Abstract
Several recent studies suggest that in the human population, a routine, short anesthetic in otherwise healthy infants is void of neurodevelopmental insult. On the other hand, many human retrospective epidemiological studies report evidence of cognitive abnormalities in children after testing those who had different anesthesia-requiring procedures in early childhood. We tested in a rat model whether post-anesthesia stressful environmental factors can contribute to developmental abnormalities that were initiated by a relatively short exposure to sevoflurane, the most widely used anesthetic in pediatric anesthesia, whose polyvalent actions include enhancement of gamma-aminobutyric acid type A receptor (GABAAR) activity.
Postnatal day 6 (P6) male Sprague-Dawley rats were anesthetized with sevoflurane for 60 min. To simulate subsequent stress, the animals were subjected to a single maternal separation for 180 min at P10. To study the role of GABAAR-mediated depolarization, subgroups of P6 rats received a single injection of the Na+-K+-2Cl− (NKCC1) inhibitor, bumetanide, prior to initiation of anesthesia with sevoflurane. Rats that were exposed to sevoflurane had decreased hypothalamic K+-2Cl− (KCC2) mRNA level (F(2,13) = 3.839, P = 0.049), increased NKCC1/KCC2 mRNA ratio (F(2,13) = 5.043, P = 0.024) and increased corticotropin-releasing hormone (CRH) mRNA level (F(2,12) = 9.450, P = 0.003) at P10, the age at which maternal separation was imposed. Adult rats, neonatally exposed to a combination of sevoflurane and maternal separation, exhibited increases in the escape latencies greater than animals exposed to sevoflurane only (P = 0.012), and only rats in the sevoflurane plus maternal separation group spent significantly less time in the target quadrant during the Morris water maze test (F(4,55) = 4.856, P = 0.002). Bumetanide ameliorated abnormalities induced by sevoflurane and a combination of sevoflurane plus maternal separation.
Neonatal exposure to sevoflurane may sensitize to stressors later in life, and post-exposure stress may exacerbate neurodevelopmental abnormalities even after a relatively short exposure to sevoflurane in rodents. The NKCC1 downregulation prior to exposure to the anesthetic may be therapeutic.
Keywords: sevoflurane, stress, developing brain, NKCC1/KCC2, neurobehavioral abnormalities, environmental factor
Introduction
Millions of preemies and neonates require anesthesia for diagnostic and operative procedures every year. Numerous studies in species as divergent as rats and rhesus monkeys provide strong support for developmental neurocognitive deficiencies induced by neonatal exposure to general anesthetics [1–10]. To what extent these data can be applied to human patients is currently the subject of vigorous debate [11, 12]. Considering the duration of anesthesia relative to a subject’s life expectancy, humans should be much less affected, if at all. In line with this possibility, a recent study analyzing the neurocognitive outcomes of a single anesthesia exposure for elective inguinal hernia surgery before the age of 3 years of otherwise healthy children found no evidence of significant differences in IQ scores in later childhood when compared with healthy siblings with no anesthesia exposure [13]. On the other hand, several human retrospective epidemiological studies report evidence of cognitive abnormalities in children after testing those who had different anesthesia-requiring procedures before 3 years of age [14–19]. It is plausible that such abnormalities are the result of a cumulative impact initially programmed by neonatal anesthesia exposure and later amplified by adverse stressful environmental factors such as diseases and/or various types of psychosocial stress. The involvement of environmental factors in modulating the neurobehavioral effects of prolonged neonatal anesthesia exposure is supported by the results of rodent studies. Thus, neonatal anesthesia-induced neurobehavioral abnormalities in rodents were ameliorated and exacerbated by housing the exposed animals in enriched and enrichment-deprived environments, respectively [20, 21]. In addition, rats neonatally exposed to sevoflurane, an anesthetic whose polyvalent actions include enhancement of gamma-aminobutyric acid type A receptor (GABAAR) activity, exhibited exacerbated endocrine corticosterone responses to stress in adulthood (with larger increases in male rats), suggesting greater vulnerability of the exposed rats to stressful environmental factors later in life [9]. Sevoflurane-enhanced GABAAR-mediated signaling, which undergoes a fundamental transition from predominantly depolarizing/stimulatory to inhibitory in the rat brain during the first two postnatal weeks, may initiate these abnormalities as the Na+-K+-2Cl− (NKCC1) Cl− importer inhibition prior to anesthesia was protective [9]. The current study has been designed to test whether developmental abnormalities programmed by a relatively short exposure of postnatal day 6 (P6) rats to sevoflurane, which may not be sufficient to induce developmental abnormalities by itself, can be further exacerbated by post-exposure stress. To receive a proof of principle, we tested whether a single episode of maternal separation for 180 min at P10, an experience which, like short exposure to sevoflurane, may not be sufficient to induce obvious developmental abnormalities, contributes to a significant combined effect of the two interventions. At P10, the rat hypothalamic GABAAR-mediated signaling already has an adult phenotype, but the stress hyporesponsive period (SHRP) is still close to its midpoint, the period of heightened vulnerability to maternal deprivation [22]. We also tested whether the effects of neonatal anesthesia and post-anesthesia stress can be ameliorated by pretreatment with bumetanide. Bumetanide, a loop diuretic, is most selective of currently available NKCC1 inhibitors and is widely used in animal and human studies to shift GABAAR-mediated signaling in immature neurons to inhibitory [22–28]. Pretreatment with bumetanide ameliorated developmental abnormalities, including exacerbated neuroendocrine responses to stress in adulthood, induced by prolonged exposures of neonatal rats to sevoflurane [2, 6–10].
Materials and Methods
Animals
The present study was approved by the Ethics Committee of Jinling Hospital, Nanjing University, China, and was performed in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, Maryland). Sprague-Dawley rats (dams with pups) were purchased from the Animal Center of Jinling Hospital, Nanjing, China, and were housed individually in standard conditions with a 12-h light/dark cycle (light from 07:00 to 19:00) at 23 ± 1°C and ad libitum access to food and water. At the age of 21 days, pups were weaned and housed in sex-matched groups of three to four for the rest of the study. At the beginning of the experiment, the pups were well nourished, as judged by their stomachs being full of milk (detectable through the transparent abdominal wall). To control for litter variability, several pups from different litters were used for each treatment condition. Multiple sets of animals were used in the experiments. The data reported in this study was collected from 76 male rats.
Treatment groups
Two cohorts of animals were studied (Table 1). Neonatal rats in cohort 1 were treated at postnatal day 6 (P6) and then sacrificed to collect brain tissue at P10, the age at which maternal separation was imposed to animals in cohort 2. Rats in cohort 2 were used for behavioral studies in adulthood. P6 male rat pups were kept in a temperature-controlled chamber (+37 °C) with a continuous supply of oxygen (1.5 L/min) during anesthesia with 6% sevoflurane (Shanghai Hengrui pharmaceutical Co. Ltd., Shanghai, China) for 3 min for anesthesia induction and 2.1% sevoflurane for 57 min for anesthesia maintenance (the Sevoflurane group). Gas monitoring was performed using a calibrated Datex side stream analyzer (Vamos, Drägerwerk AG & Co., Shanghai, China), which sampled from the interior of the animal chamber. According to Orliaguet et al, 2.1% sevoflurane lies near 0.6 minimum alveolar concentration for P6 rats [29]. At the doses of 2.1 % sevoflurane the pups did not exhibit a righting reflex. Previously we have shown that blood glucose and gas levels after anesthesia with 2.1% sevoflurane were in the normal range [2]. To simulate post-anesthesia stress, half of the animals in the Sevoflurane group were subjected to maternal separation (MS) for 180 min at P10 — the Sevoflurane plus MS10 group. To assess the effects of MS at P10, a separate group of rat pups was subjected to MS for 180 min at P10 only — the MS10 group. The control animals were subjected to animal facility rearing only — the Control group. To study the role of GABAAR-mediated depolarization, subgroups of P6 rats received a single injection of the NKCC1 inhibitor, bumetanide (1.82 mg kg−1, intraperitoneally, IP, Ben Venue Laboratories, Inc., Bedford, OH), 15 min prior to initiation of anesthesia with sevoflurane (Bumetanide plus Sevoflurane plus MS10 group). In order to control for prior anesthesia injections of bumetanide, all treatment groups except the Control group received equal volumes of saline (IP) at P6. Previously we have shown that saline at these volumes does not cause any obvious physiological responses [8, 9]. A separate group with bumetanide only has not been included. We have reported that bumetanide alone, without anesthesia, did not induce long-term behavioral effects, but ameliorated developmental effects of neonatal sevoflurane [6]. Rats in cohort 2 were evaluated in the Morris water maze (MWM) starting at P54.
Table 1.
Treatment groups.
| Cohort 1 | ||
|---|---|---|
| Group Number | Treatment | Number of animals per group |
| 1 | Facility rearing only (the Control group) | 5 |
| 2 | Anesthesia with sevoflurane for 60 min at P6 (the Sevoflurane group) | 6 |
| 3 | Bumetanide prior to anesthesia with sevoflurane for 60 min at P6 (the Bumetanide plus Sevoflurane group) | 5 |
| Cohort 2 | ||
| Group Number | Treatment | Number of animals per group |
| 1 | Facility rearing only (the Control group) | 12 |
| 2 | Anesthesia with sevoflurane for 60 min at P6 (the Sevoflurane group) | 12 |
| 3 | Maternal separation for 180 min at P10 (the MS10 group) | 12 |
| 4 | Anesthesia with sevoflurane for 60 min at P6 plus maternal separation for 180 min at P10 (the Sevoflurane plus MS10 group) | 12 |
| 5 | Bumetanide prior to sevoflurane at P6 plus maternal separation for 180 min at P10 (the Bumetanide plus Sevoflurane plus MS10 group) | 12 |
Analyses of mRNA levels for NKCC1, K+-2Cl− (KCC2) and CRH
The mRNA levels for NKCC1, KCC2, and CRH in the hypothalamus were analyzed via real-time reverse transcription-PCR (qRT-PCR) in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). RNA was extracted from the samples using an RNeasy Plus Kit (Qiagen, Valencia, CA, USA), reverse-transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad Laboratories, Hercules, CA, USA), and then analyzed via qRT-PCR. Oligonucleotide primers and Taqman probes specific for the above genes were obtained from Applied Biosystems (Carlsbad, CA, USA): NKCC1 (Rn00582505_m1), KCC2 (Rn00592624_m1), and CRH (Rn01462137_m1). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (Rn01775763_g1). Gene expression was calculated using the ΔΔCT method and data was presented as relative fold change from that of control animals.
Morris Water Maze Test
The rats’ spatial learning and memory were tested with the MWM apparatus (Shanghai Softmaze Information Technology Co. Ltd, Shanghai, China, XR-XM101). The test was performed during the light phase of a 12-h light/dark cycle between 9:00 A.M. and 4:00 P.M. The apparatus consisted of a circular tank (150 cm in diameter, 55 cm in height) filled with water (35 cm deep, temperature 23 ± 1°C). The pool was surrounded with an opaque curtain and was placed in an isolated room with four visual cues on the wall of the tank. A nontoxic paint was used to render the water opaque. The tank was divided into four equal quadrants, labeled left-top, left-bottom, right-top, and right-bottom. A 10-cm diameter platform (the escape platform) was submerged 2 cm below the surface of the water at a constant location in one of the quadrants, i.e., the left-top quadrant in this study. The rats were tested in the MWM four times per day for 6 consecutive days from P54 to P59 to search for the escape platform. The starting points for each rat were randomly chosen in one of the four quadrants, and each trial had a ceiling time of 60 s or a stopping time at which the escape platform was found. Between the trials, the rat was allowed to stay on the platform for additional 15 s to rest. If the rat failed to find the platform within 60 s, it was gently guided to the platform and allowed to rest on the platform for 15 s prior to the next trial. A video tracking system recorded the swimming tracks of the rats. Twenty-four hours later, at P60, the spatial memory test was performed. The test lasted 60 s, during which the escape platform was removed. The rat was placed in the tank in the contralateral quadrant relative to original location of the escape platform (the target quadrant). The time spent in the target quadrant was recorded.
Statistical Analysis
Values are reported as mean ± SD. SigmaPlot 13.0 software (Systat Software, Inc., Point Richmond, CA) was used for statistical analyses. Two-way repeated measures ANOVA with day and treatment as the independent variables was used to analyze the escape latency data during the 6-day training period in the MWM experiments. All other multiple comparisons among groups were analyzed using one-way ANOVA followed by the Holm-Sidak test. P < 0.05 was considered significant. The sample sizes in this study were based on previous experience with the same experimental techniques [21, 30].
Results
Anesthesia with sevoflurane for 60 min at P6 increases the NKCC1/KCC2 mRNA ratio and CRH mRNA level in the hypothalamus of P10 rat pups; an alleviating effect of pretreatment with bumetanide
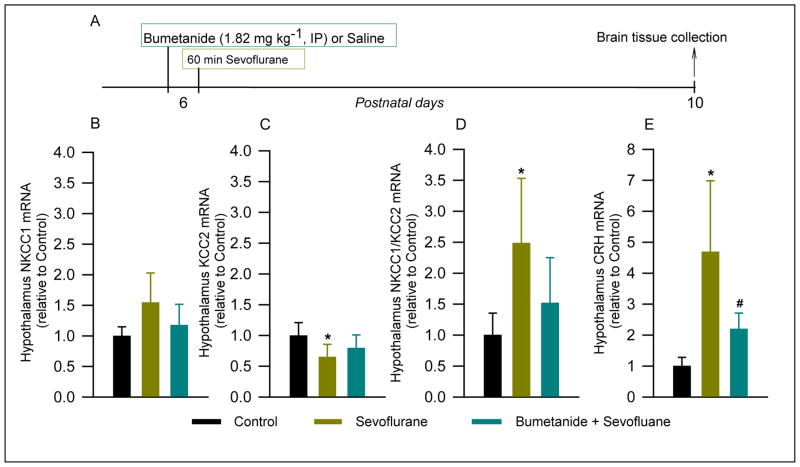
There were no significant treatment effects on hypothalamic NKCC1 mRNA levels at P10 (F(2,13) = 3.252, P = 0.072; Fig. 1A,B). Rat pups exposed to sevoflurane anesthesia at P6 exhibited reduced the KCC2 mRNA levels in the hypothalamus at P10 (F(2,13) = 3.839, P = 0.049; Fig. 1A,C), specifically in comparison to the Control group (P= 0.047). Bumetanide, administered prior to sevoflurane anesthesia at P6, normalized the hypothalamic KCC2 mRNA levels (P = 0.276 vs Control, but P = 0.264 vs Sevoflurane; Fig. 1B). The resulting NKCC1/KCC2 mRNA ratio was significantly increased at P10 in rats from the Sevoflurane group (F(2,13) = 5.043, P = 0.024; Fig. 1D), but not in those that were pretreated with bumetanide prior to exposure to sevoflurane (P = 0.32 vs Control, but P = 0.125 vs Sevoflurane; Fig. 1D).
Figure 1. Anesthesia with sevoflurane of rat pups for 60 min at postnatal day (P) 6 reduced levels of K+-2Cl− (KCC2) mRNA, increased Na+-K+-2Cl− (NKCC1)/KCC2 mRNA ratio and increased levels of corticotropin-releasing hormone (CRH) mRNA in the hypothalamus at P10. These effects were alleviated by pretreatment with bumetanide prior to anesthesia with sevoflurane.
The brain hypothalamus tissue samples were collected 4 days after the onset of anesthesia with sevoflurane for qRT-PCR analyses. (A) Illustration of the experimental protocol. Shown are the respective levels of NKCC1 mRNA (B), KCC2 mRNA (C), the resulting NKCC1/KCC2 mRNA ratios (D) and CRH mRNA (E). Data normalized against Control are means ± SD from 5–6 rats per treatment group. *P = 0.02 vs. Control; #P = 0.025 vs. Bumetanide plus Sevoflurane plus maternal separation.
One-way ANOVA analysis revealed significant differences in CRH mRNA levels in the hypothalamus of P10 rats from different treatment groups (F(2,12) = 9.450, P = 0.003; Fig. 1A,E). Post hoc pairwise comparisons found a significant increase in CRH mRNA levels in the Sevoflurane group when compared to the Control group (P = 0.003) and the Bumetanide plus Sevoflurane group (P = 0.028). In contrast to the Sevoflurane group, the Bumetanide plus Sevoflurane group had hypothalamic CRH mRNA levels not significantly different from that in the Control group (P = 0.192).
Maternal separation at P10 exacerbates impairment in the Morris water maze behavior of rats exposed to sevoflurane at P6; an alleviating effect of pretreatment with bumetanide
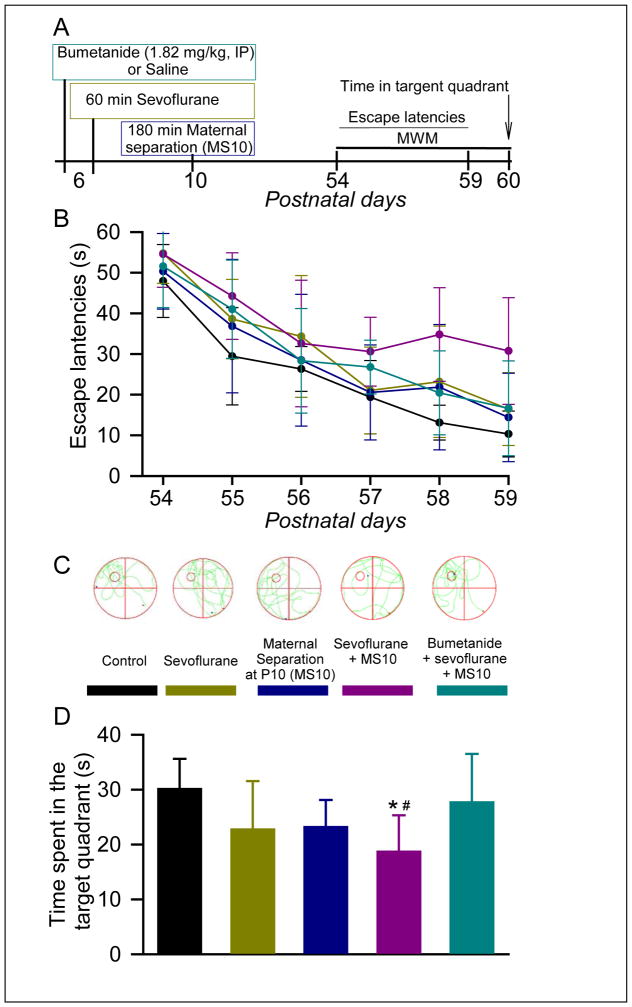
A two-way repeated measures ANOVA with training day as the within-subjects variable and group as a between-subjects variable revealed a significant main effect of day (such that escape latencies decreased across days; F(5,275)= 78.396, P< 0.001) and group (such that escape latencies differed across groups; F(4,275)= 11.445, P< 0.001), but no interaction between the two variables F(20,275)= 1.001, P= 0.462) (Fig. 2A,B). Post-hoc comparisons between treatment groups showed that 60 min of anesthesia with 2.1% sevoflurane at P6 was sufficient to induce a significant increase in escape latencies (P = 0.012 vs the Control group). Maternal separation for 180 min at P10 didn’t significantly affect the escape latencies (P = 0.394 vs the Control group). However, subsequent maternal separation at P10 of rats exposed to sevoflurane at P6 led to further increases in the escape latencies (P < 0.001 vs the Control and MS10 groups, and P = 0.012 vs the Sevoflurane only group). Pretreatment with bumetanide prior to exposure to sevoflurane at P6 of rats that were subsequently subjected to maternal separation at P10 reduced escape latencies compared to the Sevoflurane plus MS10 group (P = 0.01), but latencies were still elevated relative to the Control group (P = 0.029).
Figure 2. Maternal separation at P10 exacerbated impairment in the Morris water maze (MWM) behavior of rats exposed to sevoflurane at P6; the alleviating effects of pretreatments with bumetanide.
(A) Illustration of the experimental protocol. (B) Plots showing the values of escape latencies during the 6-day training period from P54 to P59. (C) Representative swimming tracks made by rats during the MWM test at P60. Location of the escape platform is illustrated by small circle in the left-top quadrant. (D) Histograms showing time spent in the target quadrant during the MWM test. N = 12 for each treatment group. MS10 means maternal separation at P10, See Methods for details. *P = 0.012 vs. the Control group; #P = 0.01 vs. the Bumetanide plus sevoflurane plus MS10 group. Color coding in B and D is the same as in C.
One-way ANOVA revealed significant differences in time spent in the target quadrant of the MWM test between rats from different treatment groups (F(4,55) = 4.856, P = 0.002; Fig. 2C and D). In contrast to escape time during the 6-day training period, exposure to sevoflurane only at P6 did not lead to a significant change in time spent in the target quadrant during the test at P60 (P = 0.098 vs the Control group). However, rats neonatally exposed to sevoflurane and then subjected to maternal separation at P10 (the Sevoflurane plus MS10 group) spent a significantly shorter time in the target quadrant when compared to the same parameter in the Control group (P = 0.002). Rats that were pretreated with bumetanide prior to exposure to sevoflurane and then to maternal separation at P10 spent an amount of time in the target quadrant that was not statistically different from that by rats in the Control group (P = 0.636), but significantly longer time when compared to the Sevoflurane plus MS10 group pretreated with saline (P = 0.025).
Discussion
The full range of neonatal anesthesia-induced developmental abnormalities and their underlying mechanisms remain poorly understood. The main finding of this study is that subsequent exposure to an environmental stressful factor may reinforce or even reveal developmental neurobehavioral abnormalities that were initiated by neonatal exposure to anesthesia with sevoflurane even when anesthesia exposure is too short to induce significant deficiencies by itself. Another new finding is that sevoflurane, administered to neonatal rats, may induce lasting alterations in gene expressions, as evident from the observed increases in the NKCC1/KCC2 mRNA ratio and CRH mRNA levels in the hypothalamus days after exposure to the anesthetic. These genomic effects of sevoflurane alone and long-term neurobehavioral abnormalities in rats that were neonatally exposed to a combination of neonatal anesthesia and a single episode of maternal separation, were ameliorated by pretreatment with the NKCC1 inhibitor bumetanide, suggesting that sevoflurane-enhanced GABAAR-mediated stimulatory signaling in immature neurons may represent an initial step in mechanisms involved in mediating these effects.
Only a combination of single exposure to 2.1% sevoflurane for 60 min at P6 and of subsequent maternal separation for 180 min at P10, but not these conditions taken individually, yielded significant neurobehavioral abnormalities in time spent in the target quadrant of the MWM, while a combination of neonatal anesthesia with sevoflurane and subsequent maternal separation induced significantly greater increases in escape latencies when compared to sevoflurane alone. These behavioral deficiencies, taken together with the increases in hypothalamic NKCC1/KCC2 mRNA ratio and CRH mRNA levels 4 days after exposure to sevoflurane, the age period when maternal separation was imposed, suggest a role for accumulating effects of sevoflurane and maternal separation. Sevoflurane-induced up-regulation of the NKCC1/KCC2 ratio in the hypothalamus, associated with increase in GABAAR-mediated stimulation — which may further be potentiated by stressors [31,32] — may represent a mechanistic basis for the cumulative effect of neonatal anesthesia and maternal separation observed in this study. Our recent findings in rats that were neonatally exposed to a relatively short anesthesia with etomidate, another general anesthetic that enhances GABAAR activity, and several days later to a single episode of maternal separation demonstrate that such increases in the hypothalamic NKCC1/KCC2 mRNA ratio and CRH mRNA level may persist into adulthood [34]. Similar to our current findings with sevoflurane, pretreatment with bumetanide prior to exposure to etomidate, ameliorated genetic and behavioral effects of etomidate and maternal separation [34], suggesting that common mechanisms underlie the developmental effects of these two anesthetics. The stress-like effects of enhanced GABAAR-mediated stimulation are indirectly supported by findings reported in the literature that the GABAAR agonist muscimol induced an endocrine stress response and anxiety- and depression-like behavior, with greater effects in mice treated with muscimol at P3–P5 than at P14–P16 [33]. In the future studies, it will be important to determine the cumulative neurodevelopmental effects of neonatal anesthesia and maternal separation using the anxiety and depression behavioral paradigms, such as open-field activities and forced swim test.
Our findings suggest that subsequent stressful life experiences may contribute to the developmental consequences of exposure to neonatal anesthesia. According to these findings, it is plausible that subsequent life experiences may reveal abnormalities programmed by a short early life exposure(s) to anesthesia. This may lend a plausible explanation to why many human studies found significant neurocognitive abnormalities in those who were anesthetized during the first 3 to 4 years of life despite the fact that the duration of anesthesia in young human patients is life span wise proportionately much shorter than that shown to induce developmental abnormalities in rodents. If similar mechanisms are functional in humans, we may speculate that development in healthy human patients who experience normal stress levels may be minimally if at all affected after exposure to general anesthesia, as it was shown by these recent studies [13,19]. However, most children who require general anesthesia during the early postnatal period inevitably experience a variety of significant stressors during post-anesthesia life (e.g., diseases, pain, hunger, psychological stress). Such patients may be at greater risk of developing early life anesthetic-initiated developmental abnormalities. In future studies, it will be important to determine the effects of different paradigms of postanesthesia stress that more closely model the stressful conditions human subjects may experience on developmental abnormalities induced by neonatal exposure to general anesthesia.
In summary, the results of this study provide evidence that the cumulative effect of neonatal exposure to sevoflurane and subsequent exposure to maternal separation can lead to developmental neurobehavioral abnormalities even when each condition alone may not be sufficient to induce significant alterations. Anesthetic-enhanced GABAAR-mediated depolarization during anesthesia may contribute to the mechanisms that initiate the resulting developmental neurobehavioral effects.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant nos. 81300946 and 81471105) and by the National Institutes of Health (R01GM93036 and R01NS091542 to A.E.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Dr. Nikolaus Gravenstein, Dr. Christoph Seubert, and Dr. Barry Setlow for critical reading of the manuscript and helpful suggestions.
References
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;3:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. doi: 10.1097/ALN.0b013e3181cf9138. [DOI] [PubMed] [Google Scholar]
- 3.Amrock LG, Starner ML, Murphy KL, Baxter MG. Long-term Effects of Single or Multiple Neonatal Sevoflurane Exposures on Rat Hippocampal Ultrastructure. Anesthesiology. 2015;122:87–95. doi: 10.1097/ALN.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 4.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE. Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology. 2012;117:791–800. doi: 10.1097/ALN.0b013e318266c62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seubert CN, Zhu W, Pavlinec C, Gravenstein N, Martynyuk AE. Developmental Effects of Neonatal Isoflurane and Sevoflurane Exposure in Rats. Anesthesiology. 2013;119:358–364. doi: 10.1097/ALN.0b013e318291c04e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan S, Xu C, Zhu W, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE. Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats. Anesthesiology. 2014;121:1010–1017. doi: 10.1097/ALN.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Tan S, Zhang J, Gravenstein N, Sumners C, Vasilopoulos T, Martynyuk AE. Neonatal anesthesia with sevoflurane: developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl− importer antagonists. Psychoneuroendocrinology. 2015;60:173–181. doi: 10.1016/j.psyneuen.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Xu C, Puentes DL, Seubert CN, Gravenstein N, Martynyuk AE. Role of steroids in hyperexcitatory adverse and anesthetic effects of sevoflurane in neonatal rats. Neuroendocrinology. 2016;103:440–451. doi: 10.1159/000437267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Servick K. Biomedical Research. Researchers struggle to gauge risks of childhood anesthesia. Science. 2014;346:1161–1162. doi: 10.1126/science.346.6214.1161. [DOI] [PubMed] [Google Scholar]
- 12.Rappaport BA, Suresh S, Hertz S, Evers AS, Orser BA. Anesthetic Neurotoxicity - Clinical Implications of Animal Models. N Engl J Med. 2015;372:796–797. doi: 10.1056/NEJMp1414786. [DOI] [PubMed] [Google Scholar]
- 13.Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ, Rauh V, Maxwell LG, Youn A, McGowan FX. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 16.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood. Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 17.Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanić K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ing CH, DiMaggio CJ, Malacova E, Whitehouse AJ, Hegarty MK, Feng T, Brady JE, von Ungern-Sternberg BS, Davidson AJ, Wall MM, Wood AJ, Li G, Sun LS. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;20:1319–1332. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 19.Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern BS, Sternberg, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang MQ, Ji MH, Zhao QS, Jia M, Qiu LL, Yang JJ, Peng YG, Yang JJ, Martynyuk AE. Neurobehavioural abnormalities induced by repeated exposure of neonatal rats to sevoflurane can be aggravated by social isolation and enrichment deprivation initiated after exposure to the anaesthetic. Br J Anaesth. 2015;115:752–760. doi: 10.1093/bja/aev339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacology Biochemistry and Behavior. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 23.Khazipov R, Valeeva G, Khalilov I. Depolarizing GABA and Developmental Epilepsies. CNS Neurosci Ther. 2015;21:83–91. doi: 10.1111/cns.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deidda G, Allegra M, Cerri C, Naskar S, Bony G, Zunino G, Bozzi Y, Caleo M, Cancedda L. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nat Neurosci. 2015;18:87–96. doi: 10.1038/nn.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cellot G, Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr. 2014;2:70. doi: 10.3389/fped.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 27.Lemonnier E, Robin G, Degrez C, Tyzio R, Grandgeorge M, Ben-Ari Y. Treating Fragile X syndrome with the diuretic bumetanide: a case report. Acta Paediatr. 2013;102:e288–290. doi: 10.1111/apa.12235. [DOI] [PubMed] [Google Scholar]
- 28.Lemonnier E, Lazartigues A, Ben-Ari Y. Treating schizophrenia with the diuretic bumetanide: A Case Report. Clin Neuropharmacol. 2016;39:115–117. doi: 10.1097/WNF.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 29.Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–739. doi: 10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Aguado F, Carmona MA, Pozas E, Aguiló A, Martínez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibañez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP. Chronic unpredictable mild stress induces loss of GABA inhibition in corticotrophin-releasing hormone-expressing neurons through NKCC1 upregulation. Neuroendocrinology Psychopharmacology (Berl) 2016;214:221–229. doi: 10.1159/000446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veerawatananan B, Surakul P, Chutabhakdikul N. Maternal restraint stress delays maturation of cation-chloride cotransporters and GABAA receptor subunits in the hippocampus of rat pups at puberty. Neurobiol Stress. 2015;3:1–7. doi: 10.1016/j.ynstr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salari AA, Bakhtiari A, Homberg JR. Activation of GABA-A receptors during postnatal brain development increases anxiety- and depression-related behaviors in a time- and dose-dependent manner in adult mice. Eur Neuropsychopharmacol. 2015;25:1260–1274. doi: 10.1016/j.euroneuro.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Ju LS, Yang JJ, Gravenstein N, Seubert CN, Morey TE, Sumners C, Vasilopoulos T, Yang JJ, Martynyuk AE. Role of environmental stressors in determining the developmental outcome of neonatal anesthesia. Psychoneuroendocrinology. 2017;81:96–104. doi: 10.1016/j.psyneuen.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]