Abstract
The oligometastatic state has been proposed as an intermediate stage of cancer spread between localized disease and widespread metastases. With improvements in diagnostic modalities such as functional imaging, oligometastatic prostate cancer is being diagnosed with greater frequency than ever before. Furthermore, the paradigm for treatment of advanced prostate cancers is shifting toward a more aggressive approach. Many questions surround the understanding of the process and consequences of oligometastasis, meaning that the contemporary literature offers a wide variety of definitions of oligometastatic prostate cancer. Until genomic data exist to provide a biological component to the definition of oligometastatic disease, a clinical diagnosis made on the basis of up to five extrapelvic lesions is reasonable for use. Retrospective studies suggest that interventions such as radical prostatectomy and local or metastasis-directed radiotherapy can be performed in the metastatic setting with minimal risk of toxic effects. These therapies seem to decrease the need for subsequent palliative interventions, but insufficient data are available to draw reliable conclusions regarding their effect on survival. Thus, a protocol for clinicians to manage the patient presenting with oligometastatic prostate cancer would be a useful clinical tool.
Hellman and Weichselbaum first proposed a clinically significant state of oligometastasis in 1995 (REF. 1). With data available at the time suggesting a stepwise progression of malignancy, the authors posited that tumours that are early in their chain of progression could result in an intermediate state of cancer spread between localized disease and widespread metastases2,3. The practical significance of the oligometastatic paradigm was the implication that some patients in this state could be cured with definitive directed therapies4,5.
Over the past two decades, our understanding of the oligometastatic state has continued to mature6. Meanwhile, oligometastatic disease is increasingly diagnosed — possibly owing to closer patient monitoring in the setting of clinical trials, improved detection of limited disease states with advanced imaging, and emerging therapies that have prolonged survival with a diagnosis of cancer7,8. At the same time, emerging genomic data have suggested distinct biological differences between limited metastatic lesions and widely disseminated disease for multiple primary cancers, including that of the prostate9–11. Such preliminary findings support the possibility of a true oligometastatic biology, distinct from one in which clinically apparent lesions are simply initial manifestations of a more widespread process. The ability to distinguish these disease states is crucial when considering an aggressive treatment approach in the population of patients with metastatic prostate cancer12.
At the same time, the paradigm for treatment of advanced prostate cancer is undergoing dramatic change13. Traditionally, local therapies such as radical prostatectomy and radiotherapy were offered only with the intention to cure localized disease14 and evidence of metastasis — even minimal disease such as a single positive pelvic lymph node — precluded an aggressive therapeutic approach. Such men were instead treated with systemic therapies such as androgen deprivation therapy (ADT)15,16. However, emerging data suggest that treatment of the primary tumour might provide a survival benefit to men with metastatic and lymph-node-positive disease17–21. Similar observations have been made in treatment of metastatic lesions with life-prolonging, rather than palliative, intent22. Indeed, interest in the treatment of oligometastatic prostate cancer is greater than ever before.
Treatment of oligometastatic prostate cancer is complicated by the lack of uniformity in describing the condition. Traditional definitions of oligometastatic disease have been based on the number of lesions detected by bone scan, with additional classification based on the anatomical sites of the disease. Even so, such definitions vary widely throughout the literature. Furthermore, differentiating between synchronous disease — in which the prostate is untreated (de novo or primary metastases) — and metachronous disease, in which the primary tumour was previously treated and metastases are encountered during recurrence (recurrent oligometastases), is essential. Although primary and recurrent oligometastatic disease are likely to represent distinct biological states — a fact that should be considered when initiating systemic therapies — the effect of this distinction on site-directed therapies is unclear. With these points in mind, commonly used definitions of oligometastatic prostate cancer based on contemporary reports and active clinical trials require assessment.
The curative treatment of oligometastatic prostate cancer is likely to require a three-tiered approach: firstly, local consolidative therapy of the primary tumour, secondly, metastasis-directed therapy, and thirdly, systemic chemohormonal therapy. Questions persist regarding the optimal timing and duration of ADT, but it remains a cornerstone of systemic therapy23,24, and several large prospective clinical trials have been directed at optimizing the use of chemotherapy25,26. By contrast, limited data are available that explore local and metastasis-directed interventions with nonpalliative (that is, curative) intent. These data are uniformly retrospective and subject to substantial biases, particularly that of treatment selection. These and other limitations must be noted when considering such studies. Furthermore, as many reports of local treatment did not differentiate oligometastatic from polymetastatic disease, a portion of these data are derived from heterogeneous populations. This limitation is unlikely to influence safety outcomes, but would presumably affect disease-specific survival data. Indeed, the authors of such studies acknowledge that limitations pervade the literature. Thus, the goal of this Review is not to establish expected rates of disease-specific outcomes within this patient population, but rather to generally assess whether treatment seems safe and beneficial in what is a poorly characterized setting.
Studies defining oligometastatic prostate cancer
Six original articles and ten prospective trials have explicitly defined oligometastatic prostate cancer27–42 (TABLE 1). Oligometastatic prostate cancer was defined based on the number of detectable lesions in all six original reports. Two articles (33%) limited inclusion to five or fewer metastases, one article (27%) to four or fewer, and three articles (50%) to three or fewer. Tabata et al.27 included only bony metastases in which lesions were less than 50% of the size of a vertebral body, according to extent of disease criteria previously described by Soloway and colleagues43. Two studies limited inclusion to bone or lymph node metastases. Three reports did not define oligometastasis based on site. In five of the six original reports, detection of lesions was most commonly performed using 18F-fluorodeoxyglucose (FDG) or 11C-choline PET with coregistered X-ray CT. One study did not specify the imaging modalities used for lesion detection.
Table 1.
Studies defining oligometastatic prostate cancer
| Author/Site | n | Number of metastases | Sites of metastases | Detection |
|---|---|---|---|---|
| Original studies | ||||
| Tabata et al.27 | 35 | ≤5 | Bone only; each site <50% size of vertebral body | Bone scan |
| Ahmed et al.28 | 17 | ≤5 | NS | 11C-choline PET–CT (n= 7), MRI (n= 6), biopsy (n= 1), CT (n= 1), 11C-choline PET–CT and MRI (n= 2) |
| Berkovic et al.29 | 24 | ≤3 | Bone or LN |
|
| Schick et al.30 | 50 | ≤4 | NS |
|
| Decaestecker et al.31 | 50 | ≤3 | Bone or LN |
|
| Ost et al.32 | 119 | ≤3 | Any | 18F-FDG PET–CT (n= 24) or 18F-choline PET–CT (n= 92) |
| Prospective trials (NCTI) | ||||
| University of Florida (NCT01859221)33 | NR | NS | Any except brain or CNS | — |
| Sunnybrook Health Sciences Centre (NCT02563691)34 | NR | ≤5 | Outside the prostate and pelvic lymph nodes | — |
| Sidney Kimmel Comprehensive Cancer Center (NCT02489357)35 | NR | ≤4 | Extrapelvic | — |
| Mayo Clinic (NCT01777802)36 | NR | ≤3 | NS | — |
| Grupo de Investigación Clínica en Oncología Radioterapia (NCT02192788)37 | NR | ≤4 | Bone, LN | — |
| University Hospital, Ghent (NCT01558427)38 | NR | ≤3 | NS (N1, M1a/b)* | — |
| Technische Universität Dresden (NCT02264379)39 | NR | ≤5 | NS | — |
| City of Hope Medical Center (NCT00544830)40 | NR | ≤5 | NS (N1–3, M1) | — |
| Memorial Sloan Kettering Cancer Center (NCT02020070)41 | NR | ≤10 | Bone, LN | — |
| Sidney Kimmel Comprehensive Cancer Center (ORIOLE) (NCT02680587)42 | NR | ≤3 | Bone, LN | — |
CNS, central nervous system; FDG, fluorodeoxyglucose; LN, lymph node; NCTI, national clinical trial identifier; NR, not reported; NS, not specified; ORIOLE, Phase II randomized Observation versus stereotactic ablative RadiatIon for OLigometastatic Prostate CancEr.
N1 = Metastases in regional lymph nodes; M1 = Distant metastasis; M1a = Non-regional lymph nodes; M1b = Bone; M1c = Other sites with or without bone disease.
Nine of 10 (90%) active clinical trials explicitly limited the number of metastases included34–42. Similar to published reports, the majority of trials included up to three (n= 3, 30%)36,38,42, four (n= 2, 20%)35,37, or five (n= 3, 30%)34,39,40 metastatic lesions, while one trial (10%) included up to ten41. One centre limited the number of lesions to five, but specified that no more than three tumours could be present in a given organ system. The sites of metastases were not specified in two trials36,39 and were only specified as extrapelvic in two trials34,35. Similar to retrospective reports, bone and lymph node metastases were commonly outlined when site-specific criteria were utilized.
Local consolidative therapy
Six contemporary reports of local consolidative therapy for metastatic prostate cancer are available in the literature44–49 (TABLE 2). Using the Surveillance, Epidemiology, and End Results (SEER) programme data from 2004 to 2010, Culp and colleagues44 assessed overall and cancer-specific survival in men who underwent local therapy, including radical prostatectomy or brachytherapy, compared with no local therapy. Notably, untreated patients were older (median age in no local therapy, radical prostatectomy, and brachytherapy cohorts were 72 years, 62 years, and 68 years, respectively; P<0.001 (no local therapy versus local therapy)), less likely to have Gleason score ≤7 disease (5.6%, 20.8%, 18.6%, respectively; P <0.001), and less likely to have clinical stage N0 disease (48.7%, 67.4%, 69.9%, respectively; P<0.001). Nonetheless, on multivariable competing risk regression analysis, men treated with radical prostatectomy had a 62% decreased risk of cancer-specific mortality (CSM) (subhazard ratio (SHR) 0.38, 95% CI 0.27–0.53, P<0.001) and men who underwent brachytherapy had a 32% decreased risk (SHR 0.68, 95% CI 0.49–0.93, P= 0.018). Other factors independently associated with CSM were Gleason score ≥8 (SHR 1.70, 95% CI 1.42–2.04, P <0.001), clinical stage T4 (SHR 1.25, 95% CI 1.12–1.40, P <0.001), PSA ≥20 ng/ml (SHR 1.29, 95% CI 1.20–1.40, P <0.001), clinical stage N1 (SHR 1.21, 95% CI 1.09–1.33, P <0.001), clinical stage M1b (distant metastasis of bone; SHR 1.86, 95% CI 1.55–2.24, P <0.001) or M1c (distant metastasis of other non-lymph-node sites, with or without bone disease; SHR 2.35, 95% CI 1.94–2.85, P<0.001), and year of diagnosis (SHR 0.93, 95% CI 0.91–0.95, P <0.001). Notably, radical prostatectomy was associated with decreased CSM across all M stages, and brachytherapy improved CSM in men with M1c disease.
Table 2.
Retrospective data for local consolidative therapy of the primary tumour
| Source | Study design | Inclusion | Intervention | OS* | CSS* | MVA | Additional information |
|---|---|---|---|---|---|---|---|
| Culp et al.44 | Population-based, n= 8,185, median follow-up period: 16 months | M1a–M1c |
|
|
|
SHR (CSM)
|
MVA includes: Gleason score ≥8, T4, PSA ≥20 ng/ml, AJCC N1 (versus N0), AJCC M stage (versus M1a), year of diagnosis |
| Antwi et al.45 | Population-based, n= 7,858, median follow-up period: NR | M1a–M1c |
|
|
|
aHR (CSM)
|
MVA includes: age, race, marital status, tumour grade, PSA level, and cancer registry |
| Gratzke et al.46 | Population-based, n= 1,538, median follow-up period: NR | M+ |
|
|
|
NR | Overall survival compared between RP patients and non-RP patients (including RT, ADT, and other) |
| Satkunasivam et al.47 | Population-based, n= 4,069, median follow-up period: NR |
|
|
|
|
aHR (CSM)
|
|
| Heidenreich et al.48 | Case-control, n= 61, median follow-up period:
|
Limited M1 |
|
|
|
|
Inclusion criteria: ≤3 lesions on bone scan; absence of visceral or extended LN metastases; PSA nadir <1 ng/ml after 6 months of neoadjuvant ADT |
| Cho et al.49 | Case-control, n= 140 (38 cases), median follow-up period: 34 months | M1 |
|
|
|
HR (OM)
|
MVA includes: ECOG status, site of metastasis |
ADT, androgen deprivation therapy; aHR, adjusted hazard ratio; AJCC, American Joint Committee on Cancer; BT, brachytherapy; CCI, Charlson comorbidity index; CRR, competing risk regression; CRT, conformal radiation therapy; CSM, cancer-specific mortality; CSS, cancer-specific survival; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; FDG, fluorodeoxyglucose; IMRT, intensity-modulated radiation therapy; LN, lymph node; MRI, magnetic resonance imaging; MVA, multivariable analysis; NLT, no local treatment; NR, not reported; NS, not specified; OM, overall mortality; OS, overall survival; PCSM, prostate-cancer-specific mortality; RP, radical prostatectomy; RT, radiation therapy; SHR, subhazard ratio.
In cases of unspecified time frame, values refer to proportion experiencing outcome during total follow-up period.
Antwi et al.45 subsequently assessed the SEER population using alternative methodology. To ensure that observed effects were attributable to treatment rather than baseline cohort differences50, the authors performed propensity score analysis considering age, race, marital status, tumour grade, serum PSA level, and cancer registry. Notably, these authors observed decreased CSM after radical prostatectomy or brachytherapy regardless of the extent of metastatic disease. For example, relative to the no local therapy group, the adjusted hazard ratios associated with radical prostatectomy were 0.18 (95% CI 0.07–0.50, P= 0.0008), 0.22 (95% CI 0.16–0.30, P < 0.0001), and 0.23 (95% CI 0.16–0.35, P < 0.0001) for M1a (metastasis of non-regional lymph nodes), M1b, and M1c disease, respectively. Corresponding values in the brachytherapy cohort were 0.29 (95% CI 0.13–0.64, P= 0.0024), 0.49 (0.36–0.67, P <0.0001), and 0.36 (0.24–0.54, P<0.0001). The authors of both reports acknowledged the limitations of population-based retrospective studies, such as treatment selection bias and the inability to account for unmeasured factors44,45. Furthermore, these reports described largely overlapping populations (subtle variation in inclusion criteria accounted for minor population differences), and treated men comprised <5% of the study population. Nonetheless, consistent findings were obtained using two different sophisticated analyses. Of note, patients who underwent external beam radiation therapy (EBRT) were excluded from these studies owing to the lack of organ-site-specific coding for EBRT in SEER data.
Data from Gratzke et al.46 subsequently analysed 1,538 patients in the Munich Cancer Registry with incident metastatic prostate cancer between 1998 and 2010. In total, 74 men (5%) underwent prostatectomy and demonstrated 55% overall survival at 5 years, compared with 21% in the no prostatectomy group (P<0.01). The nonsurgical group consisted of 635 men (41.3%) who underwent primary ADT, 389 men (25.3%) treated with radiation, and 440 men (28.6%) who underwent other treatment. Whether the study distinguished between definitive or palliative treatment in the radiation therapy group was not reported; this omission might explain why outcomes in the radiation group mirrored the primary ADT and other treatment groups, and why radiation therapy was not considered in the same manner as surgery. Acknowledging these considerable limitations, these data demonstrated a reproducible benefit of surgery, albeit in a highly selected sample of the population.
In a subsequent report, Satkunasivam and colleagues47 used a unique approach to address some of the limitations of previous studies. By linking the SEER database to Medicare, the authors were able to account for comorbidities and ADT use in the study population. Furthermore, they assigned a threshold of ≤15 radio-therapy claims as consistent with palliative treatment, and were, therefore, able to assess the effect of localized, nonpalliative intensity modulated radiation therapy (IMRT) and conformal radiation therapy (CRT)51–53. At baseline, the radical prostatectomy and IMRT groups were younger and had lower serum PSA levels, Gleason scores, and American Joint Committee on Cancer54 T and N stage tumours than the no local treatment and CRT groups (all P <0.001). Conversely, the radical prostatectomy and IMRT groups were less likely to receive bone radiation (radical prostatectomy 0%, IMRT 2%, CRT 7%, no local therapy 11%, P = 0.005) or ADT (radical prostatectomy 43%, IMRT 66%, CRT 88%, no local therapy 70%, P <0.001) in the 6-month period following diagnosis. On multivariable analysis including sociodemographic factors, tumour characteristics, Charlson comorbidity index, use of ADT, and bone radiation within 6 months of diagnosis, the adjusted hazard ratios for PCSM were 0.48 (95% CI 0.27–0.85, P = 0.01) for radical prostatectomy, 0.38 (95% CI 0.24–0.61, P <0.001) for IMRT, and 0.85 (95% CI 0.64–1.14, P = 0.3) for CRT.
Additionally, Heidenreich and colleagues48 reported the first case-control study to examine prostatectomy in selected men with metastatic disease. Their study was limited to 23 men with ≤3 lesions on bone scan, no visceral or extended lymph node metastases, and response to ADT (PSA nadir <1.0 ng/ml after 6 months of ADT). They identified a control cohort of 38 men with similar clinical and pathological characteristics. However, it should be noted that only 26 of the 38 control patients (68%) responded to ADT. Both groups were followed up for evidence of castration resistance (defined as biochemical progression in the presence of castrate serum testosterone levels (≤50 ng/dl)), clinical progression (defined as new symptoms due to local progression or lymphonodular or systemic metastases), and survival. After a median follow-up period of 40.6 months in the radical prostatectomy group and 44.0 months in the non-radical-prostatectomy group (P>0.05), men treated with radical prostatectomy demonstrated significantly increased time to castration resistance (median 40 months versus 29 months, P= 0.014) and freedom from clinical progression (median 38.6 months versus 26.5 months, P = 0.032). The crude proportion of overall survival (91.3% versus 78.9%, P = 0.048) was similarly improved in the radical prostatectomy cohort. The proportion of cancer-specific survival (95.6% versus 84.2%, P = 0.043) was improved with radical prostatectomy, although the median cancer-specific survival was not (47.0 versus 40.5 months, P >0.05). Given that all surgical patients were responsive to ADT, why the control group was not limited to the 26 ADT-responsive men is unclear. This additional uniformity between cases and controls could have limited the effect of selection bias, which is a substantial limitation of such reports.
Finally, Cho et al.49performed a case-control comparison in 140 men with varying extents of metastatic disease; 38 men underwent prostate radiotherapy, 39 men underwent palliative radiotherapy, and 63 men did not undergo radiotherapy. Over a median follow-up period of 34.0 months (range 1.7–108.8 months), the 3-year overall survival was 69% in those who had received prostate radiotherapy and 43% in the other groups (P = 0.004). Similarly, 3-year biochemical-failure-free survival was improved in the prostate radiation cohort (52% versus 16%, P = 0.002). The authors did observe Grade 3 thrombocytopenia in four patients (11%) and Grade 3 leukocytopenia in three patients (8%) but did not report any Grade 3 genitourinary complications.
Metastasis-directed therapy
Five studies describing metastasis-directed therapy can be found in the literature28–31,55 (TABLE 3). Muacevic et al.55 treated a total of 40 patients with one or two bony metastases using stereotactic body radiation (SBRT), 34 (85%) of whom were asymptomatic on presentation. Patients were considered for treatment regardless of their initial response to ADT. The authors observed local control (defined as lack of documented tumour growth on MRI and lack of increased tracer uptake on choline PET–CT compared with pretreatment imaging) in 95.5% of men at 2 years from treatment, including 27 men (68%) treated with ADT during the follow-up period. Adverse effects were relatively minor, including mild nausea in five men (12.5%) and a silent rib fracture in one (2.5%) patient. Ahmed et al.28 subsequently observed similar results using SBRT in a cohort of 17 men. In this study, the authors treated one liver lesion and one lymph node lesion in addition to 19 bone metastases. They observed 100% local control (defined as lack of tumour progression within the planning target volume) among evaluable lesions at a median follow-up point of 6 months, and nine (53%) patients reached an undetectable serum PSA. Interestingly, over half of the men with hormone-refractory disease had either reached an undetectable PSA level or had persistently declining PSA at the time of analysis. These authors measured safety according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE)56. After treatment of 21 lesions, the authors reported two (9.5%) Grade 1 and two (9.5%) Grade 2 events, with no Grade 3 or late toxicities observed.
Table 3.
Retrospective data for metastasis-directed radiotherapy
| Source | Study design | Treated cohort (% of lesions) | Median dose (range) and fractions | Toxicity per CTCAE (% of lesions) | Median ADT-FS (months) | Clinical outcomes* |
|---|---|---|---|---|---|---|
| Muacevic et al.55 | Case series, n= 40, 64 lesions, median follow-up period:10.2 months | Bone (100%) | 20 Gy (16.5 22), 1 fraction (80% of patients) | NR | NR | 2-year local control: 95.5% |
| Ahmed et al.28 | Case series, n= 17, 21 lesions, median follow-up period: 6 months |
|
|
|
NR |
|
| Berkovic et al.29 | Case series, n= 24, 29 lesions, median follow-up period: 24 months |
|
|
|
38 |
|
| Schick et al.30 | Case series n= 50, 79 lesions, median follow-up period: 31 months. |
|
|
|
NR |
|
| Decaestecker et al.31 | Case series, n= 50, 70 lesions, median follow-up period: 2 years |
|
|
25 |
|
ADT, androgen deprivation therapy; ADT-FS, androgen deprivation therapy-free survival; bRFS, biochemical-relapse-free survival; CPFS, clinical progression-free survival, CSS, cancer-specific survival; CTCAE, Common Terminology Criteria for Adverse Events; FFDP, failure from distant progression; LN, lymph node; NR, not reported; NS, not specified; OS, overall survival; PCSM, prostate cancer-specific mortality; PFS, progression-free survival.
In cases of unspecified time frame, values refer to proportion experiencing outcome during total follow-up period.
Percentage of patients.
In a cohort of 50 men, Schick and colleagues30 used EBRT to treat distant and/or regional lymph nodes in 33 men (66%), bone in 15 men (30%), lung in one man (2%), and both bone and lung in one man (2%). After a median follow-up duration of 31 months, they reported 3-year biochemical-recurrence-free survival of 54.5%, clinical-failure (development of new metastases)-free survival of 58.6%, and overall survival of 92%. Similar to previous studies, they observed no Grade 3 toxic effects. Berkovic et al.29 treated 24 men with up to three bone or lymph node metastases using SBRT. Again, 2-year rates of local control were 100%; clinical-progression (detection of local progression or distant disease at reassessment)-free survival was 42%. Even after 11 patients (46%) underwent a second treatment and three (12.5%) underwent a third, adverse effects were limited to Grade 2 gastrointestinal (8%) and genitourinary (6%) toxicities, with no Grade 3 toxicities. Importantly, the authors report median ADT-free survival of 38 months after treatment.
In 2014, Decaestecker et al.31 described the treatment of 50 men with lymph node (54%), bone (44%), or visceral (2%) metastases with SBRT. After median follow-up period of 2 years, local control (defined as tumour progression within the irradiated planning target volume) was observed in 100% of the cohort. Progression-free-survival rates at 1 year and 2 years after treatment were 64% and 35%, respectively. Meanwhile, median delay to ADT was 25 months, with 82% of men avoiding ADT at 1 year from treatment. Again, toxicity was limited to Grade 1 (14%) and Grade 2 (6%) events. A pooled analysis of data from these and other cohorts confirmed no Grade 3 toxicity and improved progression-free survival in the treated population32.
Imaging of oligometastatic prostate cancer
Current clinical practice is to define the extent of meta-static prostate cancer with cross-sectional imaging (CT and/or MRI) as well as 99mTc-methylene diphosphonate planar or single photon emission tomography (SPECT) bone scan57. In an attempt to improve the lower limit of prostate cancer detection — and in doing so refine the definition of oligometastatic prostate cancer — the past few years have witnessed a flurry of research into novel PET radiotracers targeting lesions of the bone and soft tissue.
18F-sodium fluoride (Na18F) is perhaps the most widely available PET radiotracer for imaging prostate cancer. Like 99mTc-methylene diphosphonate, Na18F homes to areas of bone remodelling, enabling the detection of osteoblastic metastases. Given that PET offers improved spatial resolution over planar and SPECT imaging, it is not surprising that Na18F PET–CT has consistently been shown to offer superior sensitivity for detecting osseous metastases relative to conventional bone scan58–60. However, one limitation of Na18F PET–CT is the continued reliance on CT and/or MRI to evaluate for soft tissue metastases.
PET–CT with radiotracers that directly target cancer cells offers a more efficient and potentially sensitive approach for prostate cancer imaging. Radiotracers that have received attention for this purpose include 11C-choline, 18F-fluoroethylcholine, 18F-FACBC, and a host of molecules targeting prostate-specific membrane antigen (PSMA; also known as glutamate carboxy-peptidase) such as 68Ga-PSMA-11 and 18F-DCFPyL61,62. PSMA-targeted imaging has gained particular interest, as radiotracers targeting this cell surface protein have been shown in numerous reports to offer outstanding sensitivity for detecting small-volume sites of prostate cancer that are not detectable on conventional imaging63 (FIG. 1). For example, using 68Ga-PSMA-11 PET–CT, van Leeuwen and co-workers62 observed a prostate cancer detection rate of 54% in a cohort of patients with negative conventional imaging and rising PSA values (all <1.0 ng/ml) following radical prostatectomy.
Figure 1. Recurrent prostate cancer detected with PSMA-targeted 18F-DCFPyL PET–CT.
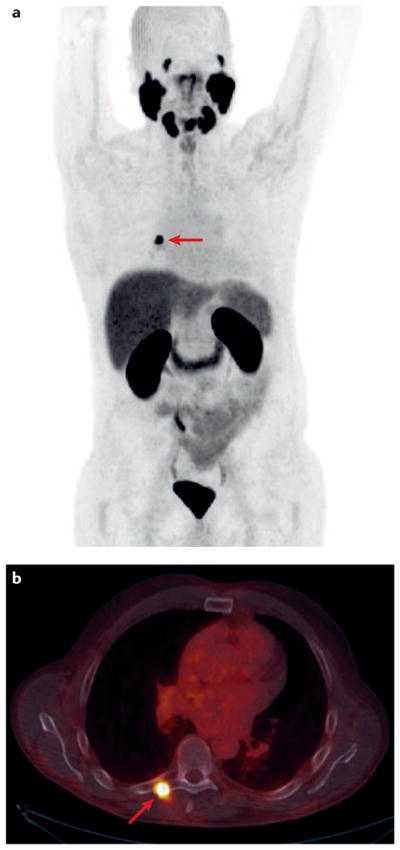
The patient presented approximately 5 years after radical prostatectomy with a serum PSA of 0.43 ng/ml and without evidence of disease on bone scan or contrast-enhanced CT. The (a) maximal intensity projection and (b) fused PET–CT axial images demonstrate recurrent disease in the transverse process of T7 (red arrows). The patient was successfully treated with stereotactic body radiation to the lesion.
The literature regarding these emerging tests for prostate cancer imaging is very promising, but remains immature at this stage. Accordingly, current guidelines and recommendations remain based on conventional imaging modalities. Given the potential for enhanced sensitivity using PET–CT, molecularly-targeted imaging will likely have an increasing role in defining the oligometastatic state as well as aiding in metastasis-directed therapy.
Considering the literature
The rationale for aggressive treatment
An aggressive approach to treating limited meta-static disease could prove beneficial. Indeed, treatment of the primary tumour seems to reduce the need for palliative interventions of locally advanced disease48,64. Furthermore, early local therapy might delay the initiation of systemic therapies such as ADT, which have a substantial effect on quality of life65. Undoubtedly, the ultimate goal of such an approach is to improve survival.
Several clinically and biologically plausible mechanisms could explain how aggressive local and metastasis-directed therapies could improve survival of men with oligometastatic prostate cancer. Improved clinical outcomes observed with local treatment of other primary cancers in the metastatic setting66–69 mean that tumour debulking could derive similar benefits in prostate cancer by prolonging the duration to a fatal tumour burden. With increased understanding of the communicating ecosystem of metastatic prostate cancer70,71, treatment of the primary tumour might also eliminate a critical site of interaction and support of metastases72–74. Other studies have discussed the potential role of the primary tumour in host immunosuppression and in creating a favourable hormonal milieu for cancer growth75–77.
Therapeutics targeting the anticancer immune response have shown great promise, particularly in melanoma, as well as bladder, lung, and other malignancies78–81. One phase III trial of ipilimumab failed to demonstrate an overall survival benefit in men with metastatic prostate cancer, but improvements in progression-free survival and overall disease-free survival were observed in men with lower tumour burden82. This outcome suggests that immunotherapies could be effective in the oligometastatic state, before disease burden is substantial enough to evade augmented anticancer immune surveillance. Accordingly, multiple trials have been opened and others are being planned to test immunotherapeutics in the oligometastatic setting. These studies include actively recruiting trials combining immunotherapy with treatment of the primary tumour, such as NCT02489357 and NCT02020070 (REFS 35,41).
Despite great promise, no clear evidence of improved survival based on an aggressive approach is currently available83, and can only be definitively obtained in the setting of randomized trials. This obstacle is augmented by the lack of a standard definition for oligometastatic prostate cancer, which must be properly set out before we can examine the effects of therapy in the oligometastatic state.
Defining the oligometastatic state
Based on earlier work of Soloway and colleagues43, Singh and et al.84 investigated survival as a function of the number of metastatic lesions observed per patient, finding that men with ≤5 lesions had similar survival to those with no metastases and significantly better survival than those with >5 lesions (P = 0.02). A number of studies subsequently adopted a definition of oligometastases based on this threshold27,28, and further studies have confirmed the role of lesion count in clinical outcomes85. In addition to lesion count, the site of metastasis has emerged as a substantial contributor to prognosis86. Review of published reports and active clinical trials confirms wide variability in how oligometastatic prostate cancer has been defined: six studies (38%) impose a limit of three metastases29,31,32,36,38,42 and five (31%) use a limit of five metastases27,28,34,39,40. Three studies (19%) include up to four metastases30,35,37, one (6%) up to 10 metastases41, and one (6%) does not strictly define an upper limit of lesions33. Eight of the 16 reports specified sites of metastasis that were considered in defining oligometastatic disease.
Indeed, lesion count and location do seem to affect clinical outcomes and, therefore, a clinical definition of metastasis such as that proposed by Rubin and colleagues12, whereby metastatic disease is designated as either a solitary metastasis (M1), two to five metastases (M2), polymetastases of a single organ (M3), or polymetastases of multiple organs (M4), seems logical. Of course, such distinctions are limited by several factors, most notably the sensitivity of imaging for detection. A more accurate definition would incorporate tumour-specific data to also provide a molecular basis for the observed phenotype. For example, Lussier and colleagues11 identified tumour microRNA expression patterns that can potentially distinguish cancers likely to remain in a stable, limited metastatic state (≤5 lesions) from those that will imminently progress to polymeta-static disease11. The authors confirmed their findings in animal models and demonstrated that one classifier — microRNA-200c — was capable of converting stable oligometastatic disease to a progressive polymetastatic state in xenograft models. Although additional testing and validation are undoubtedly necessary, such research represents great progress toward understanding the vastly different clinical states encountered87,88. As the authors suggest, the application of these methods could greatly improve patient selection for specific treatment pathways89.
Ultimately, we must consider what lesion counts and genomic classifiers are telling us — the oligometastatic state might simply be one part of an inevitable progression toward polymetastases, or, in some cases, perhaps it could represent a cancer’s final destination, whereby directed treatment of extant disease sites could prove curative. On a population level, the answer seems to encompass each of these possibilities. Indeed, some lesions will grow and metastasize to widespread disease over a short interval; others will do so gradually, such that the oligometastatic phenotype stably persists for months or years and is a clinically relevant state itself. Still others seem to lack the capacity to do so at all, such that a limited metastatic state represents its greatest potential for progression. The ability to discern these differences provides the potential to optimize the therapeutic approach for each patient.
Site-specific therapies
Regarding localized therapy
Overall, the data considering the use of local consolidative therapy for metastatic prostate cancer are quite limited. SEER-based studies, for example, lack data regarding the use of additional therapies such as chemohormonal therapy or site-specific EBRT, as well as patient comorbidities and performance status44,45. Such factors undoubtedly affect patient survival. By linking SEER with Medicare data, Satkunasivam et al.47 were able to mitigate some of these limitations; however, as a consequence their study was limited to men 65 years and older, resulting in a poorly generalizable analysis, particularly as an aggressive management approach might be more reasonable in younger men.
Acknowledging these limitations, the majority of findings suggest that local therapy could indeed improve survival in a selected group of patients. Interestingly, although the extent of metastatic disease consistently predicted cancer-specific mortality, the beneficial effect of local treatment was observed across all M stages. This observation raises the possibility that local treatment could benefit men in various metastatic states, and that overall patient health and comorbidity should be considered when using an aggressive approach90. Conversely, several studies have demonstrated that the benefit of local treatment is directly tied to the risk of cancer-specific mortality91,92. The reality is that optimal patient selection is likely to be based on a balance of both patient and disease characteristics.
Importantly, initial data suggest that prostatectomy can be performed safely in the metastatic setting. Heidenreich et al.48 reported no Clavien Grade IV or V complications in men treated with radical prostatectomy, and the proportion experiencing grades I–III complications was similar to or better than grade-specific controls. Furthermore, palliative intervention was required in 11 of 38 (28.9%) control patients, compared with zero men who underwent radical prostatectomy, and 21 (91.3%) patients reported postoperative continence, requiring either zero or one pad per day. A retrospective multi-institutional analysis of radical prostatectomy in the setting of distant metastases was similarly encouraging93. The overall rates of complications, readmission, and reoperation were 20.8%, 3.8%, and 1.9%, respectively, versus 19.4%, 3.0%, and 2.3%, respectively in open cases performed for standard indications94.
Regarding metastasis-directed therapy
With increased adoption of local ablative techniques, most notably SBRT, increasing data now describe patient outcomes after metastasis-directed therapies28–31,55. Although cancer-specific survival and overall survival outcomes are limited by short follow-up intervals, two undeniable trends can be identified in the available data. First, use of SBRT for treatment of metastatic lesions seems to be very safe. In a cohort of 141 men included in four studies using CTCAE-based reporting of adverse effects, zero (0%) Grade 3 toxicities were reported. Low-grade toxicity was generally limited to gastrointestinal effects, such as nausea, and was consistently observed in <20% of treated patients. Second, local control rates were consistently very high, including ≥95.5% in the four studies reporting this outcome (median follow-up duration 6–31 months). Establishing a minimal likelihood of substantial adverse effects is crucial when considering widespread use of these techniques.
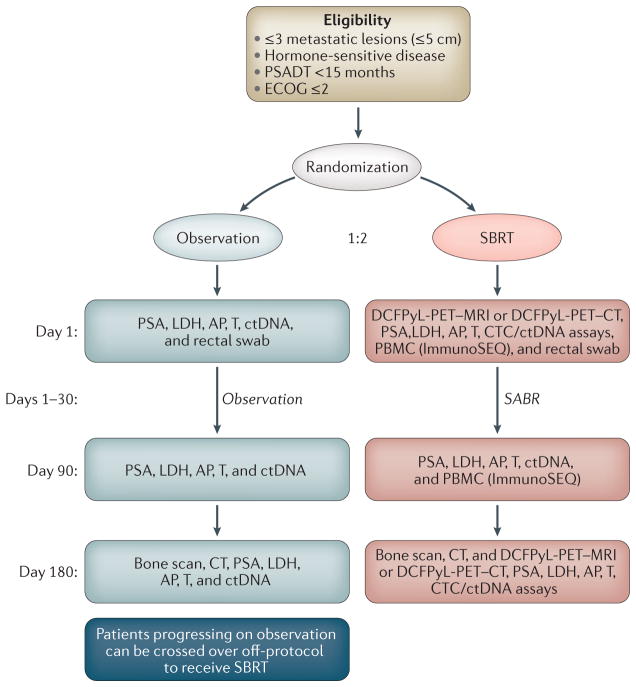
Although a cornerstone of treating metastatic prostate cancer, ADT is associated with several deleterious adverse effects and, in some patients, might decrease overall life expectancy65,95. These effects raise the question of whether consolidative treatments can prevent or delay the use of ADT. Given varying baseline rates of ADT use and varying indications for initiating ADT during follow-up monitoring, quantifying an average effect of directed therapies is difficult. Berkovic et al.29 and Decaestecker et al.29,31 reported median ADT-free survival of 38 months and 25 months, respectively, after treatment with SBRT. Three other studies calculated the proportion of treated men who ever used ADT, with values ranging from 68% to 98%28,30,55. Furthermore, several studies reported variably defined outcomes, such as clinical progression or biochemical-progression-free survival. These rates were inconsistently reported and ranged widely across studies, again leading to difficulty in deriving a general trend in outcomes. Finally, it must be acknowledged that definitive conclusions regarding more meaningful clinical outcomes cannot be derived from surrogate measures such as those reported, and these studies notably lack a comparison arm. More definitive data will become available after the completion and reporting of prospective randomized studies (TABLE 1) such as the Belgian STOMP trial96 and the Baltimore ORIOLE trial42 (FIG. 2), which primarily aims to assess clinical progression in men randomized to receive SBRT versus observation. Secondary end points include local control, ADT-free survival, toxicity, and quality-of-life outcomes.
Figure 2. Schema for the phase II Randomized Observation versus Stereotactic Ablative RadiatIon for OLigometastatic Prostate CancEr (ORIOLE) trial.
Men with metachronous hormone-naive oligometastatic disease will be enrolled and dynamically randomized to the schema as shown. AP, alkaline phosphatase; CTCs, circulating tumour cells; ctDNA, cell-free circulating tumour DNA; LDH, lactate dehydrogenase; PBMC, peripheral blood mononuclear cells; SABR, stereotactic ablative radiation; SBRT, stereotactic radiation therapy; T, serum testosterone. ORIOLE is sponsored by the National Cancer Institute (NCI) 1U01CA183031 and a Movember-PCF Challenge Award.
We would encourage future retrospective and prospective studies to clearly distinguish the number of men using ADT at baseline, and to provide median and 1-year ADT-free survival in those who are not. We propose that future studies define clinical progression as the detection of a new metastatic lesion or the occurrence of a clinically significant event (such as pain onset or fracture) in a previously detected stable lesion. Given the limited time course over which such events occur, it would be reasonable to share median, 1-year, and 3-year values for these outcomes.
Future directions and recommendations
Existing data suggest that local and metastasis-directed therapies are safe in men with metastatic prostate cancer. Whether we should take an aggressive approach to meta-static disease simply because it is available and whether this approach actually helps patients still needs addressing. Only retrospective data exist in this setting, and few of these studies provide an appropriate control group for comparison. Thus, the reality is that we are only just beginning to answer this question. In light of this limitation, we make the following recommendations for considering an aggressive treatment approach for men with known metastatic disease.
As in other settings, only those patients likely to suffer mortality or substantial morbidity due to their disease should be considered for aggressive treatment, which should only be offered in the setting of an institutional-review-board-approved clinical trial or prospective registry. Patients must be fully informed of the potential risks and benefits associated with an aggressive approach; specifically, they must be made aware that data from appropriately conducted studies to demonstrate prolonged survival as a result of treatment is lacking. Men who do undergo treatment should be assessed and treated in a multidisciplinary setting including medical oncology, radiation oncology, and urology. Clinicians managing such patients should consider establishing a prostate cancer multidisciplinary clinic if not already present at their institution97. Finally, establishment of an institutional biorepository for banking of serum, urine, stool, and tissue samples should be considered — only with the committed and coordinated efforts of the entire health-care team will we find answers to the many questions that remain.
Conclusions
In the future, oligometastatic prostate cancer is likely to be defined based on genomic and biological features in addition to pertinent clinical criteria. For now, a definition based on up to five detectable lesions is widely employed and is reasonable for use. Available data suggest that local therapies such as prostatectomy and radiotherapy can be performed safely in the presence of metastatic disease and might prevent the need for future palliative treatments. Similarly, metastasis-directed therapies such as SBRT carry a low risk of toxic effects and provide excellent local control. At this time, insufficient data are available to draw conclusions regarding the effect of aggressive therapies on overall or cancer-specific survival. Prospective, well-controlled trials are necessary and should aim to report outcomes in a consistent and systematic manner.
Key points.
Cancers presenting in the oligometastatic state likely include a spectrum of biologies
Some oligometastatic lesions quickly progress to widespread metastases, others metastasize gradually, and others lack the capacity for widespread progression, such that oligometastatic disease represents their maximum potential for progression
Preliminary genomic data support a molecular basis underlying phenotypic variability; however, for now, oligometastatic prostate cancer can be reasonably defined by up to five extrapelvic lesions
Local consolidative therapies, such as prostatectomy and radiotherapy, seem safe to perform in the metastatic setting and seem to reduce the need for palliative treatment; the effect of local therapy on survival outcomes cannot be determined conclusively using available data
Metastasis-directed approaches, such as stereotactic body radiotherapy, are associated with minimal toxicity and provide excellent local control; however, current data are insufficient for determining their effect on oncological outcomes
Aggressive treatment of oligometastatic prostate cancer should be considered only in the setting of prospective clinical trials or registries, with the patient informed of the limited evidence of benefit from such approaches
Acknowledgments
A.E.R. is supported by a DOD PRTA award (W81XWH-13-1-0445) as well as a PCF Young Investigator Award and Patrick C. Walsh Investigator Grant. P. T. Tran was funded by the Motta and Nesbitt Families, the DoD (W81XWH-11-1-0272), a Kimmel Translational Science Award (SKF-13-021), an ACS Scholar award (122688-RSG-12-196-01-TBG), the NIH (R01CA166348 & 1U01CA183031) and a Movember-PCF Challenge Award. E.M.S. is supported by NIH U01CA196390-01.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 3.Rinker-Schaeffer CW, Partin AW, Isaacs WB, Coffey DS, Isaacs JT. Molecular and cellular changes associated with the acquisition of metastatic ability by prostatic cancer cells. Prostate. 1994;25:249–265. doi: 10.1002/pros.2990250505. [DOI] [PubMed] [Google Scholar]
- 4.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 5.Pastorino U, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 6.Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6:8491–8524. doi: 10.18632/oncotarget.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoo V. Is there another bite of the cherry? The case for radical local therapy for oligometastatic disease in prostate cancer. Eur Urol. 2016;69:13–14. doi: 10.1016/j.eururo.2015.07.073. [DOI] [PubMed] [Google Scholar]
- 8.Evangelista L, et al. New clinical indications for 18F/11C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol. 2016;70:161–175. doi: 10.1016/j.eururo.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Tamoto E, et al. Gene-expression profile changes correlated with tumor progression and lymph node metastasis in esophageal cancer. Clin Cancer Res. 2004;10:3629–3638. doi: 10.1158/1078-0432.CCR-04-0048. [DOI] [PubMed] [Google Scholar]
- 10.Wuttig D, et al. Gene signatures of pulmonary metastases of renal cell carcinoma reflect the disease-free interval and the number of metastases per patient. Int J Cancer. 2009;125:474–482. doi: 10.1002/ijc.24353. [DOI] [PubMed] [Google Scholar]
- 11.Lussier YA, et al. MicroRNA expression characterizes oligometastasis(es) PLoS ONE. 2011;6:e28650. doi: 10.1371/journal.pone.0028650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol. 2006;16:120–130. doi: 10.1016/j.semradonc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Bayne CE, et al. Treatment of the primary tumor in metastatic prostate cancer: current concepts and future perspectives. Eur Urol. 2016;69:775–787. doi: 10.1016/j.eururo.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich A, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 16.Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9:S3–S8. [PMC free article] [PubMed] [Google Scholar]
- 17.James ND, et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016;2:348–357. doi: 10.1001/jamaoncol.2015.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CC, Gray PJ, Jemal A, Efstathiou JA. Androgen deprivation with or without radiation therapy for clinically node-positive prostate cancer. J Natl Cancer Inst. 2015;107:djv119. doi: 10.1093/jnci/djv119. [DOI] [PubMed] [Google Scholar]
- 19.Tward JD, Kokeny KE, Shrieve DC. Radiation therapy for clinically node-positive prostate adenocarcinoma is correlated with improved overall and prostate cancer-specific survival. Pract Radiat Oncol. 2013;3:234–240. doi: 10.1016/j.prro.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Rusthoven CG, et al. The impact of definitive local therapy for lymph node-positive prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2014;88:1064–1073. doi: 10.1016/j.ijrobp.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Aoun F, Peltier A, van Velthoven R. A comprehensive review of contemporary role of local treatment of the primary tumor and/or the metastases in metastatic prostate cancer. Biomed Res Int. 2014;2014:501213. doi: 10.1155/2014/501213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ost P, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67:852–863. doi: 10.1016/j.eururo.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2010;57:49–59. doi: 10.1016/j.eururo.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich A, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Attard G, et al. Combining enzalutamide with abiraterone, prednisone, and androgen deprivation therapy in the STAMPEDE trial. Eur Urol. 2014;66:799–802. doi: 10.1016/j.eururo.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Sweeney CJ, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabata KI, et al. Radiotherapy for oligometastases and oligo-recurrence of bone in prostate cancer. Pulm Med. 2012;2012:541656. doi: 10.1155/2012/541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed KA, et al. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2013;2:215. doi: 10.3389/fonc.2012.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berkovic P, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11:27–32. doi: 10.1016/j.clgc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Schick U, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 2013;52:1622–1628. doi: 10.3109/0284186X.2013.764010. [DOI] [PubMed] [Google Scholar]
- 31.Decaestecker K, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol. 2014;9:135. doi: 10.1186/1748-717X-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ost P, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12. doi: 10.1016/j.eururo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 33.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT01859221.
- 34.US National Library of Medicine. ClinicalTrials.gov. 2015 https://clinicaltrials.gov/ct2/show/NCT02563691.
- 35.US National Library of Medicine. ClinicalTrials.gov. 2015 https://clinicaltrials.gov/ct2/show/NCT02489357.
- 36.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT01777802.
- 37.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02192788.
- 38.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT01558427.
- 39.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02264379.
- 40.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT00544830.
- 41.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02020070.
- 42.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02680587.
- 43.Soloway MS, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 44.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058–1066. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Antwi S, Everson TM. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: a population-based, propensity score analysis. Cancer Epidemiol. 2014;38:435–441. doi: 10.1016/j.canep.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. Eur Urol. 2014;66:602–603. doi: 10.1016/j.eururo.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Satkunasivam R, et al. Radical prostatectomy or external beam radiation therapy versus no local therapy for survival benefit in metastatic prostate cancer: a SEER-Medicare analysis. J Urol. 2015;194:378–385. doi: 10.1016/j.juro.2015.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193:832–838. doi: 10.1016/j.juro.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 49.Cho Y, et al. Does radiotherapy for the primary tumor benefit prostate cancer patients with distant metastasis at initial diagnosis? PLoS ONE. 2016;11:e0147191. doi: 10.1371/journal.pone.0147191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heinze G, Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1704–1708. doi: 10.1093/eurheartj/ehr031. [DOI] [PubMed] [Google Scholar]
- 51.Wu JSY, Wong R, Johnston M, Bezjak A, Whelan T. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 52.Cameron MG, Kersten C, Guren MG, Fosså SD, Vistad I. Palliative pelvic radiotherapy of symptomatic incurable prostate cancer — a systematic review. Radiother Oncol. 2014;110:55–60. doi: 10.1016/j.radonc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Ellsworth SG, et al. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89:1100–1105. doi: 10.1016/j.ijrobp.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edge SB, et al. AJCC Cancer Staging Manual. 7. Springer; 2010. [Google Scholar]
- 55.Muacevic A, et al. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol. 2013;31:455–460. doi: 10.1016/j.urolonc.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 56.Trotti A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 57.Mohler JL, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 58.Schirrmeister H, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623–1629. [PubMed] [Google Scholar]
- 59.Poulsen MH, et al. Spine metastases in prostate cancer: comparison of technetium-99m-MDP whole-body bone scintigraphy, [18F]choline positron emission tomography(PET)/computed tomography (CT) and [18F] NaF PET/CT. BJU Int. 2014;114:818–823. doi: 10.1111/bju.12599. [DOI] [PubMed] [Google Scholar]
- 60.Apolo AB, et al. Prospective study evaluating Na18F-positron emission tomography/computed tomography (NaF-PET/CT) in predicting clinical outcomes and survival in advanced prostate cancer. J Nucl Med. 2016;57:886–892. doi: 10.2967/jnumed.115.166512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho SY, Szabo Z. Molecular imaging of urogenital diseases. Semin Nucl Med. 2014;44:93–109. doi: 10.1053/j.semnuclmed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Leeuwen PJ, et al. 68Ga-PSMA has high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–739. doi: 10.1111/bju.13397. [DOI] [PubMed] [Google Scholar]
- 63.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 64.Won ACM, Gurney H, Marx G, De Souza P, Patel MI. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 2013;112:E250–E255. doi: 10.1111/bju.12169. [DOI] [PubMed] [Google Scholar]
- 65.Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–2399. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- 66.Mickisch G, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 67.Flanigan RC, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–1076. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 68.Bristow RE. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 69.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–228. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 70.Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1:158–164. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gundem G, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coen JJ. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 73.Kim MY, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaplan RN, Rafii S, Lyden D. Preparing the ‘soil’: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Predina JD, et al. Cytoreduction surgery reduces systemic myeloid suppressor cell populations and restores intratumoral immunotherapy effectiveness. J Hematol Oncol. 2012;5:34. doi: 10.1186/1756-8722-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin XJ, et al. Tumor cytoreduction results in better response to androgen ablation — a preliminary report of palliative transurethral resection of the prostate in metastatic hormone sensitive prostate cancer. Urol Oncol. 2012;30:145–149. doi: 10.1016/j.urolonc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 77.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 81.Brahmer J, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwon ED, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klein EA. Seeing and not believing: oligometastases and the future of metastatic prostate cancer. Eur Urol. 2015;67:864–865. doi: 10.1016/j.eururo.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 84.Singh D, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol. 2004;58:3–10. doi: 10.1016/s0360-3016(03)01442-1. [DOI] [PubMed] [Google Scholar]
- 85.Ost P, et al. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate. 2014;74:297–305. doi: 10.1002/pros.22750. [DOI] [PubMed] [Google Scholar]
- 86.Gandaglia G, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. 2015;68:325–334. doi: 10.1016/j.eururo.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 87.Shindo-Okada N, Takeuchi K, Nagamachi Y. Establishment of cell lines with high- and low-metastatic potential from PC-14 human lung adenocarcinoma. Jpn J Cancer Res. 2001;92:174–183. doi: 10.1111/j.1349-7006.2001.tb01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y, et al. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630–636. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol. 2013;31:1384–1390. doi: 10.1200/JCO.2012.45.9651. [DOI] [PubMed] [Google Scholar]
- 90.Daskivich TJ, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158:709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fossati N, et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: a SEER-based study. Eur Urol. 2015;67:3–6. doi: 10.1016/j.eururo.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 92.Bradley CJ, Dahman B, Anscher M. Prostate cancer treatment and survival: evidence for men with prevalent comorbid conditions. Med Care. 2014;52:482–489. doi: 10.1097/MLR.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sooriakumaran P, et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur Urol. 2016;69:788–794. doi: 10.1016/j.eururo.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 94.Tewari A, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15. doi: 10.1016/j.eururo.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 95.Sammon JD, et al. Patterns of declining use and the adverse effect of primary androgen deprivation on all-cause mortality in elderly men with prostate cancer. Eur Urol. 2015;68:32–39. doi: 10.1016/j.eururo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 96.Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer. 2014;14:671. doi: 10.1186/1471-2407-14-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sundi D, et al. Establishment of a new prostate cancer multidisciplinary clinic: format and initial experience. Prostate. 2015;75:191–199. doi: 10.1002/pros.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]