Abstract
Epilepsy is one of the most common neurologic disorders and affects 0.5 to 1% of pregnant women. The use of antiepileptic drugs, which is usually continued throughout pregnancy, can cause in offspring mild to severe sensory deficits. Neuronal selectivity to stimulus orientation is a basic functional property of the visual cortex that is crucial for perception of shapes and borders. Here we investigate the effects of early exposure to valproic acid (Val) and levetiracetam (Lev), commonly used antiepileptic drugs, on the development of cortical neuron orientation selectivity and organization of cortical orientation columns. Ferrets pups were exposed to Val (200 mg/kg), Lev (100 mg/kg) or saline every other day between postnatal day (P) 10 and P30, a period roughly equivalent to the third trimester of human gestation. Optical imaging of intrinsic signals or single-unit recordings were examined at P42–P84, when orientation selectivity in the ferret cortex has reached a mature state. Optical imaging of intrinsic signals revealed decreased contrast of orientation maps in Val-but not Lev- or saline-treated animals. Moreover, single-unit recordings revealed that early Val treatment also reduced orientation selectivity at the cellular level. These findings indicate that Val exposure during a brief period of development disrupts cortical processing of sensory information at a later age and suggest a neurobiological substrate for some types of sensory deficits in fetal anticonvulsant syndrome.
Keywords: Valproate, Levetiracetam, Autism, Orientation selectivity, Visual cortex, Optical imaging, Fetal valproate syndrome
Introduction
Every year, pregnant women are exposed to teratogenic substances and consequently give birth to children with serious birth defects. Epilepsy is a common neurological disorder that affects 0.5 to 1% of pregnant women (Fairgrieve, 2000; Holmes et al., 2001). Many of these women require continued treatment during gestation with antiepileptic agents, even though these drugs are known teratogens and responsible for a wide spectrum of malformations and severe cognitive impairments, including learning and memory deficits, autism and hyperactivity (Adab et al., 2004; Vinten et al., 2005). These developmental problems caused by early exposure to anti-epileptic drugs are commonly termed fetal anticonvulsant syndrome (FAnS). The mechanism by which prenatal anticonvulsant exposure affects normal brain development is poorly understood and, consequently, no effective treatment for children with FAnS is currently available.
During the third trimester of gestation, there is a dramatic increase in synaptogenesis and refinement of connections, which are sculpted by activity-dependent plasticity processes (Constantine-Paton et al., 1990; Katz and Shatz, 1996). Such processes rely on a delicate balance between excitation and inhibition and can be severely impaired if the pregnant mother consumes drugs that block glutamatergic responses (reducing neuronal excitation) or drugs that enhance GABAergic responses (increasing neuronal inhibition) (Shadlen and Nwesome, 1998; Xing and Gerstein, 1996). Most anticonvulsants alter this balance, for instance, valproic acid (VAL) influences GABA synthesis and breakdown (Loscher, 2002), and reduces glutamatergic responses (Gobbi and Janiri, 2006). Levetiracetam (LEV), a more modern anticonvulsant, acts on the synaptic vesicle protein SV2A (Lynch et al., 2004) reducing the neuronal hypersynchronization observed during epileptic activity (Klitgaard et al., 2003) and it is considered “safer” than other AEDs for use in humans (Lopez-Fraile et al., 2009; Ozyurek et al., 2010; Isoherranen et al., 2003).
Neuronal selectivity to stimulus orientation (orientation selectivity, OS), a functional property that is crucial for normal vision, is regulated by neuronal activity (Weliky and Katz, 1997) and requires NMDA receptor function (Ramoa et al., 2001). This property develops perinatally (weeks before and after normal eye opening in ferrets and cats) (Chapman and Stryker, 1993), and may be particularly affected by drugs administered during this period. Recently we showed that early exposure to alcohol, a drug that also affects GABAergic and glutamatergic transmission (Costa et al., 2000), disrupts OS in the visual cortex of ferrets (Medina et al., 2005). In addition, these alcohol-treated animals showed decreased ocular dominance (OD) plasticity (Medina et al., 2003; Medina and Ramoa, 2005). Contrary to what is observed in normal animals, a few days of monocular deprivation did not result in reduction of cortical regions responsive to the deprived eye in ferrets exposed early to alcohol. Interestingly, both OS and OD plasticity could be successfully restored by treating the animals with vinpocetine, a phosphodiesterase type 1 inhibitor (Krahe et al., 2009; Medina et al., 2006). Here we examine whether early exposure to VAL and LEV affects the establishment of the OS and OD plasticity in the ferret. If we observe a disruption, then we will test whether vinpocetine can reverse this impairment.
Methods
Treatments
Lactating ferrets with litters were bought from Marshall Farms (New Rose, NY). Mothers were experimentally naïve. Ferrets were treated with valproic acid (Val; 200 mg/kg, i.p.), levetiracetam (Lev; 100 mg/kg, i.p.) or saline (Sal) every other day between postnatal day (P) 10 and P30, ending the treatment near the time of the natural eye opening (P32). Some of the developmental events observed in ferrets during this period occur in rats from approximately P4 to P10 and in humans from as early as 135 days to at least 188 days post conception (Clancy et al., 2001). All pups were weighted every other day between P10 and P30. Between P36 and P41 some Val-treated animals received 40 mg/kg of vinpocetine (oral mixed in the diet supplement Nutrical©, Vetoquinol, Buena, NJ) or only Nutrical© as a control. This treatment is proven to restore orientation selectivity in ferrets exposed to alcohol between P10 and P30 (Krahe et al., 2009). Approximately 20 days after the treatment ferrets were evaluated by optical imaging of intrinsic signals or extracellular electrophysiology (single unit recordings). All procedures described in this paper were approved by the IACUC.
Monocular deprivation
Some ferrets at P45–P48, at the peak of ocular dominance (OD) plasticity (Issa et al., 1999), had their lid of the right eye sutured closed to prevent patterned visual stimulation. After 4 days of monocular deprivation, animals were anesthetized and optical imaging of intrinsic signals or quantitative single-unit in vivo electrophysiology was performed to assess changes in OD.
Optical imaging of intrinsic signals
Experiments were conducted as described previously (Krahe et al., 2009; Medina et al., 2005). Briefly, animals received acepromazine (1 mg/kg, s.c., VEDCO, St. Joseph, MO), atropine, (0.2 mg/kg, s.c., MED-PHAREX, Pomona, CA) and dexamethasone (0.5 mg/kg, s.c., Bimeda-MTC, Ontario, Canada). Animals were anesthetized using pentobarbital (35 mg/kg, i.p., Hospira, Lake Forest, IL). A tracheal cannulation was performed, and the animal was placed on a ventilator and on a stereotaxic frame. Supplemental doses of pentobarbital (12 mg/kg) were given as needed. Nictitating membranes were retracted using phenylephrine (2.5%, Baush & Lomb, Tampa, FL), the pupilswere dilated with atropine (1%, Baush & Lomb, Tampa, FL) and contact lenses were placed on the corneas.
Optical imaging of intrinsic signals was performed with Imager 3001 VSD+ (Optical Imaging System Inc., Germantown, NY). A craniotomy was made over the left hemisphere to expose the dorsal area of the occipital cortex. The dura was reflected and the opening filled with agar (3.5% in saline) and covered with a glass coverslip. A reference image of the cortex was then obtained using a 550 nm filter. Intrinsic signals were recorded using a 700 nm filter. Visual stimulation consisted of high-contrast rectangular wave gratings (8.75° dark phase/1.25° light phase) generated by a 21-inch monitor and presented to both eyes at an angle of 0°, 45°, 90° or 135° and drifted (22.5°/s) in both directions. Each trial consisted of 4 angles and a blank screen presented to each eye for 9 s in a pseudorandom sequence, with data acquisition during the last 8 s. A total of 20 trials were performed for each eye. To create single condition for each eye, we summed the responses to each angle (0°, 45°, 90° and 135°) and subtracted from responses to a blank screen. In single condition maps, dark areas correspond to regions that are responsive to a particular stimulus' angle. To obtain differential maps (cardinal and oblique) we subtracted the optical signal at one orientation from the response obtained at its orthogonal (cardinal: 0°–90°; oblique: 45°–135°). To generate polar maps, additional angles were used (22.5°, 67.5°, 112.5°, and 157.5°). In Polar maps, intensity represents degree of selectivity and color the preferred angle.
Differential maps — contrast analysis
To quantify orientation selectivity we performed a contrast analysis on differential images (Krahe et al., 2009). Higher contrast rates would indicate higher orientation selectivity. Differential maps were mixed with the floating point files clipped at ± 3 SD from the median. The resulting 8 bit difference images were rescaled from 0 to 255 and the contrast was calculated based on the Gray-Level-Co-occurrence Matrix contrast texture technique (Haralick et al., 1973). Contrast textures were obtained by a texture analyzer plug-in (Julio E. Cabrera, http://rsbweb.nih.gov/ij/plugins/texture.html) for Image J software (National Institutes of Health, USA). Three contrast values were obtained for each differential image (248 × 60 pixels) from 4 non-overlapping ROIs (55 × 55 pixels) that included V1 and V2, covering nearly the entire map surface. The contrast values were then summed to obtain a contrast value for a single differential map. Values close to 20,000 and 10,000 indicate high and low contrast levels respectively.
Differential maps — signal strength
An estimate of signal intensity was obtained by first computing the pixel distribution along a grayscale containing 128 levels of gray in 8 bit single condition images clipped at ±3 SD from the median. In this scale, 0 represents lack of response and 127 the strongest possible response to visual stimulation. For each single condition map (0°/blank stimulus [bs], 45°/bs, 90°/bs and 135°/bs), the pixel distribution was obtained from a ROI drawn manually to include V1 and V2. To avoid sampling biases, for each animal, ROIs were outlined on maps of total visual response (0° + 45° + 90° + 135°/blank stimulus). Next, a signal intensity index (SIi) was calculated for each condition according to the following formula:
where P is the number of pixels in each one of the 128 grayscale levels, Pt is the total number of pixels in the ROI and Am is an arbitrary number ranging from 1.0 to 100.0 such that for each grayscale level there is a correspondent Am value. Thus, a SIi value of 1.0 would indicate that the entire visual cortex is maximally activated. For each animal, the SIi for each one of the 4 single conditions was averaged.
Ocular dominance maps
OD maps were created by subtracting the summed images obtained with the left eye from the summed images obtained with the right eye. In these images, dark and light areas respond best to stimulation of the right and left eye respectively. To quantify the results unfiltered OD images were first clipped at ± 3 SD and then a region of interest (ROI) outlining V1 selected. Anterior and caudal references were the contralateral eye band (White et al., 1999) and the occipital pole respectively. After defining the ROI, we created OD histograms based on the range of gray values (0–255) divided into 5 class intervals, where 0–50 and 204–255 correspond to classes containing the darkest and lightest pixels respectively. A contralateral bias index (CBI) was defined as {(P0–50-P204–255)+[(P51–101-P153–253)/2]+100}/200, where PA-B denotes the percentage of pixels with gray values between A and B. A CBI close to 1.0 indicates a prevalence of darker pixels and right eye dominance. A CBI close to 0.0 indicates a prevalence of lighter pixels and left eye dominance.
In vivo electrophysiology
Animals were premedicated, anesthetized and ventilated using similar procedures described for optical imaging. A craniotomy (3–4 mm in diameter) was performed to expose the binocular region of the left primary visual cortex. A tungsten microelectrode was lowered into the primary visual cortex at 20° to the vertical. Single-units were separated by at least 100 μm along. After the isolation of a cell, its receptive field was mapped and the optimal stimulus orientation, direction and velocity were determined qualitatively using a moving bar of light projected onto a tangent screen. To assess orientation selectivity, the moving bar of light was presented to each eye separately at four orientations centered on the optimal (0°, 45°, 90° and 135°). Spikes were collected during the 10 stimulus presentations by a computer using Spike 2 software (CED, Cambridge, UK). Spontaneous activity was determined by recording the activity in the absence of stimulation.
To quantify the orientation selectivity based on the single-unit recordings, an orientation selectivity index (OSI) was obtained for each cell by subtracting the response to 90° or 45° stimulation from the optimal (OSI90° or OSI45°) divided by the response at the optimal orientation and then subtracting the result from one. Indices of 1.0 and 0.0 indicate maximal and minimal levels of selectivity respectively.
Results
Ferrets were injected with Val (200 mg/kg) or Lev (100 mg/kg) every other day between postnatal day (P) 10 to P30, completing treatment just before eye opening (P32). Control animals received saline (Sal, i.p) during the same period. Early exposure to Val but not to Lev and Sal resulted in a reduction in weight gain (Fig. 1). An ANOVA of repetitive measures revealed an effect of age (f = 907.070, df = 2.410, p<0.001), treatment (f = 9.250, df = 2, p<0.01) and an interaction between age and treatment (f = 13.081, df = 4.819, p<0.001). Post hoc analysis showed that animals treated with Val weighted less than Sal (Bonferroni, p = 0.044) and Lev (p = 0.001). No significant difference was observed between Sal and Lev groups (p = 0.258). Approximately 20 to 35 days after the treatment, primary visual cortex functional organization was evaluated by optical imaging of intrinsic signal or single unit recordings. Table 1 shows the number of animals used in all experiments.
Fig. 1.
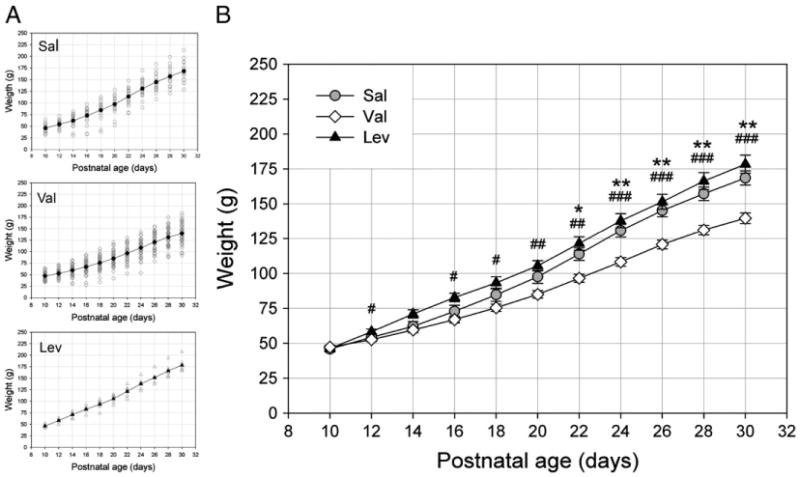
Early exposure to Val reduces weigh gain. A. Body weight of young ferrets exposed to Sal (n = 11), Val (n = 23) or Lev (n = 6) between P10 and P30. Each unfilled symbol represents an individual animal value. B. Note that animals treated with Val weighted less than Sal and Lev-treated animals. No significant difference was observed between Sal and Lev-treated animals (Sal vs Val: *P<0.05, **P<0.01; Lev vs Val: #P<0.05, ##P<0.01, ###P<0.001).
Table 1.
Experimental groups.
| Experiment | Group | Number of animals |
|---|---|---|
| OS-OI (Figs. 2,5–7) | Sal | 5 |
| Val | 9 | |
| Val + Vin | 5 | |
| Sal (young adults) | 4 | |
| Val (young adults) | 4 | |
| Val + AEO | 3 | |
| Lev | 6 | |
| OS-SU (Fig. 4) | Sal | 5 (53 cells) |
| Val | 4 (43 cells) | |
| OD (Fig. 8) | Sal | 5 |
| Val | 5 | |
| Lev | 2 | |
| Sal + MD | 3 | |
| Val + MD | 6 | |
| Lev + MD | 2 | |
| Total number of animals used | 68 |
OS-OI: orientation selectivity assessed by optical imaging of intrinsic signals. AEO: artificial eye opening; animals who had eyes manually opened at P32. OS-SU: orientation selectivity assessed by single unit recordings. OD: ocular dominance assessment. MD: monocular deprivation.
Early valproic acid exposure impairs the organization of orientation selectivity columns
To examine the effects of early anticonvulsants (Val and Lev) exposure on development of orientation selectivity columns, we conducted optical imaging of intrinsic signals. Fig. 2A illustrates the finding that Lev and Sal-treated animals had well organized, high contrast differential maps with normal spacing and pattern of domains at both cardinal and oblique orientations. Remarkably, orientation maps in Val-treated animals had reduced contrast and both cardinal and oblique orientation domains were less noticeable (Fig. 2A). Importantly, the presence of strong single condition maps indicated that the reduction in contrast did not result from poor response to visual stimulation. Fig. 2A (single condition maps) shows that Sal, Val and Lev-treated animals displayed strong cortical signal in response to visual stimulation and a clear alternation of responding (dark) and non-responding (gray areas).
Fig. 2.
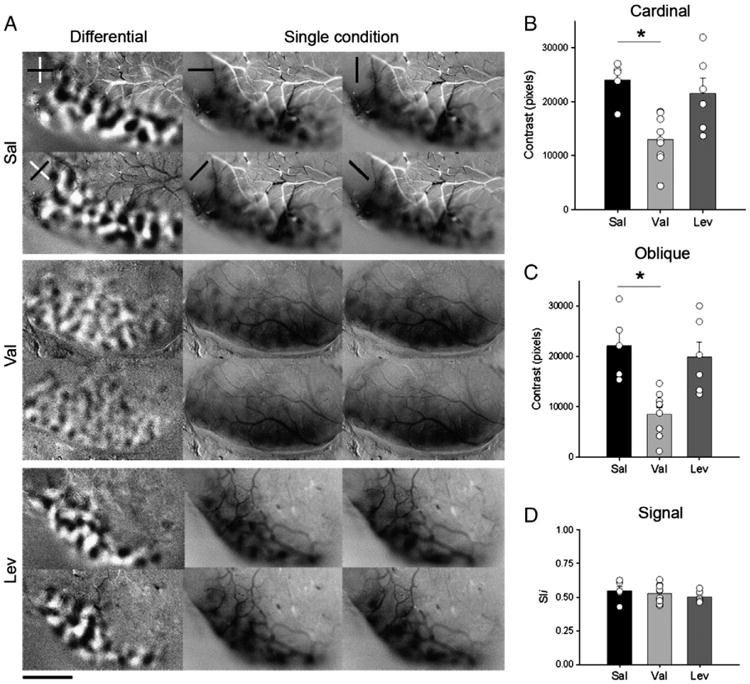
Early exposure to Val disrupts orientation selectivity. A. Analysis of orientation selectivity by optical imaging of intrinsic signals of juvenile (P42–P56) ferrets, submitted to different treatments (Sal, Val and Lev). In cardinal differential maps (first column and row from each experimental group), dark and light areas represent regions that respond preferentially to 0° (horizontal) and 90° (vertical) gratings respectively. In oblique differential maps (first column and second row from each experimental group), dark and light areas represent regions that respond preferentially to 45° and 135° gratings respectively. Note the poor contrast in both cardinal and oblique differential maps in the Val-treated animals relative to the Sal and Lev-treated animals. In single condition maps, dark areas represent regions responsive to a single angle. Stimulus orientations used were 0°, 45°, 90°, and 135°. Note that robust responses were present at every orientation tested. Scale bar = 3 mm. Quantification of contrast differences displayed for cardinal (B) and oblique (C) differential maps of Sal- Val- and Lev-treated groups. *P<0.01. Bars represent means (± SEM), and circles represent individual animal values. Note that most of the animals in the Val-group have a lower contrast than controls, while the animals in the Lev group present contrast values similar to Sal ones. D. Signal strength measured by pixel intensity. Note the similarity between groups.
To quantify the effects of anticonvulsants treatment on maturation of orientation columns, we computed the contrast of differential maps (see Methods). A value around 20,000 indicates a high contrast level (high degree of selectivity), and a value close to 10,000 indicates a low contrast level (poor degree of selectivity). An ANOVA showed a significant difference between groups (F = 9.511, df = 3, P<0.001). Post hoc analyses demonstrated that the mean contrast values (± SEM) for cardinal and oblique orientations in Val (cardinal: 12,980.36 ± 4503.92; oblique: 8775.66 ± 4281.04;n = 9 ferrets) treated animals (Figs. 2B and C) were significantly lower (Bonferroni, P<0.01 for both comparisons) than in Sal (cardinal: 23,987.34 ± 3710.96; oblique: 22,113.06 ± 6582.85; n = 5) treated animals (Figs. 2B and C). There was no difference in the mean contrast values (± SEM) for cardinal and oblique orientations in Lev (cardinal: 21,543.41 ± 6930.10; oblique: 19,899.53 ± 7249.99; n = 6 ferrets) treated animals in comparison to Sal-treated animals (Figs. 2B and C). Quantification of signal intensity (see Methods) observed in the single condition maps (Fig. 2D), revealed that the mean pixel intensity of Val (0.5291 ± 0.07; n = 9 ferrets), Lev (0.5083 ± 0.04; n = 6 ferrets) and Sal (0.5487 ± 0.08; n = 5 ferrets) treated animals were similar (ANOVA, F = 0.38, df = 3, P = 0.78), thus indicating that the response to visual stimulation in these animals was not reduced.
Fig. 3 illustrates polar maps of representative cases from Sal, Val and Lev-treated groups. Val-treated animals presented an irregular representation (uneven color distribution) of visual responses to different orientations (Fig. 3B) whereas Sal and Lev treated animals showed a homogeneous representation of visual responses (even color distribution) throughout V1 and V2 as well as defined “pinwheel” centers (Figs. 3A and C).
Fig. 3.
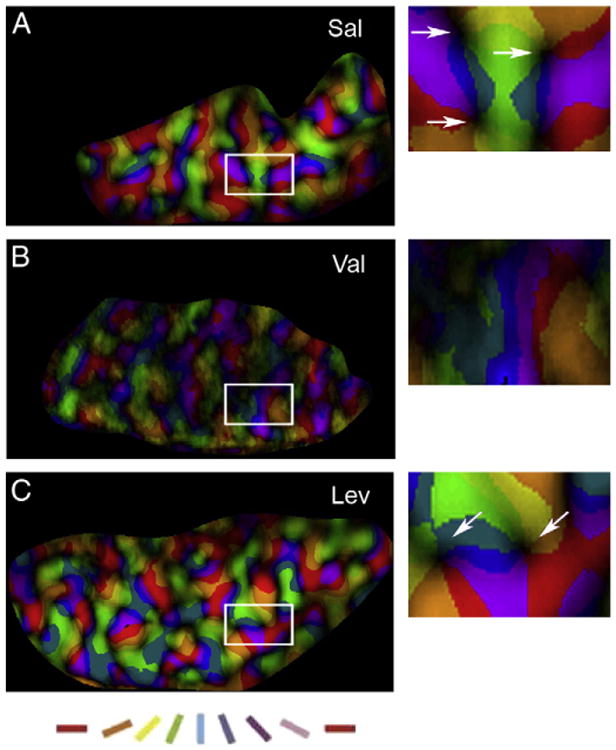
Early exposure to Val disrupts the organization of polar maps. Polar magnitude maps (left column) of Sal- Val- and Lev-treated animals (A, B, and C respectively), where brightness indicates degree of selectivity, and colors represent different orientations. Note that both Sal- and Lev-treated animals present an even representation of colors and a great number of “pinwheels” (arrows, insets). In contrast the Val-treated animals present an uneven representation of colors and do not show “pinwheels” as Sal and Lev-treated animals. Scale bar, 2.5 mm. The right column shows representative pinwheel centers (arrows) from polar maps shown in the left column.
Early valproic acid disrupts orientation selectivity at the neuronal level
While Val disrupted the organization of orientation selectivity columns, we did not know whether this disruption was also present at the neuronal level. To test this we conducted single unit recordings in the binocular region of the primary visual cortex of Val and Sal-treated animals. Figs. 4A and B show the cumulative number of cells (in percent) plotted as a function of the orientation selectivity index (OSI) in animals treated with Val (n = 4) or Sal (n = 5) from P10 to P30 and studied at P50–P63. An index of 1.0 indicates a high degree of selectivity while an index of 0.0 indicates a low degree of selectivity (see Methods). The cumulative curve for the Val-treated animals was shifted to the left of the control curves, reflecting their weaker neuronal orientation selectivity (Mann–Whitney test; OSI90°: Z = −3.799; P<0.001; OSI45°: Z = −4.730; P<0.001).
Fig. 4.
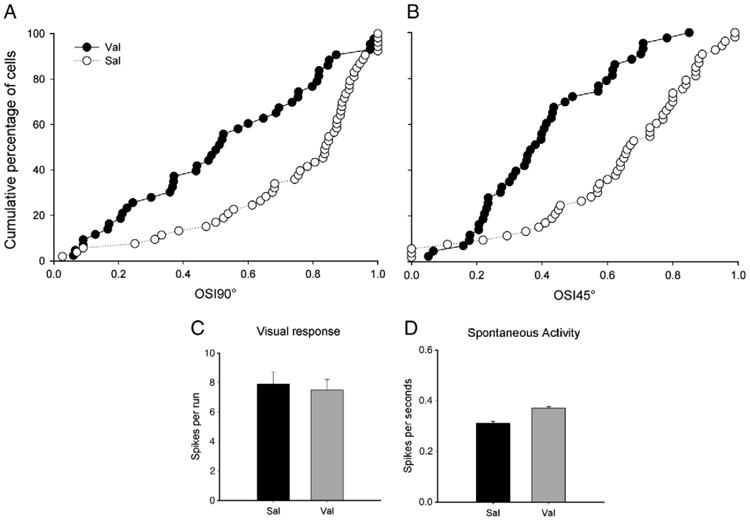
Early exposure to Val disrupt orientation selectivity at the neuronal level. Cumulative percentage of cells is plotted as a function of an orientation selectivity index (OSI, see Methods section) 90° (A) and 45° (B) in Sal (n = 53 cells, from 4 animals) and Val (n = 44 cells, from 4 animals) treated animals. In these charts, OSI values close to 0 and 1 indicate, respectively, lower or higher degrees of orientation selectivity. Note that the distribution of cells in Val-treated animals is skewed to the left indicating a higher proportion of poorly selective cells. In contrast, Sal-treated animals have their distribution skewed to the right indicating a higher proportion of highly selective cells. Note that, similar results were obtained with both indexes used (90° and 45°). C. Visual responses of striate cortical cells to a moving bar of light at the optimal orientation. Similar mean maximal response (in spikes per run) of cortical neurons to stimulation at the optimal orientation observed in Val and Sal-treated animals (P>0.05 in each case). These findings indicate that Val exposure preserved robust responses to visual stimulation. D. The average number of spikes registered when the stimulating bar was not moving was the same in Sal and Val animals (P>0.05 in each case). This finding indicates that Val exposure did not affect spontaneous activity in primary visual cortex. Bars indicate SE.
We also made use of extracellular single-unit recordings to verify whether Val treatment resulted in abnormal visually driven activity. Quantitative assessment of single-unit responses revealed similar mean maximal responses (in spikes per run) of cortical neurons to visual stimulation for both groups (Fig. 4C). The mean maximal responses (± SEM) for each group were: Val, 7.9 ± 0.8, n = 44 cells; and Sal, 7.5 ± 0.7, n = 53 cells (t-test, t = −0.35, P = 0.72). Additionally, Val treatment did not change spontaneous activity (recorded in the absence of visual stimulation) when compared to Sal-treated animals (Fig. 4D). The mean spontaneous activity (± SEM) for each group was: Val, 0.31 ± 0.08, n = 44 cells; and Sal, 0.37 ± 0.06, n = 53 cells (t-test, t = −0.68, P = 0.56).
Phosphodiesterase inhibition does not restore orientation selectivity in animals exposed to valproic acid
Recently, we showed that treatment with the phosphodiesterase type 1 inhibitor vinpocetine restored both ocular dominance plasticity (Medina et al., 2006) and orientation selectivity (Krahe et al., 2009) in animals exposed to alcohol between P10 and P30. Here we evaluated whether the same vinpocetine treatment would restore orientation selectivity in animals exposed to Val. Thus, six days after the end of the Val treatment, some animals received vinpocetine (Vin) (40 mg/kg oral, see Methods), or vehicle, everyday between P36 and P41, roughly the time of maturation of orientation selectivity in the ferret (Chapman and Stryker, 1993; Chapman et al., 1996). Optical imaging of intrinsic signal experiments was conducted around P50. Fig. 5A shows a representative case, showing poorly defined orientation selectivity maps (compare to Sal animal shown in Fig. 1A). Quantification or cardinal and oblique maps show that vinpocetine was not able to restore orientation selectivity in Val animals and lower mean contrast values (± SEM) for cardinal and oblique orientations were observed (cardinal: 10,889.61 ± 3780.27; oblique: 8442.20 ± 3535.24; n = 5 ferrets; P<0.01 for both comparisons; Fig. 5B). Signal intensity observed in the single condition maps in Val+Vin (0.5110 ± 0.11; n = 5 ferrets) treated animals was similar to that observed in the other groups (Fig. 5B).
Fig. 5.
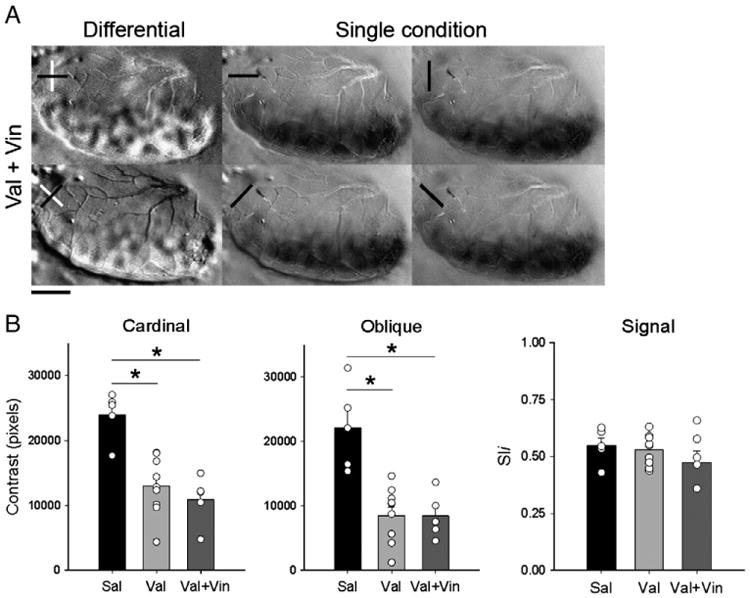
Vinpocetine does not restore orientation selectivity after early exposure to Val. A. Representative case of cardinal and oblique differential maps and single condition maps in Val+Vin-treated animals, as revealed by optical imaging of intrinsic signals. Note that, poor contrast maps can still be seen in Val + Vin treated animal after giving vinpocetine. Single condition maps show robust response at every orientation tested. Scale bar = 2.5 mm. Quantification of contrast differences (B) displayed for cardinal, oblique differential maps, and signal of Sal, Val and Val + Vin-treated groups. *P<0.01. Bars represent means (± SEM), and circles represent individual animal values. Note that animals in the Val and Val + Vin-group have a lower contrast than controls. Signal strength measured by pixel intensity. Note the similarity between groups.
Early exposure to valproic acid delays the timing of normal eye opening
We observed that exposure to Val during the third trimester equivalent of human exposure delayed the natural opening of the eyes in 4–5 days (Fig. 6A). Accordingly, the average age (in postnatal days) at eye opening was significantly higher (t-test, t = −10.154, df = 47, p<0.001) in Val (36.97 ± 0.25; n = 33 ferrets) than in Sal group (32.63 ± 0.34; n = 16 ferrets).
Fig. 6.
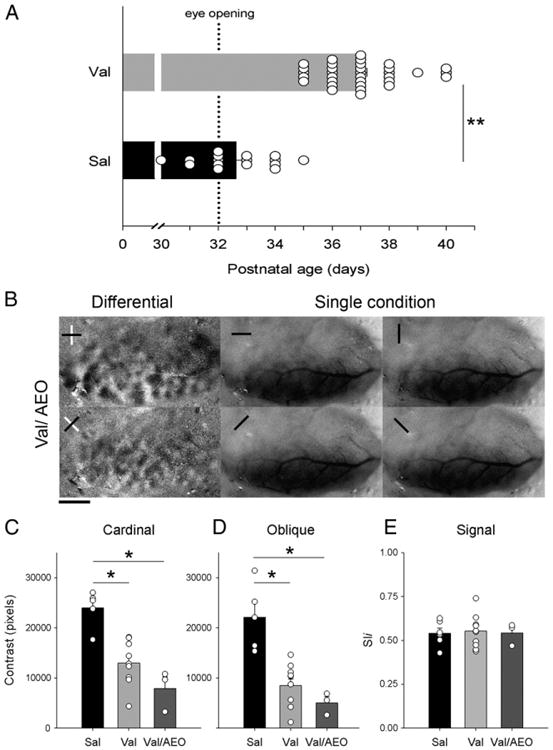
Disruption of orientation selectivity by Val cannot be explained by delayed eye opening. A. Eye opening of Sal- and Val-treated animals. Note that Val-animals have a delay of eye opening in comparison to Sal-treated animals. Circles represent an individual animal values. **P<0.001. B. Representative case of differential maps and single condition maps of a juvenile animal that was exposed to Val and had its eyes artificially opened at P32 (Val/AEO). Note that, AEO did not prevent the poor contrast of cardinal and oblique differential maps in Val-treated animals. Note that, single condition maps show robust responses at every orientation tested. Scale bar = 2.5 mm. Quantification of contrast differences displayed for cardinal (C) and oblique (D) differential maps of Sal- and Val- and Val/AEO-treated groups. *P<0.01. Bars represent means (± SEM), and circles represent an individual animal values. Note that animals in the Val and Val/AEO-group have a lower contrast than controls. (e) Signal strength measured by pixel intensity. Note the similarity between groups.
It was previously shown that early binocular lid suture leads to non-patterned visual stimulation and disrupts the establishment of orientation selectivity (White et al., 2001b). Therefore our findings on the decrease of orientation selectivity by Val could be attributed to delayed eye opening. To evaluate this possibility, we artificially opened the eyes of Val-treated animals at P32 and conducted optical imaging of intrinsic signals approximately 20 days later. Fig. 6B shows that artificial eye opening (AEO) of Val-treated animals does not prevent the disruption of orientation selectivity columns. ANOVAs showed a significant difference between groups for cardinal (F = 16.5, df = 2, P<0.001) and oblique maps (F = 16, df = 2, P<0.001; Figs. 6C and D). Post hoc analyses revealed that both Val (cardinal: 12,980.36 ± 4503.92; oblique: 8775.66 ± 4281.04; n = 9 ferrets) and Val/AEO (cardinal: 7920.02 ± 4050.60; oblique: 5029.14 ± 2183.62; n = 3 ferrets) groups presented significantly lower values (P<0.01 for both comparisons) than in Sal (cardinal: 23,987.34 ± 37.10; oblique: 22,113.06 ± 6582.85; n = 5) treated animals. Quantification of signal intensity observed in single condition maps (Fig. 6E), revealed similar mean pixel intensity (ANOVA, F = 0.135, df = 2, P = 0.875) in all groups (Val-AEO, 0.5426 ± 0.64, n = 3 ferrets; Val, 0.5291 ± 0.07, n=9; and Sal, 0.5487 ± 0.08; n = 5 ferrets).
The impairment of orientation selectivity columns in animals exposed to valproic acid is long-lasting
The maturation of orientation selectivity in ferrets occurs around P38 (Chapman and Stryker, 1993; Chapman et al., 1996). The deleterious effects of Val on the functional organization of the visual cortex of juvenile (P42–P53) ferrets observed here might be explained by a delay on the establishment of orientation selectivity. To test this possibility we examined a second group of Val-treated animals in which, orientation selectivity was assessed in young adult ferrets (P65–P84). Fig. 7A illustrates the finding that, as in juveniles, young adult Sal-treated animals have well organized high contrast differential maps with normal spacing and pattern of domains at both cardinal and oblique orientations. In contrast, orientation maps in young adult Val-treated animals showed a reduced contrast at both cardinal and oblique orientations. Single condition maps indicated that this reduction in contrast did not result from poor response to visual stimulation. Figs. 7B and C show that the mean contrast values (± SEM) for cardinal and oblique orientations in young-adult Val-treated animals (Val late; cardinal: 9609.84 ± 389.22; oblique: 7746.08 ± 650.53; n = 4 ferrets) were significantly lower than in Sal-treated animals at a later age (Sal late; cardinal: 13,811.92 ± 1388.76; oblique: 11,940.06 ± 1263.40; n = 4 ferrets) (t-test; cardinal: t = 2.914, df = 6, p<0.05; oblique: t = 2.951, df = 6, p<0.05). Quantification of signal intensity observed in the single condition maps (Fig. 7D), revealed that the mean pixel intensity of Val late (0.48 ± 0.27; n = 4 ferrets) and Sal late animals were similar (0.51 ± 0.03; n = 4 ferrets).
Fig. 7.
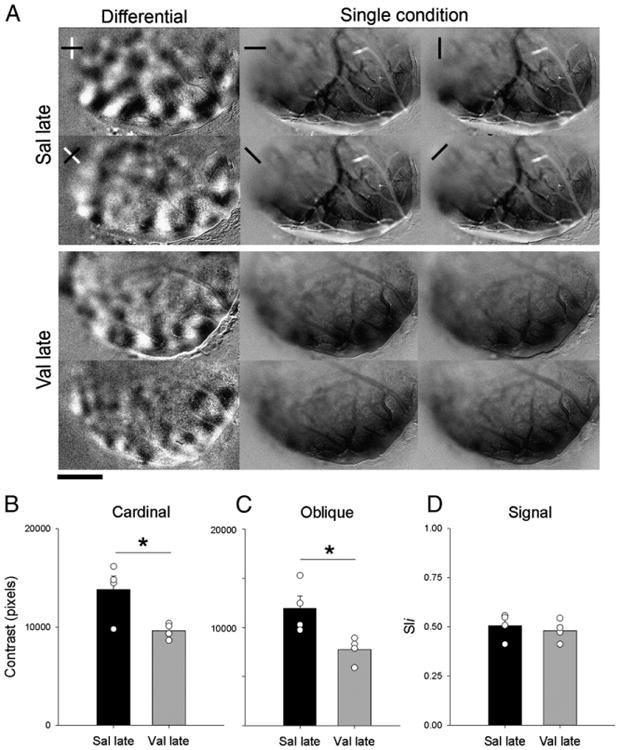
Disruption of orientation selectivity by Val is still observed at later ages. A. Representative case of cardinal and oblique differential maps and single condition maps in Sal- and Val-treated animals, as revealed by optical imaging of intrinsic signals at a later age (P65–P84). Note that, poor contrast maps can still be seen in Val-treated animal at a later age. Single condition maps show robust response at every orientation tested. Scale bar=3 mm. Quantification of contrast differences displayed for cardinal B and oblique C differential maps of Sal and Val-treated groups at late age (P65–P84). *P<0.05. Bars represent means (± SEM), and circles represent individual animal values. Note that animals in the Val-group have a lower contrast then controls. D. Signal strength measured by pixel intensity. Note the similarity between groups.
Early valproic acid exposure does not affect ocular dominance plasticity
A possible explanation to the Val effect on the functional organization of the visual cortex could be an alteration in activity-dependent neuronal plasticity, which is essential to the maturation of orientation selectivity. To test the integrity of neuronal plasticity in visual cortex of Val-treated ferrets, we evaluated the effects of 4 days of monocular deprivation (MD) on ocular dominance profile. It is quite established that 4 days of MD produces a significant change in ocular dominance maps in the contralateral visual cortex (Issa et al., 1999; Medina et al., 2003; Medina and Ramoa, 2005). Fig. 8A shows the left visual cortex of a representative case from the Val and Val + MD groups. Dark and light areas in OD (differential) maps represent regions that respond preferentially to stimulation of the right and left eyes respectively. In monocular response (single condition) maps dark regions represent areas that display strong responses to visual stimulation. Note that the nondeprived Val-treated animals (Fig. 8A) present the typical contralateral bias observed in ferrets (Issa et al., 1999; Medina et al., 2003; White et al., 1999). Four days of MD in Val-treated animals leads to a striking shift in OD towards the experienced eye (Fig. 8A). The quantification of these findings was based on the pixel distribution along a 256 gray scale, divided in five class intervals, where 0–50 and 204–255 correspond to classes containing the darkest and lightest pixels, respectively. Accordingly, non-deprived animals present histograms skewed to the left, which indicate contralateral eye dominance (Fig. 8B). In contrast, after 4 days of contralateral eye lid suture, Val animals presented OD profiles skewed to the right, indicating the dominance of the ipsilateral eye (Fig. 8C). To further quantify these findings we compared the contralateral bias indexes (CBIs) between groups (see Methods, Fig. 8D). A CBI close to 1.0 indicates a prevalence of darker pixels and right eye dominance. A CBI close to 0.0 indicates a prevalence of lighter pixels and left eye dominance. CBI comparison shows a great difference between groups (t = 8.09, df = 9, P<0.001). The mean CBI of Val + MD animals (0.35 ± 0.1; n = 3) was similar to Sal + MD animals (0.25 ± 0.05; n = 5) and consistent to ocular dominant shifts previously reported in ferrets treated with saline that were monocularly deprived for a similar time period (Medina et al., 2003; Medina and Ramoa, 2005; Medina et al., 2006; Paul et al., 2010). This shift indicates that OD plasticity is not affected after Val treatment. OD plasticity was also evaluated in 2 Lev-treated animals and no difference from controls was observed (data not shown).
Fig. 8.
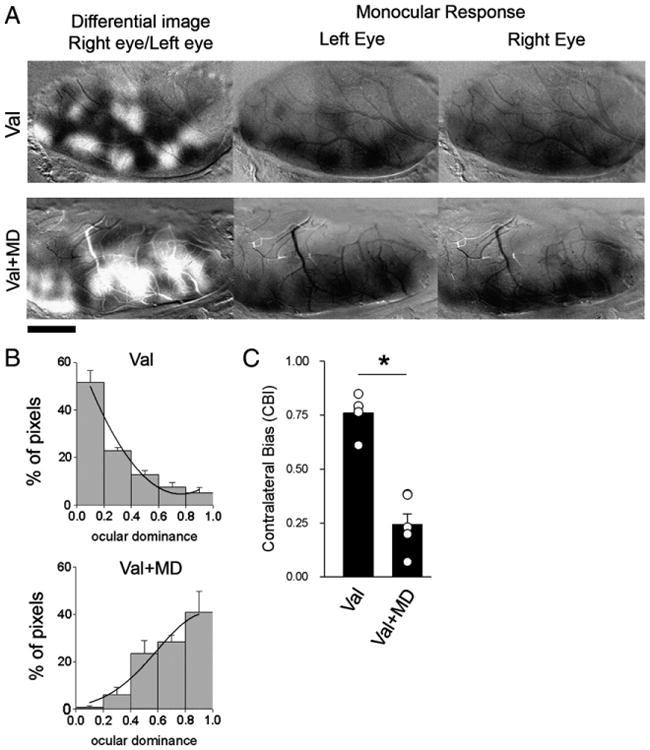
Early exposure to Val does not affect ocular dominance (OD) plasticity. A. Effects of monocular deprivation on OD maps, revealed by optical imaging of intrinsic signals. Differential maps are shown on the left column, in which dark areas respond best to gratings presented to the right eye and white areas respond best to stimulation of the left eye. Monocular response maps for the left and right eye stimulation are shown on the center and right columns, respectively. Dark areas in the monocular response maps represent visual responses. All images are from the left hemisphere, with medial represented to the right and rostral to the top of the figure. Note that, after MD was observed a predominance of light pixels in the differential map, reflecting a shift in dominance towards the left (nondeprived) eye. B. Histograms display the percentage of pixels fitting in a grayscale (0—black, 255—white). Note the predominance of black and gray pixels in Val-treated animals without MD and the predominance of lighter pixels in Val-treated animals after MD; Val (no MD), n = 5; Val + MD, n = 6. C. Mean contralateral bias indexes for both groups. Circles indicate individual animals. After 4 days of MD animals exposed to Val presented significantly lower CBIoiS than nondeprived ones. *P<0.001.
Discussion
Here we show that early exposure to Val, but not to Lev significantly affects the development of orientation columns and neuronal orientation selectivity in the visual cortex of the ferret. Optical imaging of intrinsic signals revealed that early Val exposure decreased contrast of orientation maps while preserving robust signal strength for every orientation tested. Consistently with the optical imaging results, single-unit recordings revealed that early Val exposure decreased neuronal selectivity to stimulus orientation while preserving normal strength of visual responses. These effects resemble the findings in ferrets raised under extreme disruption of sensory activity (Weliky and Katz, 1997). Here we found that exposure to VAL during the third trimester equivalent of human gestation delays the natural opening of the eyes in approximately 5 days in the ferret. However this delay cannot explain the disruption of the functional organization of the visual cortex described here, since artificially opening the eyes of Val treated animals at a proper time does not prevent the impairment of orientation selectivity columns. Furthermore, the disruption reported here is not due to delay on development (i.e. a delay in the maturation of orientation selectivity) since the orientation selectivity is impaired even in young adults Val-treated animals.
Recently, we showed that treatment with the phosphodiesterase type 1 inhibitor vinpocetine restored both ocular dominance plasticity (Medina et al., 2006) and orientation selectivity (Krahe et al., 2009) in animals exposed to alcohol between P10 and P30. Here we showed that early Val exposure did not result in an impairment of ocular dominance (OD) plasticity, and that vinpocetine was not able to restore orientation selectivity in Val animals. The comparison of these findings strongly suggests that alcohol and valproic acid disrupt orientation selectivity by two different mechanisms.
Advantages of the ferret model
Ferrets have a highly developed visual cortex characterized by ocular dominance and orientation selectivity columns (Ruthazer et al., 1999; Weliky and Katz, 1997; White et al., 2001b; White et al., 2001a), which are lacking in rodents but present in humans (Goodyear et al., 2002). The ferret model is also advantageous over rodents because their frontally placed eyes, which allow a large binocular representation in the primary visual cortex (Law et al., 1988). Another major advantage of ferrets is that their gestational period is relatively short and, most importantly, they are born relatively immature (Clancy et al., 2001; Medina et al., 2003). Moreover, a reasonably large base of knowledge has accumulated on the physiology and anatomy of the visual system in the developing ferret (Issa et al., 1999; Krahe et al., 2005; Law et al., 1988; Weliky et al., 1996; White et al., 1999; White et al., 2001a).
Effects of valproic exposure on the development of orientation selectivity
Here, ferrets were exposed to Val from P10 to P30, when lateral geniculate neurons first form synapses with layer IV neurons (Herrmann et al., 1994) and orientation selectivity of cortical neurons starts developing (Chapman and Stryker, 1993; Chapman et al., 1996; Krug et al., 2001). However, orientation selectivity matures further after the end of Val exposure until the adult state is reached approximately at P38 (Chapman et al., 1996). The establishment of orientation selectivity in the visual cortex of mammals has been extensively studied. There is a general understanding that the emergence of orientation selectivity arises from many circuits [see for review Somers et al. (1995) and White and Fitzpatrick (2007)]. Neighboring geniculate afferents, which have aligned receptive fields, converge in layer 4 neurons of V1 creating the first form of orientation selectivity (Chapman et al., 1991; Hubel and Wiesel, 1962). The orientation tuning of these layer 4 neurons is further increased due to inhibition. Cortical neurons that are tuned for a given orientation can activate interneurons which inhibit neurons that have different orientation preferences (Crook et al., 1998; Allison et al., 1995). Finally, the final tuning may be accomplished by a mechanism of recurrent cortical excitation between cells that have similar orientation selectivity (Schummers et al., 2007; Somers et al., 1995). Therefore the establishment of orientation selectivity relies on the precision of the balance between excitation and inhibition and the accuracy of the neuronal circuitry. In the light of the importance of these factors it is comprehensible why exposure to Val would disrupt orientation selectivity. Valproic acid reduces GABA degradation and increases GABA synthesis (Loscher, 2002). In addition, Val reduces NMDA-mediated excitation (Gobbi and Janiri, 2006), which could further alter the excitation/inhibition ratio. The effect of early Val exposure on neuronal circuitry was recently investigated. Rinaldi et al. showed that a single dose of 500/mg kg of Val during embryogenesis resulted in a hyperconnectivity in both excitatory and inhibitory microcircuits in the primary somatosensory cortex (Rinaldi et al., 2008). It would be interesting in further studies to investigate the anatomical basis for the effects of the early exposure to Val on the disruption of orientation selectivity reported here.
Another factor that may play a role in the disruption of orientation selectivity by Val exposure is increased neuroapoptosis. It has been extensively demonstrated that during the third trimester of development neurons are particularly susceptible to apoptosis triggered by drugs that potentiate GABA, such as alcohol, pentobarbital, isofluorene and Val (Bittigau et al., 2002; Ikonomidou et al., 2000; Brambrink et al., 2010). Therefore, it is possible that an increased cell death during a period where synapses are being formed and circuits are being refined, contributes to the disruption of orientation selectivity observed here.
Clinical relevance
Valproic acid crosses the placenta freely and its concentration is often higher in umbilical cord (or neonatal blood) than in the maternal serum (Barzago et al., 1996). The effects of Val during the first trimester of gestation have been extensively demonstrated. For instance, exposure to Val during the organogenesis has been shown to result in a constellation of alterations such as spina bifida, cranial–facial dysmorphism, cleft lip and palate and neural tube defects (Ornoy, 2009; Robert and Guibaud, 1982; Lindhout and Schmidt, 1986). In addition, animal models that receive a single injection of Val during the period of the closure of the neural tube present a delay in eye opening and many characteristics of autism (Wagner et al., 2006; Schneider and Przewlocki, 2005). In fact autistic behavior is commonly seen in cases of children that were exposed to Val during pregnancy (Rasalam et al., 2005). Recently, a multicenter study showed that children that have been exposed to valproic acid during gestation displayed a reduction of 9 points in IQ score when tested at 3 years of age (Meador et al., 2009).
The consequences of Val exposure during the late gestation are poorly known, and the lack of this knowledge may constitute a problem for the proper management of seizures in pregnant women. Here we show, in an animal model, the remarkable disruptive effect of Val exposure during the third trimester equivalent of human gestation on the functional organization of a sensory area. Surprisingly, we observed the extent of delay on natural eye opening, which is one of the features of the Val model of autism (Schneider and Przewlocki, 2005).
In conclusion, the disruption of the functional organization of a primary sensory area reported here suggests that Val acid should be avoided during the late gestation and contributes to our understanding of how this drug affects cognitive function and behavior.
Acknowledgments
This work was supported by Thrasher Fund grant 513055.
References
- Adab N, Kini U, Vinten J, Ayres J, Baker GE, Clayton-Smith J, Coyle H, Fryer A, Gorry J, Gregg J, Mawer G, Nicolaides P, Pickering L, Tunnicliffe L, Chadwick DW. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1583. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison JD, Casagrande VA, Bonds AB. Dynamic differentiation of GABAA-sensitive influences on orientation selectivity of complex cells in the cat striate cortex. Exp Brain Res. 1995;104:81–88. doi: 10.1007/BF00229857. [DOI] [PubMed] [Google Scholar]
- Barzago MM, Bortolotti A, Stellari FF, Diomede L, Algeri M, Efrati S, Salmona M, Bonati M. Placental transfer of valproic acid after liposome encapsulation during in vitro human placenta perfusion. J Pharmacol Exp Ther. 1996;277:79–86. [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospichil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13:5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Zahs KR, Stryker MP. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J Neurosci. 1991;11:1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP, Bonhoeffer T. Development of orientation preference maps in ferret primary visual cortex. J Neurosci. 1996;16:6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- Crook JM, Kisvarday ZF, Eysel UT. Evidence for a contribution of lateral inhibition to orientation tuning and direction selectivity in cat visual cortex: reversible inactivation of functionally characterized sites combined with neuroanatomical tracing techniques. Eur J Neurosci. 1998;10:2056–2075. doi: 10.1046/j.1460-9568.1998.00218.x. [DOI] [PubMed] [Google Scholar]
- Fairgrieve SD. Population based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674–675. doi: 10.1136/bmj.321.7262.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Janiri L. Sodium- and magnesium-valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psycho-pharmacology (Berl) 2006;185:255–262. doi: 10.1007/s00213-006-0317-3. [DOI] [PubMed] [Google Scholar]
- Goodyear BG, Nicolle DA, Menon RS. High resolution fMRI of ocular dominance columns within the visual cortex of human amblyopes. Strabismus. 2002;10:129–136. doi: 10.1076/stra.10.2.129.8140. [DOI] [PubMed] [Google Scholar]
- Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;3:610–621. [Google Scholar]
- Herrmann K, Antonini A, Shatz CJ. Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur J Neurosci. 1994;6:1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Harvey EA, Coull BA, Huntington KB, Khoshbin S, Hayes AM, Ryan LM. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–1138. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Isoherranen N, Spiegelstein O, Bialer M, Zhang J, Merriweather M, Yagen B, Roeder M, Tripplett AA, Schurig V, Finnell RH. Developmental outcome of levetiracetam, its major metabolite in humans, 2-pyrrolidinone N-butyric acid, and its enantiomer (R)-alpha-ethyl-oxo-pyrrolidine acetamide in a mouse model of teratogenicity. Epilepsia. 2003;44:1280–1288. doi: 10.1046/j.1528-1157.2003.21503.x. [DOI] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret's visual cortex. J Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Grimee R, Vanneste-Goemaere J, Margineanu DG. Electrophysiological, neurochemical and regional effects of levetiracetam in the rat pilocarpine model of temporal lobe epilepsy. Seizure. 2003;12:92–100. doi: 10.1016/s1059131102001930. [DOI] [PubMed] [Google Scholar]
- Krahe TE, Medina AE, Bittencourt-Navarrete RE, Colello RJ, Ramoa AS. Protein synthesis independent plasticity mediates rapid and precise recovery of deprived eye responses. Neuron. 2005;48:329–343. doi: 10.1016/j.neuron.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Krahe TE, Wang W, Medina AE. Phosphodiesterase inhibition increases CREB phosphorylation and restores orientation selectivity in a model of fetal alcohol spectrum disorders. PLoS ONE. 2009;4:e6643. doi: 10.1371/journal.pone.0006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug K, Akerman CJ, Thompson ID. Responses of neurons in neonatal cortex and thalamus to patterned visual stimulation through the naturally closed lids. J Neurophysiol. 2001;85:1436–1443. doi: 10.1152/jn.2001.85.4.1436. [DOI] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. J Comp Neurol. 1988;278:157–180. doi: 10.1002/cne.902780202. [DOI] [PubMed] [Google Scholar]
- Lindhout D, Schmidt D. In-utero exposure to valproate and neural tube defects. Lancet. 1986;1:1392–1393. doi: 10.1016/s0140-6736(86)91711-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Fraile IP, Cid AO, Juste AO, Modrego PJ. Levetiracetam plasma level monitoring during pregnancy, delivery, and postpartum: clinical and outcome implications. Epilepsy Behav. 2009;15:372–375. doi: 10.1016/j.yebeh.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjahlieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW NEAD StudyGroup. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Ramoa AS. Early alcohol exposure impairs ocular dominance plasticity throughout the critical period. Brain Res Dev Brain Res. 2005;157:107–111. doi: 10.1016/j.devbrainres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci. 2003;23:10002–10012. doi: 10.1523/JNEUROSCI.23-31-10002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol. 2005;93:1317–1325. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Restoration of neuronal plasticity by a phosphodiesterase type 1 inhibitor in a model of fetal alcohol exposure. J Neurosci. 2006;26:1057–1060. doi: 10.1523/JNEUROSCI.4177-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A. Valproic acid inpregnancy: how much arewe endangering the embryo and fetus? Reprod Toxicol. 2009;28:1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Ozyurek H, Bozkurt A, Bilge S, Ciftcioglu E, Ilkaya F, Bas DB. Effect of prenatal levetiracetam exposure on motor and cognitive functions of rat offspring. Brain Dev. 2010;32(5):396–403. doi: 10.1016/j.braindev.2009.05.003. Electronic publication ahead of print 2009 Jun 4. [DOI] [PubMed] [Google Scholar]
- Paul AP, Pohl-Guimaraes F, Krahe TE, Filgueiras CC, Lantz CL, Colello RJ, Wang W, Medina AE. Overexpression of serum response factor restores ocular dominance plasticity in a model of fetal alcohol spectrum disorders. J Neurosci. 2010;30:2513–2520. doi: 10.1523/JNEUROSCI.5840-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS, Mower AF, Liao DS, Jafri SI. Suppression of cortical NMDA receptor function prevents development of orientation selectivity in the primary visual cortex. J Neurosci. 2001;21:4299–4309. doi: 10.1523/JNEUROSCI.21-12-04299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorders. Dev Med Child Neurol. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex. 2008;18:763–770. doi: 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2 doi: 10.1016/s0140-6736(82)90908-4. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Baker GE, Stryker MP. Development and organization of ocular dominance bands in primary visual cortex of the sable ferret. J Comp Neurol. 1999;407:151–165. [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Schummers J, Cronin B, Wimmer K, Stimberg M, Martin R, Obermayer K, Koerding K, Sur M. Dynamics of orientation tuning in cat v1 neurons depend on location within layers and orientation maps. Front Neurosci. 2007;1:145–159. doi: 10.3389/neuro.01.1.1.011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Nwesome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DC, Nelson SB, Sur M. An emergent model of orientation selectivity in cat visual cortical simple cells. J Neurosci. 1995;15:5448–5465. doi: 10.1523/JNEUROSCI.15-08-05448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinten J, Adab N, Kini U, Gorry J, Gregg J, Baker JA. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954. doi: 10.1212/01.WNL.0000154514.82948.69. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK. A new neurobehavioral model of autism in mice: pre and postnatal exposure to sodium valproate. J Autism Dev Disord. 2006;36:779–793. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Disruption of orientation tuning in visual cortex by artificially correlated neuronal activity. Nature. 1997;386:680–685. doi: 10.1038/386680a0. [DOI] [PubMed] [Google Scholar]
- Weliky M, Bosking WH, Fitzpatrick D. A systematic map of direction preference in primary visual cortex. Nature. 1996;379:725–728. doi: 10.1038/379725a0. [DOI] [PubMed] [Google Scholar]
- White LE, Fitzpatrick D. Vision and cortical map development. Neuron. 2007;56:327–338. doi: 10.1016/j.neuron.2007.10.011. [DOI] [PubMed] [Google Scholar]
- White LE, Bosking WH, Williams SM, Fitzpatrick D. Maps of central visual space in ferret V1 and V2 lack matching inputs from the two eyes. J Neurosci. 1999;19:7089–7099. doi: 10.1523/JNEUROSCI.19-16-07089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Bosking WH, Fitzpatrick D. Consistent mapping of orientation preference across irregular functional domains in ferret visual cortex. Vis Neurosci. 2001a;18:65–76. doi: 10.1017/s095252380118106x. [DOI] [PubMed] [Google Scholar]
- White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature. 2001b;411:1049–1052. doi: 10.1038/35082568. [DOI] [PubMed] [Google Scholar]
- Xing J, Gerstein GL. Networks with lateral connectivity I, II, III. J Neurophysiol. 1996;75:184–232. doi: 10.1152/jn.1996.75.1.184. [DOI] [PubMed] [Google Scholar]


