Abstract
Corals host diverse microbial communities that are involved in acclimatization, pathogen defense, and nutrient cycling. Surveys of coral-associated microbes have been particularly directed toward Symbiodinium and bacteria. However, a holistic understanding of the total microbiome has been hindered by a lack of analyses bridging taxonomically disparate groups. Using high-throughput amplicon sequencing, we simultaneously characterized the Symbiodinium, bacterial, and fungal communities associated with the Caribbean coral Siderastrea siderea collected from two depths (17 and 27 m) on Conch reef in the Florida Keys. S. siderea hosted an exceptionally diverse Symbiodinium community, structured differently between sampled depth habitats. While dominated at 27 m by a Symbiodinium belonging to clade C, at 17 m S. siderea primarily hosted a mixture of clade B types. Most fungal operational taxonomic units were distantly related to available reference sequences, indicating the presence of a high degree of fungal novelty within the S. siderea holobiont and a lack of knowledge on the diversity of fungi on coral reefs. Network analysis showed that co-occurrence patterns in the S. siderea holobiont were prevalent among bacteria, however, also detected between fungi and bacteria. Overall, our data show a drastic shift in the associated Symbiodinium community between depths on Conch Reef, which might indicate that alteration in this community is an important mechanism facilitating local physiological adaptation of the S. siderea holobiont. In contrast, bacterial and fungal communities were not structured differently between depth habitats.
Keywords: Symbiosis, Coral, Fungi, Microbial community, Coral-associated microbiome, Symbiodinium
Introduction
Reef building corals harbor diverse microbial communities integral to their metabolism and physiology that are important in nutrient cycling, pathogenicity, pathogen defense, and environmental resilience (Muscatine & Porter, 1977; Rohwer et al., 2001; Lesser et al., 2004, 2007; Ritchie, 2006; Reshef et al., 2006; Wegley et al., 2007; Olson et al., 2009; Raina et al., 2009; Davy, Allemand & Weis, 2012; Ziegler et al., 2017). The interdependence of the coral host and its diverse microbiome has manifested the recognition of the coral holobiont, or the sum of all microbes and the coral host (Rohwer et al., 2001; Knowlton & Rohwer, 2003). The most well-studied symbionts, among the diverse bacteria, archaea, fungi, protista, and viruses that comprise the holobiont, are Symbiodinium, which reside intracellularly within the gastroderm. These dinoflagellates conduct photosynthesis and provide the host with fixed carbon (Muscatine & Porter, 1977). The Symbiodinium genus comprises many genetically diverse “types” or species with different phenologies that are associated with a multitude of hosts, including Foraminifera, Mollusca, Porifera, and Cnidaria (LaJeunesse, 2001; Rodriguez-Lanetty, 2003; Coffroth & Santos, 2005; Stat, Carter & Hoegh-Guldberg, 2006). The degree to which different coral species are able to associate with distinct Symbiodinium types has been termed “host flexibility” (Baker, 2003). It has been hypothesized that flexible corals are better able to adapt to changing environmental conditions (Buddenmeier & Fautin, 1993; Baker, 2003; Baird et al., 2007) and to different environmental settings along latitudinal gradients (Rodriguez-Lanetty et al., 2001; Rodriguez-Lanetty & Hoegh-Guldberg, 2003).
Recent efforts, using highly sensitive next-generation sequencing, revealed that the coral microbiome is far more diverse than previously thought (Amend, Barshis & Oliver, 2012; Lema, Willis & Bourne, 2012; Rodriguez-Lanetty et al., 2013; Bayer et al., 2013a, 2013b; Soffer, Zaneveld & Vega-Thurber, 2015; Ainsworth et al., 2015; Hernandez-Agreda et al., 2016; Brown et al., 2017). Even though understanding of the coral microbiome has developed considerably (reviewed in Rosenberg et al., 2007; Ainsworth, Vega-Thurber & Gates, 2010; Bourne, Morrow & Webster, 2016), the functional contributions of microbes associated with the host are still poorly understood. Apart from Symbiodinium and bacteria, other microbes, such as fungi, have only received occasional attention. It is, however, fairly well established that a diverse fungal community is naturally associated with the healthy coral, particularly within the skeleton (reviewed in Raghukumar, 2012; Yarden, 2014; Kendrick et al., 1982; Le Campion-Alsumard, Golubic & Priess, 1995; Domart-Coulon et al., 2004; Wegley et al., 2007; Vega-Thurber et al., 2009; Littman, Willis & Bourne, 2011; Amend, Barshis & Oliver, 2012). Symbiodinium and bacterial communities have been characterized and studied with next-generation sequencing approaches, however, this has only been applied on one occasion for fungi (Amend, Barshis & Oliver, 2012). While ecological understanding of the bacterial component of the coral holobiont is growing, the role of fungi remains understudied and enigmatic. To develop a baseline understanding of the coral microbiome ecology, it is imperative to study all microbial taxa, including fungi.
Reef building corals inhabit the upper layer of the ocean and are typically exposed to strong fluctuating environmental conditions, such as light intensity, temperature, seawater chemistry, and microbial fluxes which structure the composition of coral-associated microbial communities (Vega-Thurber et al., 2009). Corals survive over a considerable depth distribution, ranging from directly beneath the surface, to deeper and more stable mesophotic regions (Lesser, Slattery & Leichter, 2009). Microbial communities are likely key contributors to their capacity to persist in these disparate and fluctuating environments. It has been postulated that microbial succession within the microbiome could mitigate stress associated with changing environmental conditions (Reshef et al., 2006). Therefore, shifts between different microbial groups along the depth cline might support local ecological success of corals.
Given the steep decline of coral reefs globally (Gardner et al., 2003; van Hooidonk et al., 2014), an urgent need exists to develop a better understanding of the contribution of the microbiome (viruses, prokaryotes, and microeukaryotes) to holobiont resilience and adaptation. The coral selected for this study, Siderastrea siderea, is a gonochoric coral and an abundant massive coral in the Florida reef tract (Lirman & Fong, 2007). This species is considered to be among the most adaptive corals on the Caribbean reefs as it is relatively resilient to several environmental stressors (Colella et al., 2012; Castillo et al., 2014) and typified by high recovery rates from major bleaching events (Banks & Foster, 2017). Therefore, S. siderea represents a suitable species to investigate microbiome patterns between different localities on the same reef. The aim of this study was to reconstruct a detailed image of the Symbiodinium, bacterial, and fungal communities associated with S. siderea and investigate differences in microbiome diversity at the inter-domain level between two different depth habitats on the same reef. Previous studies have shown that while alpha diversity generally remains stable, coral-associated Symbiodinium and bacterial community structures (beta diversity) are variable with depth, particularly for corals occurring over a wide depth range (Klaus et al., 2007; Lee et al., 2012; Bongaerts et al., 2015; Glasl et al., 2017). For coral-associated fungal communities, the influence of depth on diversity has not yet been investigated.
We collected samples from multiple S. siderea colonies at Conch reef in the Florida Keys from two depth intervals (17 and 27 ± 1 m) and conducted high-throughput sequencing of the Symbiodinium chloroplast 23S (cp23S) hyper variable region, the V123 region of the bacterial 16S ribosomal small subunit (SSU) and the fungal internal transcribed spacer-2 (ITS2). We hypothesized that between the two depth habitats sampled in this study, S. siderea would harbor distinctive Symbiodinium and bacterial communities (i.e., beta diversity), as predicted by previous work (Klaus et al., 2007; Lee et al., 2012; Bongaerts et al., 2015; Glasl et al., 2017). We extended this hypothesis to the fungal community, reasoning that shifts in the microbiome between depths with different local environments are most likely not restricted to the Symbiodinium genus and bacterial domain alone, but act at the inter-domain level, thus including the associated fungal community. In addition, we aimed to explore shifts within the microbiome and identify depth habitat-specific microbes by performing a species indicator analysis (Caceres & Legendre, 2009) and investigate co-occurrence and mutual exclusion patterns by constructing a microbial network.
Methods
Sample collection
Siderastrea siderea colonies were sampled from 27 ± 1 m (n = 8) and 17 ± 1 m (n = 6) from Conch reef surrounding the Aquarius Reef Base (Florida International University) in the Florida reef tract, approximately 8 km offshore from Key Largo. These samples were collected by aquanauts performing a saturation mission in the Aquarius Reef Base habitat during the 20th NASA Extreme Environment Mission Operations (NEEMO) mission in July 2015. Preceding the sampling, the dive team was properly trained and familiarized with the method of collection and the operation of the sampling equipment. Diving from the saturation habitat facilitated greatly extended dives to the deeper collection areas in particular. Fragments of approximately 2 cm2 surface area were sampled from healthy colonies from 27 and 17 m using chisel and hammer. Samples were brought to the surface in seawater containing 50 mL tubes within 4 h after collection. Seawater around the sampled corals was immediately removed before fixing the samples in a sterile 20% DMSO, 0.5M EDTA NaCl-saturated solution. It should be noted that collected samples were not actively washed with sterile seawater to remove loosely attached microbes and these microbes may be likely present at background levels in our data set and contribute to the detected patterns or absence of them. Coral tissues were homogenized with high-pressure airbrushing (Rodriguez-Lanetty et al., 2013) and the resulting material was preserved in new 20% DMSO solution at −20 °C until DNA extraction.
DNA extraction, amplification, and sequencing
From each of the eight (27 m) and six (17 m) samples, DNA was isolated using the MP FastDNA spin kit for soil (MP Biomedicals, Santa Ana, CA, USA) with an additional ethanol precipitation step. All DNA samples were divided into three aliquots to allow sequencing of the bacterial 16S rRNA gene, Symbiodinium cp23S rRNA gene, and fungal ITS2 rRNA gene from the same sample.
The cp23S fragment was amplified with primers from Santos, Gutierrez-Rodriguez & Coffroth (2003; 5′-TCAGTACAAATAATATGCTG-3′ and 5′-TTATCGCCCCAATTAAACAGT-3′) and the 16S rDNA V123 region with 27F and 519R primers (5′-AGRGTTTGATCMTGGCTCAG-3′, 5′-GWATTACCGCGGCKGCTG-3′; Turner et al., 1999) using the HotStarTaq Plus Master Mix Kit (Qiagen, Hilden, Germany). The PCR programs for 16S and cp23S consisted of 3 min initial denaturation at 94 °C, 28 cycles of 30 s at 94 °C, 40 s at 53 °C and 1 min at 72 °C, and a final elongation step of 5 min at 72 °C.
To circumvent amplification of DNA from the coral host, the ITS2 region was amplified in a nested PCR using phylum-specific primers (Nikolcheva & Bärlocher, 2004). The first PCR program consisted of 2 min initial denaturation at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at 57 °C and 0:45 min at 72 °C, followed by a final elongation step of 2 min at 72 °C. PCR reactions were conducted in volumes of 20 μL, containing 0.5 μM of each primer, 10 μL 2× PCR Master Mix (Promega, Madison, WI, USA) and 10 ng DNA template. In this first PCR, the universal forward primer ITS3 (5′-GCATCGATGAAGAACGCAG-3′; White et al., 1990) was combined with reverse primers specific for Ascomycota, Basidiomycota, Chitridiomycota, Oomycota, and Zygomycota (Nikolcheva & Bärlocher, 2004). The second PCR program comprised 2 min initial denaturation at 95 °C, 10 cycles of 30 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C, and a final elongation step of 2 min at 72 °C. PCR reactions were identical to the first PCR program with 1 μL of 10−1 diluted amplicon as template. The final amplicons were generated by using the universal ITS3 forward primer and the universal ITS4 reverse primer (5′-TCCTCCGCTTATTGATATGC-3′; White et al., 1990). For all sequencing assays except the first round of the nested ITS2 PCR, primers contained Illumina adapters. Barcodes were located between adapter and forward primer. After amplification, samples were pooled per marker in equal proportions, purified using calibrated Ampure XP beads and DNA libraries were prepared following the Illumina TruSeq DNA library preparation protocol for 300 bp paired-end MiSeq sequencing at MR DNA (Shallowater, TX, USA). De-multiplexed reads were deposited in the SRA database under the accession number PRJNA356144.
Raw sequence reads were joined, de-multiplexed, quality filtered, denoised, purged of chimeras, clustered into operational taxonomic units (OTUs) using the nearest neighbor algorithm and classified with Mothur 1.36.1 software according to the MiSeq SOP (Schloss et al., 2009; Kozich et al., 2013; see Supplemental Information for the executed command steps in Mothur).
To classify the Symbiodinium cp23S sequences we used the sequences from Pochon, Putnam & Gates (2014), who conducted a multigene clade level analysis of the Symbiodinium genus and included the cp23S region using 33 unique sequences representing different types from currently all known clades (clades A–I) with the dinoflagellates Gymnodinium simplex and Polarella glacialis as outgroups. We aligned all sequences with MAFFT (Katoh & Standley, 2013) and formatted the dataset to be compatible with Mothur 1.36.1 (see Supplemental Information for the aligned, unaligned, and taxonomy files). All cp23S contigs were aligned against this dataset for denoising and OTU clustering. The same set of sequences was used to classify the contigs and remove sequences not classified as Symbiodinium. For the bacterial sequences, denoising and classification was conducted using the SILVA reference alignment (release 123; Quast et al., 2013). To effectively eliminate chloroplast, mitochondrial 16S rDNA, and unclassified sequences from the dataset, both the SILVA and GreenGenes (DeSantis et al., 2006) alignments were used. We selected the SILVA reference dataset to align and classify the 16S reads since it is both more recently updated (2015 compared to 2013 for the GreenGenes dataset) and of superior alignment quality (Schloss, 2010). Due to the high variance on the ITS2 region, a high quality reference alignment covering the full fungal kingdom does not exist. Instead, similarity distances were calculated from pairwise alignments. Sequences were initially classified with the User-friendly Nordic ITS Ectomycorrhiza (UNITE) database (Abarenkov et al., 2010), however, due to overall low similarity scores all OTU sequences were additionally compared against the full GenBank record with the basic local alignment search tool (BLAST; Altschul et al., 1990).
For each of the datasets, extremely rare OTUs (represented by 10 or less reads in the total dataset) were excluded from further analysis to prevent diversity inflation with false OTUs resulting from erroneous reads (Bokulich et al., 2013). Classification was conducted with Mothur’s implemented Wang-method and 100 bootstraps per sequence and using a criterion of a minimum of 60 bootstraps per sequence (Wang et al., 2007). 16S and cp23S reads were clustered into OTUs based on the traditional 3% difference criterion. Due to the relative high intragenomic variance on the fungal ITS2 region (Schoch et al., 2012), ITS2 reads were clustered into OTUs using a more conservative criterion of 5% difference.
Statistical analyses
Operational taxonomic unit tables were normalized with cumulative sum scaling and log transformed using the R package “metagenomeSeq” (Paulson et al., 2013). Shannon entropy indices were compared with one-way type-III ANOVAs. To test the hypothesis that microbial communities within the same depths are more similar to each other than between depths, non-parametric analysis of similarities (ANOSIMs) and permutational analysis of variances (PERMANOVAs) were calculated, using Bray–Curtis distances and 9,999 permutations. Non-metric multidimensional scaling (nMDS) plots were produced to visualize similarities in community structures.
To identify significant correlations between distributional patterns of microbes we constructed a co-occurrence network (Newman, 2006; Rodriguez-Lanetty et al., 2013). To reduce the complexity of the combined datasets, bacterial OTUs (bOTUs) were merged at the family level and Symbiodinium OTUs (sOTUs) at the type level. OTUs lacking family level classification were grouped by order or higher if all OTUs in that taxonomic level lacked a family classification. From the resulting table, we computed all possible pairwise spearman rank correlation values (ρ) and corresponding p values, corrected for a false discovery rate (Benjamini & Hochberg, 1995). Correlations were considered valid when p < 0.05 and ρ > |0.6|. The network was visualized with the software Gephi (Bastian, Heymann & Jacomy, 2009). The species indicator analysis (Caceres & Legendre, 2009) was computed from the same table as the network (i.e., containing bacteria merged at the family level and Symbiodinium at the type level) with the R package “indicspecies,” using the “multipatt” command, to test for significant associations to one of the depths and assigning bacterial families, Symbiodinium types and fungal OTUs (fOTUs) with p < 0.05 to be depth indicators.
Alignments for additionally investigated OTUs were constructed with MAFFT v. 7. (Katoh & Standley, 2013). Optimal evolutionary models were calculated with MrModelTest v. 2.3 and selected based on the AIC (Nylander, 2004). Maximum-likelihood analyses were conducted on the CIPRES Science Gateway portal (Miller, Pfeiffer & Schwartz, 2012), using RAxML v.8. (Stamatakis, 2014) with a 1,000 bootstrap iterations.
Results
Symbiodinium, bacterial, and fungal community composition
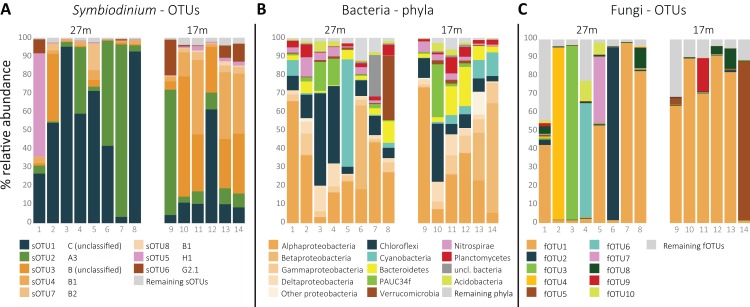
In total, 54 sOTUs were resolved from the 1,455,725 sequences remaining after quality filtering treatment with an average read count of 103,980 ± SD 90,086 per sample. OTUs were classified to types from clades A (7), B (26), C (6), D (1), F (2), G (2), and H (1). The remaining nine sOTUs lacked clade level classification by 60 bootstrap values or more. The overall most abundant, sOTU1 (39.5% average relative abundance) was classified to clade C, sOTU2 (21.7%) to clade A (A3) and sOTU3 (16.4%) and sOTU4 (10.2%) to clade B and type B1, respectively. sOTU5, classified as an H1 type, was the fifth most abundant sOTU (4.8%), and dominated in one of the 14 samples (Fig. 1A). sOTU6 was classified as a G2-1 type and was the sixth most abundant sOTU (3.3%). The one sOTU classified to clade D, type D1 (Symbiodinium trenchii), was rare and occupied the 24th position.
Figure 1. Relative distribution per sample of (A) the eight most abundant Symbiodinium OTUs, ordered by clade level classification, (B) the 10 most abundant bacterial phyla, and (C) the 10 most abundant fungal OTUs per sample.
The total amount of bOTUs after quality filtering was 714 from a total of 115,095 sequences with an average of 8,221 ± SD 4,926 reads per sample. The phylum Proteobacteria was the most abundant (49.2%), followed by Chloroflexi (14.7%), Cyanobacteria (7.4%), and Bacteriodetes (6.1%; Fig. 1B). The most abundant bOTU, bOTU1 (10.2%), was classified to the SAR116 clade (Alphaproteobacteria), followed by bOTU2 (5.7%) classified as Synechococcus (Cyanobacteria) and then bOTU3 (4.4%), classified to the clade SAR202 (Chloroflexi). bOTU4 (3.5%) and bOTU5 (3.5%) were classified to Candidatus Branchiomonas (Nitrosomonadaceae) and Candidatus Lariskella (Rickettsiales), respectively.
Despite using fungal-specific primers (Nikolcheva & Bärlocher, 2004), a relative high amount of ITS2 reads from other eukaryotes was retrieved. Only 22.2% of the 790,398 quality filtered ITS2 reads were fungal. Seventeen percent of the reads were from Cnidaria and the remaining reads from other eukaryotes. A group of fungi-like Opisthokonta (Mesomycetozoa) was also represented with 0.15% of the reads. Using the UNITE reference database in the Mothur pipeline, 184 fOTUs were classified to at least the fungal kingdom. However, additional comparison of the complete fOTU sequence set along the GenBank database showed 145 OTUs to yield high similarity scores with non-fungal sequences. These fOTUs were removed from the OTU table, resulting in 39 fOTUs with only similarity hits to fungi or Mesomycetozoa, constituting 141,706 reads in total and by average 10,122 ± SD 9,042 reads per sample. Most fOTUs belonged to Ascomycota (34). Other fOTUs were most similar to Basidiomycota (2), Entomorphthoromycota (1), and Mesomycetozoa (2). ITS2 similarity with sequences from GenBank was notably low (91.0 ± SD 8.2%). The overall most abundant fungus (fOTU1; 48.7% average relative abundance; Fig. 1C) was most similar (76%) to sequences belonging to Lulworthiales (Sordariomycetes). fOTUs 2 (7.2% average relative abundance) and 3 (6.9%) were most similar to sequences from Hyalorhinocladiella (73%; Ophiostomatales) and Physalospora (86%; Xylariales), respectively. A core set of 21 fOTUs, out of 39, was detected in >90% colonies, 15 of which were present in all samples.
Assemblage patterns in Symbiodinium, bacterial, and fungal communities
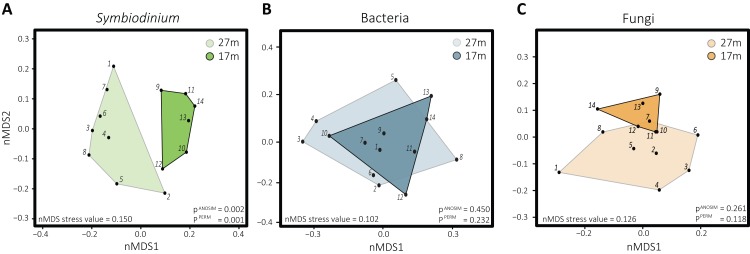
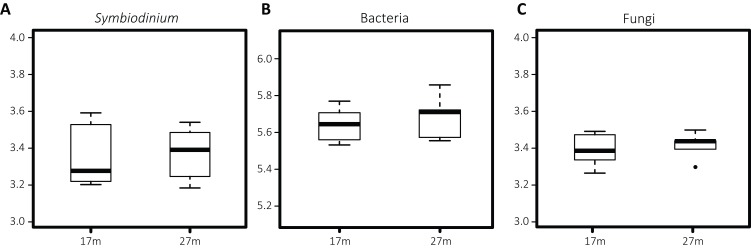
Differences in Symbiodinium community structure were detected between the two depths as visual in the nMDS plot (Fig. 2A) and statistically supported by ANOSIM and PERMANOVA analyses (p = 0.001 and 0.002, respectively). This was opposite for bacteria (pANOSIM = 0.450, pPERMANOVA = 0.232) and fungi (pANOSIM = 0.261, pPERMANOVA = 0.118) where differences between depths were not significant. While based on Shannon entropy indices no significant differences were detected in α-diversity between depths in any of the three microbial domains, the mean diversity tended to be higher in the deep habitat (27 m) across the communities (Fig. 3).
Figure 2. Non-metric multidimensional scaling plots with corresponding stress values of the distinct microbial groups associated with S. siderea: (A) Symbiodinium, (B) bacteria, (C) fungi.
Polygons are drawn around samples to visualize from which depth habitat they were collected, with 27 m (1–8) transparent and 17 m (9–15) opaque.
Figure 3. Boxplots of Shannon entropy indices for diversity in microbial groups associated with S. siderea: (A) Symbiodinium, (B) bacteria, (C) fungi.
The thick line represents the median value and boxes show the first and third quartiles. Lower and upper whiskers extend from the boxes to the extreme values or 1.5 times the inter quartile range when the extreme values are outside the range.
Co-occurrence patterns and depth indicators
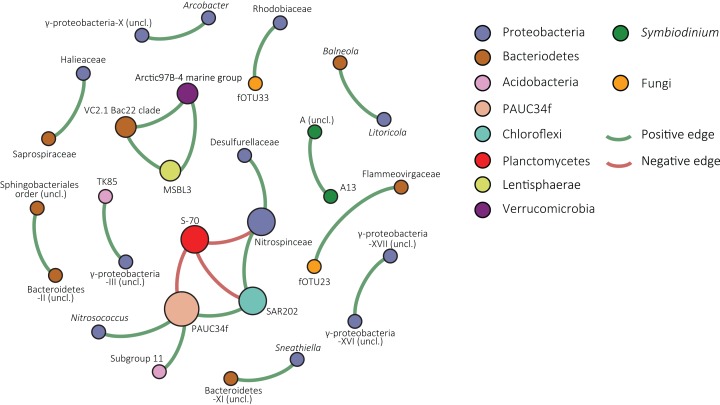
The resolved network, conducted to explore co-occurrence patterns, contained 30 nodes (fOTUs, Symbiodinium types or bacterial families) and 21 edges (significant spearman correlation values above 0.6 between nodes), three of which were negative (Fig. 4). The average degree (the average number of edges per node) was 1.4 and the average weighted degree, weighted based on spearman correlation values, was 0.94. The modularity of the network or degree to which a network tends to unfold into modules or sub-communities (Blondel et al., 2008) was 1.04.
Figure 4. Co-occurrence network of S. siderea associated microbes.
Nodes are colored based on taxonomy and green edges represent positive correlation values whereas red edges indicate negative correlations.
Within the network, one large module of co-occurring nodes was identified, containing six nodes. The module was connected with three negative edges to a node classified as S-70 (Planctomycetes). The node in the module with the most edges was an uncultured bacterial group that is designated in the SILVA database as “PAUC34f” and had three positive correlations and one negative correlation with the Planctomycete group. Other nodes were classified to Proteobacteria (Desulfurellaceae, Nitrospinaceae, and Nitrosococcus), Chloroflexi (SAR202), and Acidobacteria (Subgroup 11). In the overall network, the majority of nodes were bacterial. Symbiodinium type A13 and an unclassified clade A type co-occurred in a pair. Fungal nodes in the network were fOTUs 23 and 33, most similar to Infundibulomyces (Ascomycota; incertae sedis) and Conicomyces (Chaetosphaeriales), respectively. fOTU 23 co-occurred with the Bacteriodetes family Flammeovirgaceae and fOTU33 with Rhodobiaceae (Alphaproteobacteria; Rhizobiales).
The species indicator analysis identified Symbiodinium types C1 and C90 as indicators of the deep habitat (27 m), (Table 1). Among the bacterial groups, RSB-22 (Verrucomicrobia) was indicative for 27 m and an unclassified alphaproteobacterium and Desulvovibrio (Deltaproteobacteria) were for 17 m. fOTU 12 (with 85% ITS2 similarity to a sequence from Basidiobolus, phylum: Entomorphthoromycota) was significant for 17 m. None of the depth indicators appeared in the network.
Table 1. Depth indicators identified in this study.
| Phylum/clade | Depth (m) | p Value | |
|---|---|---|---|
| Bacteria | |||
| RSB-22 | Verrucomicrobia | 27 | 0.031 |
| Unclassified α-proteobacterium-III | Alphaproteobacteria | 17 | 0.046 |
| Desulfovibrio | Deltaproteobacteria | 17 | 0.049 |
| Symbiodinium | |||
| C90 | Clade C | 27 | 0.001 |
| C1 | Clade C | 27 | 0.037 |
| Fungi | |||
| fOTU12 | Entomorphthoromycota | 17 | 0.041 |
Note:
Symbiodinium types, fungal OTUs, and bacterial families significantly associated to one of the depth habitats (17 and 27 m).
Discussion
Different coral-associated microbial communities have been characterized with the use of high-throughput sequencing (e.g., Symbiodinium (Thomas et al., 2014; Quigley et al., 2014; Arif et al., 2014; Green et al., 2014; Klepac et al., 2015; Lucas et al., 2016; Cunning, Gates & Edmunds, 2017), bacteria (reviewed in Rosenberg et al. (2007), Ainsworth, Vega-Thurber & Gates (2010), and Bourne, Morrow & Webster (2016)), and fungi (Amend, Barshis & Oliver, 2012)). This study is the first to characterize these subcomponents of the coral microbiome simultaneously. However, our sampling was limited to only a small set of 14 colonies from two depths on one reef and although it is reasonable to assume that light conditions are considerably different between 10 m depth, the data are not supplemented with measurements of environmental parameters to verify this assumption and identify potential other differences between sites.
Siderastrea siderea associated Symbiodinium
Along the depth cline, light intensity decreases exponentially and is a major variable structuring reef diversity composition (Van den Hoek et al., 1978). In addition to overall intensity, longer wavelengths attenuate faster and as a result, the light spectrum narrows with depth. It is therefore not surprising that changes in the dominantly associated Symbiodinium type have been shown to occur over relative short depth ranges (Frade et al., 2008; Bongaerts et al., 2015). Despite the narrow range covered in this study, an exceptional diversity of Symbiodinium was detected in association with S. siderea, though it is not the first work to report high diversity of Symbiodinium associated with S. siderea (Finney et al., 2010; Bongaerts et al., 2015). Contrastingly, other work has indicated limited diversity of Symbiodinium hosted by S. siderea, though the dominant symbiont may differ (Baker, 2001; Warner et al., 2006). Siderastrea congeners have been previously shown to follow a similar reef site driven pattern of low diversity within site, but with differences between sites (Thornhill, Fitt & Schmidt, 2006; Monteiro et al., 2013).
Depth zonation in S. siderea associated Symbiodinium was reported by Bongaerts et al. (2015) where, Symbiodinium D1 was dominant at 2 m depth. For the site sampled in this study, such shallow depths are unattainable and were not included. However, sOTU24, which was classified as D1, was detected at background levels (0.012% average relative abundance) and could potentially have been more abundant at shallower depths. From 2 to 10 m in depth Bongaerts et al. (2015) detected clade B types to be dominant in a minority of sampled colonies and from 5 to 50 m the type C3 was the predominantly detected primary symbiont. Likewise, in this study we identified an unclassified clade B (sOTU3) and clade C (sOTU1) type to be the most abundant Symbiodinium types at 17 and 27 m, respectively. Although the abundance of sOTU1 was by average more than two-fold higher at 27 m compared to 17 m on average, sOTU1 (being the only unclassified clade C type) was not detected as an indicator of 27 m. However, the other clade C OTUs, C90 (sOTUs 34, 45, and 52) and C1 (sOTUs 38 and 53) were indicators of the 27 m depth.
It should be noted that we did not test for a genotype effect and that the detected Symbiodinium zonation depth patterns might reflect the existence of S. siderea genotypes specific associations. Nevertheless, to our knowledge there is no published data indicating that genotypes within coral species living under the same conditions host Symbiodinium symbionts from differing clades while on the other hand depth has been shown to influence coral-associated microbes (hence Bongaerts et al. (2015) but see also Klaus et al. (2007), Frade et al. (2008), Lee et al. (2012), and Glasl et al. (2017)).
In addition to hosting a diverse background community, varying Symbiodinium types from different clades can be the dominant colonizer. Therefore, we posit that Siderastrea have the physiological capacity for broad host flexibility, but suggest that realized flexibility is winnowed by environmental conditions. Stable conditions could select for a community strongly dominated by one type. The observation of high Symbiodinium diversity may in part result from our study being the first to employ high-throughput sequencing of S. siderea associated Symbiodinium, which has the power to detect the presence of rare symbionts. However, many of the S. siderea symbionts are present in sufficient abundance for detection through the previously employed methodologies such as cloning and DGGE. This then suggests that the historically limited sampling effort on this host species and/or a novelty specific to our study site explain the surprising diversity we report.
Symbiodinium type A3, identified as the second most abundant sOTU, is in the Caribbean known for predominance in shallow specialist hosts, including Acropora and branching Porites from 1 to 10 m (LaJeunesse, Loh & Trench, 2009). Clade A types have physiological adaptations to facilitate light tolerance (Banaszak, LaJeunesse & Trench, 2000; Reynolds et al., 2008). Despite this, Finney et al. (2010) also reported an increasing abundance of A3 with increasing depth within Siderastrea radians and Stephanocoenia intersepta hosts. While Siderastrea spp. are recognized to be more efficient at scattering light than Acropora (Marcelino et al., 2013), it seems unlikely that S. siderea from the deeper habitat are able to mimic the light environment of corals more than 10 m shallower. Thus, the A3 identified in deep Siderastrea and Stephanocoenia may represent a cryptic type or phenotypically divergent genotype as elsewhere in the A3 lineage (Parkinson et al., 2015; Pinzon et al., 2011).
To our knowledge, this is only the second study to report high abundance of Symbiodinium clade H from a scleractinian (after Bongaerts et al., 2015) and one of few to report clade G (van Oppen, Mahiny & Done, 2005; Kimes et al., 2013; Thomas et al., 2014). These clades are known to associate with foraminifera and porifera, respectively (Coffroth & Santos, 2005; Stat, Carter & Hoegh-Guldberg, 2006; Pochon, Putnam & Gates, 2014). Both H1 and G2 Symbiodinium types were present in all 14 samples and the H1 OTU was the dominant sOTUs in one of the deep habitat samples. Similarly, Bongaerts et al. (2015), detected H1 as the dominant Symbiodinium type in one Porites astreoides colony. However, further experimental study is required to characterize the nature of these associations.
Siderastrea siderea associated bacteria
The composition of the bacterial community associated to S. siderea was not structured differently between the depths covered in this study, nor did we detect differences in total Shannon diversity. The stability in alpha diversity is in line with previous environmental sequencing studies that sampled coral microbiomes from different depths (Klaus et al., 2007; Lee et al., 2012; Glasl et al., 2017).
The 16S sequence of the most abundant bacterial phylotype (bOTU1) was classified to the SAR116 clade which despite its classification under the Rickettsiales in the SILVA record, is part of the Rhodospirillaceae (Alphaproteobacteria; Rhodospirillales; Grote et al., 2011). Further investigation of this bOTU showed it was 99% similar to 16S sequences from coral holobionts (Lopez et al., 2008; Sunagawa, Woodley & Medina, 2010; Yang et al., 2013), and identical to that of a bacterium which was recently shown to be a calcifying endosymbiont of a sponge, where it lacked a cell wall and was vertically transmitted (Uriz et al., 2012; Garate et al., 2017). Garate et al. (2017) opted the presence of a “calcibacterium” clade within SAR116 mainly including sequences from sponge and coral holobionts. Based on phylogenetic analysis including sequences from Oh et al. (2010) and Garate et al. (2017) as well as all SAR116 sequences in the SILVA record (Fig. S1), bOTU1 was resolved within a highly supported clade within SAR116 that was indeed dominated by sequences from sponges and corals. As discussed in Garate et al. (2017), the presence of bacterial calcite spherules has not yet been investigated in cnidarians but the high abundance of a calcibacterium related bOTU within the S. siderea microbiome and repeated recovery of related sequences from other corals (Lopez et al., 2008; Sunagawa, Woodley & Medina, 2010; Yang et al., 2013), set the stage for further research to explore whether bacterial calcification might occur in corals as well.
The second most abundant bOTU was classified to Synechococcus, a group of cyanobacteria found in other studies as an abundant coral symbiont that fixes nitrogen utilized by Symbiodinium (Lesser et al., 2004, 2007; Carlos, Torres & Ottoboni, 2013; Morrow et al., 2015). Due to the use of different 16S rDNA regions in this study and Lesser et al. (2004), sequences cannot be directly compared. Nevertheless, the high abundance of Synechococcus in S. siderea might suggest a potential contribution of nitrogen fixation within this holobiontic association, similar to that of Montastraea cavernosa and Synechococcus (Lesser et al., 2004). Another high abundant bacterium (bOTU3) was classified to Chloroflexi SAR202. Chloroflexi are not commonly reported in coral microbiomes, however have been detected on a few occasions (Kimes et al., 2013; Lee et al., 2012), and in particular from S. siderea (Cardénas et al., 2012; Kellogg et al., 2014). Finally, the abundant bOTU5 was assigned to the Rickettsiales, and further phylogenetic investigation showed this sequence to cluster with sequences acquired from other Cnidaria (Fraune & Bosch, 2007; Sunagawa, Woodley & Medina, 2010; Kimes et al., 2013) under a fully supported node (see Fig. S2), sister to the vertically transmitted intracellular Candidatus Lariskella (Matsuura et al., 2012) and Candidatus Midichloria, a candidate genus of ecologically wide spread intracellular symbionts originally isolated from Acanthamoeba (Montagna et al., 2013).
Siderastrea siderea associated fungi
Although fungi have been studied primarily in terrestrial ecosystems, it is known that their presence in the coral holobiont is ubiquitous (Kendrick et al., 1982; Le Campion-Alsumard, Golubic & Priess, 1995; reviewed in Yarden (2014) and Raghukumar (2012)). The ecology of the coral-associated fungi, however, is poorly understood (Golubic, Radtke & Le Campion-Alsumard, 2005). While fungi within the skeleton (or endolithic fungi) may invade coral tissue during times of stress (Yarden, 2014), fungi have also been found to parasitize on endolithic algae (Le Campion-Alsumard, Golubic & Priess, 1995).
The overall low ITS2 similarity of fOTU sequences to GenBank (91.0 ± SD 8.2% average similarity) corroborates the presence of a high novel diversity associated with S. siderea’s microbiome. This agrees with Amend, Barshis & Oliver (2012), where Acropora hyacinthus associated fungi had an average similarity of 97% to sequences from GenBank, using the more conserved large ribosomal subunit (LSU) rDNA marker. A notable pattern within the fungal community was the dominance of the Lulworthiaceae related fOTU1 as the most abundant in nine of the 14 samples. In each of the remaining samples, different fungi (fOTUs 2–6) occupied this position.
Amend, Barshis & Oliver (2012) did not detect differences between fungal communities of A. hyacinthus from sites on the same reef that differed by 1.5 °C. Over this range the dominant Symbiodinium type shifted. Similarly, in this study where samples were collected from the same reef but with difference in depth instead of temperature, the dominant Symbiodinium type shifted (from the unclassified C type to a combination of B types) while such a pattern was not detected for fungal communities.
In Amend, Barshis & Oliver (2012) a core set of 11 OTUs was identified, present in >90% of the samples. Notably, 21 fOTUs in our study were detected above this threshold. Due to the use of different markers, sequences of these core-members cannot be compared between studies. However, Hortaea werneckii and Lindra sp., highlighted in their study, are related to core fOTUs from S. siderea. Among the well-classified fOTUs, fOTU35 is highly similar to H. werneckii (99%), present in 13 of the 14 samples. Lindra belongs to the Lulworthiales, of which 7 fOTUs (4 in more than 90% of the samples) yielded closest similarity hits including the most abundant fOTU1 and fOTU2. The Lulworthiales is an order of marine fungi, predominantly isolated from algae or detritus (Kohlmeyer, Spatafora & Volkmann-Kohlmeyer, 2000). Given the low similarity, the sequences acquired from S. siderea likely reflect one or more novel groups within or related to this order.
The Internal transcribed spacer region has become the standard DNA marker in fungal environmental sequencing assays (Abarenkov et al., 2010). Despite this, our data emphasize that coral-associated fungal ITS sequences are underrepresented in sequence databases, resulting in poor classification of detected OTUs. This problem was also encountered by Wainwright et al. (2017) and corroborates that marine fungi are poorly studied with respect to their terrestrial relatives (Richards et al., 2012; Peay, Kennedy & Talbot, 2016). For future attempts to characterize fungal diversity on coral reefs more conserved markers such as the SSU or LSU rRNA might be recommendable. However, the overall high degree of detected fungal novelty warrants a need for fungal isolation efforts from corals to fill the gaps in the sequence databases. Finally, the fungal-specific primers from Nikolcheva & Bärlocher (2004) used in this study failed to prevent amplification of non-fungal sequences, a problem also encountered by Amend, Barshis & Oliver (2012) on the LSU marker.
Inferences from network analysis
The network of the combined Symbiodinium, bacterial, and fOTU tables resolved predominantly co-occurrence patterns involving bacteria. However, two co-occurrences were observed between bacteria and fungi. Co-occurrence of Symbiodinium A3 and an unclassified type A likely indicates either intragenomic variability within the A3 type or niche-overlap between closely related types. The network contained one sub-community, or module, consisting of six bacterial groups and connected by three negative correlations with a group of Planctomycetes of the class Phycisphaerae. The central node, having three positive correlations and one negative included bOTUs classified in the SILVA database to the unrecognized phylum PAUC34f. This enigmatic group of bacteria has not yet been cultured and sequences in the SILVA database are solely from metagenomic studies (Quast et al., 2013).
A noteworthy member of this node is the Chloroflexi SAR202 clade, as this includes the third most abundant bOTU. The node classified as Subgroup 11 of the Acidobacteria was connected to the PAUC34f node. Acidobacteria are specialized aerobic oligotrophs (Kielak et al., 2016) obtaining energy from denitrification and known to engage in syntrophic associations with different proteobacteria (Spring et al., 2000; Meisinger et al., 2007). Proteobacteria in the module were most similar to the genus Nitrosococcus and the family Nitrospinaceae, both of which are groups of nitrifying bacteria (Kowalchuk & Stephen, 2001; Lücker & Daims, 2014). Although little is known about the majority of organisms from this module, its members have distinctive metabolisms and it seems unlikely that the co-occurrence patterns are solely a result of niche-overlap.
Conclusion
In summary, the investigation of inter-domain microbial communities of S. siderea colonies from 17 ± 1 to 27 ± 1 m depth habitats at Conch reef revealed an exceptional diversity of Symbiodinium types, including types from seven of the nine currently recognized “clades” (Pochon, Putnam & Gates, 2014). While colonies at 27 m were dominated by an unclassified clade C type, colonies at 17 m were predominantly colonized by a mixture of clade B types. In contrast, the bacterial and fungal community compositions were not structurally different between the depth habitats, however, species indicator analysis revealed the presence of depth habitat-specific taxa. Further, we constructed a network to identify correlations within the microbiome, which reflected co-occurrence is most prevalent between bacterial groups, including a variety of taxa. Overall, our data show that the Symbiodinium community associated with S. siderea can be shifted drastically between different depth habitats within the same reef, which may indicate that alteration in this community is an important mechanism to physiologically adapt to local conditions. This work further presents evidence of a diverse fungal community associated with S. siderea, containing a high degree of novelty. It also emphasizes the need for future study on the diversity and functional roles of fungi within the coral holobiont and on the reefs in general.
Supplemental Information
Maximum likelihood phylogeny with the partial 16S rRNA gene showing the phylogenetic relation between bOTU1, the top BLAST hits and all SAR116 bacteria in the SILVA reference alignment, with the exception of KJ589655, HQ673528 and KF271096 which clustered outside of the SAR116 clade. SAR116 bacterial sequences acquired from sponges or corals are indicated in bold and with stars. All other SAR116 sequences are isolated from seawater. The optimal model was selected based on the AIC criterion and found to be GTR+G+I. The maximum likelihood analysis was conducted with RAxML, using a 1000 bootstrap iterations and contained 1,344 nucleotide positions (449 for bOTU1) with Rhizobium leguminosarum (ATCC 14480) as outgroup.
Maximum likelihood phylogeny with the partial 16S rRNA gene showing the phylogenetic relation between bOTU3 and clonal sequences from corals, Candidatus Lariskella, Candidatus Midichloria and other Rickettsiales genera. The optimal model (GTR+I+G) was selected based on the AIC criterion. The maximum likelihood analysis was conducted with RAxML, using a 1,000 bootstrap iterations and contained 1,176 nucleotide positions (452 for bOTU1) with Rhizobium leguminosarum (ATCC 14480) as outgroup.
Acknowledgments
We are grateful to the Astronauts Luca Parmitano (ESA; European Space Agency), Serena Auñón (NASA), Norishige Kanai (JAXA; Japanese Aerospace Exploration Agency), and NASA Aquanaut David Coan for conducting the sample collections used in this study during the NASA Extreme Environment Mission Operations (NEEMO 20). We would also like to thank the Mission Directors Marc Reagan and Barbara Janoiko; and the entire NEEMO 20 team for their support and assistance during the coordination and planning of the marine science activities during the mission. We further would like to thank the Operations Director, Roger Garcia and the entire crew of the FIU Aquarius Reef Base for supporting and facilitating the diving and boating operations. We also express our thanks to Tanya Brown, Ellen Dow and Cindy Lewis from the IMaGeS Lab at FIU for assistance during the fieldwork. This is contribution #21 from the Marine Education and Research Center in the Institute for Water and Environment at Florida International University.
Funding Statement
This work was funded by the NEEMO mission and by a Florida International University start-up grant awarded to Mauricio Rodriguez-Lanetty. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Mauricio Rodriguez-Lanetty is an Academic Editor for PeerJ. Trevor Graff and William Todd are employees of NASA Johnson Space Center.
Author Contributions
Guido Bonthond conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Daniel G. Merselis conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Katherine E. Dougan conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Trevor Graff performed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
William Todd performed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
James W. Fourqurean contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Mauricio Rodriguez-Lanetty conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries Program (ONMS): FKNMS-2015-076-A2.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The sequence reads generated in this study have been deposited in the SRA database under the accession number PRJNA356144.
Data Availability
The following information was supplied regarding data availability:
The DNA data was deposited in NCBI database: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA356144/
References
- Abarenkov et al. (2010).Abarenkov K, Nilsson RH, Larsson K, Alexander IJ, Eberhardt U, Erland S, Hoiland K, Kjoller R, Larsson E, Pennanen T, Sen R, Taylor AFS, Tedersoo L, Ursing BM, Vralstad T, Liimatainen K, Peintner U, Koljalg U. The UNITE database for molecular identification of fungi—recent updates and future perspectives. New Phytologist. 2010;186(2):281–285. doi: 10.1111/j.1469-8137.2009.03160.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth et al. (2015).Ainsworth TD, Krause L, Bridge T, Torda G, Raina J, Zakrzewski M, Gates RD, Padilla-Gamino JL, Spalding HL, Smith C, Woolsey ES, Bourne DG, Bongaerts P, Hoegh-Guldberg O, Leggat W. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME Journal. 2015;9(10):2261–2274. doi: 10.1038/ismej.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth, Vega-Thurber & Gates (2010).Ainsworth TD, Vega-Thurber R, Gates RD. The future of coral reefs: a microbial perspective. Trends in Ecology & Evolution. 2010;25(4):233–240. doi: 10.1016/j.tree.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Altschul et al. (1990).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amend, Barshis & Oliver (2012).Amend AS, Barshis DJ, Oliver TA. Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME Journal. 2012;6(7):1291–1301. doi: 10.1038/ismej.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif et al. (2014).Arif C, Daniels C, Bayer T, Banguera-Hinestroza E, Barbrook A, Howe CJ, Lajeunesse TC, Voolstra CR. Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Molecular Ecology. 2014;23(17):4418–4433. doi: 10.1111/mec.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird et al. (2007).Baird AH, Cumbo VR, Leggat W, Rodriguez-Lanetty M. Fidelity and flexibility in coral symbioses. Marine Ecology Progress Series. 2007;347:307–309. doi: 10.3354/meps07220. [DOI] [Google Scholar]
- Baker (2001).Baker AC. Ecosystems: reef corals bleach to survive change. Nature. 2001;411(6839):765–766. doi: 10.1038/35081151. [DOI] [PubMed] [Google Scholar]
- Baker (2003).Baker AC. Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annual Review of Ecology, Evolution, and Systematics. 2003;34(1):661–689. doi: 10.1146/annurev.ecolsys.34.011802.132417. [DOI] [Google Scholar]
- Banaszak, LaJeunesse & Trench (2000).Banaszak A, LaJeunesse T, Trench R. The synthesis of mycosporine-like amino acids (MAAs) by cultured, symbiotic dinoflagellates. Journal of Experimental Marine Biology and Ecology. 2000;249(2):219–233. doi: 10.1016/S0022-0981(00)00192-1. [DOI] [PubMed] [Google Scholar]
- Banks & Foster (2017).Banks S, Foster K. Baseline levels of Siderastrea siderea bleaching under normal environmental conditions in Little Cayman. Open Journal of Marine Science. 2017;7(1):142–154. doi: 10.4236/ojms.2017.71011. [DOI] [Google Scholar]
- Bastian, Heymann & Jacomy (2009).Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media. 2009;8:361–362. [Google Scholar]
- Bayer et al. (2013a).Bayer T, Arif C, Ferrier-Pages C, Zoccola D, Aranda M, Voolstra CR. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Marine Ecology Progress Series. 2013a;479:75–84. doi: 10.3354/meps10197. [DOI] [Google Scholar]
- Bayer et al. (2013b).Bayer T, Neave MJ, Alsheikh-Hussain A, Aranda M, Yum LK, Mincer T, Hughen K, Apprill A, Voolstra CR. The microbiome of the Red Sea coral Stylophora pistillata is dominated by tissue-associated Endozoicomonas Bacteria. Applied and Environmental Microbiology. 2013b;79(15):4759–4762. doi: 10.1128/AEM.00695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini & Hochberg (1995).Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Blondel et al. (2008).Blondel VD, Guillaume J, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. Journal of Statistical Mechanics: Theory and Experiment. 2008;2008(10):P10008. doi: 10.1088/1742-5468/2008/10/p10008. [DOI] [Google Scholar]
- Bokulich et al. (2013).Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature Methods. 2013;10(1):57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaerts et al. (2015).Bongaerts P, Carmichael M, Hay KB, Tonk L, Frade PR, Hoegh-Guldberg O. Prevalent endosymbiont zonation shapes the depth distributions of scleractinian coral species. Royal Society Open Science. 2015;2(2):140297. doi: 10.1098/rsos.140297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne, Morrow & Webster (2016).Bourne DG, Morrow KM, Webster NS. Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annual Review of Microbiology. 2016;70(1):317–340. doi: 10.1146/annurev-micro-102215-095440. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2017).Brown T, Otero C, Grajales A, Rodriguez E, Rodriguez-Lanetty M. Worldwide exploration of the microbiome harbored by the cnidarian model, Exaiptasia pallida (Agassiz in Verrill, 1864) indicates a lack of bacterial association specificity at a lower taxonomic rank. PeerJ. 2017;5:e3235. doi: 10.7717/peerj.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddenmeier & Fautin (1993).Buddenmeier RW, Fautin DG. Coral bleaching as an adaptive mechanism—a testable hypothesis. Bioscience. 1993;43(5):320–326. doi: 10.2307/1312064. [DOI] [Google Scholar]
- Caceres & Legendre (2009).Caceres MD, Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90(12):3566–3574. doi: 10.1890/08-1823.1. [DOI] [PubMed] [Google Scholar]
- Cardénas et al. (2012).Cardénas A, Rodriguez-R LM, Pizarro V, Cadavid LF, Arevalo-Ferro C. Shifts in bacterial communities of two Caribbean reef-building coral species affected by white plague disease. ISME Journal. 2012;6(3):502–512. doi: 10.1038/ismej.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos, Torres & Ottoboni (2013).Carlos C, Torres TT, Ottoboni LMM. Bacterial communities and species-specific associations with the mucus of Brazilian coral species. Scientific Reports. 2013;3(1):1624. doi: 10.1038/srep01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo et al. (2014).Castillo KD, Ries JB, Bruno JF, Westfield IT. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1797):20141856. doi: 10.1098/rspb.2014.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffroth & Santos (2005).Coffroth MA, Santos SR. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist. 2005;156(1):19–34. doi: 10.1016/j.protis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Colella et al. (2012).Colella MA, Ruzicka RR, Kidney JA, Morrison JM, Brinkhuis VB. Cold-water event of January 2010 results in catastrophic benthic mortality on patch reefs in the Florida Keys. Coral Reefs. 2012;31(2):621–632. doi: 10.1007/s00338-012-0880-5. [DOI] [Google Scholar]
- Cunning, Gates & Edmunds (2017).Cunning R, Gates R, Edmunds P. Using high-throughput sequencing of ITS2 to describe Symbiodinium metacommunities in St. John, US Virgin Islands. PeerJ. 2017;5:e3472. doi: 10.7717/peerj.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy, Allemand & Weis (2012).Davy SK, Allemand D, Weis VM. Cell biology of cnidarian–dinoflagellate symbiosis. Microbiology and Molecular Biology Reviews. 2012;76(2):229–261. doi: 10.1128/MMBR.05014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis et al. (2006).DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domart-Coulon et al. (2004).Domart-Coulon IJ, Sinclair CS, Hill RT, Tambutte S, Puverel S, Ostrander GK. A basidiomycete isolated from the skeleton of Pocillopora damicornis (Scleractinia) selectively stimulates short-term survival of coral skeletogenic cells. Marine Biology. 2004;144(3):583–592. doi: 10.1007/s00227-003-1227-0. [DOI] [Google Scholar]
- Finney et al. (2010).Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, LaJeunesse TC. The relative significance of host-habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microbial Ecology. 2010;60(1):250–263. doi: 10.1007/s00248-010-9681-y. [DOI] [PubMed] [Google Scholar]
- Frade et al. (2008).Frade P, De Jongh F, Vermeulen F, Van Bleijswijk J, Bak R. Variation in symbiont distribution between closely related coral species over large depth ranges. Molecular Ecology. 2008;17(2):691–703. doi: 10.1111/j.1365-294X.2007.03612.x. [DOI] [PubMed] [Google Scholar]
- Fraune & Bosch (2007).Fraune S, Bosch TCG. Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(32):13146–13151. doi: 10.1073/pnas.0703375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garate et al. (2017).Garate L, Sureda J, Agell G, Uriz MJ. Endosymbiotic calcifying bacteria across sponge species and oceans. Scientific Reports. 2017;7:43674. doi: 10.1038/srep43674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner et al. (2003).Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301(5635):958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- Glasl et al. (2017).Glasl B, Bongaerts P, Elisabeth NH, Hoegh-Guldberg O, Herndl GJ, Frade PR. Microbiome variation in corals with distinct depth distribution ranges across a shallow–mesophotic gradient (15–85 m) Coral Reefs. 2017;36(2):447–452. doi: 10.1007/s00338-016-1517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubic, Radtke & Le Campion-Alsumard (2005).Golubic S, Radtke G, Le Campion-Alsumard T. Endolithic fungi in marine ecosystems. Trends in Microbiology. 2005;13(5):229–235. doi: 10.1016/j.tim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Green et al. (2014).Green EA, Davies SW, Matz MV, Medina M. Quantifying cryptic Symbiodinium diversity within Orbicella faveolata and Orbicella franksi at the Flower Garden Banks, Gulf of Mexico. PeerJ. 2014;2:e386. doi: 10.7717/peerj.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote et al. (2011).Grote J, Bayindirli C, Bergauer K, de Moraes PC, Chen H, D’Ambrosio L, Edwards B, Fernández-Gómez B, Hamisi M, Logares R. Draft genome sequence of strain HIMB100, a cultured representative of the SAR116 clade of marine Alphaproteobacteria. Standards in Genomic Sciences. 2011;5(3):269–278. doi: 10.4056/sigs.1854551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Agreda et al. (2016).Hernandez-Agreda A, Leggat W, Bongaerts P, Ainsworth TD. The microbial signature provides insight into the mechanistic basis of coral success across reef habitats. mBio. 2016;7(4):e00560-16. doi: 10.1128/mBio.00560-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg et al. (2014).Kellogg CA, Piceno YM, Tom LM, DeSantis TZ, Gray MA, Andersen GL. Comparing bacterial community composition of healthy and dark spot-affected Siderastrea siderea in Florida and the Caribbean. PLOS ONE. 2014;9(10):e108767. doi: 10.1371/journal.pone.0108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick et al. (1982).Kendrick B, Risk MJ, Michaelides J, Bergman K. Amphibious microborers—bio-eroding fungi isolated from live corals. Bulletin of Marine Science. 1982;32:862–867. [Google Scholar]
- Kielak et al. (2016).Kielak AM, Barreto CC, Kowalchuk GA, van Veen JA, Kuramae EE. The ecology of Acidobacteria: moving beyond genes and genomes. Frontiers in Microbiology. 2016;7:744. doi: 10.3389/fmicb.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimes et al. (2013).Kimes NE, Johnson WR, Torralba M, Nelson KE, Weil E, Morris PJ. The Montastraea faveolata microbiome: ecological and temporal influences on a Caribbean reef-building coral in decline. Environmental Microbiology. 2013;15(7):2082–2094. doi: 10.1111/1462-2920.12130. [DOI] [PubMed] [Google Scholar]
- Klaus et al. (2007).Klaus JS, Janse I, Heikoop JM, Sanford RA, Fouke BW. Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environmental Microbiology. 2007;9(5):1291–1305. doi: 10.1111/j.1462-2920.2007.01249.x. [DOI] [PubMed] [Google Scholar]
- Klepac et al. (2015).Klepac CN, Beal J, Kenkel CD, Sproles A, Polinski JM, Williams MA, Matz MV, Voss JD. Seasonal stability of coral-Symbiodinium associations in the subtropical coral habitat of St. Lucie Reef, Florida. Marine Ecology Progress Series. 2015;532:137–151. doi: 10.3354/meps11369. [DOI] [Google Scholar]
- Knowlton & Rohwer (2003).Knowlton N, Rohwer F. Multispecies microbial mutualisms on coral reefs: the host as a habitat. American Naturalist. 2003;162(S4):S51–S62. doi: 10.1086/378684. [DOI] [PubMed] [Google Scholar]
- Kohlmeyer, Spatafora & Volkmann-Kohlmeyer (2000).Kohlmeyer J, Spatafora JW, Volkmann-Kohlmeyer B. Lulworthiales, a new order of marine Ascomycota. Mycologia. 2000;92(3):453–458. doi: 10.2307/3761504. [DOI] [Google Scholar]
- Kowalchuk & Stephen (2001).Kowalchuk G, Stephen J. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annual Review of Microbiology. 2001;55(1):485–529. doi: 10.1146/annurev.micro.55.1.485. [DOI] [PubMed] [Google Scholar]
- Kozich et al. (2013).Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and Environmental Microbiology. 2013;79(17):5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse (2001).LaJeunesse TC. Investigating the biodiversity, ecology and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a ‘species’ level marker. Journal of Phycology. 2001;37(5):866–880. doi: 10.1046/j.1529-8817.2001.01031.x. [DOI] [Google Scholar]
- LaJeunesse, Loh & Trench (2009).LaJeunesse TC, Loh W, Trench RK. Do introduced endosymbiotic dinoflagellates ‘take’ to new hosts? Biological Invasions. 2009;11(4):995–1003. doi: 10.1007/s10530-008-9311-5. [DOI] [Google Scholar]
- Le Campion-Alsumard, Golubic & Priess (1995).Le Campion-Alsumard T, Golubic S, Priess K. Fungi in corals: symbiosis or disease? Interaction between polyps and fungi causes pearl-like skeleton biomineralization. Marine Ecology Progress Series. 1995;117:137–147. doi: 10.3354/meps117137. [DOI] [Google Scholar]
- Lee et al. (2012).Lee OO, Yang J, Bougouffa S, Wang Y, Batang Z, Tian R, Al-Suwailem A, Qian P. Spatial and species variations in bacterial communities associated with corals from the Red Sea as revealed by pyrosequencing. Applied and Environmental Microbiology. 2012;78(20):7173–7184. doi: 10.1128/AEM.01111-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema, Willis & Bourne (2012).Lema KA, Willis BL, Bourne DG. Corals form characteristic associations with symbiotic nitrogen-fixing bacteria. Applied and Environmental Microbiology. 2012;78(9):3136–3144. doi: 10.1128/AEM.07800-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser et al. (2007).Lesser MP, Falcon LI, Rodriguez-Roman A, Enriquez S, Hoegh-Guldberg O, Iglesias-Prieto R. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Marine Ecology Progress Series. 2007;346:143–152. doi: 10.3354/meps07008. [DOI] [Google Scholar]
- Lesser et al. (2004).Lesser MP, Mazel CH, Gorbunov MY, Falkowski PG. Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science. 2004;305(5686):997–1000. doi: 10.1126/science.1099128. [DOI] [PubMed] [Google Scholar]
- Lesser, Slattery & Leichter (2009).Lesser MP, Slattery M, Leichter JJ. Ecology of mesophotic coral reefs. Journal of Experimental Marine Biology and Ecology. 2009;375(1–2):1–8. doi: 10.1016/j.jembe.2009.05.009. [DOI] [Google Scholar]
- Lirman & Fong (2007).Lirman D, Fong P. Is proximity to land-based sources of coral stressors an appropriate measure of risk to coral reefs? An example from the Florida Reef Tract. Marine Pollution Bulletin. 2007;54(6):779–791. doi: 10.1016/j.marpolbul.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Littman, Willis & Bourne (2011).Littman R, Willis BL, Bourne DG. Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environmental Microbiology Reports. 2011;3(6):651–660. doi: 10.1111/j.1758-2229.2010.00234.x. [DOI] [PubMed] [Google Scholar]
- Lopez et al. (2008).Lopez JV, Ranzer L, Ledger A, Schoch B, Duckworth A, McCarthy P, Kerr R. Comparison of bacterial diversity within the coral reef sponge, Axinella corrugata, and the encrusting coral Erythropodium caribaeorum. Proceedings of the 11th International Coral Reef Symposium; 2008. p. 1362. [Google Scholar]
- Lucas et al. (2016).Lucas MQ, Stat M, Smith MC, Weil E, Schizas NV. Symbiodinium (internal transcribed spacer 2) diversity in the coral host Agaricia lamarcki (Cnidaria: Scleractinia) between shallow and mesophotic reefs in the Northern Caribbean (20–70 m) Marine Ecology. 2016;37(5):1079–1087. doi: 10.1111/maec.12367. [DOI] [Google Scholar]
- Lücker & Daims (2014).Lücker S, Daims H. The family Nitrospinaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes. Heidelberg: Springer; 2014. pp. 231–237. [Google Scholar]
- Marcelino et al. (2013).Marcelino LA, Westneat MW, Stoyneva V, Henss J, Rogers JD, Radosevich A, Turzhitsky V, Siple M, Fang A, Swain TD, Fung J, Backman V. Modulation of light-enhancement to symbiotic algae by light-scattering in corals and evolutionary trends in bleaching. PLOS ONE. 2013;8(4):e61492. doi: 10.1371/journal.pone.0061492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura et al. (2012).Matsuura Y, Kikuchi Y, Meng XY, Koga R, Fukatsu T. Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Applied and Environmental Microbiology. 2012;78(12):4149–4156. doi: 10.1128/AEM.00673-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger et al. (2007).Meisinger DB, Zimmermann J, Ludwig W, Schleifer K, Wanner G, Schmid M, Bennett PC, Engel AS, Lee NM. In situ detection of novel Acidobacteria in microbial mats from a chemolithoautotrophically based cave ecosystem (Lower Kane Cave, WY, USA) Environmental Microbiology. 2007;9(6):1523–1534. doi: 10.1111/j.1462-2920.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- Miller, Pfeiffer & Schwartz (2012).Miller MA, Pfeiffer W, Schwartz T. The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources. Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the eXtreme to the campus and beyond, Chicago, IL, USA; New York: AMC; 2012. p. 39. [Google Scholar]
- Montagna et al. (2013).Montagna M, Sassera D, Epis S, Bazzocchi C, Vannini C, Lo N, Sacchi L, Fukatsu T, Petroni G, Bandi C. “Candidatus Midichloriaceae” fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular Alphaproteobacteria. Applied and Environmental Microbiology. 2013;79(10):3241–3248. doi: 10.1128/AEM.03971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro et al. (2013).Monteiro JG, Costa CF, Gorlach-Lira K, Fitt WK, Stefanni SS, Sassi R, Santos RS, LaJeunesse TC. Ecological and biogeographic implications of Siderastrea symbiotic relationship with Symbiodinium sp C46 in Sal Island (Cape Verde, East Atlantic Ocean) Marine Biodiversity. 2013;43(4):261–272. doi: 10.1007/s12526-013-0153-8. [DOI] [Google Scholar]
- Morrow et al. (2015).Morrow KM, Bourne DG, Humphrey C, Botte ES, Laffy P, Zaneveld J, Uthicke S, Fabricius KE, Webster NS. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME Journal. 2015;9(4):894–908. doi: 10.1038/ismej.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatine & Porter (1977).Muscatine L, Porter JW. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience. 1977;27(7):454–460. doi: 10.2307/1297526. [DOI] [Google Scholar]
- Newman (2006).Newman MEJ. Modularity and community structure in networks. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(23):8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolcheva & Bärlocher (2004).Nikolcheva LG, Bärlocher F. Taxon-specific fungal primers reveal unexpectedly high diversity during leaf decomposition in a stream. Mycological Progress. 2004;3(1):41–49. doi: 10.1007/s11557-006-0075-y. [DOI] [Google Scholar]
- Nylander (2004).Nylander J. MrModeltest v2. Sweden: Evolutionary Biology Centre, Uppsala University; 2004. Program distributed by the author. [Google Scholar]
- Oh et al. (2010).Oh HM, Kwon KK, Kang I, Kang SG, Lee JH, Kim SJ, Cho JC. Complete genome sequence of “Candidatus Puniceispirillum marinum” IMCC1322, a representative of the SAR116 clade in the Alphaproteobacteria. Journal of Bacteriology. 2010;192(12):3240–3241. doi: 10.1128/JB.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson et al. (2009).Olson ND, Ainsworth TD, Gates RD, Takabayashi M. Diazotrophic bacteria associated with Hawaiian Montipora corals: diversity and abundance in correlation with symbiotic dinoflagellates. Journal of Experimental Marine Biology and Ecology. 2009;371(2):140–146. doi: 10.1016/j.jembe.2009.01.012. [DOI] [Google Scholar]
- Parkinson et al. (2015).Parkinson JE, Banaszak AT, Altman NS, LaJeunesse TC, Baums IB. Intraspecific diversity among partners drives functional variation in coral symbioses. Scientific Reports. 2015;5(1):15667. doi: 10.1038/srep15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson et al. (2013).Paulson JN, Stine CO, Corrada Bravo H, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nature Methods. 2013;10(12):1200–1202. doi: 10.1038/nmeth.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peay, Kennedy & Talbot (2016).Peay KG, Kennedy PG, Talbot JM. Dimensions of biodiversity in the Earth mycobiome. Nature Reviews Microbiology. 2016;14(7):434–447. doi: 10.1038/nrmicro.2016.59. [DOI] [PubMed] [Google Scholar]
- Pinzon et al. (2011).Pinzon JH, Devlin-Durante MK, Weber MX, Baums IB, LaJeunesse TC. Microsatellite loci for Symbiodinium A3 (S. fitti) a common algal symbiont among Caribbean Acropora (stony corals) and Indo-Pacific giant clams (Tridacna) Conservation Genetics Resources. 2011;3(1):45–47. doi: 10.1007/s12686-010-9283-5. [DOI] [Google Scholar]
- Pochon, Putnam & Gates (2014).Pochon X, Putnam HM, Gates RD. Multi-gene analysis of Symbiodinium dinoflagellates: a perspective on rarity, symbiosis, and evolution. PeerJ. 2014;2:e394. doi: 10.7717/peerj.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast et al. (2013).Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research. 2013;41(D1):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley et al. (2014).Quigley KM, Davies SW, Kenkel CD, Willis BL, Matz MV, Bay LK. Deep-sequencing method for quantifying background abundances of Symbiodinium types: exploring the rare Symbiodinium biosphere in reef-building corals. PLOS ONE. 2014;9(4):e94297. doi: 10.1371/journal.pone.0094297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghukumar (2012).Raghukumar C. Biology of Marine Fungi. Berlin: Springer-Verlag Berlin Heidelberg; 2012. [Google Scholar]
- Raina et al. (2009).Raina J, Tapiolas D, Willis BL, Bourne DG. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Applied and Environmental Microbiology. 2009;75(11):3492–3501. doi: 10.1128/AEM.02567-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef et al. (2006).Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E. The coral probiotic hypothesis. Environmental Microbiology. 2006;8(12):2068–2073. doi: 10.1111/j.1462-2920.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- Reynolds et al. (2008).Reynolds JM, Bruns BU, Fitt WK, Schmidt GW. Enhanced photoprotection pathways in symbiotic dinoflagellates of shallow-water corals and other cnidarians. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(36):13674–13678. doi: 10.1073/pnas.0805187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards et al. (2012).Richards TA, Jones MD, Leonard G, Bass D. Marine fungi: their ecology and molecular diversity. Annual Review of Marine Science. 2012;4(1):495–522. doi: 10.1146/annurev-marine-120710-100802. [DOI] [PubMed] [Google Scholar]
- Ritchie (2006).Ritchie KB. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Marine Ecology Progress Series. 2006;322:1–14. doi: 10.3354/meps322001. [DOI] [Google Scholar]
- Rodriguez-Lanetty (2003).Rodriguez-Lanetty M. Evolving lineages of Symbiodinium-like dinoflagellates based on ITS1 rDNA. Molecular Phylogenetics and Evolution. 2003;28(1):152–168. doi: 10.1016/S1055-7903(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lanetty et al. (2013).Rodriguez-Lanetty M, Granados-Cifuentes C, Barberan A, Bellantuono AJ, Bastidas C. Ecological inferences from a deep screening of the complex bacterial consortia associated with the coral, Porites astreoides. Molecular Ecology. 2013;22(16):4349–4362. doi: 10.1111/mec.12392. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lanetty & Hoegh-Guldberg (2003).Rodriguez-Lanetty M, Hoegh-Guldberg O. Symbiont diversity within the widespread scleractinian coral Plesiastrea versipora, across the north western Pacific. Marine Biology. 2003;143(3):501–509. doi: 10.1007/s00227-003-1105-9. [DOI] [Google Scholar]
- Rodriguez-Lanetty et al. (2001).Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O. Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Marine Biology. 2001;138(6):1175–1181. doi: 10.1007/s002270100536. [DOI] [Google Scholar]
- Rohwer et al. (2001).Rohwer F, Breitbart M, Jara J, Azam F, Knowlton N. Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs. 2001;20(1):85–91. doi: 10.1007/s003380100138. [DOI] [Google Scholar]
- Rosenberg et al. (2007).Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nature Reviews Microbiology. 2007;5(5):355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- Santos, Gutierrez-Rodriguez & Coffroth (2003).Santos SR, Gutierrez-Rodriguez C, Coffroth MA. Phylogenetic identification of symbiotic dinoflagellates via length heteroplasmy in domain V of chloroplast large subunit (cp23S)—ribosomal DNA sequences. Marine Biotechnology. 2003;5(2):130–140. doi: 10.1007/s10126-002-0076-9. [DOI] [PubMed] [Google Scholar]
- Schloss (2010).Schloss PD. The effects of alignment quality, distance calculation method, sequence filtering, and region on the analysis of 16S rRNA gene-based studies. PLOS Computational Biology. 2010;6(7):e1000844. doi: 10.1371/journal.pcbi.1000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss et al. (2009).Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch et al. (2012).Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffer, Zaneveld & Vega-Thurber (2015).Soffer N, Zaneveld J, Vega-Thurber R. Phage–bacteria network analysis and its implication for the understanding of coral disease. Environmental Microbiology. 2015;17(4):1203–1218. doi: 10.1111/1462-2920.12553. [DOI] [PubMed] [Google Scholar]
- Spring et al. (2000).Spring S, Schulze R, Overmann J, Schleifer K. Identification and characterization of ecologically significant prokaryotes in the sediment of freshwater lakes: molecular and cultivation studies. FEMS Microbiology Reviews. 2000;24(5):573–590. doi: 10.1111/j.1574-6976.2000.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stat, Carter & Hoegh-Guldberg (2006).Stat M, Carter D, Hoegh-Guldberg O. The evolutionary history of Symbiodinium and scleractinian hosts—symbiosis, diversity, and the effect of climate change. Perspectives in Plant Ecology Evolution and Systematics. 2006;8(1):23–43. doi: 10.1016/j.ppees.2006.04.001. [DOI] [Google Scholar]
- Sunagawa, Woodley & Medina (2010).Sunagawa S, Woodley CM, Medina M. Threatened corals provide underexplored microbial habitats. PLOS ONE. 2010;5(3):e9554. doi: 10.1371/journal.pone.0009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas et al. (2014).Thomas L, Kendrick GA, Kennington WJ, Richards ZT, Stat M. Exploring Symbiodinium diversity and host specificity in Acropora corals from geographical extremes of Western Australia with 454 amplicon pyrosequencing. Molecular Ecology. 2014;23(12):3113–3126. doi: 10.1111/mec.12801. [DOI] [PubMed] [Google Scholar]
- Thornhill, Fitt & Schmidt (2006).Thornhill DJ, Fitt WK, Schmidt GW. Highly stable symbioses among western Atlantic brooding corals. Coral Reefs. 2006;25(4):515–519. doi: 10.1007/s00338-006-0157-y. [DOI] [Google Scholar]
- Turner et al. (1999).Turner S, Pryer KM, Miao VP, Palmer JD. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. Journal of Eukaryotic Microbiology. 1999;46(4):327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- Uriz et al. (2012).Uriz MJ, Agell G, Blanquer A, Turon X, Casamayor EO. Endosymbiotic calcifying bacteria: a new cue to the origin of calcification in metazoa? Evolution. 2012;66(10):2993–2999. doi: 10.1111/j.1558-5646.2012.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoek et al. (1978).Van den Hoek C, Breeman A, Bak R, Van Buurt G. The distribution of algae, corals and gorgonians in relation to depth, light attenuation, water movement and grazing pressure in the fringing coral reef of Curaçao, Netherlands Antilles. Aquatic Botany. 1978;5:1–46. doi: 10.1016/0304-3770(78)90045-1. [DOI] [Google Scholar]
- van Hooidonk et al. (2014).Van Hooidonk R, Maynard JA, Manzello D, Planes S. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Global Change Biology. 2014;20(1):103–112. doi: 10.1111/gcb.12394. [DOI] [PubMed] [Google Scholar]
- van Oppen, Mahiny & Done (2005).Van Oppen MJH, Mahiny A, Done T. Geographic distribution of zooxanthella types in three coral species on the Great Barrier Reef sampled after the 2002 bleaching event. Coral Reefs. 2005;24(3):482–487. doi: 10.1007/s00338-005-0487-1. [DOI] [Google Scholar]
- Vega-Thurber et al. (2009).Vega-Thurber R, Willner‐Hall D, Rodriguez‐Mueller B, Desnues C, Edwards RA, Angly F, Dinsdale E, Kelly L, Rohwer F. Metagenomic analysis of stressed coral holobionts. Environmental Microbiology. 2009;11(8):2148–2163. doi: 10.1111/j.1462-2920.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- Wainwright et al. (2017).Wainwright BJ, Zahn GL, Spalding HL, Sherwood AR, Smith CM, Amend AS. Fungi associated with mesophotic macroalgae from the ‘Au‘au Channel, west Maui are differentiated by host and overlap terrestrial communities. PeerJ. 2017;5:e3532. doi: 10.7717/peerj.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2007).Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner et al. (2006).Warner ME, LaJeunesse TC, Robison JD, Thur RM. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnology and Oceanography. 2006;51(4):1887–1897. doi: 10.4319/lo.2006.51.4.1887. [DOI] [Google Scholar]
- Wegley et al. (2007).Wegley L, Edwards R, Rodriguez-Brito B, Liu H, Rohwer F. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environmental Microbiology. 2007;9:2707–2719. doi: 10.1111/j.1462-2920.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- White et al. (1990).White TJ, Bruns T, Lee S, Taylor J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Yang et al. (2013).Yang S, Sun W, Zhang F, Li Z. Phylogenetically diverse denitrifying and ammonia-oxidizing bacteria in corals Alcyonium gracillimum and Tubastraea coccinea. Marine Biotechnology. 2013;15(5):540–551. doi: 10.1007/s10126-013-9503-6. [DOI] [PubMed] [Google Scholar]
- Yarden (2014).Yarden O. Fungal association with sessile marine invertebrates. Frontiers in Microbiology. 2014;5:228. doi: 10.3389/fmicb.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler et al. (2017).Ziegler M, Seneca FO, Yum LK, Palumbi SR, Voolstra CR. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nature Communications. 2017;8:14213. doi: 10.1038/ncomms14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum likelihood phylogeny with the partial 16S rRNA gene showing the phylogenetic relation between bOTU1, the top BLAST hits and all SAR116 bacteria in the SILVA reference alignment, with the exception of KJ589655, HQ673528 and KF271096 which clustered outside of the SAR116 clade. SAR116 bacterial sequences acquired from sponges or corals are indicated in bold and with stars. All other SAR116 sequences are isolated from seawater. The optimal model was selected based on the AIC criterion and found to be GTR+G+I. The maximum likelihood analysis was conducted with RAxML, using a 1000 bootstrap iterations and contained 1,344 nucleotide positions (449 for bOTU1) with Rhizobium leguminosarum (ATCC 14480) as outgroup.
Maximum likelihood phylogeny with the partial 16S rRNA gene showing the phylogenetic relation between bOTU3 and clonal sequences from corals, Candidatus Lariskella, Candidatus Midichloria and other Rickettsiales genera. The optimal model (GTR+I+G) was selected based on the AIC criterion. The maximum likelihood analysis was conducted with RAxML, using a 1,000 bootstrap iterations and contained 1,176 nucleotide positions (452 for bOTU1) with Rhizobium leguminosarum (ATCC 14480) as outgroup.
Data Availability Statement
The following information was supplied regarding data availability:
The DNA data was deposited in NCBI database: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA356144/