Abstract
The recent hype surrounding the antimelanogenic properties of glutathione has resulted in physicians frequently administering it as a “wonder” drug for skin lightening and treatment of hyperpigmentation, especially in ethnic populations with darker skin tones. This phenomenon has seen a recent surge owing to aggressive marketing and capitalization of pharma-cosmeceutical companies. However, the unbridled and prodigal use of it, especially as a parenteral formulation, seems unjustified, given the lacunae in our knowledge about its antimelanogenic potential, limited clinical evidence favoring its role in skin lightening, and the statutory ban/advisory issued by certain federal agencies. Even though parenteral glutathione is approved only for severe liver disorders and for prevention of chemotherapy associated neurotoxicity, the lack of statutory laws governing the use of systemic glutathione in most countries has contributed to its unchecked use for skin lightening. The current clinical evidence of intravenous glutathione for skin lightening is limited to a single study with a dubious study design and apparently flawed analysis of results, casting doubt on the drug’s efficacy and reported adverse effects. Two studies evaluating oral/sublingual administration and one trial involving the use of topical glutathione reported good safety profile and appreciable but reversible results on skin tone. In this article, we shall review and discuss the current status of glutathione as a skin lightening agent and address the sundry unanswered queries regarding the dosage, duration of use and longevity of accrued effects based on clinical evidence and recent insights into its antimelanogenic mechanism.
Keywords: glutathione, skin lightening, intravenous, GSH, GSSG
Introduction
The preoccupation with exploration of treatment options that may help attain a lighter skin tone or fairer complexion has been an ongoing phenomenon in people with skin of color (SOC). The direct implication of this craze is the exploitation of topical agents originally developed for treatment of hyperpigmentation, such as skin lightening therapies. Topicals containing hydroquinone, alpha and beta hydroxy acids, tretinoin, mequinol, arbutin, vitamin C, soy extracts and concoctions of multiple ingredients, including newer cosmeceuticals, are now in vogue for treatment of facial melanoses especially melasma [1] and for general skin lightening, at least of the face, neck and other exposed parts. The local adverse effects of these agents [1] and the quantity required for large surface area application constitute major limitations of this approach. Understandably, the effect of such locally applied topicals remains limited to the application site alone without any notable systemic skin lightening effect. The quest for a systemic skin-whitening agent ensues. Oral antioxidants, such as vitamin C, vitamin E, tranexamic acid, flavonoids, and various botanical extracts have been tried in melasma and disorders of hyperpigmentation, but none has proven to provide an overall skin lightening effect [1,2].
Glutathione, being a strong antioxidant with additional anti-melanogenic properties, has recently become the most popular “systemic skin lightening molecule.” The most “popular” and controversial route of administration of glutathione for skin lightening has been intravenous (IV). Glutathione (GSH), a low molecular weight thiol-tripeptide is central to the maintenance of intracellular redox balance [3]. Currently, to the best of our knowledge, IV GSH has been approved by different statutory drug regulatory authorities for specific systemic disorders. The indications approved by the Central Drugs Standard Control Organization (CDSCO) in India are: 1) alcoholic fatty liver, 2) alcoholic liver fibrosis, 3) alcoholic liver cirrhosis, and 4) alcoholic hepatitis [4]. The Philippines Food and Drug Administration (FDA) has approved its use as an adjunctive treatment to reduce neurotoxicity associated with cisplatin chemotherapy [5]. In addition to it being one of the richest antioxidants, it is being promoted as a skin-lightening agent, following the discovery of its antimelanogenic properties [7]. Amongst the many mechanisms postulated to contribute to its antimelanogenic properties (Figure 1), inhibition of tyrosinase enzyme, skewing of melanogenesis from the darker eumelanin to the lighter phaeomelanin, and scavenging of free radicals seem to be the most important [8]. Unfortunately, there is a clear contradiction between the exact evidence supporting its efficacy and safety, and the hype around its depigmentary properties, with pharma-cosmeceuticals inundating dermatology therapeutics with glutathione tablets, capsules, topical preparations and parenteral preparations across the globe [9].
Figure 1.
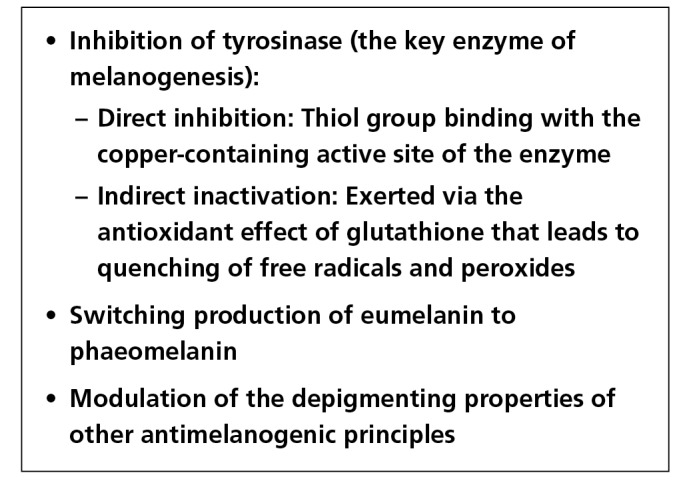
Mechanisms postulated to be responsible for the skin lightening effect of glutathione. [Copyright: ©2018 Sonthalia et al.]
Hailed for generations as a “magical skin whitening” molecule in countries like the Republic of Philippines, glutathione has seen a rapid spread in its popularity across the globe in a short duration of time. This has been the outcome of ardent manufacturer supported media campaigns about the almost preposterous effects of this molecule as a wonder drug for not only disorders of hyperpigmentation such as melasma, but also for general “skin whitening.” This article is meant to update healthcare professionals about the current status of efficacy, safety, and evidence of different formulations of glutathione for skin tone lightening. For more detailed background information regarding the basic and applied physiology of glutathione, readers may refer to a previously published exhaustive article on this aspect [6]. It is important to know that glutathione exists in a reduced form (GSH) and an oxidized form (GSSG). The reduced form, GSH, seems to be instrumental in the depigmenting properties of this unique molecule. Apart from these two major forms, GSH may be esterified to form glutathione esters [7].
An evidence-update on glutathione as a skin-lightening agent
At the time of authoring this article, there were only four published studies that evaluated the efficacy of oral, topical, and parenteral glutathione as a skin-whitening agent (Table 1) [10–13]. The two trials on oral GSH, conducted in Thai population by Arjinpathana and Asawanonda, and in Filipino women by Handog et al. involved administering 500 mg/day of GSH in two divided doses to the study population, the difference being the use of a buccal lozenge (instead of oral capsules) in the latter study to enhance systemic absorption of glutathione [10,11]. The primary efficacy outcome in both the trials was to evaluate the pre- and post-treatment melanin indices. Both trials employed a Mexameter MX 18 (Courage+Khazaka electronic GmbH, Cologne, Germany) to evaluate the primary efficacy outcome. The randomized, double-blind, two-arm, placebo-controlled study conducted by Arjinpathana and Asawanonda in 60 healthy medical students showed a consistent reduction in the melanin indices at all the six sites evaluated in the GSH group subjects, with a statistically significant reduction over placebo at two sites [10]. The open-label, single-arm pilot study conducted by Handog et al. in 30 healthy Filipino women (aged 22–42 years) with Fitzpatrick skin types IV or V, using buccal lozenges instead of capsules of GSH, reported significant reduction in melanin index at both sun-exposed and sun-protected sites in all the subjects and moderate skin lightening observed by 90% of the subjects on global evaluation [11]. The tolerance to GSH was excellent in both the studies. The singular randomized, double-blind, placebo-controlled clinical trial by Watanabe et al., conducted in 30 healthy Filipino women aged 30–50 years has provided virginal evidence favoring efficacy of topical GSSG 2% lotion (applied twice daily for 10 weeks) in producing temporary skin whitening [12]. The results of this split-face, protocol-based study revealed statistically significant reduction of skin melanin index with glutathione compared to placebo, with no adverse drug effects. However, the results of these studies need to be interpreted with caution owing to certain limitations in their study design.
TABLE 1.
Evidence of Glutathione as a Skin Lightening Agent: Current Summary of Studies Conducted to Date
| Glutathione Formulation | Topical (GSSG cream) | Oral (Capsules) | Oral / Buccal Lozenges | Intravenous |
|---|---|---|---|---|
| Authors | Watanabe et al. [12] | Arjinpathana & Asawanonda [10] | Handog et al. [11] | Zubair et al. [15] |
| Study subjects |
|
|
|
|
| Study design | Randomized, double-blind, placebo-controlled, Split-face study | Randomized, double-blind, placebo-controlled study | Open-label, single-arm, pilot study | Open-label, placebo-controlled study |
| Methodology | Split-face study; application of 2% (w/w) GSSG lotion and placebo lotion, twice daily for 10 weeks | Oral glutathione (500 mg) or placebo capsules daily, in 2 divided doses on an empty stomach for 4 weeks | One buccal lozenge (500 mg) per day, for 8 weeks. | Injection glutathione 1200 mg or normal saline (placebo) injected over 30 minutes |
| Frequency of evaluation | Baseline; weekly for 10 weeks | Baseline; and at 4 weeks | Baseline, twice weekly for 8 weeks | Baseline, twice weekly for 8 weeks |
| Primary outcome | Melanin index—by Mexameter MX18 | Melanin index—by Mexameter | Melanin index—by Mexameter | Visual Taylor hyperpigmentation scale |
| Subjective parameters | Global evaluation on a 7-point rating scale | Global evaluation s on a 4-point rating scale | Global evaluation on a 5-point rating scale | None |
| Results | Melanin index
|
Melanin index
|
Melanin index
|
Taylor scale
|
| Tolerance & Safety |
|
|
|
|
| Follow-up After Completion of Study | None | None | None | Done on 3 occasions—2nd, 4th, 6th month after treatment completion |
| Study Limitations |
|
|
|
|
Modified from Table 1 published in: Sonthalia S, Daulatabad D, Sarkar R. Glutathione as a skin whitening agent: facts, myths, evidence and controversies. Indian J Dermatol Venereol Leprol. 2016;82:262.
The major limitations of these studies included: small sample size, cohort consisting of healthy volunteers, extremely short study period with an even shorter follow-up, and lack of measurement of blood levels of glutathione [10–12]. Details of the inclusion criteria, methodology, results and specific limitations of these studies have been comprehensively catalogued in Table 1.
Despite the rampant use of intravenous (IV) glutathione injections for skin lightening in certain countries, evidence favoring such a practice remains elusive. For years, the strong lobby of proponents of IV glutathione for skin lightening, including manufacturers, distributors, many skin clinics, and med spas, have been recommending arbitrary dosage schedules, despite complete lack of evidence [13,14]. It is only recently, that Zubair et al. studied the efficacy and safety of IV GSH for skin tone lightening in 25 patients of Pakistani origin in a placebo-controlled trial (1,200 mg given IV twice a week for 6 weeks in the treatment group versus normal saline in control group) [15]. Although the results from this singular trial did not favor IV glutathione as an effective or lasting treatment for skin tone lightening, the inherent flaws of the study design mandate cautious analysis. The small sample size (n=25 in each group), complicated with a high dropout rate from the treatment group (9 out of 25) in this trial exhorts a cautious interpretation of the results. The methodology was flawed due to the employment of a highly subjective visual Taylor scale for pre- and post-treatment evaluation of change in skin hue and tone and lack of mention of statistical tests applied. The Taylor scale is an unreliable tool for observing subtle changes in skin pigmentation with a very high inter-investigator variability in its interpretation [16]. Results based on Taylor scale, rather than a reliable objective parameter like the melanin index using a Mexameter MX-18, at best, are speculative. Further, the major adverse effect reported in the IV GSH treated group was liver dysfunction, which was neither qualified nor quantified. Development of liver dysfunction in healthy individuals receiving IV GSH is surprising, since the medication is approved by the CDSCO for the treatment of various liver disorders mentioned earlier [4]. The researchers also did not evaluate baseline or post-treatment renal nor thyroid function of the subjects, both of which were reported to be adversely affected by IV glutathione in the position paper by the FDA, Department of Health, Republic of the Philippines [5].
The controversy surrounding absorption of oral glutathione and adverse effects of IV glutathione and statutory status
Glutathione-based oral dietary supplements have been accorded the status of “Generally Recognized as Safe (GRAS)” consistent with Section 201(s) of the Federal Food, Drug, and Cosmetic Act of the United States Food and Drug Administration (US-FDA) [16]. There is no restriction on its availability in this form in the US, Philippines and Japan. Oral GSH is also easily available over-the-counter (OTC) in India and many other Asian countries. Since oral GSH is known to have a low bioavailability in humans [9], manufacturers of IV injections of GSH “recommend” this route of administration to achieve desired therapeutic levels in the blood and skin rapidly to produce “instant” skin whitening results. However, as emphasized above, the literature evaluating the efficacy of IV GSH is still lacking. Furthermore, the duration of therapy and long-term efficacy is yet to be established. Despite the lack of evidence, manufacturers of IV GSH have been “recommending” a dose of 600–1200 mg, to be injected weekly or twice a week, with no specified net duration of the therapy [8].
Although the overall safety of IV GSH, extrapolated from studies evaluating its use for male infertility and liver disorders seems to be convincing [18,19], several adverse effects of IV GSH have been documented in the Philippines (Figure 2), detailed in the position paper by the FDA, Department of Health, Republic of the Philippines [5] with a warning for the public on the subject of the safety of the off-label use of glutathione solution for injection (Figure 3). The reported adverse effects include adverse cutaneous eruptions including potentially fatal Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), severe abdominal pain, thyroid dysfunction, renal dysfunction, and lethal complications such as air embolism, or potentially fatal sepsis due to incorrect/unsterile method of IV administration and use of counterfeit GSH [5,9]. Apart from the lack of evidence favoring IV GSH for skin lightening, the extremely high cost of injection vials constitutes another compelling deterrent to its use. The important limitations of IV GSH have been enumerated in Figure 4.
Figure 2.
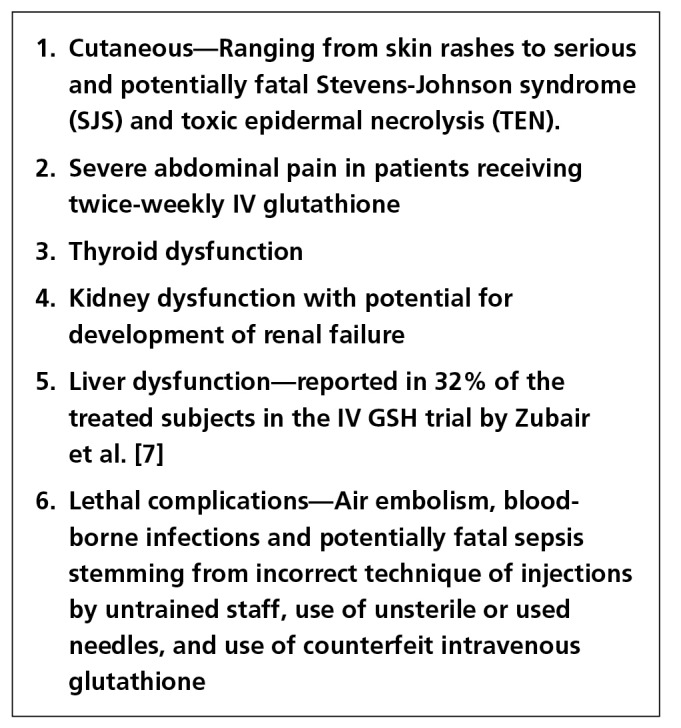
Adverse effects reported with intravenous glutathione injections by the Food and Drug Administration, Department of Health, Republic of the Philippines [5] and the intravenous GSH trial by Zubair et al. [15] [Copyright: ©2018 Sonthalia et al.]
Figure 3.

Public warning issued by the Food and Drug Administration, Department of Health, Republic of the Philippines [5] (May 12, 2011). [Copyright: ©2018 Sonthalia et al.]
Figure 4.

Limitations of intravenous glutathione as a skin lightening agent. [Copyright: ©2018 Sonthalia et al.]
The pharmacological game changer: recent insights from research on the effect of GSH versus esterified GSH in tyrosinase inhibition
A reasonable intracellular concentration of GSH and its unimpeded transportation into the melanosomes are essential for GSH to inhibit tyrosinase and switch melanogenesis from eumelanin to pheomelanin. Trans-melanosomal transportation can be achieved through a membrane channel or diffusion, both of which seem to be lacking for GSH, a fact well established in previous research [20,21]. Chung et al. recently evaluated the in vitro antimelanogenic effects and cytotoxicity of GSH and its three esterified derivatives—GSH monoethyl ester (GSH-MEE), GSH diethyl ester (GSH-DEE), and GSH monoisopropyl ester (GSH-MIPE)—in three cell culture lines [22]. The results of their research demonstrated significant inhibitory effect of GSH-MEE and GSH-MIPE, but not GSH, on intracellular tyrosinase activity and melanin production. The authors attributed this effect to the lipophilicity of the esterified derivatives of GSH. Of the three esters, GSH-DEE and GSH-MIPE demonstrated additional cytotoxic activity, rendering them unsuitable for clinical use. Given in vitro efficacy and lack of cytotoxicity of GSH-MEE, the researchers suggested the development of GSH-MEE, instead of GSH, as an efficacious and safe molecule for the treatment of hyperpigmentation [22]. However, these results need further validation in both in vitro and clinical trials before drawing definitive conclusions.
Conclusion
There is little convincing evidence in favor of glutathione as a therapy for hyperpigmentation at the present time, and there are many unresolved controversies that surround its use (Figure 5). The trials available to date that have evaluated the role of glutathione in skin lightening administered through different modes have numerous limitations. Although the safety of topical and oral GSH seems to be good, their efficacy (especially long-term) remains questionable. The extant evidence to support or discourage use of IV GSH as a therapeutic modality for improving skin tone or pigmentation is minimal and contradictory; notwithstanding the austere concern regarding the potential adverse effects associated with this mode of administration. More evidence in the form of high quality trials with better study design, larger sample size, and long-term follow-up is vital, before our patients are subjected to glutathione-based treatments.
Figure 5.

Controversial aspects of glutathione as a potential skin lightening therapy. [Copyright: ©2018 Sonthalia et al.]
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
References
- 1.Sarkar R, Chugh S, Garg VK. Newer and upcoming therapies for melasma. Indian J Dermatol Venereol Leprol. 2012;78:417–428. doi: 10.4103/0378-6323.98071. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson DA, Forman HJ. Glutathione in defense and signaling: Lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 3.Murray RK. Metabolism of xenobiotics. In: Murray RK, Bender DA, Botham KM, Kennelly PJ, Rodwell VW, Weil PA, editors. Harper’s Illustrated Biochemistry. 28th ed. Michigan: McGraw-Hill; 2009. pp. 612–613. [Google Scholar]
- 4.CDSCO list of approved drug from 01-01-2011 to 31-12-2011. [Accessed on April 26, 2017]. http://www.cdsco.nic.in/writereaddata/LISTOF-APPROVED-DRUG-FROM-2011.pdf.
- 5.Lazo SH. Safety on the off-label use of glutathione solution for injection (IV) Food and Drug Administration, Department of Health; Republic of the Philippines: 2011. [Accessed on February 26, 2017]. http://www.doh.gov.ph/sites/default/files/Advisories_cosmetic_DOH-FDA%20Advisory%20No.%202011-004.pdf. [Google Scholar]
- 6.Sonthalia S, Sarkar R. Glutathione for skin lightening: an update. Pigment Int. 2017;4:3–6. [Google Scholar]
- 7.Exner R, Wessner B, Manhart N, Roth E. Therapeutic potential of glutathione. Wien Klin Wochenschr. 2000;112:610–616. [PubMed] [Google Scholar]
- 8.Villarama CD, Maibach HI. Glutathione as a depigmenting agent: an overview. Int J Cosmet Sci. 2005;27:147–153. doi: 10.1111/j.1467-2494.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 9.Sonthalia S, Daulatabad D, Sarkar R. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian J Dermatol Venereol Leprol. 2016;82:262–272. doi: 10.4103/0378-6323.179088. [DOI] [PubMed] [Google Scholar]
- 10.Arjinpathana N, Asawanonda P. Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat. 2012;23:97–102. doi: 10.3109/09546631003801619. [DOI] [PubMed] [Google Scholar]
- 11.Handog EB, Datuin MS, Singzon IA. An open-label, single-arm trial of the safety and efficacy of a novel preparation of glutathione as a skin-lightening agent in Filipino women. Int J Dermatol. 2016;55:153–157. doi: 10.1111/ijd.12999. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe F, Hashizume E, Chan GP, Kamimura A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: a double-blind and placebo-controlled clinical trial in healthy women. Clin Cosmet Investig Dermatol. 2014;7:267–274. doi: 10.2147/CCID.S68424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OSkin Med Spa website. [Accessed on September 8, 2017]. http://www.oskinmedspa.com/portfolio/iv-glutathione-injections/
- 14.Magic Beauty website. [Accessed on September 8, 2017]. http://www.magicbeauty.in/Product/GSH-Ultima-1500mg.
- 15.Zubair S, Hafeez S, Mujtaba G. Efficacy of intravenous glutathione vs. placebo for skin tone lightening. J Pak Ass Dermatol. 2016;26:177–181. [Google Scholar]
- 16.Taylor SC, Arsonnaud S, Czernielewski J Hyperpigmentation Scale Study Group. The Taylor Hyperpigmentation Scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270–274. [PubMed] [Google Scholar]
- 17.GRAS notice for Glutathione. 2009. [Accessed on April 26, 2017]. URL http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-foods-gen/documents/document/ucm269318.pdf.
- 18.Lenzi A, Lombardo F, Gandini L, Culasso F, Dondero F. Glutathione therapy for male infertility. Arch Androl. 1992;29:65–68. doi: 10.3109/01485019208987710. [DOI] [PubMed] [Google Scholar]
- 19.Cook GC, Sherlock S. Results of a controlled clinical trial of glutathione in cases of hepatic cirrhosis. Gut. 1965;6:472–476. doi: 10.1136/gut.6.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potterf SB, Virador V, Wakamatsu K, et al. Cysteine transport in melanosomes from murine melanocytes. Pigment Cell Res. 1999;12:4–12. doi: 10.1111/j.1600-0749.1999.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 21.Wellner VP, Anderson ME, Puri RN, Jensen GL, Meister A. Radioprotection by glutathione ester: Transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci USA. 1984;81:4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung BY, Choi SR, Moon IJ, Park CW, Kim YH, Chang SE. The glutathione derivative, GSH monoethyl ester, may effectively whiten skin but GSH does not. Int J Mol Sci. 2016;17:629. doi: 10.3390/ijms17050629. [DOI] [PMC free article] [PubMed] [Google Scholar]


