Abstract
BACKGROUND: Despite many publications about cerebral cavernous malformations (CCMs), controversy remains regarding diagnostic and management strategies.
OBJECTIVE: To develop guidelines for CCM management.
METHODS: The Angioma Alliance (www.angioma.org), the patient support group in the United States advocating on behalf of patients and research in CCM, convened a multidisciplinary writing group comprising expert CCM clinicians to help summarize the existing literature related to the clinical care of CCM, focusing on 5 topics: (1) epidemiology and natural history, (2) genetic testing and counseling, (3) diagnostic criteria and radiology standards, (4) neurosurgical considerations, and (5) neurological considerations. The group reviewed literature, rated evidence, developed recommendations, and established consensus, controversies, and knowledge gaps according to a prespecified protocol.
RESULTS: Of 1270 publications published between January 1, 1983 and September 31, 2014, we selected 98 based on methodological criteria, and identified 38 additional recent or relevant publications. Topic authors used these publications to summarize current knowledge and arrive at 23 consensus management recommendations, which we rated by class (size of effect) and level (estimate of certainty) according to the American Heart Association/American Stroke Association criteria. No recommendation was level A (because of the absence of randomized controlled trials), 11 (48%) were level B, and 12 (52%) were level C. Recommendations were class I in 8 (35%), class II in 10 (43%), and class III in 5 (22%).
CONCLUSION: Current evidence supports recommendations for the management of CCM, but their generally low levels and classes mandate further research to better inform clinical practice and update these recommendations. The complete recommendations document, including the criteria for selecting reference citations, a more detailed justification of the respective recommendations, and a summary of controversies and knowledge gaps, was similarly peer reviewed and is available on line www.angioma.org/CCMGuidelines.
Keywords: Cavernous, Angioma, Malformation, Guidelines, Recommendations
ABBREVIATIONS
- CCM
cerebral cavernous malformations
- CI
confidence interval
- CRE
cerebral cavernous malformation-related epilepsy
- CT
computed tomography
- DVA
developmental venous anomaly
- FND
focal neurological deficit
- HR
hazard ratio
- ICH
intracranial hemorrhage
- MRC
Medical Research Council
- MRI
magnetic resonance imaging
- mRS
modified Rankin score
- NSAID
nonsteroidal anti-inflamatory drug
- OHS
Oxford Handicap Scale
- SRS
stereotactic radiosurgery
A remarkable number of papers focusing on the clinical management of cerebral cavernous malformations (CCMs) have been published in the peer-reviewed literature, mostly with greater recognition of the disease upon the advent of magnetic resonance imaging (MRI). Opinions guiding clinical practice have been expressed based on selected information from the literature, but these have not been synthesized into consensus recommendations for disease management based on systematic review of all available evidence.
The Cavernoma Alliance UK, a patient support group based in the United Kingdom commissioned a scientific advisory panel to develop guidelines based on high-quality evidence published before January 1st, 2011. They found few published studies of the diagnosis and treatment of CCM of level 1 or 2 quality according to the Centre for Evidence-Based Medicine's 2011 criteria and were therefore unable to make many specific recommendations.1
Expert opinions have been proposed to fill the gap that exists between research and clinical practice.2 Expert opinions on CCM management have been assembled in 3 published monographs to date3-5 and in a project by invited Italian experts in 2009.6 These efforts did not use a methodology of systematic literature review.
The current project was initiated by the Angioma Alliance (www.angioma.org), the patient support group in the United States advocating on behalf of patients and research in CCM. The scope and goals of this project were developed in consultation between the Angioma Alliance Scientific Advisory Board and the patient community through the Angioma Alliance Board of Directors and committees, which developed a range of relevant clinical questions. The project aimed to develop expert consensus guidelines guided by a systematic analysis of the peer-reviewed literature with regard to relevant clinical questions impacting CCM management. It further aimed to define levels of evidence, areas of current consensus and controversy, and knowledge gaps in the diagnosis (imaging, genetic testing, etc.), monitoring (surveillance strategies, lifestyle decisions, etc.), and treatment (medical, surgical resection and radiosurgery) of CCM and its associated clinical manifestations. These consensus recommendations are intended to define recommended care options and to guide clinical decisions in community and referral care settings, based on the available literature and current understanding of the disease by its leading experts. It is also hoped that these recommendations would provide a roadmap for future clinical research based on relevant knowledge gaps and areas of equipoise and controversy. The process for guideline development followed recommendations of the US Preventive Services Taskforce [https://www.uspreventiveservicestaskforce.org] and the Standards for Developing Trustworthy Clinical Practice Guidelines of the U.S. National Academy of Medicine [https://www.ncbi.nlm.nih.gov/books/NBK209539/] with regard to multidisciplinary writing group composition, input by the patient community, topic-focused systematic review of the literature, prespecified methodology for justifying recommendations, the standardized rating of recommendations, and a transparent process of consensus development regarding recommendations.
METHODS
Writing Group and Development of the Project Outline
A multidisciplinary writing group (“Writing Group”) including clinician members of the Angioma Alliance Scientific Advisory Board, and invited experts were assembled to help summarize the existing literature related to the clinical care of CCM, focusing on 5 key topics: (1) epidemiology and natural history, (2) genetic testing and counseling, (3) diagnostic criteria and radiology standards, (4) neurosurgical considerations, and (5) neurological considerations. For each topic, specific questions were formulated by the writing group with input from the Angioma Alliance patient community, and these were developed into a proposed outline of the sections addressing the 5 key topics. These were used to generate specific key words for the literature search (Table 1). Members of the Writing Group were assigned to each of the 5 respective topics (“Topic Authors”) based on their areas of expertise, each with a lead topic author.
TABLE 1.
Literature Search Terms and Topics
| Literature search terms for CCM (combined with the Boolean operator “OR”) |
| Cavernous angioma, cavernous malformation, cavernous hemangioma, or cavernoma |
| Literature search terms for the topics (combined with terms for CCM with the Boolean operator “AND”) |
| Prevalence, incidence, natural history, presentation, epidemiology, genetics, genotype, phenotype, sporadic CCM, single lesion, familial CCM, multiple lesion, spinal CCM, pregnancy, pediatric, imaging, MRI, CAT scan, CT, acquisition sequences, hemorrhage, bleeding, epilepsy, seizure, headache, antithrombotic, hormone, head injury, incidental findings, surgery, craniotomy, radiosurgery, postoperative care, therapeutics, cerebral, spinal, brainstem, deep, hemorrhagic stroke, and stroke |
| Epidemiology and natural history formulated questions/topics |
| Disease prevalence and incidence |
| Comment about rarity |
| Relevant outcome measures |
| Bleed risk per CCM, per patient, rebleed vs first bleed |
| Impact of interventions |
| Summary of knowledge gaps and controversies |
| Genetic testing and counseling formulated questions/topics |
| Review of the genetic basis of CCM (including relative frequencies of CCM1, CCM2, and CCM3 genotypes) |
| Genotype/phenotype correlation and CCM3 syndrome |
| Genetic testing |
| Benefits/advantages of genetic testing |
| Confirming diagnosis |
| Family screening |
| Who should be tested? |
| Screening of children |
| Prenatal testing |
| Summary of knowledge gaps and controversies |
| Diagnostic criteria and radiology standards formulated questions/topics |
| What are the standard criteria for MRI acquisition sequences and reporting to properly diagnose CCM of the brain and/or spinal cord? |
| Frequency of routine/follow-up MRI |
| Appropriate use/caution of CAT scans |
| Imaging parameters for prospective studies |
| New technologies and novel imaging biomarkers |
| Summary of knowledge gaps and controversies |
| Neurosurgical considerations formulated questions/topics |
| Indications for CCM resection—surgery vs conservative management |
| Thresholds for surgical intervention per CCM location and rates of complication |
| Surgery for CCM associated with seizures |
| In what situations is radiosurgery preferable to CCM microsurgical resection? |
| Special considerations for radiosurgery and familial CCM |
| Special considerations in solitary vs multifocal CCMs, associated venous anomalies |
| How to manage incidental CCMs? |
| Summary of knowledge gaps and controversies |
| Neurosurgical considerations formulated questions/topics |
| How to manage hemorrhage in cases of single and multiple CCMs? |
| How to manage seizures in cases of single and multiple CCMs? |
| How to manage head pain in cases of single and multiple CCMs? |
| How to manage incidental CCM? |
| Recommendations for CCM management during pregnancy |
| Special considerations for childhood onset |
| Influence of select medications (antithrombics, hormonal agents, etc.) |
| What pain medications can be safely used and for which indications? |
| Contraindicated activities and potential for head injury |
| Summary of knowledge gaps and controversies |
CCM = cerebral cavernous malformation.
Systematic Literature Review and Cataloging of Selected References
The literature searched for publications in the English language appearing between January 1, 1983 and September 31, 2014 with key words for the condition (linked by the word “OR”): cavernous angioma, cavernous malformation, cavernous hemangioma, or cavernoma. Key text words for the intervention or clinical feature (linked by the word “AND” to the key words for the condition) prevalence, incidence, natural history, presentation, epidemiology, genetics, genotype, phenotype, sporadic CCM, single lesion, familial CCM, multiple lesion, spinal CCM, pregnancy, and pediatric were searched by the AA and KD group. Imaging, MRI, computed tomography (CT) scan, acquisition sequences, hemorrhage, bleeding, epilepsy, seizure, headache, antithrombotic, hormone, head injury, sports, contraindicated activities, incidental findings, surgery, craniotomy, radiosurgery, postoperative care, therapeutics, cerebral, spinal, brainstem, and deep were searched by IAA and TR. The key words had been selected by the Writing Groups based on questions identified by the lay group and scientific advisory Board (Table 1). This search yielded 1270 publications which were screened at the abstract level, and grouped into 5 topic areas (some articles were listed in more than 1 topic area).
In order to practically limit the number of cited papers, the broad lists of topic-related references were then narrowed down for preferential citation using prespecified criteria detailed in the full Guidelines document [www.angioma.org/CCMGuidelines]. In addition to the list of references selected for preferential citation (n = 98, 17-26 per topic area), the Topic Authors were given wide leeway in citing references from the broader list, other and newer references (appearing after September 2014 date of systematic literature review) that they felt were critical for articulating a specific recommendation. For topic questions without published peer-reviewed articles, we sought book chapters that refer to expert opinion on those topics in the 3 published textbooks on cavernous malformations.3-5 Ultimately, 136 references were cited in support of the recommendations.
Process of Manuscript Assembly and Approval
Reference lists were catalogued by the 5 key topics (some articles were assigned to more than 1 topic), and were distributed to the Topic Authors. The respective Topic Authors (excepting the section on Epidemiology and Natural History) were asked to grade the quality of evidence for class (size of effect) and level (estimate of certainty) using the American Heart Association scoring system (Table 2).7 Authors were tasked to summarize, within assigned manuscript length limits, the current knowledge reflected in the literature addressing the previously outlined topic items, justify the respective recommendations by citing supporting evidence or lack thereof, and to identify areas of controversy and knowledge gaps. The writing group used the Delphi technique8 to formulate expert opinion consensus where high-level evidence is lacking. Anonymous voting on the levels and classes of evidence was repeated 3 times, achieving agreement among all authors regarding every recommendation. There was no attempt in these guidelines to assess the potential bias in individual studies or across studies, nor the impact that bias might have on the recommended guidelines.
TABLE 2.
Definition of Classes and Levels of Evidence Used in American Heart Association/American Stroke Association Recommendations. Table Reprinted With Permission. Stroke.2015;46:2032-2060 ©American Heart Association, Inc.
| Class I | Conditions for which there is evidence for and/or general agreement that the procedure or treatment is useful and effective |
| Class II | Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment |
| Class IIa | The weight of evidence or opinion is in favor of the procedure or treatment |
| Class IIb | Usefulness/efficacy is less well established by evidence or opinion |
| Class III | Conditions for which there is evidence and/or general agreement that the procedure or treatment is not useful/effective and in some cases may be harmful |
| Therapeutic recommendations | |
| Level of evidence A | Data derived from multiple randomized clinical trials or meta-analyses |
| Level of evidence B | Data derived from a single randomized trial or nonrandomized studies |
| Level of evidence C | Consensus opinion of experts, case studies, or standard of care |
| Diagnostic recommendations | |
| Level of evidence A | Data derived from multiple prospective cohort studies using a reference standard applied by a masked evaluator |
| Level of evidence B | Data derived from a single grade A study or 1 or more case-controlled studies, or studies using a reference standard applied by an unmasked evaluator |
| Level of evidence C | Consensus opinion of experts |
Topic drafts were circulated for comments by all the Writing Group, and these were included in revisions and manuscript assembly conducted by AA and IAA. The assembled manuscript was circulated for further comments and ultimate approval by all members of the Writing Group. We herein publish a synopsis of the recommendations, including their detailed methodology, and a list of recommendations with their respective classes and levels of evidence, and justifying reference citations. The complete recommendations document, including the criteria for selecting reference citations, a more detailed justification of the respective recommendations, and a summary of controversies and knowledge gaps, was similarly peer reviewed and is available online [www.angioma.org/CCMGuidelines].
EPIDEMIOLOGY AND UNTREATED CLINICAL COURSE
CCM is also referred to in the literature as cavernous angioma, hemangioma, or cavernoma (Online Mendelian Inheritance in Man #116860). Disease prevalence is estimated at 0.16%9 to 0.5%,10,11 and a population-based annual detection rate of CCM has been estimated at 0.56 per 100 000 per year for adults >16 years of age.12 The most common clinical manifestations of CCM include seizures (50%), intracranial hemorrhage (ICH; 25%), and focal neurological deficits (FND) without radiographic evidence of recent hemorrhage (25%).13 However, a significant fraction of cases (20%-50%) have no symptoms and are discovered incidentally due to widespread availability and utilization of brain MRI.9,14
CCMs can occur in either a sporadic or familial form, and can also appear de novo15 or after radiation therapy.16 Approximately 20% of cases present with multiple CCMs,13,17 many with a positive family history consistent with autosomal dominant inheritance. The diagnosis of familial CCM can be confirmed by genetic testing for mutations in 3 genes: CCM1 (KRIT1), CCM2 (MGC4607), or CCM3 (PDCD10; see genetic testing section for more details). CCM has been reported in all race/ethnicities; however, Hispanic-Americans from the Southwest region of the US and northern states of Mexico have a higher prevalence of CCM18,19 due to a founder mutation in CCM1 (Q455X or “Common Hispanic Mutation”) that explains the majority of cases in this ethnic group.20
Symptomatic ICH is the most feared complication of CCM, and the primary reason for treating them. Previous CCM natural history studies calculating ICH rates have reported a wide range of frequencies, partly due to differences in definition of ICH. Thus, CCM hemorrhage was standardized in 2008 as “requiring acute or subacute onset symptoms (any of headache, epileptic seizure, impaired consciousness, or new/worsened FND referable to the anatomic location of the CCM) accompanied by radiological, pathological, surgical, or rarely only cerebrospinal fluid evidence of recent extra- or intralesional hemorrhage. The definition includes neither an increase in CCM diameter without other evidence of recent hemorrhage, nor the existence of a hemosiderin halo.”21
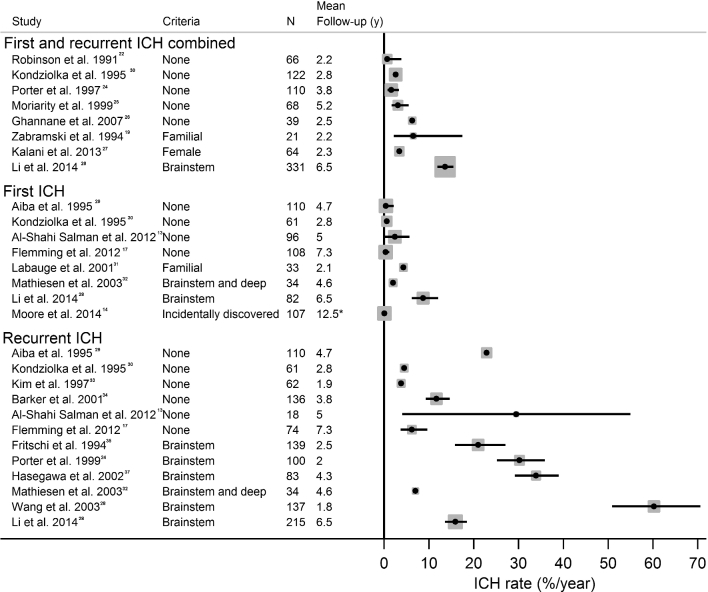
The authors updated a systematic review of studies published in 201213 that (a) included 20 or more CCM patients, (b) presented annual hemorrhage rates per-patient year, and (c) had at least 1 year of follow-up. Figure summarizes annual hemorrhage rates per patient-year by combined first and recurrent hemorrhage,19,22-28 followed by first hemorrhage13,14,17,28-32 and then recurrent hemorrhage.13,17,28-30,32-38 Two meta-analysis studies have been conducted; one used aggregate data from studies,39 but the most recent used individual patient data from 7 cohorts and report a 5-year ICH risk of 15.8% (13.7%-17.9%) overall.40 Two studies and the recent individual patient data meta-analysis also showed that the annual risk of recurrent ICH significantly declined over time,13,17,40 which has long-term clinical implications when weighing treatment decisions for CCM patients. Further, the risk of first hemorrhage was very low (0.08% per patient-year) among CCM cases identified incidentally.14
FIGURE.
Annual ICH per patient-year by combined, first, or recurrent ICH. Studies are ordered by selection criteria and date. ICH rates and 95% CI (when available) from published papers are plotted. Figure adapted and updated from Al-Shahi Salman et al,13 available at http://www.sciencedirect.com/science/article/pii/S1474442212700042, and licensed under the Creative Commons Attribution License (CC BY).
Initial CCM presentation with hemorrhage (hazard ratio [HR] 5.6, 95% confidence interval [CI] 3.2-9.7) and CCM location in the brainstem (HR 4.4, 95% CI 2.3-8.6) are the 2 risk factors for future CCM hemorrhage that have been identified by many individual studies, and conclusively by the individual patient data meta-analysis.40 Patients with CCM located in the brainstem have higher rates of hemorrhage in the untreated course (ranging from 2% to 60%, Figure).28,32,35-38,40
Other than this, female sex, CCM size, and CCM multiplicity have all been reported as risk factors for hemorrhage with inconsistent results.39 Al-Holou et al11 specifically examined risk among 56 CCM cases ≤25 years of age (identified by screening 14 936 records at their institution over a 12-year period), and found comparable hemorrhage rates of 1.6% per patient-year, which was much higher in the symptomatic group (8.0%) compared to the incidentally discovered group (0.2%). These results suggest that there is not an increased annual risk of bleeding in children and younger adults with CCM when indirectly compared to rates reported in older adults. However, younger age at ICH is observed in some familial cases of CCM, and lifetime hemorrhage risk is probably greater in younger patients.
Data available on natural history of spinal cord cavernous malformations are sparse.41 Badhiwala et al42 recently performed a meta-analysis of 40 studies, totaling 632 cases of intramedullary spinal cord cavernous malformations, and reported an annual hemorrhage rate of 2.1% (95% CI: 1.3%-3.3%). Associated CCM occurred in 17% and family history of CCM in 12%.42
Data across familial CCM studies generally report higher annual ICH rates per patient-year than for sporadic cases (4.3-6.5%, Figure).19,31 Because of multiple CCMs in familial cases, hemorrhage rates per CCM-year are also typically reported (0.6%-1.1% per CCM-year, Figure), 19,31,43 which are similar to sporadic cases. For cases with repeat scans, the rate of new CCM formation per patient-year can also be calculated, which ranges from 0.4 to a high of 2.7 new CCMs per patient-year in CCM3 cases,39,43 demonstrating the variable and dynamic nature of familial CCMs.
Seizures related to CCM are thought to be induced by recurrent microhemorrhages, resulting in surrounding blood (hemosiderin), perilesional gliosis, and inflammation.44 There has been only 1 study examining seizures as an endpoint in CCM. Josephson et al45 performed a prospective population-based study of 139 adults diagnosed with CCM and found that a 5-year risk of first-ever seizure was 6% (95% CI: 0%-14%) in 38 CCM patients presenting with ICH/FND and 4% (95% CI: 0%-10%) in 57 CCM patients presenting incidentally. Among adults who never experienced ICH/FND and presented with or developed epilepsy, the proportion achieving 2-year seizure freedom over 5 years was 47% (95% CI: 27%-67%). Thus, adults with CCM may have a high risk of epilepsy after first-ever seizure and roughly half achieve 2-year seizure freedom over 5 years after an epilepsy diagnosis.
There is no standardized tool for assessing functional outcome in CCM studies,46 and many derivatives of the modified Rankin score (mRS) exist, such as the Oxford Handicap Scale (OHS), which has been used in some CCM studies.47 Li et al28 calculated 5-year complete recovery rates (final mRS scores of 0) in 331 brainstem CCM patients seen at their hospital between 1985 and 2012, and found significant reduction in recovery across groups experiencing no hemorrhages (37%), 1 hemorrhage (18%), or more than 1 prospective hemorrhage event (11%). Overall, the complete recovery rate was 30.3% at 2 years, which primarily occurred within the first 18 months after the most recent hemorrhage. Moultrie et al47 reported clinical outcomes in 109 conservatively managed CCM patients from a prospective, population-based study conducted in Scotland between 1999 and 2003. Poor outcome was defined as at least 2 successive ratings of the OHS scores between 2 and 6. During 5 years of follow-up, 37% (95% CI: 28%-46%) of the conservatively managed group experienced poor OHS outcome. Cordonnier et al48 reported that functional impairment from hemorrhage is milder at initial presentation for CCM than other types of intracranial vascular malformation.
GENETIC TESTING AND COUNSELING
The genetic basis of CCM has been established. Familial CCM, typified by multifocal CCMs and/or a family history, is caused by loss of function mutations in 1 of 3 genes, CCM1 (KRIT1), CCM2 (MGC4607), and CCM3 (PDCD10).49,50 The functions of these genes continue to be investigated; all are involved in signaling networks responsible for the maintenance of junctional integrity between neighboring vascular endothelial cells.51,52 Biallelic somatic mutations of the same genes in CCM endothelial cells likely contribute to CCM genesis in both familial and sporadic CCM.53,54 Approximately 20% of cases are estimated to be familial with autosomal dominant inheritance,50 although estimating risks is complicated by incomplete penetrance and variable presentation even within families.55 The vast majority of familial cases have multiple CCMs. The remaining 80% of CCM cases are sporadic and present most often with solitary CCMs, often associated with a developmental venous anomaly (DVA) and without germline mutation of any CCM gene.53,56 Multiple CCMs in immediate association with a DVA and/or due to localized radiation are occasionally seen in sporadic cases.56,57
Genetic testing of familial cases should include direct sequencing and deletion analysis of CCM1-3.58 Following this protocol results in a mutation detection rate of at least 75% of all cases with multiple CCMs,54,59-61 with approximately 53% to 65% of cases are due to mutations in CCM1, 20% in CCM2, and 10% to 16% in CCM3.59,62-64 The majority of mutations in CCM1-3 are loss of function mutations including nonsense, frameshift, and splice site, leading to a premature stop codon and an unstable mRNA. Larger deletion and duplications of multiple exons and the entire gene have been recognized, emphasizing the importance of screening for these types of mutations when utilizing genetic testing.65 The inherited mutation is an inherited risk, but not sufficient for CCM genesis. It is hypothesized that a “second hit” or somatic mutation is required for malformation development and, consistent with this, a second mutation has been described in cases where somatic tissue is tested.50,53,54
Clinical severity is highly variable, but CCM1 gene mutations may cause the least severe clinical course, and PDCD10 (CCM3) mutations are associated with more severe disease manifestations.43,63 CCM3 mutation carriers have a greater chance of spontaneous mutation, an increased CCM burden, and a younger mean age of presentation, which is often associated with clinical hemorrhage. There is also a significant association with other manifestations including skin CCMs, scoliosis, spinal cord cavernous malformations, cognitive disability, and benign brain tumor including meningioma, vestibular schwannoma, and astrocytoma.43 Genotype does not entirely explain CCM clinical variability; investigation of possible genetic and environment modifiers is currently underway.
Recommendations for Genetic Testing and Counseling
Obtain a 3-generation family history at the time of a new diagnosis, focusing on symptoms of headache, stroke, abnormal MRI scan, or other neurological complication. (class I, level C).
Consider genetic testing of CCM1-3 genes by Sanger or NextGen sequencing followed by deletion/duplication analysis, in the setting of multiple CCM without an associated DVA or history of brain radiation or with a positive family history. (class I, level B).
In the setting of a positive mutation in a proband, counsel the individual and family about autosomal dominant inheritance and identify at-risk individuals based on the pedigree. Genetic testing of adult at-risk family members can be offered; however, genetic screening of asymptomatic individuals raises ethical issues that should be taken into account. Asymptomatic individuals should be provided information on the possible psychological consequences of a positive test before they make their decision (class I, level C).
IMAGING CCMs AND REPORTING STANDARDS
CT is insensitive for detection of small CCMs, with suggestive but not specific findings, such as multifocal calcifications.66 CT is widely available, and is suitable for emergent identification of acute hematoma, mass effect, and hydrocephalus. However, small risks do accompany use of ionizing radiation as it may promote CCM formation, and CCM patients may need repeated imaging.67 The suspicion of CCM on CT should be followed by MRI.68 MRI is the imaging test of choice for detection and characterization of CCMs, with near-perfect sensitivity and great specificity.69,70 Other differential diagnostic considerations can include hemorrhagic or calcified neoplasms, especially hemorrhagic metastases (melanoma, renal cell, others), oligodendrogliomas, and pleomorphic xanthoastrocytomas.71 The hallmark of CCMs on MRI reflects particularly blood breakdown products within and surrounding the CCMs.21 Gradient echo or susceptibility sequences may reveal smaller CCMs not visible on conventional MRI sequences, particularly in association with familial or radiation-induced CCMs.19,72 A variety of conditions, especially hypertension and cerebral amyloid angiopathy in the elderly, can cause multifocal small hemorrhages, including microhemorrhages only visible on gradient-based techniques, mimicking CCMs. It is unusual (but not impossible) for large numbers of small CCMs to occur without the presence of some additional larger, more typical CCMs.43,57
Brain imaging should be performed as soon as possible after the onset of clinical symptoms to demonstrate hemorrhage or new CCM formation.7,21 A CT scan may be performed emergently, but should be followed ideally with MRI, when assessing clinical change in CCM patients. The role of angiography in CCM diagnosis is limited.73 An associated DVA is usually readily seen on contrast enhanced or susceptibility-weighted MRI sequences.57,74
Because of the importance of detecting blood breakdown products of varying stages, both T1-weighted and T2-weighted sequences are important. It is critical for MRI detection of CCMs to include susceptibility-sensitive sequences. T2-weighted gradient-echo sequences are much more sensitive for detection of hemosiderin than fast spin-echo sequences, and susceptibility-weighted imaging techniques using volume acquisition, thin slices, and postprocessing offers still greater sensitivity (first demonstrated with susceptibility-weighted imaging, although similar techniques such as SWAN and VenoBOLD are likely to offer similar sensitivity).57,66,75 Sensitivity to blood breakdown products also increases with higher field. At a minimum, MRI for evaluation of suspected CCMs must include a gradient-based sequence with T2 weighting or susceptibility-weighted sequences as noted above.
T1 with gadolinium contrast is mostly useful for evaluation of possible associated DVAs or capillary telangiectasias,56,76 to exclude neoplasm as differential diagnosis,71 or to detect neoplasms in association with some forms of familial CCMs.43,58 Use of gadolinium should be carefully weighed in light of recent recognition of gadolinium retention in the globi pallidi and dentate nuclei in some patients, although the clinical significance of this is not yet known77,78 and the consideration of gadolinium administration should follow any updated current guidelines by the United States Food and Drug Administration.79 For presurgical planning, other factors such as location of overlying veins and the anticipated CCM vascularity at surgery may be important to the surgeon and may increase the importance of gadolinium administration.
Routine follow-up of CCMs is not well established and is dependent upon insurance, patient preferences, and neurological and/or neurosurgical practitioner's practice standards. Repeat imaging is precipitated by changes in neurological status, in particular the development of new neurological symptoms suggestive of CCM hemorrhage, changed or worsening epilepsy, or changes in the neurological exam. Optimal timing and indications for surveillance or follow-up scans are currently based primarily on clinical judgment, and relatively little evidence is available to make recommendations.
There is no evidence to justify routine spinal imaging in patients with brain CCMs in the absence of pain or other myelopathic symptoms, especially when no intervention is recommended for asymptomatic spinal cavernomas (see section on Neurosurgical Considerations).
Advanced imaging techniques may offer advantages for specific purposes, including functional MRI and tractography,69 quantitative susceptibility mapping,80 permeability imaging using dynamic contrast-enhanced MRI,81-83 or potential use of Ferumoxytol.84
Recommendations Regarding Imaging
Brain MRI is recommended for the diagnosis and clinical follow-up of suspected or known CCM (class I, level B evidence).
Brain MRI for CCM should include gradient echo or susceptibility-weighted sequences to establish whether there is 1, or many, CCM (class I, level B).
Catheter angiography is not recommended in the evaluation of CCM, unless a differential diagnosis of arteriovenous malformation is being considered (class III, level C).
Follow-up imaging in CCM should be considered to guide treatment decisions or to investigate new symptoms. Brain imaging should be performed as soon as possible after the onset of clinical symptoms suspicious of hemorrhage. CT may be used within 1 week of symptom onset, but MRI should be used thereafter (ideally within 2 weeks of symptom onset). Repeat MRI should be performed in conjunction with new or worsened symptoms to assess for any new CCM or new hemorrhage (class I, level C).
Reporting standards have been subjective and commonly inconsistent. However, based on input from neurologists, neurosurgeons, neuroradiologists, and patients, recommendations may be offered for consideration so as to enhance interpretation and comparability in clinical practice (Table 3).
TABLE 3.
Suggested MRI Reporting Standards for Cerebral Cavernous Malformations
| • Magnet field strength and pulse sequences are especially valuable to include in the report when CCMs are likely. This conveys to the informed reader useful information about sensitivity of the study for blood breakdown products. |
| • When a single CCM is detected, presence or absence of an associated DVA should be noted. Several CCMs around the periphery of a DVA should still be considered part of a single vascular complex and are consistent with sporadic (unlikely genetic) disease. Multiple hemorrhagic lesions with features of CCMs are likely due to a genetic mutation, with or without a family history. As with other imaging findings, it is appropriate with either single or multiple lesions to include differential diagnosis, depending on the degree of confidence in characteristic vs unusual features that would suggest alternative possibilities. |
| • Signal characteristics, size, location, and unusual features are helpful to report. For larger CCMs that are generally round, a single largest diameter measurement may be adequate; for more asymmetric CCMs, orthogonal measurements may be more appropriate. Measurements should be based on spin echo (or fast- or turbo-spin echo) sequences to avoid the “blooming” that accompanies gradient echo sequences. Detailed descriptions are warranted for CCMs in the brainstem and in unusual locations including spinal cord, cranial nerves, cavernous sinus, and intraventricular extension. Evidence of possible acute or subacute hemorrhage, extralesional recent hemorrhage or perilesional edema can be important. |
| • Small numbers of CCMs can be described in detail. Large numbers are a challenge, but estimates (eg, “approximately 20-30 small CCMs” or “greater than 50 in each cerebral hemisphere) are more helpful than “too numerous to count.” Especially as patient portals to the electronic medical record become more common, the description of “too numerous to count” CCMs can have a dramatic psychological impact on the affected patient. It is useful to note that the presence of multiple small CCMs, visible only on gradient echo or SWI sequences, is seen in many patients with familial CCM and does not necessarily correlate with a worse clinical outcome. In addition, the gradient echo technique, for technical reasons, causes the CCMs to appear larger on the MRI images than they actually are in the brain. Higher field strength may result in more CCMs to be apparent on MRI than on a study previously performed on a lower field strength magnet, and apparent differences in numbers of CCMs must be interpreted carefully. Thinner slices and less interslice gap also increase sensitivity. |
| • The discovery of a CCM on a study done for an unrelated purpose should be described. However, the clinical relevance may depend on further historical or physical examination information. Terms such as “incidental” are therefore best used carefully and, ideally, in a clinical context. |
NEUROSURGICAL CONSIDERATIONS
Despite decades of neurosurgical experience in this field, evidence supporting surgical resection of CCM remains conflicting. Reviews including at least 20 symptomatic CCM patients could not identify high-quality studies that show dramatic benefit or harm of surgery, only a few studies showed beneficial effects of surgical resection of CCM induced seizures, and most studies were deemed to be biased.47,85 A recent, nonrandomized population-based study comparing surgical excision to conservative management revealed poorer outcome over the subsequent 5 years, and higher risk of symptomatic bleeds and focal neurological deficits in the surgical group.49 However, the baseline health of the surgical arm was not stated and patients more severely affected by the CCM were in the excision group. In addition, with CCMs that have previously bled, and those in deep and infratentorial locations behaving more aggressively,44 it is important to weigh the risk of surgery vs the natural history of the CCM in specific clinical scenarios and CCM locations. Management of intracerebral and intraventricular hemorrhage associated with CCM should follow evidence-based guidelines7 for these entities, including early blood pressure control, reversal of coagulopathy, control of intracranial pressure, and the evacuation of hemorrhages causing impending herniation or posterior fossa mass effect.7
Case series generally advocate conservative management of asymptomatic incidentally identified CCM.86 A recent systematic review documented an overall risk of death or nonfatal stroke of 6% after CCM resection.85 This exceeds the analogous natural risk (2.4% over 5 years) of a CCM that has never bled. The same postoperative risk becomes more favorable compared to the risk associated with recurrent ICH after a first CCM bleed (29.5% over 5 years).85 The risk of resection varies greatly with CCM location, and this influences surgical decisions. Resection is generally recommended for symptomatic easily accessible CCMs given the increased risk of rebleed after first hemorrhage, and the low morbidity associated with surgery.87,88 Other considerations are needed for CCMs involving the visual pathways,89,90 and those involving the lateral ventricle.91
Deeper CCMs located in the insula, basal ganglia, and thalamus require a more technically cautious surgery because of the presence of critical neuronal pathways packed in smaller areas and the risk of injury of the small perforating arteries. In spite of careful technique, the rate of postoperative morbidity for these CCMs is 5% to 18%, and a mortality approaching 2%, but many patients achieving recovery from severe preoperative disability.88,92 Surgery for brainstem CCMs is associated with significant early morbidity in nearly one-half of cases, but most patients recover over time.93,94 Technical adjuncts including image guidance,95,96 neurophysiologic monitoring,97 and laser assisted technique98,99 are thought to improve outcome of surgical resection strategies in eloquent areas, but there are limited controlled studies to support specific modalities. Much of the reported literature on surgical outcomes is from specialized centers, and hence it may not necessarily be translated to community settings without equivalent experience.
In the case of supratentorial noneloquent region CCMs, the risk of new neurological sequelae is equivalent to living with the CCM for 1 to 2 years after a first bleed.47 On the other hand, surgery in more eloquent locations is associated with higher risk, equivalent to living with the CCM for 5 to 10 years after a first bleed.
Spinal CCMs pose a significant challenge, with most reports documenting surgical outcomes similar to brainstem CCMs, and advocating similar treatment decisions.42 There remains significant controversy about whether surgical risk is justified by the natural history.100
Medically refractory seizures due to CCM can be safely controlled by surgical resection.101,102 Several studies showed that pure lesionectomy results in postoperative seizure control of 70% to 90% in patients with sporadic seizures or those with seizure duration less than 1 year.103,104 There is a lower chance of seizure control after surgery in cases with longer preoperative duration of seizures.105 As a result, some authors argue for performing early surgery in patients who fail 1 drug therapy, even if they do not satisfy criteria for medically refractory epilepsy due to the CCM.102 Recent report has suggested a role for laser fiber ablation of cavernous malformation as a potentially promising treatment of associated epilepsy.106 Further studies are needed on epilepsy outcome in comparison to the more established approach of lesionectomy.
An associated DVA is thought related to CCM genesis in many sporadic cases.107,108 There is conflicting data on resection of DVA associated with the CCM, with most authors advocating avoiding DVA dissection to prevent serious complications such as edema, hemorrhage, and/or venous infarcts.94,102
Stereotactic radiosurgery (SRS) has been proposed as an alternative treatment for symptomatic CCM in eloquent areas.94,109 A recent meta-analysis identified 4 out of 5 studies revealing statistically significant decline in the yearly hemorrhage rate 2 years after SRS of brainstem CCM. The mortality rate was 5.61%, 11.8% developed new focal neurological deficits,110,94 and there is ongoing debate as to whether the effects of SRS merely reflect the CCM's natural history.111,108 Guidelines for SRS have been proposed by Niranjan et al112 advocating to select patients depending on age, location, risk of hemorrhage, risk of surgical resection, and previous hemorrhage. Radiosurgery in brain locations considered high risk for resection may be associated with morbidity, and may have no immediate effect on the CCM. There is legitimate concern over whether any radiation exposure may enhance the genesis of new CCMs in familial cases. The SRS optimal dose to reduce hemorrhage is not known, although there are dose prescription recommendations for safety.113
Recommendations for Surgical Treatment
Surgical resection is not recommended for asymptomatic CCM, especially if located in eloquent, deep, or brainstem areas, nor in cases with multiple asymptomatic CCMs (class III, level B).
Surgical resection may be considered in solitary asymptomatic CCM if easily accessible in noneloquent area, to prevent future hemorrhage, because of psychological burden, expensive and time-consuming follow-ups, to facilitate lifestyle or career decisions, or in patients who might need to be on anticoagulation (class IIb, level C).
Early surgical resection of CCM causing epilepsy should be considered, especially when medically refractory epilepsy, in the absence of uncertainty about CCM epileptogenicity (class IIa, level B).
Surgery may be considered in symptomatic easily accessible CCMs, with mortality and morbidity equivalent to living with the CCM for about 2 years (class IIb, level B).
Surgical resection may be considered in deep CCMs if symptomatic or after prior hemorrhage, with mortality and morbidity equivalent to living with the CCM for 5-10 years (class IIb, level B).
After reviewing the high risks of early postoperative mortality and morbidity and impact on quality of life, it may be reasonable to offer surgical resection of brainstem CCM after a second symptomatic bleed as those CCMs might have a more aggressive course (class IIb, level B).
Indications for resection of brainstem CCM after a single disabling bleed, or for spinal cavernous malformations are weaker (class IIb, level C).
Radiosurgery may be considered in solitary CCMs with previous symptomatic hemorrhage if the CCM lies in eloquent areas that carry an unacceptable high surgical risk (class IIb, level B).
Radiosurgery is not recommended for asymptomatic CCMs, for CCMs that are surgically accessible, nor in familial CCM because of concern about de novo CCM genesis (class III, level C).
NEUROLOGICAL CONSIDERATIONS
Definitions for the relationship of epilepsy to the CCM have been proposed (Table 4).102 In definite CCM-related epilepsy (CRE), antiepileptic treatment is generally recommended.45,114 There has never been a clinical trial assessing early surgery vs antiepileptic oral therapy. In clinical practice it is common to start with antiepileptic medication. Surgery may be considered early to reduce future hemorrhage risk if seizures were associated with a hemorrhagic CCM or in patients who may not be compliant with medications. Approximately 50% to 60% of patients will become seizure free on medication after the first diagnosis of CRE.45,102,115,116 Patients with a known seizure disorder should avoid medications and activities that may lower the seizure threshold or could potentially result in harm. In addition, patients should follow the individual state law or other governing jurisdiction about seizures and driving.
TABLE 4.
Proposed Definitions for the Relationship of Cerebral Cavernous Malformations and Epilepsya
| Type | Definition |
|---|---|
| Definite CRE | Epilepsy in patients with at least 1 CCM and evidence of a seizure onset zone in the immediate vicinity of the CCM |
| Probable CRE | Epilepsy in a patient with at least 1 CCM and with evidence that the epilepsy is focal and arises from same hemisphere as the CCM |
| Cavernomas unrelated to epilepsy | Epilepsy in a patient with at least 1 CCM with evidence that the CCM and the epilepsy are not causally related. Eg, patient with juvenile myoclonic epilepsy or absence epilepsy and CCM |
CRE = CCM-related epilepsy.
aText reprinted from Rosenow et al.102
The incidence of headache in the CCM population has been poorly studied, but may be as high as 52%.117 In patients meeting criteria for migraine who happen to also have a CCM, standard migraine therapy is recommended. In very small case series, nonsteroidal anti-inflamatory drugs (NSAIDs) were safe, but large numbers of patients have not been prospectively followed.117 With the increasing use of MRI for various neurological symptoms, CCM may be identified incidentally. Symptomatic hemorrhage risk in these cases is low.14 The seizure risk in patients with incidental CCM is also low (<1% per year),14,102 hence justifying conservative management.86
Management of CCM in Children
Approximately one-fourth of sporadic and familial CCMs occur in pediatric age groups.59 Literature specific to pediatrics is largely based on case reports or series publications reporting giant CCM, or the natural history and surgical outcomes of CCM of specific location: brainstem,28,118,119 spinal cord,120 and basal ganglia.121 Imaging in young children (typically under age 6 years or those with developmental disability) requires sedation for accurate results, which presents some additional risk to children.
Of special interest in pediatrics is the eventual fate of small dot-like CCMs based on radiological features118,119,122,123 with mean annual hemorrhage rate of 1.3%. Larger CCMs not seen exclusively on susceptibility-weighted imaging that did not have surgery had a higher prospective hemorrhage rate.124
Based on the response of infantile hemangiomas (a distinct condition) to propranolol, and the treatment of diffuse or multifocal infantile hemangiomatosis involving brain and spinal cord, propranolol has been used clinically in cases of CCM. Case reports and case series report limited treatment success on pediatric and adult cases without genetic confirmation of CCM mutations.125,126 Controlled studies of propranolol have not yet been performed in CCM, so its use for this indication cannot be currently recommended.
Children may develop CCM in response to therapeutic radiation over 300 Gy in the first decade of life and without pre-existing sporadic or familial CCMs127,128 increasing concern from patients receiving frequent CT scans in the first decade or dental radiographs and in the setting of carriers of CCM mutations.
Management of CCM During Pregnancy
Several large series have suggested that the risk of CCM clinical symptoms and hemorrhage rate is no different than the nonpregnant state,27,129 although some controversy remains.130
In patients with multiple CCMs, genetic counseling may be discussed with the patient contemplating pregnancy. In patients with a seizure disorder due to CCM, discussion of the appropriate antiepileptic drug to reduce teratogenic side effects and folate supplementation should occur prior to the patient becoming pregnant, if possible. If focal neurological deficits, an acute, severe headache, or a flare-up in seizures occur during pregnancy, MRI scan without contrast should be considered. If a patient has a brain hemorrhage during pregnancy, the severity of symptoms and risk of recurrent hemorrhage need to be weighed against the risk of surgical intervention at that point in the pregnancy. It is generally agreed upon that vaginal delivery is appropriate in most patients unless there is a neurological deficit that precludes such or recent hemorrhage.
Safety of Anticoagulation
Most studies suggest the likely safety of antiplatelet medication,131 and a low risk of bleeding from an existing CCM in patients placed on antithrombotic.132 We must caution that these studies were uncontrolled, with less likely treatment of patients with recent hemorrhage. Erdur and colleagues133 report no significant difference in symptomatic ICH and parenchymal hemorrhage rate when comparing 9 patients with CCM compared to 341 patients without CCM undergoing thrombolysis for expected cerebral ischemia. The safety of other medications including estrogens, NSAIDs, triptans, other potential blood-thinning agents (novel anticoagulants, vitamin E, fish oil, selective serotonin reuptake inhibitors) has not been studied or sufficiently studied in patients with CCM to make recommendations. And there is no data on more powerful antiplatelet therapy and novel anticoagulants.
Physical Activity
There are some activities that pose theoretical risks in CCM patients with134 and without associated seizures135 (Table 5). Flemming et al131 did not find any relationship to physical activity at the time of hemorrhage due to CCM.
TABLE 5.
Situations that Theoretically Pose Risk to Patients With Cerebral Cavernous Malformationsa
| Activity | Theoretical mechanism | Clinical studies or direct evidence in relationship to CCM |
|---|---|---|
| Mountain climbing above 10 000 feet | Hypoxia results in changes of VEGF, an important factor in angiogenesis and vascular permeability. | None |
| Smoking | Similar to above | None |
| Water activity | Patients at risk for seizure should not swim alone as a seizure in the water could be fatal. | b |
| Scuba diving | Scuba diving is not recommended for people with seizure disorder | b |
| Contact sports | Head trauma may result in an increased risk of seizure disorder | b |
| Strenuous exercise (aggressive aerobic activity, power weight lifting) | Strenuous exercise could result in impaired venous return resulting in increased peripheral venous pressures. | None |
| Other (caving, skydiving, surfing, solo airplane flying) | Activities that could result in potential injury should a seizure occur during that activity | b |
VEGF = vascular endothelial growth factor.
aModified from Berg and Vay.135
bExtrapolated from Epilepsy Foundation recommendations regarding seizures, in general.
Potentially Beneficial Medications
Statins have been suggested in laboratory and preclinical studies as potential therapy for CCM, but their risk and benefit have not been carefully evaluated. Patients with CCM should receive statins for approved cholesterol lowering and cardiovascular indications, with close monitoring of the CCMs. Statins should not be used for the purpose of treating CCM in the absence of evidence from clinical trials.
There is biological evidence of benefit of vitamin D in the treatment of CCM from laboratory studies. Recent report from Girard et al136 showed an association of vitamin D deficiency with historically aggressive CCM disease behavior. There is no evidence that vitamin D supplementation prevents future CCM disease manifestations.
Laboratory studies are identifying potential targets for pharmacologic therapy aimed at stabilizing CCMs or preventing CCM genesis. These await careful clinical assessment of potential safety and effectiveness.
Recommendations Regarding Neurological Management
Antiepileptic therapy for first seizure thought to be due to a CCM is reasonable (class I, level B).
Patients with familial or multifocal CCM may consider genetic counseling prior to pregnancy (class I, level C).
Patients may be counseled that the risk of neurological symptoms during pregnancy is likely not different than the nonpregnant state (class IIa, level B).
MRI should be considered in patients with CCM that develop new neurological symptoms during pregnancy (class IIa, level C).
Few data are available on the risk of antithrombotic medication use in the general population of CCM patients (class III, level C).
The safety of thrombolytic therapies in patients with CCM and concomitant cerebral ischemia is unclear (class III, level C).
The influence of physical activity on CCM behavior is largely unknown (class IIb, level C).
Disclosures
No funding was received to support the development of these guidelines. Dr Akers received salary support as Chief Scientific Officer of Angioma Alliance and Dr Al-Shahi Salman was funded by an Medical Research Council (MRC) senior clinical fellowship. The authors have no personal, institution, or financial interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors would like to acknowledge Dr Michel Berg for his contribution toward earlier drafts of imaging and radiology standards.
COMMENTS
Guidelines are useful tools to assist with standardization of care for complex pathologies. Therefore, the contribution of the Angioma Alliance is a welcome addition to the literature on triage, workup and management of cavernous malformations. Akin to the Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury1 and Guidelines for the Management of Severe Traumatic Brain Injury,2 both published in the journal, these guidelines provide a roadmap for practitioners who may not regularly treat cavernous malformations. Although generally thought of as rare lesions, cavernous malformations have an incidence of 1 in 200 to 1 in 400, meaning that 0.25% to 0.5% of the population harbors a cavernous malformation3 and many practitioners are faced with the challenge of recommending a course of action for these lesions. As with any complex pathology, however, guidelines are merely a starting point for decision making and treatment. In most cases the decision to suggest treatment and the timing of treatment are complicated by the neurological status of the patient, multiplicity and location of lesions, surgical experience of the treating team, and patient and family wishes. Furthermore, although currently the only treatment decision tree is between observation or surgical management, several potentially promising medical alternatives, such as propranolol,4 Rho kinase inhibitors5 and statins,6 are under active investigation and may complement surgery as treatment options in the near future. The authors are to be commended for accumulating this wealth of information and summarizing it for the neurosurgical community.
M. Yashar S. Kalani
Salt Lake City, Utah
- 1. Neurosurgery. 2013March;72Suppl 2:1 doi: 10.1227/NEU.0b013e318276ee7e. Updated Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury. Resnick DK1. [DOI] [PubMed] [Google Scholar]
- 2. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition Carney N, Totten AM, OReilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Neurosurgery. 2016September20. [DOI] [PubMed] [Google Scholar]
- 3. Zabrmaski J., Kalani M. Y. Natural History of Cerebral Cavernous Malformations. Youmans and Winn Neurological Surgery, 4-Volume Set, 7th Edition, 2016. [Google Scholar]
- 4. Propranolol Treatment of Cavernous Malformations with Symptomatic Hemorrhage. Zabramski JM, Kalani MY, Filippidis AS, Spetzler RF. World Neurosurg. 2016April;88:631–9. [DOI] [PubMed] [Google Scholar]
- 5. McDonald D.A., Shi C., Shenkar R., Stockton R.A., Liu F., Ginsberg M.H. et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke, 43 (2012), pp. 571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shenkar R, Shi C, Austin C et al. RhoA Kinase Inhibition With Fasudil Versus Simvastatin in Murine Models of Cerebral Cavernous Malformations. Stroke. 2017; 48(1):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
The authors provide a comprehensive review of the literature on cavernous malformations (CM). These timely guidelines provide valuable guidance for the management of CMs. The following facts and recommendations are worth summarizing here. The prevalence of CMs is estimated at 0.16% to 0.5%. Up to 50% of patients are asymptomatic and are diagnosed with CMs incidentally identified by CTs and/or MRIs. Up to 20% of patients with CMs have multiple lesions. The 2 main features associated with future hemorrhagic presentations are an initial presentation with a symptomatic hemorrhage and the location of the lesion in the brainstem. The natural history of spinal cord CMs is poorly understood given the paucity of literature on the subject. There are no randomized controlled trials comparing surgical resection to conservative treatment of CMs. It has become apparent that CMs outside the brainstem rarely present with catastrophic symptoms. Therefore, most neurosurgeons currently reserve surgical treatment for increasingly symptomatic lesions that are easily accessible. CMs in the insula, basal ganglia, and thalamus are associated with a postoperative morbidity of up to 18% and a mortality approaching 2%. Surgical treatment of brainstem CMs is associated with an even higher early morbidity of nearly 50%. Outcomes associated with CMs in these locations should be taken into account when considering microsurgical resection. There is controversy concerning microsurgical treatment of spinal cord CMs. Control of medically refractory seizures after CM resection is favorable, and ranges from 70 to 90% in patients with sporadic seizure and also in those with seizures present for less than 1 year. Practically all CMs are associated with developmental venous anomalies (DVAs), and most neurosurgeons agree that the DVA should not be disturbed during resection of the adjacent CM, as it is a normal venous drainage structure. Stereotactic radiosurgery of brainstem CMs has been shown to result in a decline in the annual rate of hemorrhage years after treatment. With regards to pregnancy, several studies suggest the risk of hemorrhage or symptomatic presentation of CMs is no different during pregnancy as compared to that of the non-pregnant state. Most practitioners do not place restrictions on vaginal delivery in patients with CMs. Finally, there is not much data on the risk of treatment with antiplatelet agents or anticoagulants in patients with CMs. In my practice, however, I do not view the presence of a CM as a contraindication to the use of antiplatelet agents or anticoagulants if medically necessary. In summary, these authors are to be congratulated for this timely and thoughtful document on the epidemiology and management of CMs is an important contribution to the clinical management of CMs.
Rafael J. Tamargo
Baltimore, Maryland
REFERENCES
- 1. Samarasekera N, Poorthuis M, Kontoh K et al. Guidelines for the Management of Cerebral Cavernous Malformations in Adults, 2005. Available at: http://www.geneticalliance.org.uk/docs/managementofCCM.pdf Accessed January 2015.
- 2. Tonelli MR. In defense of expert opinion. Acad Med. 1999;74(11):1187–1192. [DOI] [PubMed] [Google Scholar]
- 3. Awad I, Barrow DL. Cavernous Malformations. United States: American Association of Neurological Surgeons; 1993. [Google Scholar]
- 4. Rigamonti D. Cavernous Malformations of the Nervous System. Cambridge, England: Cambridge University Press; 2011. [Google Scholar]
- 5. Lanzino G. Cavernous Malformations of the Brain and Spinal Cord. New York, NY: Thieme; 2011. [Google Scholar]
- 6. Battistini S, Catapano D, Cerase A et al. Angiomi cavernosi (o Cavernomi) cerebrali consensus diagnostico-terapeutico. CCM Italia. 2009. Available at: http://www.ccmitalia.unito.it/attachments/060_AREA MEDICI-CONSEN-SUS DIAGNOSTICO-TERAPEUTICO.pdf Accessed January 2015. [Google Scholar]
- 7. Hemphill JC 3rd, Greenberg SM, Anderson CS et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. [DOI] [PubMed] [Google Scholar]
- 8. Hsu C, Stanford B. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12:(10)1–8. [Google Scholar]
- 9. Morris Z, Whiteley WN, Longstreth WT Jr et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Otten P, Pizzolato GP, Rilliet B, Berney J. 131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies. Neurochirurgie. 1989;35(2):82–83, 128-131. [PubMed] [Google Scholar]
- 11. Al-Holou WN, O’Lynnger TM, Pandey AS et al. Natural history and imaging prevalence of cavernous malformations in children and young adults. J Neurosurg Pediatr. 2012;9(2):198–205. [DOI] [PubMed] [Google Scholar]
- 12. Al-Shahi R, Bhattacharya JJ, Currie DG et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34(5):1163–1169. [DOI] [PubMed] [Google Scholar]
- 13. Al-Shahi Salman R, Hall JM, Horne MA et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol. 2012;11(3):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore SA, Brown RD Jr, Christianson TJ, Flemming KD. Long-term natural history of incidentally discovered cavernous malformations in a single-center cohort. J Neurosurg. 2014;120(5):1188–1192. [DOI] [PubMed] [Google Scholar]
- 15. Flemming KD, Bovis GK, Meyer FB. Aggressive course of multiple de novo cavernous malformations. J Neurosurg. 2011;115(6):1175–1178. [DOI] [PubMed] [Google Scholar]
- 16. Gastelum E, Sear K, Hills N et al. Rates and characteristics of radiographically detected intracerebral cavernous malformations after cranial radiation therapy in pediatric cancer patients. J Child Neurol. 2015;30(7):842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flemming KD, Link MJ, Christianson TJ, Brown RD Jr.. Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. 2012;78(9):632–636. [DOI] [PubMed] [Google Scholar]
- 18. Rigamonti D, Hadley MN, Drayer BP et al. Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med. 1988;319(6):343–347. [DOI] [PubMed] [Google Scholar]
- 19. Zabramski JM, Wascher TM, Spetzler RF et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994;80(3):422–432. [DOI] [PubMed] [Google Scholar]
- 20. Gunel M, Awad IA, Finberg K et al. A founder mutation as a cause of cerebral cavernous malformation in Hispanic Americans. N Engl J Med. 1996;334(15):946–951. [DOI] [PubMed] [Google Scholar]
- 21. Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Scientific Advisory Board. Stroke. 2008;39(12):3222–3230. [DOI] [PubMed] [Google Scholar]
- 22. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75(5):709–714. [DOI] [PubMed] [Google Scholar]
- 23. Kondziolka D, Monaco EA 3rd, Lunsford LD. Cavernous malformations and hemorrhage risk. Prog Neurol Surg. 2013;27:141–146. [DOI] [PubMed] [Google Scholar]
- 24. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87(2):190–197. [DOI] [PubMed] [Google Scholar]
- 25. Moriarity JL, Wetzel M, Clatterbuck RE et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery. 1999;44(6):1166–1171; discussion 1172-1163. [PubMed] [Google Scholar]
- 26. Ghannane H, Khalil T, Sakka L, Chazal J. Analysis of a series of cavernomas of the central nervous system: 39 non operated cases, 39 operated cases, 1 dead. Neurochirurgie. 2007;53(2-3 pt 2):217–222. [DOI] [PubMed] [Google Scholar]
- 27. Kalani MY, Zabramski JM. Risk for symptomatic hemorrhage of cerebral cavernous malformations during pregnancy. J Neurosurg. 2013;118(1):50–55. [DOI] [PubMed] [Google Scholar]
- 28. Li D, Hao SY, Jia GJ, Wu Z, Zhang LW, Zhang JT. Hemorrhage risks and functional outcomes of untreated brainstem cavernous malformations. J Neurosurg. 2014;121(1):32–41. [DOI] [PubMed] [Google Scholar]
- 29. Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83(1):56–59. [DOI] [PubMed] [Google Scholar]
- 30. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83(5):820–824. [DOI] [PubMed] [Google Scholar]
- 31. Labauge P, Brunereau L, Laberge S, Houtteville JP. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology. 2001;57(10):1825–1828. [DOI] [PubMed] [Google Scholar]
- 32. Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. 2003;99(1):31–37. [DOI] [PubMed] [Google Scholar]
- 33. Kim DS, Park YG, Choi JU, Chung SS, Lee KC. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997;48(1):9–17; discussion 17-18. [DOI] [PubMed] [Google Scholar]
- 34. Barker FG 2nd, Amin-Hanjani S, Butler WE et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49(1):15–24; discussion 24-25. [DOI] [PubMed] [Google Scholar]
- 35. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir. 1994;130(1-4):35–46. [DOI] [PubMed] [Google Scholar]
- 36. Porter RW, Detwiler PW, Spetzler RF et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90(1):50–58. [DOI] [PubMed] [Google Scholar]
- 37. Hasegawa T, McInerney J, Kondziolka D, Lee JY, Flickinger JC, Lunsford LD. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50(6):1190–1197; discussion 1197-1198. [DOI] [PubMed] [Google Scholar]
- 38. Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol. 2003;59(6):444–454; discussion 454. [DOI] [PubMed] [Google Scholar]
- 39. Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011;30(6):E24. [DOI] [PubMed] [Google Scholar]
- 40. Horne MA, Flemming KD, Su IC et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol. 2016;15(2):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen-Gadol AA, Jacob JT, Edwards DA, Krauss WE. Coexistence of intracranial and spinal cavernous malformations: a study of prevalence and natural history. J Neurosurg. 2006;104(3):376–381. [DOI] [PubMed] [Google Scholar]
- 42. Badhiwala JH, Farrokhyar F, Alhazzani W et al. Surgical outcomes and natural history of intramedullary spinal cord cavernous malformations: a single-center series and meta-analysis of individual patient data. J Neurosurg Spine. 2014;21(4):662–676. [DOI] [PubMed] [Google Scholar]
- 43. Shenkar R, Shi C, Rebeiz T et al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with PDCD10 mutations. Genet Med. 2015;17(3):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus. 2010;29(3):E7. [DOI] [PubMed] [Google Scholar]
- 45. Josephson CB, Leach JP, Duncan R, Roberts RC, Counsell CE, Al-Shahi Salman R. Seizure risk from cavernous or arteriovenous malformations: prospective population-based study. Neurology. 2011;76(18):1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. [DOI] [PubMed] [Google Scholar]
- 47. Moultrie F, Horne MA, Josephson CB et al. Outcome after surgical or conservative management of cerebral cavernous malformations. Neurology. 2014;83(7):582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cordonnier C, Al-Shahi Salman R, Bhattacharya JJ et al. Differences between intracranial vascular malformation types in the characteristics of their presenting haemorrhages: prospective, population-based study. J Neurol Neurosurg Psychiatry. 2008;79(1):47–51. [DOI] [PubMed] [Google Scholar]
- 49. Cavalcanti DD, Kalani MY, Martirosyan NL, Eales J, Spetzler RF, Preul MC. Cerebral cavernous malformations: from genes to proteins to disease. J Neurosurg. 2012;116(1):122–132. [DOI] [PubMed] [Google Scholar]
- 50. Riant F, Bergametti F, Ayrignac X, Boulday G, Tournier-Lasserve E. Recent insights into cerebral cavernous malformations: the molecular genetics of CCM. FEBS J. 2010;277(5):1070–1075. [DOI] [PubMed] [Google Scholar]
- 51. Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med. 2013;19(5):302–308. [DOI] [PubMed] [Google Scholar]
- 52. Yadla S, Jabbour PM, Shenkar R, Shi C, Campbell PG, Awad IA. Cerebral cavernous malformations as a disease of vascular permeability: from bench to bedside with caution. Neurosurg Focus. 2010;29(3):E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McDonald DA, Shi C, Shenkar R et al. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: evidence for a common biochemical pathway for CCM pathogenesis. Hum Mol Genet. 2014;23(16):4357–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18(5):919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haasdijk RA, Cheng C, Maat-Kievit AJ, Duckers HJ. Cerebral cavernous malformations: from molecular pathogenesis to genetic counselling and clinical management. Eur J Hum Genet. 2012;20(2):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petersen TA, Morrison LA, Schrader RM, Hart BL. Familial versus sporadic cavernous malformations: differences in developmental venous anomaly association and lesion phenotype. AJNR Am J Neuroradiol. 2010;31(2):377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Champfleur NM, Langlois C, Ankenbrandt WJ et al. Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery. 2011;68(3):641–647; discussion 647-648. [DOI] [PubMed] [Google Scholar]
- 58. Riant F, Cecillon M, Saugier-Veber P, Tournier-Lasserve E. CCM molecular screening in a diagnosis context: novel unclassified variants leading to abnormal splicing and importance of large deletions. Neurogenetics. 2013;14(2):133–141. [DOI] [PubMed] [Google Scholar]
- 59. Spiegler S, Najm J, Liu J et al. High mutation detection rates in cerebral cavernous malformation upon stringent inclusion criteria: one-third of probands are minors. Mol Genet Genomic Med. 2014;2(2):176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Denier C, Labauge P, Brunereau L et al. Clinical features of cerebral cavernous malformations patients with KRIT1 mutations. Ann Neurol. 2004;55(2):213–220. [DOI] [PubMed] [Google Scholar]
- 61. Cigoli MS, Avemaria F, De Benedetti S et al. PDCD10 gene mutations in multiple cerebral cavernous malformations. PLoS One. 2014;9(10):e110438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. D’Angelo R, Marini V, Rinaldi C et al. Mutation analysis of CCM1, CCM2 and CCM3 genes in a cohort of Italian patients with cerebral cavernous malformation. Brain Pathol. 2011;21(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gault J, Sain S, Hu LJ, Awad IA. Spectrum of genotype and clinical manifestations in cerebral cavernous malformations. Neurosurgery. 2006;59(6):1278–1284; discussion 1284-1275. [DOI] [PubMed] [Google Scholar]
- 64. Mondejar R, Solano F, Rubio R et al. Mutation prevalence of cerebral cavernous malformation genes in Spanish patients. PLoS One. 2014;9(1):e86286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cave-Riant F, Denier C, Labauge P et al. Spectrum and expression analysis of KRIT1 mutations in 121 consecutive and unrelated patients with cerebral cavernous malformations. Eur J Hum Genet. 2002;10(11):733–740. [DOI] [PubMed] [Google Scholar]
- 66. Liu XW, Wang SH, Chi ZF, Su LJ, Zhao XH, Wang SJ. The value of T(2) (*)-weighted gradient echo imaging for detection of familial cerebral cavernous malformation: A study of two families. Exp Ther Med. 2013;5(2):448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Golden M, Saeidi S, Liem B, Marchand E, Morrison L, Hart B. Sensitivity of patients with familial cerebral cavernous malformations to therapeutic radiation. J Med Imaging Radiat Oncol. 2015;59(1):134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987;67(4):518–524. [DOI] [PubMed] [Google Scholar]
- 69. Campbell PG, Jabbour P, Yadla S, Awad IA. Emerging clinical imaging techniques for cerebral cavernous malformations: a systematic review. Neurosurg Focus. 2010;29(3):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rigamonti D, Johnson PC, Spetzler RF, Hadley MN, Drayer BP. Cavernous malformations and capillary telangiectasia: a spectrum within a single pathological entity. Neurosurgery. 1991;28(1):60–64. [PubMed] [Google Scholar]
- 71. Sze G, Krol G, Olsen WL et al. Hemorrhagic neoplasms: MR mimics of occult vascular malformations. AJR Am J Roentgenol. 1987;149(6):1223–1230. [DOI] [PubMed] [Google Scholar]
- 72. Lehnhardt FG, von Smekal U, Ruckriem B et al. Value of gradient-echo magnetic resonance imaging in the diagnosis of familial cerebral cavernous malformation. Arch Neurol. 2005;62(4):653–658. [DOI] [PubMed] [Google Scholar]
- 73. Dillon WP. Cryptic vascular malformations: controversies in terminology, diagnosis, pathophysiology, and treatment. AJNR Am J Neuroradiol. 1997;18(10):1839–1846. [PMC free article] [PubMed] [Google Scholar]
- 74. Wilms G, Marchal G, Van Hecke P, Van Fraeyenhoven L, Decrop E, Baert AL. Cerebral venous angiomas. MR imaging at 1.5 tesla. Neuroradiology. 1990;32(2):81–85. [DOI] [PubMed] [Google Scholar]
- 75. de Souza JM, Domingues RC, Cruz LC Jr, Domingues FS, Iasbeck T, Gasparetto EL. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with t2-weighted fast spin-echo and gradient-echo sequences. AJNR Am J Neuroradiol. 2008;29(1):154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meng G, Bai C, Yu T et al. The association between cerebral developmental venous anomaly and concomitant cavernous malformation: an observational study using magnetic resonance imaging. BMC Neurol. 2014;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McDonald RJ, McDonald JS, Kallmes DF et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772–782. [DOI] [PubMed] [Google Scholar]
- 78. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–841. [DOI] [PubMed] [Google Scholar]
- 79. Cenzato M, Stefini R, Ambrosi C, Giovanelli M. Post-operative remnants of brainstem cavernomas: incidence, risk factors and management. Acta Neurochir. 2008;150(9):879–886; discussion 887. [DOI] [PubMed] [Google Scholar]
- 80. Tan H, Liu T, Wu Y et al. Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Invest Radiol. 2014;49(7):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hart BL, Taheri S, Rosenberg GA, Morrison LA. Dynamic contrast-enhanced MRI evaluation of cerebral cavernous malformations. Transl Stroke Res. 2013;4(5):500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mikati AG, Tan H, Shenkar R et al. Dynamic permeability and quantitative susceptibility: related imaging biomarkers in cerebral cavernous malformations. Stroke. 2014;45(2):598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mikati AG, Khanna O, Zhang L et al. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab. 2015;35(10):1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hamilton BE, Nesbit GM, Dosa E et al. Comparative analysis of ferumoxytol and gadoteridol enhancement using T1- and T2-weighted MRI in neuroimaging. AJR Am J Roentgenol. 2011;197(4):981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Poorthuis M, Samarasekera N, Kontoh K et al. Comparative studies of the diagnosis and treatment of cerebral cavernous malformations in adults: systematic review. Acta Neurochir (Wien). 2013;155(4):643–649. [DOI] [PubMed] [Google Scholar]
- 86. Dalyai RT, Ghobrial G, Awad I et al. Management of incidental cavernous malformations: a review. Neurosurg Focus. 2011;31(6):E5. [DOI] [PubMed] [Google Scholar]
- 87. Bilginer B, Narin F, Hanalioglu S, Oguz KK, Soylemezoglu F, Akalan N. Cavernous malformations of the central nervous system (CNS) in children: clinico-radiological features and management outcomes of 36 cases. Childs Nerv Syst. 2014;30(8):1355–1366. [DOI] [PubMed] [Google Scholar]
- 88. Pasqualin A, Meneghelli P, Giammarusti A, Turazzi S. Results of surgery for cavernomas in critical supratentorial areas. Acta Neurochir Suppl. 2014;119:117–123. [DOI] [PubMed] [Google Scholar]
- 89. Kivelev J, Koskela E, Setala K, Niemela M, Hernesniemi J. Long-term visual outcome after microsurgical removal of occipital lobe cavernomas. J Neurosurg. 2012;117(2):295–301. [DOI] [PubMed] [Google Scholar]
- 90. Liu JK, Lu Y, Raslan AM, Gultekin SH, Delashaw JB Jr.. Cavernous malformations of the optic pathway and hypothalamus: analysis of 65 cases in the literature. Neurosurg Focus. 2010;29(3):E17. [DOI] [PubMed] [Google Scholar]
- 91. Carrasco R, Pedrosa M, Pascual JM, Navas M, Liberal R, Sola RG. Cavernous angiomas of the lateral ventricles. Acta Neurochir. 2009;151(2):149–154. [DOI] [PubMed] [Google Scholar]
- 92. Gross BA, Batjer HH, Awad IA, Bendok BR. Cavernous malformations of the basal ganglia and thalamus. Neurosurgery. 2009;65(1):7–18; discussion 18-19. [DOI] [PubMed] [Google Scholar]
- 93. Li D, Zhang J, Hao S et al. Surgical treatment and long-term outcomes of thalamic cavernous malformations. World Neurosurg. 2013;79(5-6):704–713. [DOI] [PubMed] [Google Scholar]
- 94. Gross BA, Batjer HH, Awad IA, Bendok BR, Du R. Brainstem cavernous malformations: 1390 surgical cases from the literature. World Neurosurg. 2013;80(1-2):89–93. [DOI] [PubMed] [Google Scholar]
- 95. Flores BC, Whittemore AR, Samson DS, Barnett SL. The utility of preoperative diffusion tensor imaging in the surgical management of brainstem cavernous malformations. J Neurosurg. 2015;122(3):653–662. [DOI] [PubMed] [Google Scholar]
- 96. Winkler D, Lindner D, Strauss G, Richter A, Schober R, Meixensberger J. Surgery of cavernous malformations with and without navigational support–a comparative study. Minim Invasive Neurosurg. 2006;49(1):15–19. [DOI] [PubMed] [Google Scholar]
- 97. Zhou H, Miller D, Schulte DM et al. Transsulcal approach supported by navigation-guided neurophysiological monitoring for resection of paracentral cavernomas. Clin Neurol Neurosurg. 2009;111(1):69–78. [DOI] [PubMed] [Google Scholar]
- 98. Choudhri O, Karamchandani J, Gooderham P, Steinberg GK. Flexible omnidirectional carbon dioxide laser as an effective tool for resection of brainstem, supratentorial, and intramedullary cavernous malformations. Neurosurgery. 2014;10(suppl 1):34–34; discussion 43-35. [DOI] [PubMed] [Google Scholar]
- 99. Steinberg GK, Marks MP, Shuer LM, Sogg RL, Enzmann DR, Silverberg GD. Occult vascular malformations of the optic chiasm: magnetic resonance imaging diagnosis and surgical laser resection. Neurosurgery. 1990;27(3):466–470. [PubMed] [Google Scholar]
- 100. Kharkar S, Shuck J, Conway J, Rigamonti D. The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery. 2007;60(5):865–872; discussion 865-872. [DOI] [PubMed] [Google Scholar]
- 101. Brelie C, von Lehe M, Raabe A et al. Surgical resection can be successful in a large fraction of patients with drug-resistant epilepsy associated with multiple cerebral cavernous malformations. Neurosurgery. 2014;74(2):147–153; discussion 153. [DOI] [PubMed] [Google Scholar]
- 102. Rosenow F, Alonso-Vanegas MA, Baumgartner C et al. Cavernoma-related epilepsy: review and recommendations for management–report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2013;54(12):2025–2035. [DOI] [PubMed] [Google Scholar]
- 103. Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G. Cerebral cavernomas and seizures: a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci. 2006;26(6):390–394. [DOI] [PubMed] [Google Scholar]
- 104. Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. 1995;83(2):237–242. [DOI] [PubMed] [Google Scholar]
- 105. von der Brelie C, Kuczaty S, von Lehe M. Surgical management and long-term outcome of pediatric patients with different subtypes of epilepsy associated with cerebral cavernous malformations. J Neurosurg Pediatr. 2014;13(6):699–705. [DOI] [PubMed] [Google Scholar]
- 106. McCracken DJ, Willie JT, Fernald BA et al. Magnetic resonance thermometry-guided stereotactic laser ablation of cavernous malformations in drug-resistant epilepsy: imaging and clinical results. Oper Neurosurg. 2016;12(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang P, Liu L, Cao Y, Wang S, Zhao J. Cerebellar cavernous malformations with and without associated developmental venous anomalies. BMC Neurol. 2013;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee SH, Choi HJ, Shin HS, Choi SK, Oh IH, Lim YJ. Gamma knife radiosurgery for brainstem cavernous malformations: should a patient wait for the rebleed? Acta Neurochir. 2014;156(10):1937–1946. [DOI] [PubMed] [Google Scholar]
- 109. Sager O, Beyzadeoglu M, Dincoglan F et al. Evaluation of linear accelerator (LINAC)-based stereotactic radiosurgery (SRS) for cerebral cavernous malformations: a 15-year single-center experience. Ann Saudi Med. 2014;34(1):54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lu XY, Sun H, Xu JG, Li QY. Stereotactic radiosurgery of brainstem cavernous malformations: a systematic review and meta-analysis. J Neurosurg. 2014;120(4):982–987. [DOI] [PubMed] [Google Scholar]
- 111. Lunsford LD, Khan AA, Niranjan A, Kano H, Flickinger JC, Kondziolka D. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J Neurosurg. 2010;113(1):23–29. [DOI] [PubMed] [Google Scholar]
- 112. Niranjan A, Lunsford LD. Stereotactic radiosurgery guidelines for the management of patients with intracranial cavernous malformations. Prog Neurol Surg. 2013;27:166–175. [DOI] [PubMed] [Google Scholar]
- 113. Kondziolka D, Lunsford LD, Coffey RJ, Bissonette DJ, Flickinger JC. Stereotactic radiosurgery of angiographically occult vascular malformations: indications and preliminary experience. Neurosurgery. 1990;27(6):892–900. [DOI] [PubMed] [Google Scholar]
- 114. Awad I, Jabbour P. Cerebral cavernous malformations and epilepsy. Neurosurg Focus. 2006;21(1):e7. [DOI] [PubMed] [Google Scholar]
- 115. Stavrou I, Baumgartner C, Frischer JM, Trattnig S, Knosp E. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery. 2008;63(5):888–896; discussion 897. [DOI] [PubMed] [Google Scholar]
- 116. Batra S, Lin D, Recinos PF, Zhang J, Rigamonti D. Cavernous malformations: natural history, diagnosis and treatment. Nat Rev Neurol. 2009;5(12):659–670. [DOI] [PubMed] [Google Scholar]
- 117. Leigh R, Wityk RJ. Special problems in cavernous malformations: migraine, pregnancy, hormonal replacement, anticoagulation, NSAIDs. In: Rigamonti D. Cavernous Malformations of the Central Nervous System. Cambridge: Cambridge University Press; 2011:185–191. [Google Scholar]
- 118. Li H, Ju Y, Cai BW, Chen J, You C, Hui XH. Experience of microsurgical treatment of brainstem cavernomas: report of 37 cases. Neurol India. 2009;57(3):269–273. [DOI] [PubMed] [Google Scholar]
- 119. Li D, Hao SY, Tang J et al. Surgical management of pediatric brainstem cavernous malformations. J Neurosurg Pediatr. 2014;13(5):484–502. [DOI] [PubMed] [Google Scholar]
- 120. Choi H, Kim CH, Lee KY, Lee YJ, Koh SH. A probable cavernoma in the medulla oblongata presenting only as upbeat nystagmus. J Clin Neurosci. 2011;18(11):1567–1569. [DOI] [PubMed] [Google Scholar]
- 121. Gross BA, Smith ER, Scott RM. Cavernous malformations of the basal ganglia in children. J Neurosurg Pediatr. 2013;12(2):171–174. [DOI] [PubMed] [Google Scholar]
- 122. Li D, Hao SY, Tang J et al. Clinical course of untreated pediatric brainstem cavernous malformations: hemorrhage risk and functional recovery. J Neurosurg Pediatr. 2014;13(5):471–483. [DOI] [PubMed] [Google Scholar]
- 123. Nikoubashman O, Wiesmann M, Tournier-Lasserve E et al. Natural history of cerebral dot-like cavernomas. Clini Radiol. 2013;68(8):e453–e459. [DOI] [PubMed] [Google Scholar]
- 124. Gross BA, Du R, Orbach DB, Scott RM, Smith ER. The natural history of cerebral cavernous malformations in children. J Neurosurg Pediatr. 2015:1–6[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 125. Consales A, Piatelli G, Ravegnani M et al. Treatment and outcome of children with cerebral cavernomas: a survey on 32 patients. Neurol Sci. 2010;31(2):117–123. [DOI] [PubMed] [Google Scholar]
- 126. Bigi S, Capone Mori A, Steinlin M, Remonda L, Landolt H, Boltshauser E. Cavernous malformations of the central nervous system in children: presentation, treatment and outcome of 20 cases. Eur J Paediatr Neurol. 2011;15(2):109–116. [DOI] [PubMed] [Google Scholar]
- 127. Faraci M, Morana G, Bagnasco F et al. Magnetic resonance imaging in childhood leukemia survivors treated with cranial radiotherapy: a cross sectional, single center study. Pediatr Blood Cancer. 2011;57(2):240–246. [DOI] [PubMed] [Google Scholar]
- 128. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002;94(12):3285–3291. [DOI] [PubMed] [Google Scholar]
- 129. Witiw CD, Abou-Hamden A, Kulkarni AV, Silvaggio JA, Schneider C, Wallace MC. Cerebral cavernous malformations and pregnancy: hemorrhage risk and influence on obstetrical management. Neurosurgery. 2012;71(3):626–630; discussion 631. [DOI] [PubMed] [Google Scholar]
- 130. Yamada S, Nakase H, Nakagawa I, Nishimura F, Motoyama Y, Park YS. Cavernous malformations in pregnancy. Neurol Med Chir (Tokyo). 2013;53(8):555–560. [DOI] [PubMed] [Google Scholar]
- 131. Flemming KD, Link MJ, Christianson TJ, Brown RD Jr.. Use of antithrombotic agents in patients with intracerebral cavernous malformations. J Neurosurg. 2013;118(1):43–46. [DOI] [PubMed] [Google Scholar]
- 132. Schneble HM, Soumare A, Herve D et al. Antithrombotic therapy and bleeding risk in a prospective cohort study of patients with cerebral cavernous malformations. Stroke. 2012;43(12):3196–3199. [DOI] [PubMed] [Google Scholar]
- 133. Erdur H, Scheitz JF, Tutuncu S et al. Safety of thrombolysis in patients with acute ischemic stroke and cerebral cavernous malformations. Stroke. 2014;45(6):1846–1848. [DOI] [PubMed] [Google Scholar]
- 134. Kumar S, Puri V, Malik R, Gupta S. Cerebral cavernous angioma. Indian Pediatr. 1991;28(6):675–678. [PubMed] [Google Scholar]
- 135. Berg MJ, Vay T. Clinical features and medical management of cavernous malformations. In: Rigamonti D, ed. Cavernous Malformations of the Nervous System. Vol. 2011 Cambridge, England: Cambridge University Press; 2011:65–78. [Google Scholar]
- 136. Girard R, Khanna O, Shenkar R et al. Peripheral plasma vitamin D and non-HDL cholesterol reflect the severity of cerebral cavernous malformation disease. Biomark Med. 2016;10(3):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]