Abstract
Tumor suppressor candidate 2 (TUSC2; also known as FUS1) was identified in 2000 as a candidate tumor suppressor gene located in a region on chromosome 3p21.3 that is homozygously deleted in some lung and breast cancers. The deletion is rare in lung and breast cancers, but is frequent in malignant pleural mesothelioma. Evidence to date indicates that TUSC2 behaves as a tumor suppressor in lung cancer; however, its role as a tumor suppressor for other tumor types has not been fully established. Loss of TUSC2 expression at the mRNA and protein levels has been reported in various cancers. While the mechanisms underlying the loss are still not well understood, several microRNAs have been reported to downregulate TUSC2 protein expression. TUSC2 elicits its anti-tumor effects through regulating G1 cell cycle progression, apoptosis, calcium homeostasis, gene expression, and the activity of various protein tyrosine kinases and Ser/Thy kinases, albeit the precise mechanisms that TUSC2 utilizes to regulates these cellular processes and signaling molecules are still elusive. TUSC2 restoration has been exploited as an anti-cancer therapy in various cancers in preclinical models, and clinically in patients with lung cancer. The first-in-human Phase I trial demonstrated desirable safety outcomes. Phase I/II trials are being conducted to evaluate the efficacy of combining TUSC2-nanoparticles with Erlotinib, an FDA-approved EGFR inhibitor. This review summarizes recent publications that advanced our understanding of TUSC2 as a novel tumor suppressor and a therapeutic opportunity for treating TUSC2-deficient cancers.
Introduction
Tumor suppressor candidate 2 (TUSC2; also known as FUS1) was identified as a candidate tumor suppressor gene located in a region on chromosome 3p21.3 that is homozygously deleted in some lung and breast cancers (Lerman & Minna, 2000). However, 3p21.3 deletion was later found to be very rare in lung cancer (1.1%; TCGA) and most cancer types, except for malignant pleural mesothelioma (36%) (Ivanova et al., 2009). In lung cancer, no evidence of methylation was found in the TUSC2 gene promoter region (Kondo et al., 2001). In contrast, TUSC2 promoter region was reported to be partially methylated in oral tumors and normal salivary rinses but unmethylated in normal mucosa (Demokan et al., 2013).
In lung cancer for which TUSC2 is considered as a tumor suppressor gene, loss of TUSC2 mRNA expression has been observed in ~80% of the tumors and the loss was attributed to 3p21.3 deletion (Lerman & Minna, 2000) and post-transcriptional repression by microRNAs (Du et al., 2009; Lee et al., 2007). TUSC2 somatic mutations have not been found in any cancer specimens according to TCGA, albeit infrequent mutations have been reported in lung cancer cell lines (Kondo et al., 2001).
The TUSC2 protein is consisted of 110 amino acids and located predominately in mitochondria and cytoplasm (Ivanova et al., 2007; Kondo et al., 2001). Myristoylation is the only post-translational modification of the TUSC2 protein that has been reported. Using surface-enhanced laser desorption/ ionization mass spectrometric analysis on an anti-TUSC2 antibody-capture ProteinChip array, Uno et al identified TUSC2 as an N-myristoylated protein. Myristoylation is required for its tumor suppressive activity in human lung cancer cells (Uno et al., 2004). A myristoylation-deficient TUSC2 mutant lost the ability to inhibit cell growth and induce apoptosis in lung cancer, in vitro and in vivo, and is susceptible to rapid proteasome-dependent degradation.
The exact functions of TUSC2 are still unclear. Published studies suggest that TUSC2 may have a wide range of functions. TUSC2 has been shown to induce G1 cell cycle arrest and apoptosis (Ji & Roth, 2008; Kondo et al., 2001; Li et al., 2014), regulate calcium signaling (Uzhachenko et al., 2014), modulate tyrosine kinases (Lin et al., 2007), and affect gene expression (Ivanova et al., 2009). TUSC2-knockout mice were not embryonic lethal but displayed complex immunological phenotype and developed spontaneous vascular tumors (Ivanova et al., 2007). Restoration of TUSC2 expression has been explored as an anti-cancer therapy using cell lines, xenograft mouse models and in a Phase I clinical trial of lung cancer (Lu et al., 2012).
TUSC2 Expression in Normal versus Cancerous Tissues
Loss of TUSC2 mRNA expression has been observed in ~80% of lung tumors (Lerman & Minna, 2000). Loss and reduction of TUSC2 protein expression was detected in 82% of non-small cell lung cancers (NSCLCs) and 100% of small cell lung carcinomas (Prudkin et al., 2008). This loss of TUSC2 expression has been shown to occur early in lung cancer pathogenesis (Prudkin et al., 2008). Bronchial squamous metaplastic and dysplastic lesions expressed lower levels of TUSC2 protein compared to normal and hyperplastic epithelia (Prudkin et al., 2008). In NSCLCs, loss of TUSC2 protein, determined by immunohistochemical staining, was associated with significantly worse overall survival (Prudkin et al., 2008).
In malignant pleural mesothelioma (MPM), an aggressive inflammatory cancer associated with exposure to asbestos, TUSC2 mRNA was downregulated in approximately 84% of 30 MPM specimens while loss of 3p21.3 region observed in approximately 36% of MPMs including stage 1 tumors (Ivanova et al., 2009). In gliomas, TUSC2 mRNA was reported to be reduced in high-grade tumors compared to low-grade tumors; however, normal brain tissues were not examined in this study (Xin et al., 2015). Interestingly, TUSC2 mRNA expression was detected in almost all sarcoma cell lines, benign bone and soft-tissue sarcoma tissues, and healthy tissues (Li et al., 2011). In contrast, TUSC2 protein expression was undetected in the majority of tumor tissues frequently lost in sarcoma cells and sarcoma tissues (Li et al., 2011).
TUSC2 Expression Regulation by MicroRNAs
MicroRNAs (miRNAs) are short non-coding RNAs that regulate gene expression post-transcriptionally. MiRNAs bind to the 3′-UTR (untranslated region) of their target mRNAs and repress protein production by destabilizing the mRNA and translational silencing. TUSC2 protein expression has been shown to be negatively regulated by several miRNAs. MiR-93, miR-98, and miR-197 were reported to target the 3′-UTR region of the TUSC2 transcript and that individual deletion of the three miRNA target sites in the TUSC2 3-′UTR restores the expression of TUSC2 protein in lung cancer (Du et al., 2009). Elevated miR-93 and miR-197 expression was found to correlate with reduced TUSC2 expression in NSCLC tumor specimens (Du et al., 2009).
MiRNA-378 binds to 3′-UTR of the TUSC2 transcript and represses its translation (Lee et al., 2007). Expression of miR-378 enhances cell survival, reduces caspase-3 activity, and promotes tumor growth and angiogenesis of U87 glioblastoma cells (Lee et al., 2007). Consistent with these observations, miR-378 transfection has been shown to promote mesenchymal stem cell survival and vascularization under hypoxic-ischemic condition, in part, through downregulating TUSC2 expression (Xing et al., 2014). Furthermore, miR-584 can target TUSC2 expression in thyroid cancer cells (Orlandella et al., 2016).
In addition to 3′-UTR, the 5′-UTR of the TUSC2 transcript may be subjected to miRNA regulation (Haiman et al., 2011). Interestingly, TUSC2 pseudogenes (TUSC2P), found on chromosomes X and Y, are homologous to the 3′-UTR of TUSC2. 3′-UTRs of TUSC2P and TUSC2 share many common miRNA-binding sites (Rutnam et al., 2014). The authors found that ectopic expression of TUSC2P and the TUSC2 3′-UTR inhibited cell proliferation, survival, migration, and invasion, and increased tumor cell death. By interacting with endogenous miRNAs, TUSC2P and TUSC2 3′-UTR arrest the functions of these miRNAs, resulting in increased translation of TUSC2. These observations indicated that TUSC2P can sequester miRNAs so that they can’t inhibit translation of TUSC2 (Rutnam et al., 2014).
TUSC2 Functions
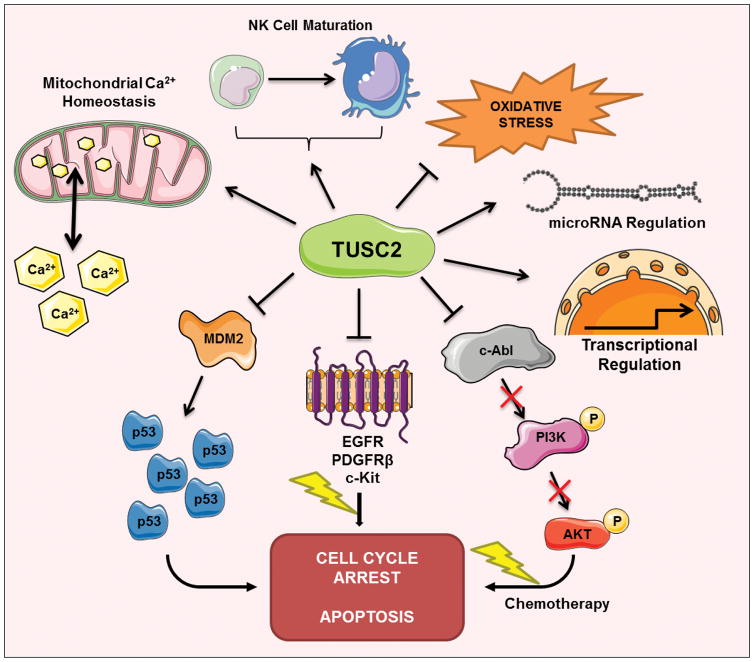
Exact functions of TUSC2 are still not clear. TUSC2 is associated with several pathways and cellular processes (Fig. 1). Forced TUSC2 expression can decrease cell growth, induce G1 cell cycle arrest, and promote apoptosis in lung cancer in vitro and in vivo (Kondo et al., 2001; Li et al., 2014). TUSC2 appeared to affect transcription profile of MPM (Ivanova et al., 2009). The authors conducted gene expression arrays with TUSC2-transfected MPM cells and found TUSC2 transfection led to increased expression of multiple genes with tumor suppressor properties and reduced expression of pro-tumorigenic genes.
Figure 1. TUSC2 regulation of cellular processes and signaling pathways.
TUSC2 has been show to regulate diverse cellular processes, including cell cycle arrest, apoptosis, calcium homoeostasis, oxidative stress response, immune response, miRNA expression, transcriptional regulation, p53/MDM pathway, and tyrosine and Ser/Thr kinases.
TUSC2 has been shown to regulate protein tyrosine and Serine/Threonine kinases. A TUSC2 peptide inhibits activity of Abl tyrosine kinase in NSCLC cells (Lin et al., 2007). Forced expression of full-length TUSC2 decreased levels of activated c-Abl and inhibited its tyrosine kinase activity, suggesting c-Abl is a possible target of TUSC2 in NSCLC. Additional evidence suggest that TUSC2 may inhibit the function of protein tyrosine kinases and Ser/Thr kinases including EGFR, PDGFR, Akt, c-Abl, FGFR and c-Kit (Dai et al., 2015; Hood et al., 2013; Ji & Roth, 2008; Meng et al., 2013). TUSC2 inhibits mTOR activation (Dai et al., 2015). TUSC2 transient expression in LKB1-defective NSCLC cells significantly stimulated AMP-activated protein kinase (AMPK) phosphorylation and enzymatic activity (Meng et al., 2013). A recent study reported that TUSC2 can upregulate miR-197 expression in human glioblastoma cells although the underlying mechanism is not known (Xin et al., 2015).
TUSC2 can regulate mitochondrial calcium handling (Uzhachenko et al., 2014). Since TUSC2 possesses putative calcium-binding and myristoyl-binding domains, the authors found that TUSC2 regulates mitochondrial calcium handling and calcium-coupled processes. TUSC2 loss led to reduced mitochondrial calcium uptake in calcium-loaded epithelial cells, splenocytes, and activated CD4(+) T cells. Ex vivo analysis of activated CD4(+) T cells showed TUSC2-dependent changes in calcium-regulated processes, such as surface expression of CD4 and PD1/PD-L1, proliferation, and Th polarization. In contract, TUSC2-knockout T cells showed increased of calcium-dependent NF-kappaB and NFAT targets but were unable to fully activate these pathways after stimulation (Uzhachenko et al., 2014). Since mitochondrial calcium homeostasis is an important contributing factor to premature aging and aging-associated pathologies, the same group (Uzhachenko et al., 2017) observed that TUSC2-KO mice developed multiple early aging signs including lordokyphosis, lack of vigor, inability to accumulate fat, reduced ability to tolerate stress, and premature death, as well as, low sperm counts, compromised ability of adult stem cells to repopulate tissues, and chronic inflammation. In addition to altered mitochondrial calcium homeostasis, TUSC2-KO cells have low reserve respiratory capacity, suggesting that energy homeostasis is also controlled by TUSC2 (Uzhachenko et al., 2017).
TUSC2 Restoration as Cancer Therapy
TUSC2 restoration is an attractive strategy to inhibit tumor growth and progression given its tumor suppressive effects. Overexpression of TUSC2 has been shown to have anti-tumor effects in lung cancer (Ito et al., 2004; Kondo et al., 2001), glioblastoma (Xin et al., 2015), and oesophageal carcinoma (Zhang et al., 2013), and thyroid cancer (Orlandella et al., 2016). In NSCLC models, exogenous restoration of TUSC2 expression sensitized EGFR-expressing lung cancer cells to EGFR inhibitors (Dai et al., 2015). The authors reported that combination treatment with intravenous TUSC2 nanovesicles and erlotinib (EGFR inhibitor) synergistically inhibited tumor growth and metastasis, and increased apoptotic activity of NSCLC xenografts. TUSC2 inhibits mTOR activation, and TUSC2 restoration rendered tumors more responsive to the mTOR inhibitor rapamycin in combination with erlotinib. A more recent study further reported that increasing reactive oxygen species (ROS) via addition of the thioredoxin reductase 1 inhibitor auranofin enhanced therapeutic efficacy of the combination of TUSC2 with erlotinib in EGFR-expressing lung cancer (Xiaobo et al., 2016).
TUSC2 restoration also sensitized LKB1-defective NSCLC to an Akt inhibitor MK2206 (Meng et al., 2013). In contrast, TUSC2 did not affect the response to MK2206 treatment for two LKB1-proficient NSCLC cell lines. Systemic TUSC2 delivery by nanoparticle gene transfer, combined with MK2206, inhibited growth LKB1-defective lung cancer xenografts. Interestingly, TUSC2 transient expression in LKB1-defective cells significantly activated AMPK phosphorylation and enzymatic activity. AMPK gene knockdown abrogated the TUSC2-MK2206 cooperation, suggesting TUSC2 restoration as a viable strategy to overcome tumor resistance to Akt-targeted therapy (Meng et al., 2013). More recently, the same group explored the use of combined gene therapy with LKB1 and TUSC2, two tumor suppressors, as a cancer therapy for lung cancer (Li et al., 2014). The results indicated that intratumoral administration of TUSC2-LKB1 liposomes led to inhibition of subcutaneous lung tumor xenograft while intravenous injections resulted in a reduction of metastatic tumor modules.
Co-expression of TUSC2 with another tumor suppressor p53 also synergistically suppressed lung tumor growth (Deng et al., 2007). Co-expression of TUSC2 with human IL-12 inhibits lung tumor growth and metastasis (Ren et al., 2014). The co-expression was found to induce strong antitumor immune response by secreting much higher levels of interferon-gamma and IL-15, enhancing expression of MHC-I and Fas, increasing infiltration of activated CD4+ and CD8+ T lymphocytes. TUSC2-hIL-12 coexpression could also obviously induce tumor cell apoptosis and inhibit tumor cell proliferation partly by higher activation of STAT1 signal pathway and upregulation of p53 (Ren et al., 2014).
TUSC2 restoration can also potentiate the effects of cisplatin in NSCLC (Deng et al., 2008). Systemic administration of TUSC2 nanoparticle sensitized NSCLC tumors to cisplatin. TUSC2-enhanced chemosensitivity is associated with the downregulation of MDM2, accumulation of p53 and activation of the Apaf-1-dependent apoptosis pathway. Interestingly, TUSC2 is associated with radioprotection of normal tissues (Yazlovitskaya et al., 2013; Yazlovitskaya et al., 2015).
A Phase I clinical study with systemically administered TUSC2 nanoparticles was conducted in NSCLC patients (NCT00059605)(Lu et al., 2012). Thirty one patients with recurrent and/or metastatic lung cancer previously treated with platinum-based chemotherapy were enrolled in this study. Patients were treated with six escalating doses of intravenous N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTAP):cholesterol nanoparticles encapsulating a TUSC2 expression plasmid (DOTAP:chol-TUSC2) every 3 weeks. The MTD was determined to be 0.06 mg/kg. Five patients achieved stable disease (2.6–10.8 months, including 2 minor responses). The authors concluded that DOTAP:chol-TUSC2 can be safely administered intravenously in lung cancer patients. This encouraging outcome has led to ongoing Phase I/II trials with stage IV lung cancer patients (NCT01455389). In the Phase I trial, TUSC2-nanoparticles combined with Erlotinib (EGFR inhibitor) will be tested to determine MTD. The goal of the Phase II trial is to examine whether the combination of TUSC2-nanoparticles and erlotinib can help to control NSCLC.
Conclusions
Evidence to date indicates that TUSC2 behaves as a tumor suppressor in lung cancer; however, its role as a tumor suppressor for other tumor types has not been fully established. Loss of TUSC2 expression at the mRNA and protein levels has been reported in various cancers. The mechanisms underlying the loss are still not well understood. Additional efforts are needed to elucidate how TUSC2 expression is reduced or lost in tumors that lack 3p21.3 homozygous deletion. Also unclear is how TUSC2 elicits its anti-tumor effects. Although TUSC2 is involved in G1 cell cycle arrest, apoptosis, calcium homeostasis, gene expression and regulation of the activity of various protein tyrosine kinases and Ser/Thy kinases, the precise mechanisms that TUSC2 utilizes to regulate these cellular processes and signaling molecules are still not well understood. Additional efforts are needed to address these knowledge gaps. TUSC2 restoration has been exploited as an anti-cancer therapy in various cancers in preclinical models, and clinically in patients with lung cancer. The first-in-human Phase I trial demonstrated desirable safety outcomes and subsequent Phase I/II trials are being conducted to determine the efficacy of combining TUSC2-nanoparticles with Erlotinib. Taken together, TUSC2 has been shown to play an important tumor suppressive role in lung cancer and may potentially be an important tumor suppressor for other cancer types.
Acknowledgments
This study is supported by the NIH/NINDS grants (7R01NS087169 to HWL), 7R01NS087169-4S1 (to HWL and AH), and the DoD grant (W81XWH-17-1-0044/BC160850 to HWL).
Footnotes
Disclosure Statement:
The authors report no conflicts of interest.
References
- Dai B, Yan S, Lara-Guerra H, Kawashima H, Sakai R, Jayachandran G, Majidi M, Mehran R, Wang J, Bekele BN, Baladandayuthapani V, Yoo SY, Wang Y, Ying J, Meng F, Ji L, Roth JA. Exogenous Restoration of TUSC2 Expression Induces Responsiveness to Erlotinib in Wildtype Epidermal Growth Factor Receptor (EGFR) Lung Cancer Cells through Context Specific Pathways Resulting in Enhanced Therapeutic Efficacy. PLoS One. 2015;10(6):e0123967. doi: 10.1371/journal.pone.0123967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demokan S, Chuang AY, Chang X, Khan T, Smith IM, Pattani KM, Dasgupta S, Begum S, Khan Z, Liegeois NJ, Westra WH, Sidransky D, Koch W, Califano JA. Identification of guanine nucleotide-binding protein gamma-7 as an epigenetically silenced gene in head and neck cancer by gene expression profiling. Int J Oncol. 2013;42(4):1427–1436. doi: 10.3892/ijo.2013.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WG, Kawashima H, Wu G, Jayachandran G, Xu K, Minna JD, Roth JA, Ji L. Synergistic tumor suppression by coexpression of FUS1 and p53 is associated with down-regulation of murine double minute-2 and activation of the apoptotic protease-activating factor 1-dependent apoptotic pathway in human non-small cell lung cancer cells. Cancer Res. 2007;67(2):709–717. doi: 10.1158/0008-5472.CAN-06-3463. [DOI] [PubMed] [Google Scholar]
- Deng WG, Wu G, Ueda K, Xu K, Roth JA, Ji L. Enhancement of antitumor activity of cisplatin in human lung cancer cells by tumor suppressor FUS1. Cancer Gene Ther. 2008;15(1):29–39. doi: 10.1038/sj.cgt.7701094. [DOI] [PubMed] [Google Scholar]
- Du L, Schageman JJ, Subauste MC, Saber B, Hammond SM, Prudkin L, Wistuba Ii, Ji L, Roth JA, Minna JD, Pertsemlidis A. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol Cancer Res. 2009;7(8):1234–1243. doi: 10.1158/1541-7786.MCR-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman CA, Chen GK, Vachon CM, Canzian F, Dunning A, Millikan RC, Wang X, Ademuyiwa F, Ahmed S, Ambrosone CB, Baglietto L, Balleine R, Bandera EV, Beckmann MW, Berg CD, Bernstein L, Blomqvist C, Blot WJ, Brauch H, Buring JE, et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011;43(12):1210–1214. doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Uzhachenko R, Boyd K, Skaar EP, Ivanova AV. Loss of mitochondrial protein Fus1 augments host resistance to Acinetobacter baumannii infection. Infect Immun. 2013;81(12):4461–4469. doi: 10.1128/IAI.00771-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I, Ji L, Tanaka F, Saito Y, Gopalan B, Branch CD, Xu K, Atkinson EN, Bekele BN, Stephens LC, Minna JD, Roth JA, Ramesh R. Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates potent antitumor activity against human lung cancer in vivo. Cancer Gene Ther. 2004;11(11):733–739. doi: 10.1038/sj.cgt.7700756. [DOI] [PubMed] [Google Scholar]
- Ivanova AV, Ivanov SV, Pascal V, Lumsden JM, Ward JM, Morris N, Tessarolo L, Anderson SK, Lerman MI. Autoimmunity, spontaneous tumourigenesis, and IL-15 insufficiency in mice with a targeted disruption of the tumour suppressor gene Fus1. J Pathol. 2007;211(5):591–601. doi: 10.1002/path.2146. [DOI] [PubMed] [Google Scholar]
- Ivanova AV, Ivanov SV, Prudkin L, Nonaka D, Liu Z, Tsao A, Wistuba I, Roth J, Pass HI. Mechanisms of FUS1/TUSC2 deficiency in mesothelioma and its tumorigenic transcriptional effects. Mol Cancer. 2009;8:91. doi: 10.1186/1476-4598-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Roth JA. Tumor suppressor FUS1 signaling pathway. J Thorac Oncol. 2008;3(4):327–330. doi: 10.1097/JTO.0b013e31816bce65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Ji L, Kamibayashi C, Tomizawa Y, Randle D, Sekido Y, Yokota J, Kashuba V, Zabarovsky E, Kuzmin I, Lerman M, Roth J, Minna JD. Overexpression of candidate tumor suppressor gene FUS1 isolated from the 3p21.3 homozygous deletion region leads to G1 arrest and growth inhibition of lung cancer cells. Oncogene. 2001;20(43):6258–6262. doi: 10.1038/sj.onc.1204832. [DOI] [PubMed] [Google Scholar]
- Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104(51):20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000;60(21):6116–6133. [PubMed] [Google Scholar]
- Li G, Kawashima H, Ji L, Ogose A, Ariizumi T, Umezu H, Xu Y, Hotta T, Endo N. Frequent absence of tumor suppressor FUS1 protein expression in human bone and soft tissue sarcomas. Anticancer Res. 2011;31(1):11–21. [PubMed] [Google Scholar]
- Li L, Yu C, Ren J, Ye S, Ou W, Wang Y, Yang W, Zhong G, Chen X, Shi H, Su X, Chen L, Zhu W. Synergistic effects of eukaryotic coexpression plasmid carrying LKB1 and FUS1 genes on lung cancer in vitro and in vivo. J Cancer Res Clin Oncol. 2014;140(6):895–907. doi: 10.1007/s00432-014-1607-5. [DOI] [PubMed] [Google Scholar]
- Lin J, Sun T, Ji L, Deng W, Roth J, Minna J, Arlinghaus R. Oncogenic activation of c-Abl in non-small cell lung cancer cells lacking FUS1 expression: inhibition of c-Abl by the tumor suppressor gene product Fus1. Oncogene. 2007;26(49):6989–6996. doi: 10.1038/sj.onc.1210500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Stewart DJ, Lee JJ, Ji L, Ramesh R, Jayachandran G, Nunez MI, Wistuba Ii, Erasmus JJ, Hicks ME, Grimm EA, Reuben JM, Baladandayuthapani V, Templeton NS, Mcmannis JD, Roth JA. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS One. 2012;7(4):e34833. doi: 10.1371/journal.pone.0034833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Majidi M, Fang B, Ji L, Bekele BN, Minna JD, Roth JA. The tumor suppressor gene TUSC2 (FUS1) sensitizes NSCLC to the AKT inhibitor MK2206 in LKB1-dependent manner. PLoS One. 2013;8(10):e77067. doi: 10.1371/journal.pone.0077067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandella FM, Di Maro G, Ugolini C, Basolo F, Salvatore G. TWIST1/miR-584/TUSC2 pathway induces resistance to apoptosis in thyroid cancer cells. Oncotarget. 2016;7(43):70575–70588. doi: 10.18632/oncotarget.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudkin L, Behrens C, Liu DD, Zhou X, Ozburn NC, Bekele BN, Minna JD, Moran C, Roth JA, Ji L, Wistuba Ii. Loss and reduction of FUS1 protein expression is a frequent phenomenon in the pathogenesis of lung cancer. Clin Cancer Res. 2008;14(1):41–47. doi: 10.1158/1078-0432.CCR-07-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Yu C, Wu S, Peng F, Jiang Q, Zhang X, Zhong G, Shi H, Chen X, Su X, Luo X, Zhu W, Wei Y. Cationic liposome mediated delivery of FUS1 and hIL-12 coexpression plasmid demonstrates enhanced activity against human lung cancer. Curr Cancer Drug Targets. 2014;14(2):167–180. doi: 10.2174/1568009614666140113115651. [DOI] [PubMed] [Google Scholar]
- Rutnam ZJ, Du WW, Yang W, Yang X, Yang BB. The pseudogene TUSC2P promotes TUSC2 function by binding multiple microRNAs. Nat Commun. 2014;5:2914. doi: 10.1038/ncomms3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno F, Sasaki J, Nishizaki M, Carboni G, Xu K, Atkinson EN, Kondo M, Minna JD, Roth JA, Ji L. Myristoylation of the fus1 protein is required for tumor suppression in human lung cancer cells. Cancer Res. 2004;64(9):2969–2976. doi: 10.1158/0008-5472.can-03-3702. [DOI] [PubMed] [Google Scholar]
- Uzhachenko R, Boyd K, Olivares-Villagomez D, Zhu Y, Goodwin JS, Rana T, Shanker A, Tan WJ, Bondar T, Medzhitov R, Ivanova AV. Mitochondrial protein Fus1/Tusc2 in premature aging and age-related pathologies: critical roles of calcium and energy homeostasis. Aging (Albany NY) 2017;9(3):627–649. doi: 10.18632/aging.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzhachenko R, Ivanov SV, Yarbrough WG, Shanker A, Medzhitov R, Ivanova AV. Fus1/Tusc2 is a novel regulator of mitochondrial calcium handling, Ca2+-coupled mitochondrial processes, and Ca2+-dependent NFAT and NF-kappaB pathways in CD4+ T cells. Antioxid Redox Signal. 2014;20(10):1533–1547. doi: 10.1089/ars.2013.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaobo C, Majidi M, Feng M, Shao R, Wang J, Zhao Y, Baladandayuthapani V, Song J, Fang B, Ji L, Mehran R, Roth JA. TUSC2(FUS1)-erlotinib Induced Vulnerabilities in Epidermal Growth Factor Receptor(EGFR) Wildtype Non-small Cell Lung Cancer(NSCLC) Targeted by the Repurposed Drug Auranofin. Sci Rep. 2016;6:35741. doi: 10.1038/srep35741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J, Zhang XK, Xin DY, Li XF, Sun DK, Ma YY, Tian LQ. FUS1 acts as a tumor-suppressor gene by upregulating miR-197 in human glioblastoma. Oncol Rep. 2015;34(2):868–876. doi: 10.3892/or.2015.4069. [DOI] [PubMed] [Google Scholar]
- Xing Y, Hou J, Guo T, Zheng S, Zhou C, Huang H, Chen Y, Sun K, Zhong T, Wang J, Li H, Wang T. microRNA-378 promotes mesenchymal stem cell survival and vascularization under hypoxic-ischemic conditions in vitro. Stem Cell Res Ther. 2014;5(6):130. doi: 10.1186/scrt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazlovitskaya EM, Uzhachenko R, Voziyan PA, Yarbrough WG, Ivanova AV. A novel radioprotective function for the mitochondrial tumor suppressor protein Fus1. Cell Death Dis. 2013;4:e687. doi: 10.1038/cddis.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazlovitskaya EM, Voziyan PA, Manavalan T, Yarbrough WG, Ivanova AV. Cellular oxidative stress response mediates radiosensitivity in Fus1-deficient mice. Cell Death Dis. 2015;6:e1652. doi: 10.1038/cddis.2014.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xu X, Qi Z, Peng L, Qiu B, Huo X. The FUS1 gene inhibits EC109 cell growth mediated by a lentivirus vector. Br J Biomed Sci. 2013;70(1):22–26. doi: 10.1080/09674845.2013.11669925. [DOI] [PubMed] [Google Scholar]