Abstract
Glucagon-like peptide 1 receptor agonists (GLP-1RA) are the newest treatment for diabetes mellitus (DM). These drugs can down-regulate fasting glucose more than oral drugs and lead to more constant glucose levels compared to regular insulin. Acute pancreatitis is a serious condition that may have a fatal outcome. It has been shown that long term and high doses of GLP-1RA can cause pancreatic changes in animals, but no connection has been proven in humans. We present the case of a 67 years old man with DM treated with oral drugs for 10 years until 3 months before, when GLP-1RA was added. He presented progressive abdominal pain, vomiting, and increased level of serum lipase and amylase were found. Ultrasonography and computed tomography found pancreatic and peripancreatic fatty tissue inflammation (inflammation score 2, necrosis score 0). All the etiologies of acute pancreatitis (lithiasis, alcohol, autoimmune, or trauma) were excluded. After GLP-1RA cessation and supportive treatment the evolution was self-limited with full recovery within 5 days. We concluded that acute pancreatitis can be considered a side effect of the GLP-1 treatment.
Keywords: acute pancreatitis, diabetes mellitus, GLP-1 agonist, lixisenatide
Introduction
Diabetes mellitus (DM) has become a common health problem of the industrialized countries. Oral treatments are available, but insulin tends to have the best response in well-conducted treatments. The newest alternative for DM treatment is the class of glucagon-like peptide receptors agonists (GLP-1RA) through binding glucagon and by stimulating beta-cells insulin secretion [1].
Lixisenatide, a member of GLP-1-RA class, was first approved for human use in type 2 DM in 2013 in the USA. A single 20 mg dose injected daily came to reduce both postprandial and fasting glucose, with the main advantage of an easier monitoring of the dose related effects (as hypoglycemia) compared to regular insulins [2]. In animal experimental studies (necropsy findings) pancreas related acute or chronic diseases were described as side-effects of GLP-1RA. Clinical studies failed to demonstrate a correlation in humans so far [3]. A few cases of acute pancreatitis have been described until now during Lixisenatide treatment, but their occurrence was not at a higher rate compared to placebo [4].
Case report
A 67 years old patient was admitted to the emergency room for intense epigastric pain slightly irradiating towards the right hypochondrium. No pain relief was obtained after WHO step 1 and 2 pain treatment. He also presented nausea, vomiting (3–4 times a day), and alteration of the general status in the last 5 days. He had been diagnosed with type 2 DM and hypertension 10 years ago. Chronic treatment was conducted with lisinopril 5mg/day, metformin 1000 mg/day, and lixisenatide 10mg/day. The GLP-1 receptor agonist treatment was started 3 months prior to presentation and oral antidiabetic medication 10 years ago. No other drugs or alcohol were used.
Physical examination revealed normal temperature (36.7°C), normal blood pressure (110/60 mmHg), dehydrated skin, and abdominal obesity. The abdomen was painful at palpation in the upper half, mainly in the epigastric region and right hypochondrium, with diminished bowel sounds.
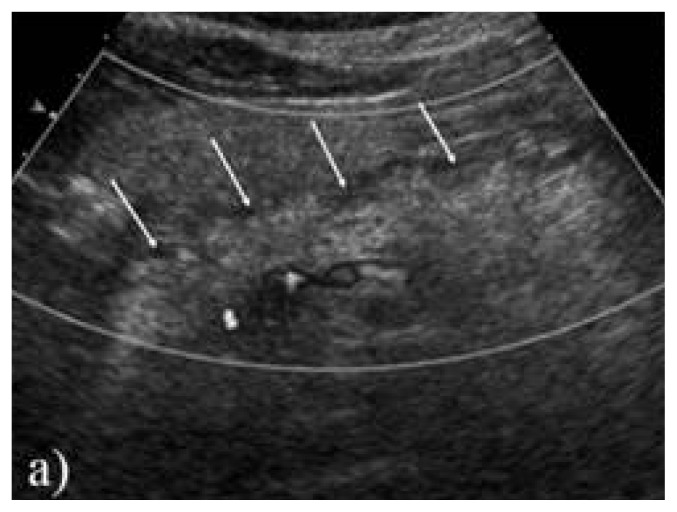
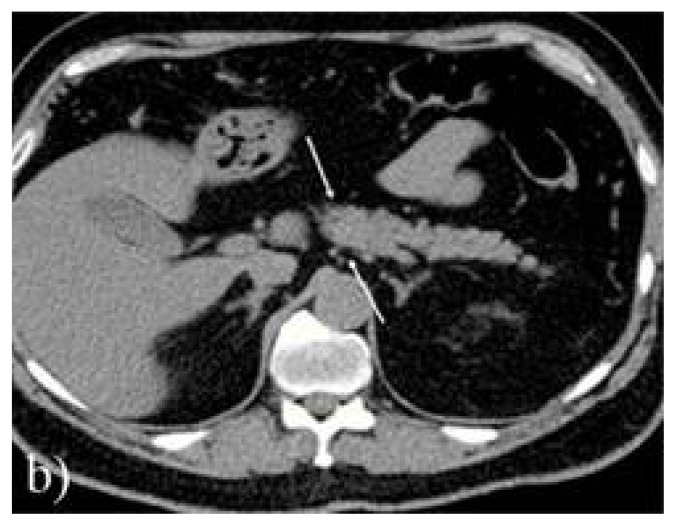
Biological exams showed high lipasis levels (665 U/L – normal value <60 U/L), high amylasemia (240 U/dL, normal values <100 U/L) along with high urinary amylase (1856 U/24 hours, normal ranges 25–400 U/24 hours), lactate dehydrogenase 180 U/L (normal range), inflammatory syndrome (erythrocyte sedimentation ratio of 34 mm/h, CRP=1.82mg/dL), slightly low hemoglobin (11.2 g/dL) and a total calcium level of 2.05 mmol/L (normal range 2.1–2.5 mmol/L). Also, glycemia was high (up to 16.6 mmol/L). It was interpreted as a mild acute pancreatitis and abdominal ultrasonography was performed. The diameters of the pancreas and Wirsung duct were found slightly increased and edema in the peripancreatic fatty tissue was found. No gallbladder stones or intra/extrahepatic biliary ducts dilatation were detected. Computed tomography confirmed the peripancreatic fatty tissue and pancreatic edema, but no anatomic abnormalities or gallbladder stones were found. No necrosis or cysts were detected either (Figure 1).
Figure 1.
a) Ultrasonography longitudinal scan of the pancreas edema of the fatty tissue around the pancreas (arrows).
The case was interpreted as an acute pancreatitis (Ranson Score 2). After the exclusion of all the other possible causes for acute pancreatitis (metabolic- normal lipids serum levels, and calcemia, no prior trauma, no current alcohol or other toxics consumption, no signs for autoimmune pathology, no anatomic abnormalities such pancreas divisum), or other disease to explain the patient’s symptomatology (such as viral/bacterial infections) the pathology was related to the GLP-1 RA treatment. The lixisenatide treatment was interrupted; oral and parenteral rehydration was administrated as the patient was put to fasting for the first 48 hours, proton pump inhibitor and antispasmodic drugs (drotaverine) were administered. After 24 hours, lipasis serum levels started to decrease (normalization after 3 days), blood sugar level were within normal range (under metformin treatment with fast acting insulin administration as needed), and hemoglobin increased slowly (12.3 g/dL). Clinical status progressively improved, with no pain or vomiting from the second day. At one month follow up, the patient had normal blood sugar values, normal biological status, and no clinical symptoms.
Discussion
Our case is a patient who recently started the GLP-1 RA treatment for a type 2 DM treated by the time only with oral drugs. Clinical description of the pain was not a classical one for the acute pancreatitis, considering the slow onset time (several days), abnormal localization (right hypochondrium), and the mild intensity. Laboratory evaluation found abnormal levels of pancreatic lipase and amylase and imaging techniques confirmed the pancreatic inflammation. As we excluded the other possible causes for acute pancreatitis, and the evolution after GLP-1 RA treatment interruption was clearly favorable, we concluded the case as being an acute pancreatitis related to the side effect of the lixisenatide treatment.
Incretins (substances that can lower the blood sugar by insulin production in beta pancreatic cells) have been suspected to relate to pancreatitis. Some animal studies showed no important pancreatic alterations in diabetic rats (DM being considered an important cause of acute pancreatitis itself) during treatment with exenatide [5]. A meta-analysis carried by USA FDA on more than 200 human subjects showed a small but possible connection between incretins treatment and acute pancreatitis or even pancreatic cancer [6]. Liraglutide treatment was studied by pharmaceuticals companies and only 8 cases of acute pancreatitis (all as mild forms) in over 6000 patients treated were reported [7].
In the last decade many efforts have been made to study new treatments in DM. Since introducing the insulin treatment, no other drug came to ameliorate blood sugar levels as the GLP-1 RA did. Large human studies have not finished yet or partial results have only presented (phase IV in progress: ELIXA trials, Get Goal Duo- II) [8,9]. Compared to other insulin secretagogues as sulfonylureas or glinides, the risk for hypoglycemia of the GLP-1 RA is low [10]. Other drugs can block the postprandial glucagon secretion (gliptins) and increase beta-cells insulin production, but GLP-1 receptor agonists are the most potent ones. Animal studies have been conducted showing pancreatic alterations during GLP-1 treatment. Several studies concluded that acute pancreatitis - chronic pancreatitis – cancer sequence is possible during oral antidiabetic medication as well, but human cases were considered “notoriety bias” [11]. Also, the risk of pancreatic events was and is still considered low compared to glycemic control obtained by the new treatments [12].
In conclusion acute pancreatitis during GLP-1RA treatment can be underdiagnosed due to lack of classical symptomatology or natural evolution (self-limited forms are commonly found). Only animal morphopathologic pancreatic changes can bring in our attention the side effects of the new treatments. Considering pancreatitis a rare secondary effect of GLP1-RA treatment, new DM treatment drugs can be considered for long-term treatment and may replace insulin treatment, with higher adherence. Only an active pharmacovigilance can report the actual prevalence of pancreatic involvement during new treatments.
Figure 1.
b) computed tomography scan showing pancreatic edema between arrows (Balthazar C inflammation score).
References
- 1.Kuc RE, Maguire JJ, Siew K, Patel S, Derksen DR, Margaret Jackson V, et al. Characterization of [125I]GLP-1(9–36), a novel radiolabeled analog of the major metabolite of glucagon-like peptide 1 to a receptor distinct from GLP1-R and function of the peptide in murine aorta. Life Sci. 2014;102(2):134–138. doi: 10.1016/j.lfs.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Christensen M, Knop FK, Holst JJ, Vilsboll T. Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. IDrugs. 2009;12(8):503–513. [PubMed] [Google Scholar]
- 3.Consoli A, Formoso G. Potential side effects to GLP-1 agonists: understanding their safety and tolerability. Expert Opin Drug Saf. 2015;14(2):207–218. doi: 10.1517/14740338.2015.987122. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 5.Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, et al. Pancreatic safety of incretin-based drugs-FDA and EMA assessment. N Engl J Med. 2014;370(9):794–797. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]
- 6.Engel SS, Round E, Golm GT, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther. 2013;4(1):119–145. doi: 10.1007/s13300-013-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen TM, Saha K, Steinberg WM. Is there a link between liraglutide and pancreatitis? A post hoc review of pooled and patient-level data from completed liraglutide type 2 diabetes clinical trials. Diabetes Care. 2015;38(6):1058–1066. doi: 10.2337/dc13-1210. [DOI] [PubMed] [Google Scholar]
- 8.Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36(9):2497–2503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ELIXA trial show CV safety of lixisenatide. Available from: https://www.escardio.org/The-ESC/Press-Office/Press-releases/elixa-trial-shows-cv-safety-of-lixisenatide.
- 10.American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauck MA, Friedrich N. Do GLP-1–based therapies increase cancer risk? Diabetes Care. 2013;36(Supp 2):S245–S252. doi: 10.2337/dcS13-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryder RE. The potential risks of pancreatitis and pancreatic cancer with GLP-1-based therapies are far outweighed by the proven and potential (cardiovascular) benefits. Diabet Med. 2013;30(10):1148–1155. doi: 10.1111/dme.12301. [DOI] [PubMed] [Google Scholar]