Abstract
Objective
To characterize puberty in girls with Turner syndrome (TS) and determine whether specific patient characteristics are associated with the timing of menarche. We also sought to compare spontaneous versus induced puberty in these patients.
Methods
Medical records of girls followed in our Pediatric Endocrine clinic for TS from 2007 to 2015 were reviewed.
Results
Fifty-three girls were included, of whom 10 (19%) achieved menarche spontaneously and 43 (81%) received hormone replacement therapy (HRT). Of girls receiving HRT, a younger age at estrogen initiation correlated with a longer time to menarche (P = .02), and a mosaic karyotype was associated with a shorter time to menarche (P = .02), whereas no relationship was seen for body mass index, estrogen regimen, or maternal age at menarche. Nineteen girls (44%) receiving HRT had bleeding on estrogen alone at a wide dose range and were more likely to be on transdermal than oral preparations (P = .01). Girls with spontaneous puberty achieved menarche at a younger age (P<.01) and were more likely to have mosaic TS (P = .02).
Conclusion
Significant variability in the timing of menarche exists among girls with TS. However, age at pubertal induction and karyotype were significantly correlated with age at menarche in our patients. A wide range of estrogen doses is seen in girls who bleed prior to progesterone, suggesting extreme variability in estrogen sensitivity among patients with TS. Girls achieving spontaneous menarche are younger and more likely to have a mosaic karyotype than those with induced menarche. Large-scale prospective studies are needed to confirm these results.
INTRODUCTION
Turner syndrome (TS) is one of the most common chromosomal abnormalities in females, with an annual incidence of 1 in 2,500 live births (1). First described in 1938, the most common phenotypic features of TS are short stature and primary ovarian failure (2). On average, 50% of girls with TS are monosomy X, whereas the remainder have a variety of karyotypes, including mosaicism and partial deletions of one X chromosome (3). Primary ovarian failure occurs in >80% of girls with TS, and estrogen therapy is required for pubertal development with the subsequent addition of progesterone to induce menses (4). While standardized algorithms for hormone replacement therapy (HRT) in girls with TS are currently lacking, the goal is to simulate the timing and tempo of normal puberty as much as possible in order to achieve optimal psychosexual outcomes (1,5). It is currently unknown whether individual patient or treatment characteristics influence the time from the initiation of estrogen to menarche in these patients. Furthermore, minimal information exists regarding how girls with TS who go through puberty on their own and achieve spontaneous menarche differ from those in whom menarche is induced. Thus, the objectives of this study were to characterize puberty in a cohort of girls with TS and to compare those with spontaneous versus induced puberty. We also investigated whether individual factors were related to the timing of menarche in girls with TS who were treated with HRT.
METHODS
After ethical approval from the Indiana University institutional review board (IRB), medical records of girls followed for TS in the pediatric endocrine clinic at Riley Hospital for Children between January 2007 and May 2015 were reviewed. Records were initially identified using International Classification of Diseases, ninth revision (ICD-9) code 758.6, which includes TS, gonadal dysgenesis, and ovarian dysgenesis. As no personal identifying patient information was recorded, informed consent was not required by the IRB and a waiver was granted. Historical information collected included age at diagnosis of TS, additional medical problems, karyotype, maternal age at menarche, patient age at menarche, and whether puberty was spontaneous or induced. For girls with induced puberty, additional variables analyzed included age at the initiation of estrogen as well as the dose, formulation, and escalation schedule used, and whether bleeding occurred prior to the addition of progesterone. Body mass index (BMI) at the time that initial breast development was documented (in girls with spontaneous puberty), and at initiation of estrogen (in girls with induced puberty) and at menarche (in both) was also recorded.
Statistical Analysis
Descriptive statistics were calculated for subjects with spontaneous menarche and those with induced menarche. BMI Z-scores were derived using a web-based BMI calculator based on the Clinical Growth Charts of the Centers for Disease Control and Prevention, published in 2000 (6). Subjects with spontaneous menarche were compared to those with induced menarche. Data are expressed as mean and standard deviation for continuous variables and frequencies with percentages for categorical variables. Student’s t tests were used to determine P values for continuous variables, and Fisher’s exact tests were used for categorical variables. Subjects who received HRT for pubertal induction were analyzed based on whether they experienced menarche prior to initiation of progesterone. Parameter estimates were slope and standard error for regression analyses for continuous variables and odds ratios with 95% confidence intervals for logistic regression analyses for categorical variables. Calculations were performed using SAS version 9.4 (SAS Institute, Cary, NC) and SPSS Statistics version 23.0 (IBM Corp, Armonk, NY). The α level was set at .05, and P values ≤.05 were considered significant.
RESULTS
Of 191 charts initially identified by ICD-9 code 758.6, 112 were excluded after manual chart review revealed a diagnosis other than TS, leaving 79 evaluable patients with TS. Thirty-six of these were excluded because the girls had not yet completed puberty. The remaining 53 patients were included in the analysis. Of these, 43 girls (81%) had induced menarche, and 10 (19%) had spontaneous menarche. The average age of diagnosis of TS was 6.4 ± 5.6 years (range, prenatally to 16.8 years). The diagnosis of TS was made prenatally in 4 patients (7.8%) and postnatally in the remaining 49 (92.2%). A 45,X karyotype was found in 32.1% of patients, while the remaining 62.3% had a mosaic karyotype. Two of the patients with a prenatal diagnosis were 45,X, while the other 2 patients had mosaic karyotypes. Over half the patients (54.7%) had additional medical diagnoses, including hypothyroidism, hearing loss, developmental delay, and attention deficit hyperactivity disorder. Maternal age at menarche was 13.2 ± 1.4 years (range, 11 to 17 years). A summary of baseline patient characteristics is provided in Table 1.
Table 1.
Baseline Patient Characteristics
| Patient population (N) | 53 |
| Age at diagnosis (years, ±SD) | 6.4 ± 5.6 |
| Age range, years | 0–16.8 |
| Prenatal (n, %) | 4 (7.8) |
| Postnatal (n, %) | 49 (92.2) |
| Maternal age at menarche (years, ±SD) | 13.2 ± 1.4 |
| Age range, years | 11–17 |
| 45,X (n, %) | 17 (32.1) |
| Mosaic (n, %) | 33 (62.3) |
| Additional diagnoses (n, %) | 24 (45.3) |
| Induced puberty (n, %) | 43 (81) |
| Spontaneous menarche (n, %) | 10 (19) |
For girls with primary ovarian failure, the mean age at estrogen initiation was 13.9 ± 1.9 years (range, 11.3 to 19 years), and the mean age at menarche was 15.7 ± 1.9 years (range, 11 to 19.5 years). None had initial pubertal development that subsequently arrested. The mean BMI Z-score was 0.65 ± 1.15 at estrogen initiation and 0.80 ± 1.01 at menarche. The initial estrogen dose ranged from 0.00175 mg transdermal to 0.625 mg oral, and the estrogen dose at menarche ranged from 0.025 mg transdermal to 2 mg oral. Specific estrogen formulations used included transdermal ethinyl estradiol, oral conjugated estrogen, and oral ethinyl estradiol. The average time from estrogen initiation to menarche was 2.4 ± 1.1 years (range, 0.5 to 4.75 years). As shown in Figure 1, a younger age at estrogen initiation correlated with a longer time to menarche (P = .02). A mosaic karyotype correlated with a shorter time to menarche (P = .02), whereas no relationship was seen for BMI Z-score, estrogen route or dose, maternal age at menarche, or prenatal versus postnatal diagnosis. The relationship between patient variables and time to menarche is seen in Table 2.
Fig. 1.

Correlation between age at induction of puberty and time to menarche.
Table 2.
Relationships Between Patient Variables and Time to Menarche
| Slope (standard error) | P value | |
|---|---|---|
| Age at initiation of estrogen | −0.20 (0.08) | .02a |
| Mother’s age at menarche | −0.13 (0.15) | .39 |
| BMI at menarche | −0.00 (0.03) | .91 |
| BMI Z-score at menarche | −0.06 (0.18) | .73 |
| Karyotype | ||
| Mosaic | 1.82 (0.89) | .02a |
| Mosaic with Y | 3.20 (1.57) | |
| Structural abnormality | 2.05 (0.72) | |
| 45,X | 2.75 (0.95) | |
| Estrogen regimen | ||
| Oral | 2.42 (0.82) | .8 |
| Patch | 2.33 (1.21) | |
| Timing of TS diagnosis | 0.06 (0.56) | |
| Prenatally | .92 | |
| Postnatally |
Abbreviations: BMI = body mass index; TS = Turner syndrome.
Significant difference.
Within the group that underwent induced menarche, 19 girls (44.2%) had bleeding on estrogen alone, and 24 (55.8%) were treated with progesterone prior to experiencing menarche. A greater proportion of girls who bled on estrogen alone were treated with transdermal estrogen (P = .01). No other differences in regards to individual patient characteristics were seen between these girls and girls treated with progesterone to precipitate menarche, as shown in Table 3.
Table 3.
Predictors of Bleeding on Estrogen Alone
| “Yes” bleeding estrogen only (n = 19) | “No” bleeding estrogen only (n = 24) | P value | |
|---|---|---|---|
| Age at initiation/estrogen, years | 13.5 (1.8) | 14.2 (1.9) | .23 |
| Mother’s age at menarche, years | 13.2 (1.4) | 13.4 (1.4) | .69 |
| BMI at menarche, kg/m2 | 24.5 (4.7) | 27.1 (6.9) | .14 |
| BMI Z-score at menarche | 0.79 (0.90) | 1.00 (0.98) | .47 |
| Karyotype (2 groups) (n, %) | |||
| Mosaic | 12 (66.7) | 12 (52.2) | .52 |
| 45,X | 6 (33.3) | 11 (47.8) | |
| Estrogen regimen (n, %) | |||
| Oral | 3 (15.8) | 13 (54.2) | |
| Patch | 16 (84.2) | 11 (45.8) | .01a |
| Timing of TS diagnosis (n, %) | |||
| Prenatally | 2 (10.5) | 0 | .13 |
| Postnatally | 17 (89.5) | 24 (100) |
Abbreviations: BMI = body mass index; TS = Turner syndrome.
Significant difference.
Girls who completed puberty spontaneously were significantly younger at menarche than those who required pubertal induction (P<.01). This finding is in line with results from a 2014 study assessing pubertal timing in girls with TS and is likely influenced by the timing of puberty induction (7). In our cohort, girls with spontaneous menarche were also significantly more likely to have mosaic karyotypes than girls with ovarian failure (P = .02). These results are seen in Figures 2 and 3.
Fig. 2.
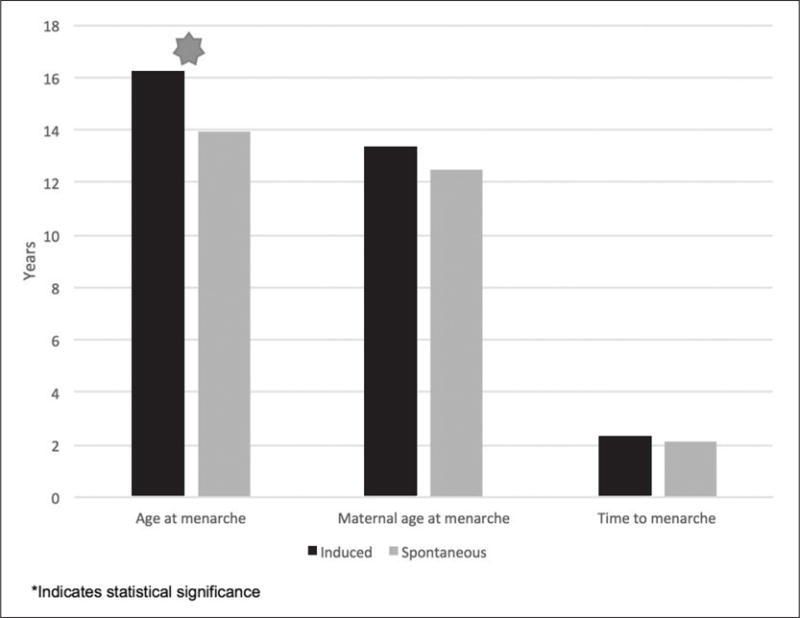
Side-by-side comparison of girls with induced and spontaneous menarche by age at menarche, maternal age at menarche, and time from induction of puberty to menarche.
Fig. 3.

Comparison of karyotype in girls with induced versus spontaneous menarche.
DISCUSSION
Estrogen therapy is required for pubertal development and progression in girls with TS and primary ovarian failure. Although clinical practice guidelines recommend estrogen initiation in a manner that mimics the physiologic progression of normal puberty (1), the optimal age and developmental stage at which to begin pubertal induction in girls with TS have not been defined, and clinical practice varies widely (8). Additionally, it is unknown whether the dose or form of estrogen administration has any bearing on the timing of menarche or tempo of pubertal development in this patient population.
In girls without TS, the average age at the onset of puberty has traditionally been thought to be 10.2 years, with menarche occurring approximately 2.3 years on average after initial breast development (9). Studies have demonstrated that the typical tempo of puberty in female adolescents is related to the age of onset, with a longer time to menarche in girls who enter puberty at an earlier age. An analysis of 163 girls subgrouped by age of puberty onset found that duration of puberty was inversely proportional to the age of onset (10). This is similar to our results, which identified that a younger age at estrogen initiation was associated with a longer time to menarche in our group of patients with TS who required pubertal induction. Our results also demonstrated a shorter time from induction to menarche in girls with mosaic karyotypes. It has been demonstrated that prepubertal girls with TS have significantly lower serum estradiol levels than girls without TS, even before the onset of puberty (11). It is not known, however, whether estradiol levels of girls with TS and ovarian failure vary in relation to karyotype. The current guidelines do not recommend routine measurement of estradiol levels in pediatric patients with TS, and therefore, these were not regularly obtained in our cohort. It may be that girls with mosaic karyotypes have higher endogenous estradiol levels and thus require less exogenous estrogen to initiate and complete pubertal development. As more sensitive estradiol assays are developed, additional research in this area could further explore this hypothesis. Another potential physiologic explanation for this finding could be related to greater tissue sensitivity to estrogen in girls with a mosaic versus monosomy X karyotype. However, data to support this hypothesis are similarly lacking, and further studies are needed.
Interestingly, we observed a wide range of estrogen doses at the time of menarche in our patients receiving HRT. Some girls in our study had vaginal bleeding on doses of estrogen as low as 0.025 mg transdermal, while other patients required estrogen doses as high as 2 mg oral before menarche occurred. Another interesting finding was that 44% of patients with induced puberty had bleeding on estrogen therapy alone on a wide range of doses, indicating extreme variability in estrogen sensitivity among these patients.
Girls who bled on estrogen alone prior to the addition of progesterone for cycling were significantly more likely to have been treated with transdermal, rather than oral, estrogen. This is likely related to physiologic differences between transdermal versus oral formulations of estrogen. In 2013, a randomized trial comparing transdermal to oral estrogen was conducted in 40 adolescent girls with TS to determine whether the mode of estrogen delivery affected metabolic markers (12). Although individuals treated with oral estrogen were noted to have higher levels of estrone, estrone sulfate, and sex hormone–binding globulin, levels of free estradiol were similar between the two groups. Moreover, the girls treated with transdermal estrogen were more likely to have levels of both estrogen precursors and estradiol similar to those in age- and sex-matched girls without TS, suggesting that transdermal estrogen delivers more physiologic effects.
Another rationale for using transdermal rather than oral estrogen therapy for pubertal induction in girls with TS comes from a multicenter study performed in Sweden. In this study, 54 girls with hypogonadism were treated with escalating doses of transdermal estrogen ranging from 0.05 to 0.15 μg/kg, administered nocturnally only. Multiple sequential estradiol levels were measured and compared to those of girls without hypogonadism in similar stages of pubertal development (13). A subgroup analysis was performed in the 31 girls with TS which demonstrated a linear relationship between estrogen dose and serum estradiol levels, with lower starting doses more closely resembling estradiol levels in normal girls in corresponding stages of puberty. Transdermal estrogen has also been linked to higher bone mineral density and increased uterine volumes compared with oral conjugated estrogen in a pilot study of girls with TS (14). Given the cumulative evidence supporting preferential use of the transdermal route, it is not surprising that all but one of the girls in our study treated from 2009 on have been treated with this modality.
In our study, nearly 20% of girls with TS entered puberty and achieved menarche spontaneously, without the need for exogenous HRT. All of these girls had mosaic karyotypes. When evaluating the subgroup of girls with mosaic karyotypes, nearly 38% experienced spontaneous menarche. This is a higher proportion than that seen in a previously published assessment of 40 patients with mosaic TS, in which 15% had spontaneous menarche (15). Molecular-cytogenic analyses were performed to determine the degree of X chromosome mosaicism, and women with mosaicism of at least 10% were found to be significantly more likely to undergo spontaneous menarche than those with lower levels. Another larger study of 522 women with TS reported a spontaneous menarche rate of 16%, with 70% of those women having mosaic karyotypes (4). Taken together, these studies indicate that when counseling families and patients with TS, a spontaneous menarche rate of 15 to 20% can be reasonably estimated, with potentially higher rates in patients with mosaic karyotypes.
Several strengths of our study include the relatively large number of patients with TS, the availability of long-term clinical and laboratory assessments, and clear documentation of estrogen doses and adjustments. Our study also has several limitations. As this was a retrospective study, we were not able to control for potential confounding factors, including variable patient follow-up, patient adherence to therapy, and the multitude of disparate treatment regimens used, making it difficult to compare girls to each other. Although the R2 was significant, the value of 0.143 suggests that there are other variables affecting timing of menarche in our patient population. The additional factors mentioned above likely contribute to the time span from puberty induction to menarche and should be further evaluated. Decisions regarding the timing and pace of pubertal induction in girls with TS are complex and must take into consideration numerous variables, including height potential and patient preferences concerning prioritization of height versus feminization. To our knowledge, ours is the first study to systematically characterize puberty in girls with TS, and our results may facilitate management decisions, especially in those with mosaic karyotypes.
CONCLUSION
Our findings emphasize the broad spectrum of pubertal development in girls with TS, ranging from complete ovarian failure to spontaneous puberty and menarche. They also confirm that girls with mosaic TS are significantly more likely to undergo spontaneous menarche than those with 45,X karyotypes. Furthermore, the wide range of estrogen doses being prescribed at the time of menarche in girls with induced puberty emphasizes the extreme variability in estrogen sensitivity in these patients. This suggests that estrogen titration should be guided in part by individual responses and evaluated on an ongoing basis in conjunction with patients and families over time. Our results should help to inform decisions regarding timing of estrogen initiation and provide important prognostic information for families and providers who care for girls with TS.
Acknowledgments
Dr. Folsom was supported by a grant from the National Institutes of Health (No. 2 T32DK065549).
Abbreviations
- BMI
body mass index
- HRT
hormone replacement therapy
- TS
Turner syndrome
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
References
- 1.Bondy CA, Turner Syndrome Study Group Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 2.Turner HH. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology. 1938;23:566–574. [PubMed] [Google Scholar]
- 3.Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- 4.Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in Turner’s syndrome. Italian Study Group for Turner’s Syndrome. J Clin Endocrinol Metab. 1997;82:1810–1813. doi: 10.1210/jcem.82.6.3970. [DOI] [PubMed] [Google Scholar]
- 5.Carel JC, Elie C, Ecosse E, et al. Self-esteem and social adjustment in young women with Turner syndrome-influence of pubertal management and sexuality: population-based cohort study. J Clin Endocrinol Metab. 2006;91:2972–2979. doi: 10.1210/jc.2005-2652. [DOI] [PubMed] [Google Scholar]
- 6.Pediatric percentile calculator for height, weight, BMI, and blood pressure. Secondary Pediatric percentile calculator for height, weight, BMI, and blood pressure. 2013 Available at: http://www.quesgen.com/BMIPedsCalc.php. Accessed May 1, 2017.
- 7.Negreiros LP, Bolina ER, Guimarães MM. Pubertal development profile in patients with Turner syndrome. J Pediatr Endocrinol Metab. 2014;27:845–849. doi: 10.1515/jpem-2013-0256. [DOI] [PubMed] [Google Scholar]
- 8.Nabhan ZM, Eugster EA. Medical care of girls with Turner syndrome: where are we lacking? Endocr Pract. 2011;17:747–752. doi: 10.4158/EP11059.OR. [DOI] [PubMed] [Google Scholar]
- 9.Robert L. Rosenfield DWC, Sally Radovick Puberty and its disorders in the female. In: Sperling MA, editor. Pediatric Endocrinology. 3rd. Philadelphia, PA: Saunders Elsevier; 2008. pp. 562–563. [Google Scholar]
- 10.Martí-Henneberg C, Vizmanos B. The duration of puberty in girls is related to the timing of its onset. J Pediatr. 1997;131:618–621. doi: 10.1016/s0022-3476(97)70073-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CA, Heinrichs C, Larmore KA, et al. Estradiol levels in girls with Turner’s syndrome compared to normal prepubertal girls as determined by an ultrasensitive assay. J Pediatr Endocrinol Metab. 2003;16:91–96. doi: 10.1515/jpem.2003.16.1.91. [DOI] [PubMed] [Google Scholar]
- 12.Torres-Santiago L, Mericq V, Taboada M, et al. Metabolic effects of oral versus transdermal 17beta-estradiol (E2): a randomized clinical trial in girls with Turner syndrome. J Clin Endocrinol Metab. 2013;98:2716–2724. doi: 10.1210/jc.2012-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ankarberg-Lindgren C, Kriström B, Norjavaara E. Physiological estrogen replacement therapy for puberty induction in girls: a clinical observational study. Horm Res Paediatr. 2014;81:239–244. doi: 10.1159/000356922. [DOI] [PubMed] [Google Scholar]
- 14.Nabhan ZM, Dimeglio LA, Qi R, Perkins SM, Eugster EA. Conjugated oral versus transdermal estrogen replacement in girls with Turner syndrome: a pilot comparative study. J Clin Endocrinol Metab. 2009;94:2009–2014. doi: 10.1210/jc.2008-2123. [DOI] [PubMed] [Google Scholar]
- 15.Castronovo C, Rossetti R, Rusconi D, et al. Gene dosage as a relevant mechanism contributing to the determination of ovarian function in Turner syndrome. Hum Reprod. 2014;29:368–379. doi: 10.1093/humrep/det436. [DOI] [PMC free article] [PubMed] [Google Scholar]


