Abstract
BACKGROUND
White matter hyperintensities (WMH), the hallmark of vascular cognitive impairment, are associated with vascular risk factors (VRF). WMH can also be associated with blood-brain barrier (BBB) disruption. The purpose of this study was to look for associations between VRF and BBB disruption in stroke patients with WMH.
METHODS
MRIs of stroke patients were reviewed for the presence of WMH. Blood-brain permeability images were retrospectively generated. The degree of BBB permeability was compared with the presence of VRFs using logistic regression. Patterns and extent of WMH were classified using Fazekas scores.
RESULTS
65 patients were included in this study. None of the VRF tested were associated with an increase in BBB disruption. Hypertension was significantly associated with less BBB disruption (p=0.04). Non-hypertensive patients in our study had a different pattern of WMH than hypertensive patients, with less involvement of the periventricular white matter.
CONCLUSIONS
We found that in stroke patients with WMH, those with hypertension had less BBB disruption and greater involvement of the periventricular white matter when compared with patients that did not have a history of hypertension. Further investigation is need to determine if the development of WMH in stroke patients with a history of hypertension has a different pathophysiology from patients who develop WMH in the absence of hypertension.
Keywords: White matter hyperintensities, Blood-brain barrier, Hypertension, Vascular cognitive impairment, Permeability imaging
INTRODUCTION
The clinical significance and pathogenesis of white matter hyperintensities (WMH), detected on T2-weighted MRI sequences, remain unclear. WMH occur with normal aging; when progressive, they are associated with cognitive decline [1]. WMH are also associated with vascular risk factors [2]; untreated hypertension is associated with WMH progression [3]. However, vascular risk factors account for only a small fraction of WMH pathogenesis [4], suggesting WMH may be a final common pathway for multiple etiologies. A recent consensus statement on subcortical small vessel disease drew a distinction between WMH caused by recurrent lacunar stroke and WMH that accumulates as part of an insidious process [5].
In some patients, WMH are associated with blood-brain barrier (BBB) disruption [6]. Such BBB disruption is often found at the edge of the WMH involving the normal appearing white matter (NAWM) [7]. It has been hypothesized that BBB disruption reflects an active chronic inflammatory process and may precede WMH progression [8]. The relationship between vascular risk factors and BBB disruption is not known. Therefore, the purpose of this study was to examine a cohort of stroke patients with WMH to look for associations between vascular risk factors and BBB disruption.
METHODS
Population
Pre-treatment MRIs of patients presenting with acute ischemic stroke who were enrolled in the IRB-approved NIH Natural History of Stroke Study from 1999 to 2009 were reviewed for the presence of WMH on FLAIR sequences. MRIs of untreated patients during that time period were also reviewed if their NIH stroke scale was >3. Patients were included in this study if: 1) their FLAIR scan demonstrated confluent WMH, and 2) they had a dynamic susceptibility contrast (DSC) sequence (typically used in MRI to generate perfusion weighted imaging, PWI) of adequate quality to perform the BBB permeability analysis.
Fazekas score grading
Although all patients in this study were initially identified due to confluent WMH, after assembling the dataset, all of the FLAIR MRIs were reviewed again by a board certified neurologist (N.G.) and graded based on the Fazekas scoring system [9]. A Fazekas score is based on WMH severity graded in two locations: periventricular white matter (PVWM) and deep white matter (DWM). Each location is graded from 0 to 3 and thus, when added together, the maximum score of 6 indicates the most severe distribution of WMH.
Blood-Brain Permeability Imaging (BBPI)
To measure BBB disruption for this study, a method for extracting BBB permeability from DSC images was employed [10]. A DSC image sequence acquires serial imaging during the injection of gadolinium. The typical clinical application of DSC MRI is to generate PWI, such as time-to-peak (TTP) maps, mean-transit-time (MTT) maps and cerebral blood volume (CBV) maps. PWI assumes that gadolinium stays in the vascular compartment and does not cross the BBB. However, when there is disruption of the BBB, gadolinium begins to accumulate in the brain parenchyma, which affects the signal recorded with DSC imaging. Thus, in the setting of BBB disruption, DSC images reflect changes due to both intravascular flow of gadolinium and extravascular accumulation of gadolinium. These two processes can be separated, allowing for the isolation of the effect caused by BBB disruption [11].
BBB permeability maps used for this study were generated by processing the DSC source images originally collected for purpose of PWI. DSC source images are primarily T2* weighted but have some T1 weighting as well. Intravascular gadolinium causes the recorded signal to temporarily drop, due to T2* effects, as the bolus passes through the voxel. In the setting of BBB disruption, gadolinium leaks out of the vasculature and into the parenchyma causing an increase in the recorded signal due to a T1 effect. By comparing regions with an intact BBB with regions that have BBB disruption, a relative measure of BBB disruption can be obtained. An arrival time correction is performed to account for regional differences in cerebral perfusion [11]. Normal tissue was identified using an automated process that excludes regions of BBB disruption based on several signal characteristics. Only a small amount of normal tissue is needed to create the reference curve; thus this automated approach excludes regions that show any signal changes that may be associated with BBB disruption. This method has been internally validated against manual selection in a separate dataset.
The BBB permeability model generates a measure that can be expressed as a percent leak ranging from 0 to 100. This permeability value is most easily understood as the fractional decrease in the recorded cerebral blood volume that is due to gadolinium leakage. The end result is a voxel-by-voxel permeability map. However the ability to extract BBB information from DSC images depends on the signal-to-noise ratio (SNR) of the image acquisition which varies from patient to patient. In the setting of low SNR detection of BBB disruption is not possible. Thus, in order to be included in this study, the average curve-fit (r2) of the model had to be greater than 0.85. This excludes patients with noisy DSC acquisitions (low SNR) from this analysis due to poor curve fitting of the model. The BBB measurement method used has been validated in acute stroke [12] and chronic cerebrovascular disease [13], has been shown to be sensitive to both subtle and overt BBB disruption [14], and is designed to be independent of regional difference in blood flow [11]. This method has also been independently validated in brain tumor patients [15].
Image Analysis
The sequence parameters in this study varied over time, however all patients had a fluid attenuated inversion recovery (FLAIR) sequence and a single-shot echo planar DSC sequence acquired during the injection of a weight-based dose of gadolinium. Patients with a Fazekas score of 2 or greater in the deep white matter and/or the periventricular region were included. Permeability measures were calculated from axial slices beginning above the basal ganglia and ending with the first slice of the centrum semiovale. This region of interest (ROI) was selected to avoid artifacts from mineralization in the basal ganglia (below the ROI) and signal from superficial cortical veins (above the ROI). Cortex was manually removed. Areas of acute ischemia (time-to-peak > 4 seconds beyond normal) were also removed from the ROI. The resulting ROI restricted analysis to BBB disruption within the white matter, including both WMH and the adjacent NAWM, as demonstrated in figure 1. The mean permeability derangement (MPD) was calculated from the mean of all voxels in the ROI whose permeability was above the noise threshold of 1%.
Figure 1.
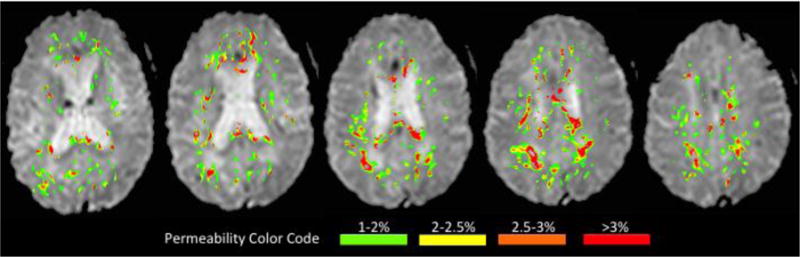
An example of the blood-brain permeability imaging (BBPI) used in this study is displayed. The permeability map (in color) is overlain on the T2* weighted DSC source images (black and white). Only slices above the basal ganglia extending to the first slice of the centrum semiovale are included in the analysis. BBB permeability is restricted to the white matter (WMH+NAWM). Increasing BBB disruption is color-coded green->yellow->orange->red.
Statistical Analysis
Logistic regression was used to compare MPD as a continuous variable with risk factors as binary variables. Risk factors included hypertension (HTN), Diabetes Mellitus (DM), Hyperlipidemia (HLD), coronary artery disease (CAD), atrial fibrillation (Afib) and tobacco use (TOB). The association between age (independent variable) and MPD (dependent variable) was assessed with linear regression. The associations between two binary variables were assessed with Pearson’s chi-squared test. A p-value <= 0.05 was considered significant. Statistical analysis was performed using the STATA 13 software package.
RESULTS
Of the 1193 patients screened for this study, 247 had confluent WMH. Of those patients 92 had PWI source images. Of those patients, 65 had adequate BBB modeling (average r2 > 0.85) and were included in the study. The mean age of the cohort was 79, and it was predominantly female (78%). The median NIHSS was 8, and 26% of them were treated with IV tPA. Of note, MRI scans used for this analysis were all obtained prior to any treatment. All patients had a Fazekas score in the range of 4 to 6, indicating that they scored either a 2 or a 3 in the two regions graded.
Table 1 shows p-values for the associations of the risk factors with mean permeability derangement (MPD). Only HTN (p=0.04) had significant association; a history of HTN was associated with less BBB disruption. Figure 2 shows boxplots comparing MPD of the hypertensive and non-hypertensive groups. The association between HTN and BBB disruption remained significant when age was added to the model (p=0.039).
Table 1.
The mean age, percentage female, and prevalence of the risk factors for the population are shown. The logistic regression p-values for association with mean permeability derangement of BBB are shown for each. (HTN=hypertension, DM=Diabetes Mellitus, HLD=Hyperlipidemia, CAD=coronary artery disease, Afib=atrial fibrillation, TOB=tobacco use)
| Age | Female sex | HTN | DM | HLD | CAD | Afib | TOB | |
|---|---|---|---|---|---|---|---|---|
| Prevalence or mean | 79 | 78% | 81% | 22% | 51% | 11% | 35% | 5% |
| p-value | 0.209 | 0.96 | 0.04 | 0.386 | 0.353 | 0.951 | 0.218 | 0.407 |
Figure 2.
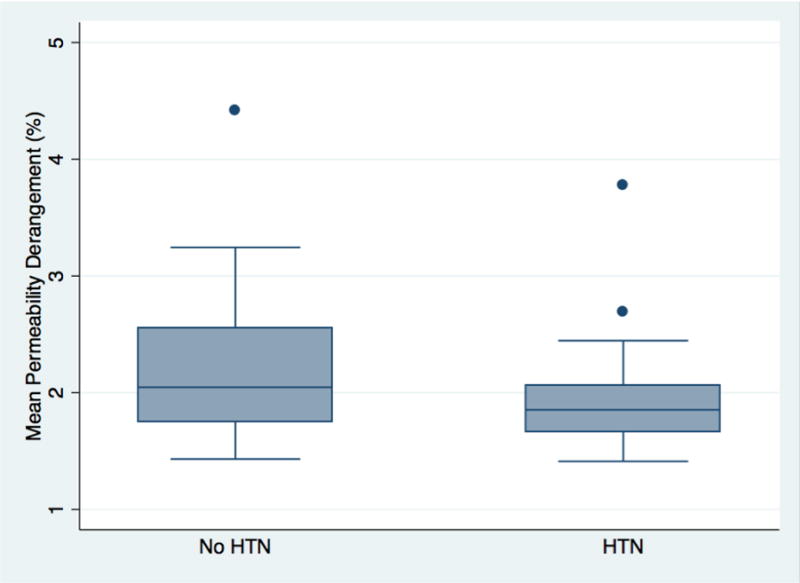
Box plots compare the mean permeability derangement (MPD) of the patients with and without a history of hypertension (HTN).
To further evaluate this finding, we separated the population into two groups based on whether or not they had a history of hypertension to look for other variables that might be different between the groups. Table 2 shows the demographics and clinical variables for these two groups. We found that the pattern of WMH was also different between the groups. Patients with a history of hypertension had more severe WMH in the periventricular regions compared with non-hypertensive patients (p=0.027), while for the deep white matter regions, there was no difference detected (p=0.355). Multivariate analysis found lower MPD (p=0.040) and higher periventricular Fazekas score (p=0.038) to be independently associated with hypertension.
Table 2.
Demographics and imaging findings are shown for the whole group and the hypertension sub-groups. p-values reflect differences between the sub-groups based on logistic regression for continuous variables and Pearson’s chi-squared test for binary variables.
| All Patients (n=65) |
Patients without Hypertension (n=12) |
Patients with Hypertension (n=53) |
P Value | |
|---|---|---|---|---|
| Mean Age | 79 | 80 | 79 | p=0.933 |
| Sex (Female) | 78% | 70% | 80% | p=0.271 |
| Diabetes mellitus | 22% | 17% | 23% | p=0.649 |
| Hyperlipidermia | 51% | 25% | 57% | p=0.048 |
| Coronary artery disease | 11% | 0% | 13% | p=0.183 |
| Atrial fibrillation | 35% | 25% | 38% | p=0.405 |
| Hypertension | 82% | 0% | 100% | |
| IV tPA | 26%α | 25% | 26%β | p=0.942 |
| Median Pretreatment NIHSS | 8 | 8.5 | 7.5 | p=0.357 |
| Fazekas Score, Periventricular White Matter – 2 | 24%* | 42% | 20%# | p=0.027 |
| Fazekas Score, Periventricular White Matter – 3 | 76%* | 58% | 80%# | |
| Fazekas Score, Deep White Matter – 2 | 17%* | 8% | 20%# | p=0.355 |
| Fazekas Score, Deep White Matter – 3 | 83%* | 92% | 80%# | |
| Mean Permeability Derangement | 1.9% | 2.3% | 1.9% | p=0.04 |
n=62,
n=50,
n=63,
n=51.
DISCUSSION
We screened several vascular risk factors to see if they were associated with an increase in BBB disruption in stroke patients with WMH. We found no positive associations; however, in this population of patients selected due to the presence of WMH, BBB disruption was less severe in patients with a history of hypertension. Patients with a history of hypertension also had a different distribution of WMH, with greater involvement of the periventricular regions than patients without hypertension.
These findings are exploratory and need further validation. However, this is the first study looking at the relationship between WMH associated BBB disruption and VRF. Although our sample size was not large (n=65) it is larger than other studies looking at diffuse BBB disruption in stroke patients [7, 16, 17]. Our findings bring up the possibility that patients with WMH and hypertension may have a different underlying pathophysiology than patients with WMH who do not suffer from hypertension. WMH on MRI probably represent a non-specific biomarker for multiple underlying pathologies [5, 18]. Poorly controlled HTN is a major cause of stroke, including lacunar stroke. Patients with HTN and recurrent lacunar strokes can develop an MRI appearance similar to WMH caused by an insidious progressive process [19].
In the absence of lacunar stroke, progressive WMH is thought to be due to chronic hypoperfusion, which, in the setting of increased demand for blood flow, results in intermittent hypoxia [20]. Deep white matter is predominantly affected since it is part of the watershed region, falling in between the major vascular territories. It is hypothesized that hypoxia leads to an inflammatory reaction, initially characterized by opening of the BBB and subsequently resulting in demyelination and eventually leading to the formation of WMH [5]. This presumed pathophysiology is supported by the observation that BBB disruption is a migrating process while WMH is an accumulating process [7]. It has been hypothesized that BBB disruption in the NAWM precedes the development of WMH. For this study, we did not differentiate between BBB disruption occurring in the WMH vs. BBB disruption occurring in the NAWM. It is possible that the NAWM in non-hypertensives has higher permeability than that in hypertensive patients while WMH has the same permeability in both groups. This could account for the observation that non-hypertensives have greater mean permeability derangement. If this is the case, MRI measures of BBB disruption may be able to serve as a biomarker for disease activity.
In our study we found that BBB disruption was more prominent in non-hypertensive patients, and that these patients had greater involvement of deep white matter (watershed) than of the periventricular region. These findings are in line with the idea that WMH may be the final common pathway for two processes: a stepwise accumulation of lacunar infarctions, and an insidious, progressive, possibly inflammatory, injury to the brain. This may have clinical implications, given that the former may respond to secondary stroke prevention while the latter may not.
There was a skew towards female gender in this study that is not present in the Natural History of Stroke Study cohort in general. There is no reason the methods of patient selection for this study should have introduced bias toward including female subjects, other than the presence of confluent WMH. These findings support prior findings that women have more WMH progression [21, 22]. While the neurobiological basis for this difference is not known, it has been hypothesized to be due to postmenopausal estrogen withdrawal [23]. This sex difference has been reported to be mostly due to WMH in the deep white matter rather than the periventricular WMH [21]. Our study did not detect any difference in the BBB disruption between the sexes.
There are several limitations to this study. It is a retrospective analysis of data collected over a decade. Scan parameters changed over time. The MRI scans were collected as part of an acute stroke evaluation. Although areas of acute ischemia were excluded from the analysis, there may be remote BBB effects caused by diaschisis. Ideally this study should be repeated in a population of patients who are not suffering an acute event. Despite these limitations, this study supports the hypothesis that BBB disruption is a biomarker for an etiology of WMH that may be distinct from hypertension-related WMH. A prospective investigation would be needed to confirm this hypothesis.
Acknowledgments
Sources of funding:
All of the authors are supported by the Intramural Program of NIH, NINDS.
Appendix
This research was possible because of contributions from the NIH Natural History of Stroke Investigators: Richard T. Benson, Amie W. Hsia, Lawrence L. Latour, Richard Leigh, Marie Luby, John K. Lynch, Jose G. Merino, Zurab Nadareishvili, and Steven J. Warach.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have nothing to disclose.
References
- 1.De Groot JC, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52(3):335–41. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 2.Lechner H, et al. Nuclear magnetic resonance image white matter lesions and risk factors for stroke in normal individuals. Stroke. 1988;19(2):263–5. doi: 10.1161/01.str.19.2.263. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman RF, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41(1):3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82(15):1331–8. doi: 10.1212/WNL.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg GA, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30(3):337–52. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Huisa BN, et al. Long-Term Blood-Brain Barrier Permeability Changes in Binswanger Disease. Stroke. 2015 doi: 10.1161/STROKEAHA.115.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardlaw JM, et al. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke. 2013;44(2):525–7. doi: 10.1161/STROKEAHA.112.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazekas F, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 10.Zaharchuk G. Theoretical basis of hemodynamic MR imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR Am J Neuroradiol. 2007;28(10):1850–1858. doi: 10.3174/ajnr.A0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leigh R, et al. Arrival Time Correction for Dynamic Susceptibility Contrast MR Permeability Imaging in Stroke Patients. PLoS One. 2012;7(12):e52656. doi: 10.1371/journal.pone.0052656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leigh R, et al. Pretreatment blood-brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology. 2016 doi: 10.1212/WNL.0000000000002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arba F, et al. Blood brain barrier leakage increases with small vessel disease in acute ischemic stroke. Neurology. 2017 doi: 10.1212/WNL.0000000000004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpkins AN, et al. Identification of Reversible Disruption of the Human Blood-Brain Barrier Following Acute Ischemia. Stroke. 2016;47(9):2405–8. doi: 10.1161/STROKEAHA.116.013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluge A, et al. Analysis of three leakage-correction methods for DSC-based measurement of relative cerebral blood volume with respect to heterogeneity in human gliomas. Magn Reson Imaging. 2015 doi: 10.1016/j.mri.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Wardlaw JM, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65(2):194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 17.Topakian R, et al. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81(2):192–7. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 18.Spilt A, et al. Not all age-related white matter hyperintensities are the same: a magnetization transfer imaging study. AJNR Am J Neuroradiol. 2006;27(9):1964–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Duering M, et al. Incident lacunes preferentially localize to the edge of white matter hyperintensities: insights into the pathophysiology of cerebral small vessel disease. Brain. 2013;136(Pt 9):2717–26. doi: 10.1093/brain/awt184. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke. 2014;45(5):1531–8. doi: 10.1161/STROKEAHA.113.004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Heuvel DM, et al. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63(9):1699–701. doi: 10.1212/01.wnl.0000143058.40388.44. [DOI] [PubMed] [Google Scholar]
- 22.Schulz UG, et al. Leukoaraiosis and increased cerebral susceptibility to ischemia: lack of confounding by carotid disease. J Am Heart Assoc. 2013;2(4):e000261. doi: 10.1161/JAHA.113.000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt R, et al. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J Am Geriatr Soc. 1996;44(11):1307–13. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]


