) tumors: atypical proliferative (borderline) tumors, intraepithelial, microinvasive, and invasive carcinomas. Am J Surg Pathol. 2002;26:1529–1541. doi: 10.1097/00000478-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Vang R., Gown A.M., Barry T.S., Wheeler D.T., Ronnett B.M. Ovarian atypical proliferative (borderline) mucinous tumors: gastrointestinal and seromucinous (endocervical-like) types are immunophenotypically distinctive. Int J Gynecol Pathol. 2006;25:83–89. doi: 10.1097/01.pgp.0000177125.31046.fd. [DOI] [PubMed] [Google Scholar]
- 62.Mok S.C., Bell D.A., Knapp R.C., Fishbaugh P.M., Welch W.R., Muto M.G., Berkowitz R.S., Tsao S.W. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–1492. [PubMed] [Google Scholar]
- 63.Cuatrecasas M., Villanueva A., Matias-Guiu X., Prat J. K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79:1581–1586. doi: 10.1002/(sici)1097-0142(19970415)79:8<1581::aid-cncr21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 64.Seidman J.D., Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: a study of 120 tumors. Arch Pathol Lab Med. 2008;132:1753–1760. doi: 10.5858/132.11.1753. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Wu R.C., Shwartz L.E., Haley L., Lin M.T., Shih I.M., Kurman R.J. Clonality analysis of combined Brenner and mucinous tumours of the ovary reveals their monoclonal origin. J Pathol. 2015;237:146–151. doi: 10.1002/path.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piek J.M., van Diest P.J., Zweemer R.P., Jansen J.W., Poort-Keesom R.J., Menko F.H., Gille J.J., Jongsma A.P., Pals G., Kenemans P., Verheijen R.H. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 67.Piek J.M., van Diest P.J., Zweemer R.P., Kenemans P., Verheijen R.H. Tubal ligation and risk of ovarian cancer. Lancet. 2001;358:844. doi: 10.1016/S0140-6736(01)05992-X. [DOI] [PubMed] [Google Scholar]
- 68.Piek J.M., Verheijen R.H., Kenemans P., Massuger L.F., Bulten H., van Diest P.J. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol. 2003;90:491. doi: 10.1016/s0090-8258(03)00365-2. [DOI] [PubMed] [Google Scholar]
- 69.Finch A., Beiner M., Lubinski J., Lynch H.T., Moller P., Rosen B., Murphy J., Ghadirian P., Friedman E., Foulkes W.D., Kim-Sing C., Wagner T., Tung N., Couch F., Stoppa-Lyonnet D., Ainsworth P., Daly M., Pasini B., Gershoni-Baruch R., Eng C., Olopade O.I., McLennan J., Karlan B., Weitzel J., Sun P., Narod S.A., Hereditary Ovarian Cancer Clinical Study Group Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 70.Medeiros F., Muto M.G., Lee Y., Elvin J.A., Callahan M.J., Feltmate C., Garber J.E., Cramer D.W., Crum C.P. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 71.Kindelberger D.W., Lee Y., Miron A., Hirsch M.S., Feltmate C., Medeiros F., Callahan M.J., Garner E.O., Gordon R.W., Birch C., Berkowitz R.S., Muto M.G., Crum C.P. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 72.Przybycin C.G., Kurman R.J., Ronnett B.M., Shih IeM., Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 73.Morrison J.C., Blanco L.Z., Jr., Vang R., Ronnett B.M. Incidental serous tubal intraepithelial carcinoma and early invasive serous carcinoma in the nonprophylactic setting: analysis of a case series. Am J Surg Pathol. 2015;39:442–453. doi: 10.1097/PAS.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 74.Gilks C.B., Irving J., Kobel M., Lee C., Singh N., Wilkinson N., McCluggage W.G. Incidental nonuterine high-grade serous carcinomas arise in the fallopian tube in most cases: further evidence for the tubal origin of high-grade serous carcinomas. Am J Surg Pathol. 2015;39:357–364. doi: 10.1097/PAS.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 75.Hirst J.E., Gard G.B., McIllroy K., Nevell D., Field M. High rates of occult fallopian tube cancer diagnosed at prophylactic bilateral salpingo-oophorectomy. Int J Gynecol Cancer. 2009;19:826–829. doi: 10.1111/IGC.0b013e3181a1b5dc. [DOI] [PubMed] [Google Scholar]
- 76.Rabban J.T., Garg K., Crawford B., Chen L.M., Zaloudek C.J. Early detection of high-grade tubal serous carcinoma in women at low risk for hereditary breast and ovarian cancer syndrome by systematic examination of fallopian tubes incidentally removed during benign surgery. Am J Surg Pathol. 2014;38:729–742. doi: 10.1097/PAS.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 77.Tang S., Onuma K., Deb P., Wang E., Lytwyn A., Sur M., Daya D. Frequency of serous tubal intraepithelial carcinoma in various gynecologic malignancies: a study of 300 consecutive cases. Int J Gynecol Pathol. 2012;31:103–110. doi: 10.1097/PGP.0b013e31822ea955. [DOI] [PubMed] [Google Scholar]
- 78.Kuhn E., Kurman R.J., Vang R., Sehdev A.S., Han G., Soslow R., Wang T.L., Shih I.M. TP53 mutations in serous tubal intraepithelial carcinoma (STIC) and concurrent pelvic high-grade serous carcinoma- evidence supporting their clonal relationship. J Pathol. 2012;226:421–426. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuhn E., Meeker A., Wang T.L., Sehdev A.S., Kurman R.J. Shih Ie-M: Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. Am J Surg Pathol. 2010;34:829–836. doi: 10.1097/PAS.0b013e3181dcede7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perets R., Wyant G.A., Muto K.W., Bijron J.G., Poole B.B., Chin K.T., Chen J.Y., Ohman A.W., Stepule C.D., Kwak S., Karst A.M., Hirsch M.S., Setlur S.R., Crum C.P., Dinulescu D.M., Drapkin R. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Falconer H., Yin L., Gronberg H., Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/dju410. [DOI] [PubMed] [Google Scholar]
- 82.McAlpine J.N., Hanley G.E., Woo M.M., Tone A.A., Rozenberg N., Swenerton K.D., Gilks C.B., Finlayson S.J., Huntsman D.G., Miller D.M., Ovarian Cancer Research Program of British Columbia Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol. 2014;210:471.e1–471.e11. doi: 10.1016/j.ajog.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Visvanathan K., Vang R., Shaw P., Gross A., Soslow R., Parkash V., Shih IeM., Kurman R.J. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am J Surg Pathol. 2011;35:1766–1775. doi: 10.1097/PAS.0b013e31822f58bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jarboe E., Folkins A., Nucci M.R., Kindelberger D., Drapkin R., Miron A., Lee Y., Crum C.P. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 85.Espinosa I., Catasus L., Canet B., D'Angelo E., Munoz J., Prat J. Gene expression analysis identifies two groups of ovarian high-grade serous carcinomas with different prognosis. Mod Pathol. 2011;24:846–854. doi: 10.1038/modpathol.2011.12. [DOI] [PubMed] [Google Scholar]
- 86.Gagner J.P., Mittal K. Malignant mixed Mullerian tumor of the fimbriated end of the fallopian tube: origin as an intraepithelial carcinoma. Gynecol Oncol. 2005;97:219–222. doi: 10.1016/j.ygyno.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 87.Jacobs I.J., Menon U., Ryan A., Gentry-Maharaj A., Burnell M., Kalsi J.K. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buys S.S., Partridge E., Black A., Johnson C.C., Lamerato L., Isaacs C., Reding D.J., Greenlee R.T., Yokochi L.A., Kessel B., Crawford E.D., Church T.R., Andriole G.L., Weissfeld J.L., Fouad M.N., Chia D., O'Brien B., Ragard L.R., Clapp J.D., Rathmell J.M., Riley T.L., Hartge P., Pinsky P.F., Zhu C.S., Izmirlian G., Kramer B.S., Miller A.B., Xu J.L., Prorok P.C., Gohagan J.K., Berg C.D., PLCO Project Team Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 89.Kobel M., Madore J., Ramus S.J., Clarke B.A., Pharoah P.D., Deen S. Evidence for a time-dependent association between FOLR1 expression and survival from ovarian carcinoma: implications for clinical testing. An Ovarian Tumour Tissue Analysis consortium study. Br J Cancer. 2014;111:2297–2307. doi: 10.1038/bjc.2014.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinde I., Bettegowda C., Wang Y., Wu J., Agrawal N., Shih IeM., Kurman R., Dao F., Levine D.A., Giuntoli R., Roden R., Eshleman J.R., Carvalho J.P., Marie S.K., Papadopoulos N., Kinzler K.W., Vogelstein B., Diaz L.A., Jr. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5:167ra4. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bijron J.G., Seldenrijk C.A., Zweemer R.P., Lange J.G., Verheijen R.H., van Diest P.J. Fallopian tube intraluminal tumor spread from noninvasive precursor lesions: a novel metastatic route in early pelvic carcinogenesis. Am J Surg Pathol. 2013;37:1123–1130. doi: 10.1097/PAS.0b013e318282da7f. [DOI] [PubMed] [Google Scholar]
- 92.Fishman D.A., Cohen L., Blank S.V., Shulman L., Singh D., Bozorgi K., Tamura R., Timor-Tritsch I., Schwartz P.E. The role of ultrasound evaluation in the detection of early-stage epithelial ovarian cancer. Am J Obstet Gynecol. 2005;192:1214–1221. doi: 10.1016/j.ajog.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 93.Powell C.B., Swisher E.M., Cass I., McLennan J., Norquist B., Garcia R.L., Lester J., Karlan B.Y., Chen L. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecol Oncol. 2013;129:364–371. doi: 10.1016/j.ygyno.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conner J.R., Meserve E., Pizer E., Garber J., Roh M., Urban N., Drescher C., Quade B.J., Muto M., Howitt B.E., Pearlman M.D., Berkowitz R.S., Horowitz N., Crum C.P., Feltmate C. Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations. Gynecol Oncol. 2014;132:280–286. doi: 10.1016/j.ygyno.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parker W.H., Feskanich D., Broder M.S., Chang E., Shoupe D., Farquhar C.M., Berek J.S., Manson J.E. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstet Gynecol. 2013;121:709–716. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wethington S.L., Park K.J., Soslow R.A., Kauff N.D., Brown C.L., Dao F., Otegbeye E., Sonoda Y., Abu-Rustum N.R., Barakat R.R., Levine D.A., Gardner G.J. Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC) Int J Gynecol Cancer. 2013;23:1603–1611. doi: 10.1097/IGC.0b013e3182a80ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V., Doll R., Hermon C., Peto R., Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 98.Habis M., Wroblewski K., Bradaric M., Ismail N., Yamada S.D., Litchfield L., Lengyel E., Romero I.L. Statin therapy is associated with improved survival in patients with non-serous-papillary epithelial ovarian cancer: a retrospective cohort analysis. PLoS One. 2014;9:e104521. doi: 10.1371/journal.pone.0104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi Y., Kashima H., Wu R.C., Jung J.G., Kuan J.C., Gu J., Xuan J., Sokoll L., Visvanathan K., Shih I.M., Wang T.L. Mevalonate pathway antagonist suppresses formation of serous tubal intraepithelial carcinoma and ovarian carcinoma in mouse models. Clin Cancer Res. 2015;21:4652–4662. doi: 10.1158/1078-0432.CCR-14-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., Biedrzycki B., Donehower R.C., Zaheer A., Fisher G.A., Crocenzi T.S., Lee J.J., Duffy S.M., Goldberg R.M., de la Chapelle A., Koshiji M., Bhaijee F., Huebner T., Hruban R.H., Wood L.D., Cuka N., Pardoll D.M., Papadopoulos N., Kinzler K.W., Zhou S., Cornish T.C., Taube J.M., Anders R.A., Eshleman J.R., Vogelstein B., Diaz L.A., Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bitler B.G., Aird K.M., Garipov A., Li H., Amatangelo M., Kossenkov A.V., Schultz D.C., Liu Q., Shih IeM., Conejo-Garcia J.R., Speicher D.W., Zhang R. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen J., Peng Y., Wei L., Zhang W., Yang L., Lan L., Kapoor P., Ju Z., Mo Q., Shih IeM., Uray I.P., Wu X., Brown P.H., Shen X., Mills G.B., Peng G. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5:752–767. doi: 10.1158/2159-8290.CD-14-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J.F., Barry W.T., Birrer M., Lee J.M., Buckanovich R.J., Fleming G.F., Rimel B., Buss M.K., Nattam S., Hurteau J., Luo W., Quy P., Whalen C., Obermayer L., Lee H., Winer E.P., Kohn E.C., Ivy S.P., Matulonis U.A. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H., Thornton A., Norquist B.M., Casadei S., Nord A.S., Agnew K.J., Pritchard C.C., Scroggins S., Garcia R.L., King M.C., Swisher E.M. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]