Abstract
Excimer laser coronary atherectomy (ELCA) is a long-established adjunctive therapy that can be applied during percutaneous coronary intervention (PCI). Technical aspects have evolved and there is an established safety and efficacy record across a number of clinical indications in contemporary interventional practice where complex lesions are routinely encountered. The role of ELCA during PCI for thrombus, non-crossable or non-expandable lesions, chronic occlusions and stent under-expansion are discussed in this review. The key advantage of ELCA over alternative atherectomy interventions is delivery on a standard 0.014-inch guidewire. Additionally, the technique can be mastered by any operator after a short period of training. The major limitation is presence of heavy calcification although when rotational atherectomy (RA) is required but cannot be applied due to inability to deliver the dedicated RotaWireTM (Boston Scientific), ELCA can create an upstream channel to permit RotaWire passage and complete the case with RA – the RASER technique.
Keywords: Excimer laser coronary atherectomy (ELCA), percutaneous coronary intervention (PCI), non-crossable lesions, chronic total occlusions (CTO), intra-coronary thrombus, under-expanded stents, rotational atherectomy (RA)
The introduction of lasers for the treatment of vascular atherosclerosis began in the 1980s, initially for the treatment of critical limb ischaemia,[1] followed by trials that supported its use in coronary circulation.[2–5] However, catheters and technique were rudimentary and associated with complications.[6,7] Refinements in catheter technology[8] and introduction of safe lasing techniques[9,10] have led to improvements in clinical outcomes.[11]
The aim of this article is to describe the principles and practice of Excimer laser coronary atherectomy (ELCA), illustrating with case examples and relevant clinical data.
Excimer Laser Coronary Atherectomy
Excimer lasers are pulsed gas lasers that use a mixture of a rare gas and halogen as an active medium to generate pulses of short wavelength, high-energy ultraviolet (UV) light (see Figure 1). The depth of laser penetration is directly related to its wavelength, with UV laser (shorter wavelength) having less depth of penetration, less heat production and less unwanted tissue damage (see Table 1).
Figure 1: Spectrum of Light for Therapeutic Laser use.
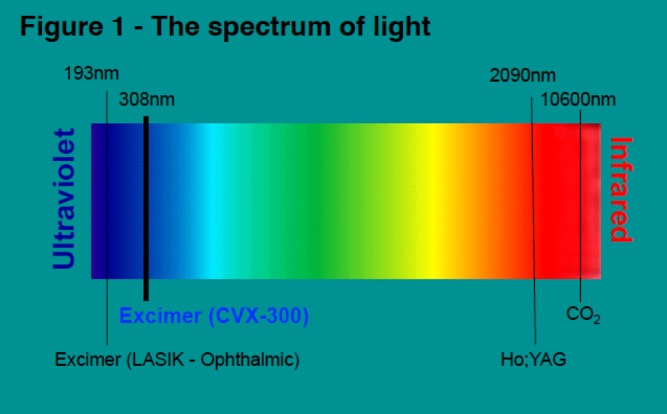
The wavelength of light emitted from a laser is determined by the lasing medium and it is an important factor in determining the properties of the system. Laser light emitted from the Spectranectics CVX-300 excimer (XeCl) laser system is ‘Cool’ (308 nm), which is similar to laser light employed for laser-assisted in situ keratomileusis (LASIC; 193.3 nm) used in ophthalmic surgery. In contrast to infrared lasers, the Excimer laser has a shallow penetration of depth (50 µm) and ablates tissue precisely without excessive heat production and minimises inadvertent tissue damage. Ho:YAG = holmium yttrium aluminium garnet.
Table 1: Types of Laser Categorised by Emitted Light Wavelength.
| Laser type | Wavelength(nm) | Absorption depth (mm) | Absorption mechanism |
|---|---|---|---|
| XeCl (Excimer) | 308 | 0.05 | Protein-lipids |
| Nd:YAG | 1,060 | 2.0 | Protein-water |
| 1,320 | 1.25 | Water | |
| Dye | 480 | 0.5 | Protein |
| Argon | 488 | 0.5 | Protein |
| Ho:YAG | 2,060 | 0.3 | Water |
HO:YAG = holmium yttrium aluminium garnet; Nd:YAG = neodymium-doped yttrium aluminium garnet; XeCl = xenon chloride.
Excimer laser tissue ablation is mediated through three distinct mechanisms: photochemical, photo-thermal and photomechanical. UV laser light is absorbed by intra-vascular material and breaks carbon–carbon bonds (photochemical). It elevates the temperature of intra-cellular water, causing cellular rupture and generates a vapour bubble at the catheter tip (photo-thermal). Expansion and implosion of these bubbles disrupts the obstructive intra-vascular material (photomechanical). The fragments released are <10 μm in diameter, avoiding microvascular obstruction as they are absorbed by the reticulo-endothelial system.
The threshold energy required for the penetration of UV light into tissue and the creation of a steam bubble is called ‘fluence’ (range: 30–80 mJ/mm2). The number of pulses emitted during a 1–second period is the ‘pulse repetition rate’. The duration of each pulse is termed a ‘pulse width’, which is modified according to the nature of the treated lesion for example fibro-calcific lesions require higher fluence and repletion rate for effective ablation (see Figure 2).
Figure 2: The Spectranetics CVX-300 Excimer Laser System.
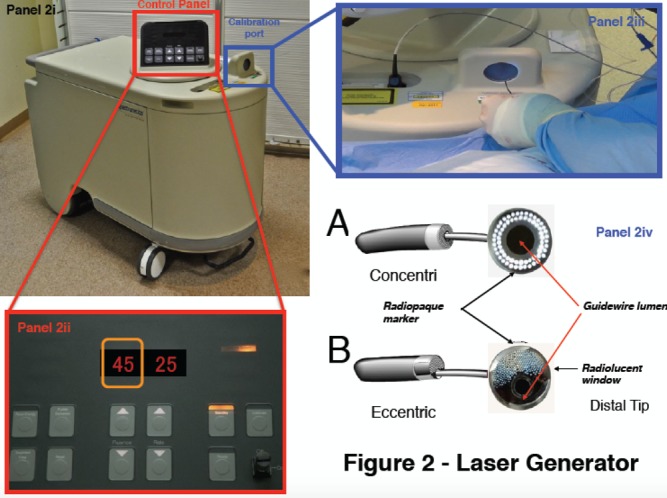
Panel 2i illustrates the pulse generator. The controls are located on the top of the device, and illustrated in panel 2ii. Two numbers are visible: fluence on the left (indicated by the orange box, in this case set at 45 mJ/mm2), and pulse repetition frequency on the right (in this example, 25 Hz). Panel 2iv illustrates the two available configurations of laser catheter – concentric and eccentric – referring to the orientation of the laser fibres within the catheter. These are directed at the calibration port (panel 2iii) before being introduced into the body.
Excimer Laser Equipment and General Technique
The CVX-300 cardiovascular laser Excimer system (Spectranetics; see Figure 2) uses Xenon chloride (XeCl) as the active medium. The light emitted has a wavelength of 308 nm (in the UVB spectrum) with a tissue penetration depth between 30–0 μm. It is the only coronary laser-emitting device currently approved by the US Food and Drug Administration.
It is essential that safety procedures should be observed when performing laser atherectomy. Prior to activation, all persons in the catheter lab, including the patient, must wear protective tinted spectacles to minimise the risk of retinal exposure to the UV light. All windows should be covered and doors locked. Following this safety checklist, the laser unit is warmed up and the selected catheter is connected and calibrated prior to being introduced into the body. Even when the catheter is in vivo, all staff in the vicinity should wear eye protection in case the catheter housing breaks, which could release UV light.
ELCA catheters are advanced on a short monorail segment (30 mm), compatible with any standard 0.014-inch Q2 guidewire. This is a major advantage over alternative coronary atherectomy techniques that require dedicated guidewires that are often more difficult to deliver distally. Coronary catheters are available in four diameters (0.9, 1.4, 1.7, 2.0 mm; see Table 2) and those most commonly used have a concentric array of laser fibres at the tip. The laser fibres of eccentric laser catheters are focused toward one hemisphere. These devices are primarily used for eccentric lesions or for extensive debulking of in-stent restenosis (ISR). The larger diameter laser catheters (1.7, 2.0 mm) are primarily used in straight sections of vessels with a diameter >3.0 mm and require 7F and 8F guide catheters, respectively. The 0.9- and 1.4-mm devices are used via a 6F guiding system. Care should be taken to select a guiding catheter that provides adequate support and that remains coaxial during lasing. Laser catheter size selection is primarily based on: (a) the severity of the lesion; (b) the reference vessel diameter and; (c) consistency of the target material[12] (see Table 2). The 0.9-mm X80 catheter is used in non-crossable, non-dilatable fibrocalcific lesions, due to its enhanced delivery and ability to emit laser energy at high power (80 mJ/mm2) at the highest repletion rate (80 Hz).
Table 2: Indications for Excimer Laser Coronary Atherectomy (ELCA) and the Preferred Laser Catheter.
| ELCA indication | Preferred laser catheter (mm) |
|---|---|
| Acute myocardial infarct, intra-coronary thrombus |
0.9–1.4 |
| Uncrossable lesions | 0.9 X 80 |
| Chronic total occlusions | 0.9 X 80 |
| Under-expanded stent | 0.9 X 80 |
| In-stent restenosis | 0.9–2.0 (concentric or eccentric) |
| Saphenous vein grafts | 0.9–2.0 |
Saline Infusion Technique
Both blood and iodinated contrast media contain non-aqueous cellular macromolecules, such as proteins, and these absorb the majority of delivered Excimer laser energy creating cavitating microbubbles, which form at the site of energy delivery, increasing the likelihood of traumatic dissection.[13] By contrast, saline permits passage of light from the catheter tip to the tissue without any interference so no microbubbles are formed in this milieu. Therefore, a saline flush/infusion technique is used to safely control energy delivery and minimise dissection risk.[14,15] Application of laser in blood or contrast media is rarely performed in certain specific situations – such as treatment of an under-expanded stent – and should only be undertaken by experienced laser percutaneous coronary intervention (PCI) operators.[16]
To clear blood from the catheter–tissue interface, a 1-l bag of 0.9 % saline solution is connected to the manifold via a three-way tap, and a clean 20-ml Luer-Lok™ (Becton Dickinson) syringe replaces the contrast syringe. Once the system has been purged of contrast, confirmed by screening, 5 ml of saline solution should be infused followed by continued injection – at a rate of 1–2 ml/second – throughout laser activation. The guide catheter should be well intubated and coaxial within the artery, ensuring saline delivery to the catheter tip. For the standard coronary catheters activation will automatically cease after 5 seconds with a 10-second rest period. An audible alarm sounds at the end of the rest period to signal when to commence the next laser train. The 0.9-mm X80 catheter permits 10 seconds activation and 5 seconds rest, reflecting its use in more complex lesions.
The pulses of laser energy are delivered as the catheter is slowly (0.5 mm/second) advanced through the lesion, allowing adequate absorption and ablation. If the catheter is advanced too rapidly the tissue does not have time to absorb the light energy and ablation will be sub-optimal. On completion of several anterograde trains, retrograde lasing can be performed, particularly in severe lesions when there is anterograde resistance.
Contraindications and Avoiding Complications – Tips and Tricks (see Figure 3)
Figure 3: Tips and Tricks for Successful Laser Atherectomy.
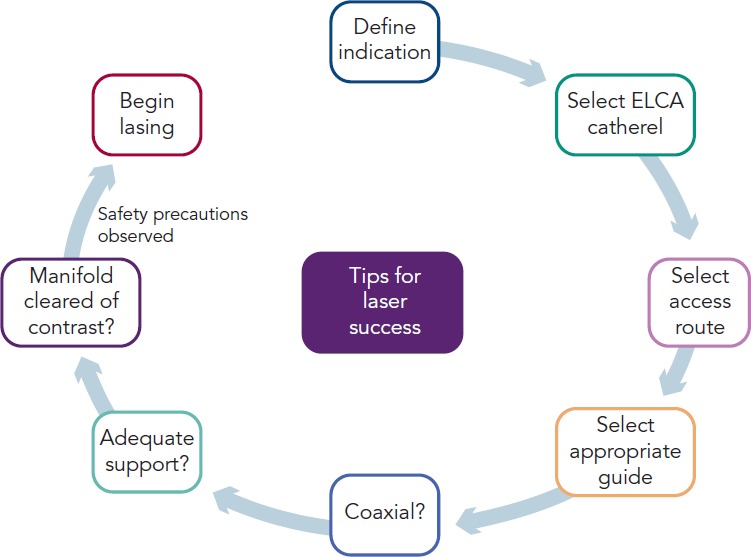
Once the indication for laser has been defined and the appropriate catheter selected, the access route can be determined. Almost all interventions can be delivered via the radial route, although delivery of an 8F catheter (or equivalent sheathless guide) requires care and many need techniques such as balloon tracking. Once an appropriate coaxial guide has been selected, that maintains good co-coaxial support, then excimer laser coronary atherectomy (ELCA) can be undertaken once adequate safety precautions have been employed.
Other than lack of informed consent and unprotected left main disease (a relative contraindication) there are no absolute coronary contraindications for ELCA. ELCA complications are similar to those encountered during routine PCI. Specific issues may arise from interruption of the saline flush or contamination with contrast, which can generate excessive heat and increase the risk of vascular perforation. ELCA is not recommended when the operator is aware that there is a long length of sub-intimal guidewire positioning as may exist during hybrid PCI techniques for chronic total occlusions (CTOs).
Clinical Indications for Excimer Laser Coronary Atherectomy
As the application of ELCA has been refined, a number of indications have emerged for the technique:
1. Acute Coronary Syndromes and Myocardial Infarction (see Figure 4)
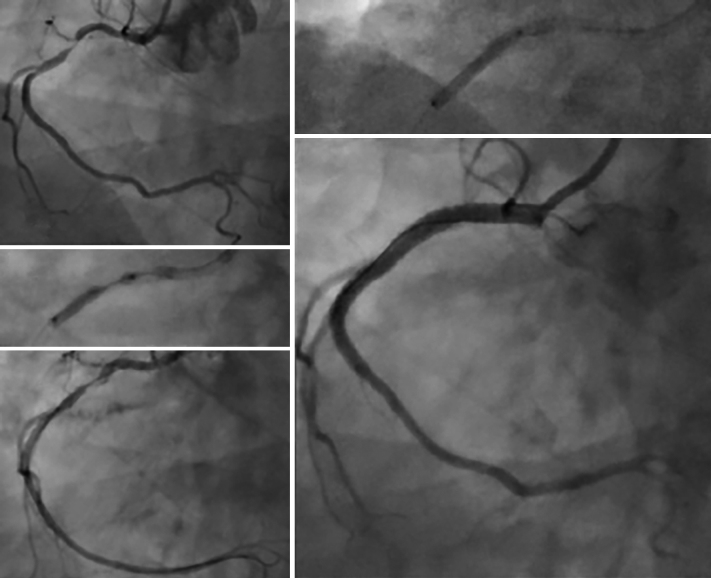
Figure 4: The Use of Excimer Laser Coronary Atherectomy in Intra-coronary Thrombus.
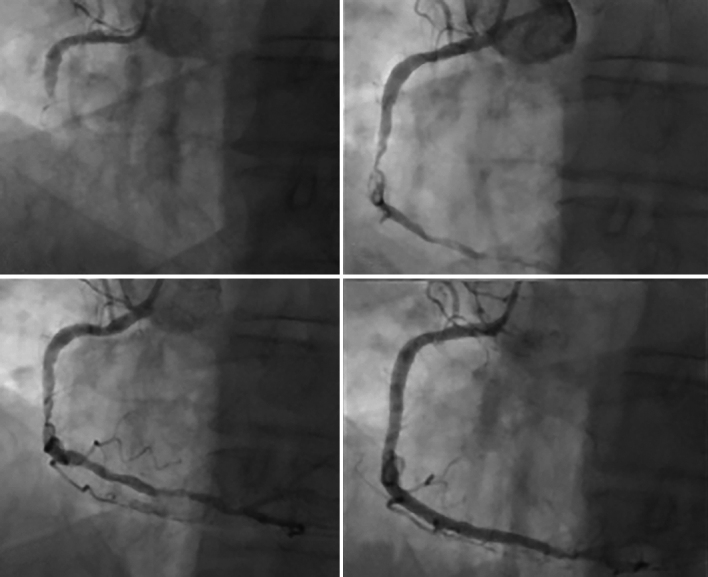
A 73-year-old man presented inferior ST elevation with a thrombotically occluded right coronary artery (A). Initial thrombectomy restored Thrombolysis In Myocardial Infarction (TIMI) 2 flow, but a large volume of thrombus remained in the artery despite repeated passage. excimer laser coronary atherectomy was therefore undertaken, using a 1.4-mm 6F compatible catheter; 4,500 pulses were delivered over 15 trains with a dramatic improvement in thrombotic burden and obstructive stenosis. The case was completed with overlapping drug-eluting stent with an excellent final angiographic result.
The recommended treatment for acute myocardial infarction (AMI) associated with electrocardiogram (ECG) ST segment elevation is primary PCI.[17,18] ELCA may be a beneficial given its potential for effective thrombus removal,[19] promotion of fibrinolysis,[20] platelet-stunning effects[21] and concomitant plaque debulking.[22] We have published case reports of how effective ELCA can be at dealing with a large burden of intra-coronary thrombus providing excellent immediate and long-term results.[23,24]
However, clinical data supporting the use of ELCA in AMI remain limited. The largest study to date, the Cohort of Acute Revascularization of Myocardial infarction with Excimer Laser (CARMEL) multicentre registry, enrolled 151 AMI patients, 65 % of whom had large thrombus burden in the culprit artery.[25] Following ELCA, Thrombolysis In Myocardial Infarction (TIMI) flow grade was significantly increased (1.2 to 2.8), with an associated reduction in angiographic stenosis (83 to 52 %).[25] There was a low rate (8.6 %) of major adverse coronary events (MACE). The maximal effect was observed in arteries with a large angiographic thrombus burden.
A single randomised trial, the Laser AMI study, has been conducted. This study included 66 patients and sought to demonstrate safety and feasibility. They used optimal lasing technique (saline flushing with slow advancement [0.2–0.5 mm/second]) using the CVX-300 Excimer laser system and treated the majority of lesions with a laser-stent strategy (only two patients required balloon angioplasty prior to stenting). Primary angiographic endpoints were myocardial blush grade, TIMI flow and length-adjusted TIMI frame count. The TIMI score increased from 0.2±0.4 at baseline to 2.65±0.5 post-laser to 2.9±0.3 post-stent (both p<0.01 versus baseline). Similarly, myocardial blush grade increased from 0.12±0.4 to 2.5±0.6 post-laser, and to 2.8±0.4 post-stent. No reflow was observed in 11 % of cases after laser and a major dissection occurred in one case. There were no intra-procedural deaths and 95 % event-free survival at 6 months with LV remodelling occurring in 8 % patients.[26] A second, larger study is ongoing and due to report in 2016.
2. Excimer Laser Coronary Atherectomy for Non-crossable/Non-dilatable Lesions (Balloon Failure; see Figure 5)
Figure 5: The use of Excimer Laser Coronary Atherectomy in a Calcific Undilatable Lesion.
A 69-year-old man with a severe calcific lesion in the proximal portion of a dominant right coronary artery (A). Despite use of a 3.0 x 20-mm noncompliant balloon at high pressure, there was clear failure of lesion expansion (B). Excimer laser atherectomy was undertaken with a 0.9- mm X80 catheter, delivering 5,000 pulses over 15 trains. This caused a small proximal localised dissection, but allowed balloon expansion (C) and subsequent treatment with overlapping drug-eluting stents (D).
Balloon failure occurs when a lesion cannot be crossed with a low-profile device, or when the balloon inadequately expands with dilatation. This is a situation where ELCA may be applied, with a high success rate in un-crossable or un-dilatable stenoses. However, in cases of significant calcification, the response is less favourable (calcified 79 % versus non-calcified 96 %; p<0.05).[27,28] This is because the ablative effects of ELCA on calcium are minimal and success relies on the ablation of more pliable tissue within the calcific lesion, which will vary accordingly.
In heavily calcified coronary lesions the default technique for the majority of PCI operators remains rotational atherectomy (RA), even among proficient ELCA users. RA requires delivery of a dedicated 0.009-inch guidewire (Rotawire™) into the distal coronary vessel. This wire is less deliverable directly, and it may not be possible either independently or through a micro-catheter. When this situation arises, ELCA can be used to modify the lesion to create a channel through which a Rotawire™ can subsequently be delivered distally (usually via a microcatheter), to permit RA and case completion. We termed the combination of ELCA and RA the RASER technique and have developed this combination of atherectomy devices in a number of challenging cases.[29–31] This combined use of atherectomy devices is particularly effective for non-crossable, non-dilatable calcified stenosis frequently encountered in daily PCI practice. The outcome is predictable and is associated with a low complication rate in experienced hands.[29–31]
The 0.9-mm X80 catheter is selected in the vast majority of balloon failure cases since this catheter provides the widest range of power and repetition rate to maximise the chances of procedural success. Given that this catheter only requires a 6F guiding system, the application of such techniques can easily be achieved through the transradial approach in keeping with contemporary PCI practice. Other devices that can be used to facilitate the PCI in these situations include the use of the GuideLiner® (Vascular Solutions) for the delivery of the ELCA, although care should be taken to retract the device prior to commencing lasing since the obstruction to blood flow may lead to ischaemia.[32] Support strategies that require the use of additional wires (e.g., anchor wires, balloons, etc.) can also be used given that it is possible to safely laser with a second wire in place.
3. Excimer Laser Coronary Atherectomy for Chronic Total Occlusions
The role of ELCA in the treatment of CTOs is for resistant lesions: when equipment is unable to cross the lesion or proximal cap despite attaining distal wire position. It may also offer additional benefits as its ablative effect is transmitted through the lesion architecture, potentially weakening bonds between the constituent components of the CTO. In addition, the antithrombotic[19,20] and platelet-suppressive[21] effects of ELCA may reduce the risk of thrombotic complications during disobilteration. A success rate of 86–90 % for ELCA in CTO cases has been reported.[16,29,33] From a technical perspective, saline is often not used at the laser–lesion interface for CTO cases as anterograde injections are usually avoided to prevent extending areas of dissection. In addition, it is unlikely that saline would reach the laser–tissue interface.
4. Excimer Laser Coronary Atherectomy in Under-expanded Stents (see Figure 6)
Figure 6: Excimer Laser Coronary Atherectomy for Stent Underexpansion.
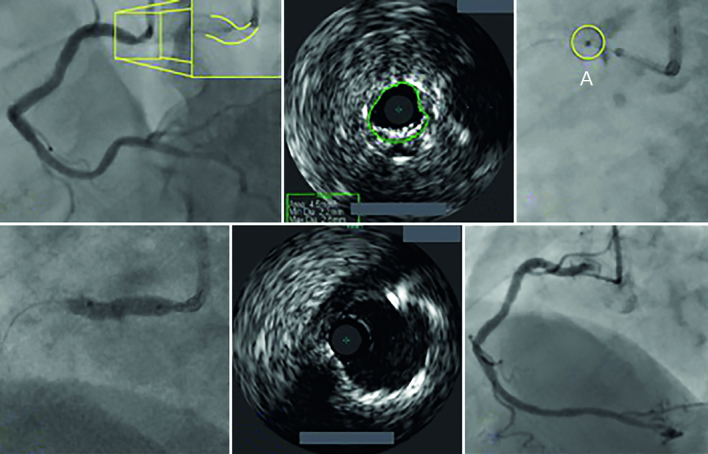
An illustration of severe stent under-expansion in the ostium of a large dominant right coronary artery from a female patient who has previously undergone percutaneous coronary intervention a few weeks before in another institution (A). The minimal lumen area (B). From the right radial artery, using a 7F guiding catheter along with a guideliner extension, the lesion was treated with 0.9-mm X80 Excimer laser coronary atherectomy catheter. Seven thousand pulses were delivered and this facilitated balloon angioplasty, with full expansion of a balloon (D). The final angiographic and intravenous ultrasound result is shown in (F) and (E), respectively, with full stent expansion having been achieved.
Stent under-expansion poses a significant risk for stent thrombosis. There are few PCI options available when this occurs. Maximal balloon dilatation (both diameter and pressure) has often already been undertaken, and RA risks metal fragment embolisation and burr stalling. ELCA remains the only technique that is able to modify the underlying resistant atheroma by delivering energy to the abluminal stent surface without disrupting the stent architecture.[34,35] While having no impact on the calcification itself, ELCA modifies the plaque behind the stent, which weakens the overall resistance, thus enabling subsequent complete stent expansion.[36–38]
We have found that delivering high power laser energy (80 mJ/mm2/80 Hz), using the 0.9-mm X80 catheter in the absence of saline, or with contrast injection, amplifies the ablative effect. Within a stented environment ‘contrast-mileu’ lasing appears to be safe, facilitating high-pressure balloon stent expansion.[36–39]
This technique has been evaluated in the ELLEMENT registry of 28 patients.[40] Procedural success was achieved in 96.4 % (27/28) of cases, using an increase of either 1 cm2 on intravenous ultrasound (IVUS) or 10 % using quantitative coronary angiography derived minimal stent diameter as a definition. This confirmed efficacy, with a low associated MACE rate.[40]
5. In-stent Restenosis
ISR remains a major limitation of PCI following stent implantation with rates of restenosis reported in 10–50 % of patients receiving a bare-metal stent,[41] although considerably less in the DES era.[42] ELCA is a safe and effective technique in the treatment of ISR.[43] Excimer laser did not alter stainless-steel stent endurance or liberate any significant material when five types of stainless-steel stents were subjected to 1,000 pulses of laser energy from a 2.0-mm eccentric Excimer laser catheter.[35] In a clinical study, the examination of 107 re-stentoic lesions in 98 patients demonstrated that lesions treated with ELCA compared with balloon angioplasty alone, had a greater IVUS cross-sectional area and luminal gain, with more intimal hyperplasia ablation. There was a non-significant trend towards a less frequent need for target vessel revascularisation at 6 months (21 versus 38 %; p=0.083).[43]
We have observed, using optical coherence tomography (OCT) and optical frequency domain imaging, that during treatment of the restenotic segment, the laser therapy ablates both the luminal and abluminal atherosclerotic material.[44] Therefore, if the mechanism of restenosis is in part stent under-expansion, ELCA increases the likelihood of achieving greater stent expansion and more durable longer-term outcomes.
6. Saphenous Vein Grafts
Occlusions in old saphenous vein grafts (SVGs) frequently consist of degenerative diffuse plaques often containing thrombus[45,46] and prone to distal embolisation.[47] Hence, distal protection devices (DPDs) are advocated when attempting SVG-PCI,[46] but their bulky nature may prevent distal device delivery. ELCA is a safer alternative, allowing predictable debulking during SVG-PCI.[48,49] The low rate of distal embolisation during ELCA of degenerative bypass grafts (1–5 %) may preclude the need for routine DPD in the majority of cases.[48] However, OCT images post-SVG ELCA make it is clear that there remains friable fragments that could embolise and cause no-reflow.[44] Therefore, when using ELCA for SVG-PCI, it is advisable to stent on a DPD system to prevent no-reflow.[44] Given advances in CTO success in recent years, SVG-PCI is likely to be less frequently undertaken as operators choose to treat the occluded native vessel. Nonetheless, if SVG-PCI is considered necessary, ELCA remains a useful adjunctive therapeutic intervention.
7. Bifurcations
Generally PCI for coronary bifurcation lesions is best treated with a main vessel (MV)-only stenting approach with preservation of side branch (SB), rather than an upstream two-stent strategy. However, in large vessels involving extensive SB disease it may be necessary to stent SB as well. ELCA could potentially be of value in these cases by debulking the SB lesion to permit more predictable success with the MV-only approach. However, in the few cases in our practice in which we have used this technique we have discovered SB dissection because of vessel angulation, which has necessitated SB stenting – thereby defeating the purpose of using ELCA. We have used ELCA more successfully in rare cases of SB restensosis (often due to stent under-expansion) guided by intra-coronary imaging with durable results.
Conclusion
The current indications for the use of Excimer laser atherectomy in modern interventional practice are described in this article. A detailed description of the ELCA technique and its potential pitfalls has been illustrated with complex interventional cases. This technology provides a solution to a variety of problems that may be encountered, including massive intra-coronary thrombus, un-crossable lesions and stent under-expansion. Careful case selection, proper use of equipment and safe, efficacious lasing technique all play crucial roles in successful ELCA interventions.
References
- 1.Choy DSJ. History and state-of-the-art of lasers in cardiovascular disease. Laser Medicine & Surgery News & Advances. 1988. pp. 34–8.
- 2.Cook SI, Eigler NL, Shefer A et al. Percutaneous excimer laser coronary angioplasty of lesions not ideal for balloon angioplasty. Circulation. 1991;84:632–3. doi: 10.1161/01.cir.84.2.632. [DOI] [PubMed] [Google Scholar]
- 3.Koster R, Kahler J, Brockhoff C et al. Laser coronary angioplasty: history, present and future. Am J Cardiovasc Drugs. 2002;2:197–207. doi: 10.2165/00129784-200202030-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bittl JA, Sanborn TA, Tcheng JE. Clinical success, complications and restenosis rates with excimer laser coronary angioplasty. Am J Cardiol. 1992;70:1553–9. doi: 10.1016/0002-9149(92)90453-6. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind HJ, Dubois-Rande JL, Zelinsky R et al. Percutaneous coronary mid-infrared laser angioplasty. Am Heart J. 1991;122:552–8. doi: 10.1016/0002-8703(91)91015-f. [DOI] [PubMed] [Google Scholar]
- 6.Bittl JA, Ryan TJ, Keaney JF. Coronary artery perforation during excimer laser coronary angioplasty. J Am Coll Cardiol. 1993;21:1158–65. doi: 10.1016/0735-1097(93)90240-2. [DOI] [PubMed] [Google Scholar]
- 7.Topaz O. Whose fault is it? Notes on “true” versus “pseudo” laser failure. Editorial. Cath Cardiovasc Diagn. 1995;36:1–4. doi: 10.1002/ccd.1810360102. [DOI] [PubMed] [Google Scholar]
- 8.Taylor K, Reiser C. Large eccentric laser angioplasty catheter. In Proceedings of lasers in surgery: advanced characterization, therapeutics and systems. SPIE. 1997;2970:34–41. [Google Scholar]
- 9.Tcheng JE. Saline infusion in excimer laser coronary angioplasty. Semin Intervent Cardiol. 1996;1:135–41. [PubMed] [Google Scholar]
- 10.Topaz O. A new safer lasing technique for laser facilitated coronary angioplasty. J Intervent Cardiol. 1993;6:297–306. doi: 10.1111/j.1540-8183.1993.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 11.Topaz O. Textbook of Interventional Cardiology. Philadelphia, PA: WB Saunders,; 1995. Coronary laser angioplasty. In: Topol EJ (editor). ; pp. 235–55. [Google Scholar]
- 12.Topaz O, Safian RD. Manual of Interventional Cardiology. 3rd edition. Royal Oaks, MI: Physicians Press; 2001. Freed MS (editors). pp. 681–91. Excimer laser coronary angioplasty. In: Safian RD. [Google Scholar]
- 13.Baumbach A, Haase KK, Rose C et al. Formation of pressure waves during in vitro excimer laser irradiation in whole blood and the effect of dilution with contrast media and saline. Lasers Surg Med. 1994;14:3–6. doi: 10.1002/lsm.1900140104. [DOI] [PubMed] [Google Scholar]
- 14.Tcheng JE, Wells LD, Phillips HR et al. Development of a new technique for reducing pressure pulse generation during 308-nm excimer laser coronary angioplasty. Cathet Cardiovasc Diagn. 1995;34:15–22. doi: 10.1002/ccd.1810340306. [DOI] [PubMed] [Google Scholar]
- 15.Deckelbaum LI, Natarajan MK, Bittl JA et al. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty: a randomized trial. The percutaneous excimer laser coronary angioplasty (PELCA) investigators. J Am Coll Cardiol. 1995;26:1264–9. doi: 10.1016/0735-1097(95)00330-4. [DOI] [PubMed] [Google Scholar]
- 16.Topaz O. Chronic Total Occlusions: A Guide to Recalization. Hoboken, NJ: Wiley-Blackwell,; 2009. Saito S (editors). Laser for total occlusion recanalization. In: Waksman R. [Google Scholar]
- 17.The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. 10.1093/eurheartj/ehs215 [DOI] [PubMed] [Google Scholar]
- 18.American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2013;61:E78–140. doi: 10.1016/j.jacc.2012.11.019. 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 19.Dahm JB, Topaz O, Woenckhaus C et al. Laser facilitated thrombectomy: a new therapeutic option for treatment of thrombus–laden coronary lesions. Cath Cardiovasc Intervent. 2002;56:365–72. doi: 10.1002/ccd.10200. [DOI] [PubMed] [Google Scholar]
- 20.Topaz O, Minisi AJ, Morris C et al. Photoacoustic fibrinolysis: pulsed-wave, mid infrared laser-clot interaction. J Thrombo Thrombolysis. 1996;3:209–14. doi: 10.1007/BF00181663. [DOI] [PubMed] [Google Scholar]
- 21.Topaz O, Minisi AJ, Bernardo NL et al. Alterations of platelet aggregation kinetics with ultraviolet laser emission: the “stunned platelet“ phenomenon. Thromb Haemost. 2001;86:1087–93. [PubMed] [Google Scholar]
- 22.Topaz O, Bernardo NL, Shah R et al. Effectiveness of excimer laser coronary angioplasty in acute myocardial infarction or in unstable angina pectoris. Am J Cardiol. 2001;87:849–55. doi: 10.1016/s0002-9149(00)01525-3. [DOI] [PubMed] [Google Scholar]
- 23.Rawlins J, Sambu N, O’Kane P. Strategies for the management of massive intra-coronary thrombus in acute myocardial infarction. Heart. 2013;99:510. doi: 10.1136/heartjnl-2012-303370. 10.1136/heartjnl-2012-303370 [DOI] [PubMed] [Google Scholar]
- 24.Whittaker A, Rawlins J. O’Kane P. Contemporary therapy of intracoronary thrombus: laser and bioresorbable scaffold. Cardiovasc Interv Ther. 2015;30:277–8. doi: 10.1007/s12928-014-0280-6. 10.1007/s12928-014-0280-6 [DOI] [PubMed] [Google Scholar]
- 25.Topaz O, Ebersole D, Das T et al. Excimer laser angioplasty in acute myocardial infarction [the CARMEL multicenter study]. Am J Cardiol. 2004;93:694–701. doi: 10.1016/j.amjcard.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Dorr M, Vogelgesang D, Hummel A et al. Excimer laser thrombus elimination for prevention of distal embolisation and no-reflow in patients with acute ST elevation myocardial infarction: results from the randomised LaserAMI study. Int J Cardiol. 2007;116:20–6. doi: 10.1016/j.ijcard.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Bittl JA. Clinical results with excimer laser coronary angioplasty. Semin Intervent Cardiol. 1996;1:129–34. [PubMed] [Google Scholar]
- 28.Bilodeau L, Fretz EB, Taeymans Y et al. Novel use of a high energy excimer laser catheter for calcified and complex coronary artery lesions. Cath Cardiovasc Interv. 2004;62:155–61. doi: 10.1002/ccd.20053. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez JP, Hobson AR, McKenzie D et al. Beyond the balloon: excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expansible coronary lesions. EuroIntervention. 2013;9:243–50. doi: 10.4244/EIJV9I2A40. 10.4244/EIJV9I2A40 [DOI] [PubMed] [Google Scholar]
- 30.McKenzie DB, Talwar S, Jokhi PP et al. How should I treat severe coronary artery calcification when it is not possible to inflate a balloon or deliver a RotaWire? Eurointervention. 2011;6:779–83. doi: 10.4244/EIJV6I6A132. 10.4244/EIJV6I6A132 [DOI] [PubMed] [Google Scholar]
- 31.Fernandez JP, Hobson AR, McKenzie D et al. Treatment of calcific coronary stenosis with the use of excimer laser coronary atherectomy and rotational atherectomy. Int Card. 2010;2:801–06. 10.2217/ica.10.83 [Google Scholar]
- 32.Sambu N, Fernandez J, Shah NC. O’Kane P. The Guideliner®: an interventionist’s experience of their first 50 cases: ‘the mostly good, rarely bad, beware of the ugly!’. Int Card. 2013;5:389–402. 10.2217/ica.13.37 [Google Scholar]
- 33.Holmes DR Jr, Forrester JS, Litvack F et al. Chronic total obstructions and short term outcome: the excimer laser angioplasty registry experience. Mayo Clin Proc. 1993;68:5–10. doi: 10.1016/s0025-6196(12)60012-3. [DOI] [PubMed] [Google Scholar]
- 34.Papaioannou T, Yadegar D, Vari S et al. Excimer laser (308 nm) recanalisation of in-stent restenosis: thermal considerations. Lasers Med Sci. 2001;16:90–100. doi: 10.1007/pl00011348. [DOI] [PubMed] [Google Scholar]
- 35.Burris N, Lippincott RA, Elfe A et al. Effects of 308 nanometer excimer laser energy on 316 L stainless-steel stents: implications for laser atherectomy of in-stent restenosis. J Invasive Cardiol. 2000;12:555–9. [PubMed] [Google Scholar]
- 36.Sunew J, Chandwaney RH, Stein DW et al. Excimer laser facilitated percutaneous coronary intervention of a nondilatable coronary stent. Cath Cardiovasc Intervent. 2001;53:513–7. doi: 10.1002/ccd.1212. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez JP, Hobson AR, Mckenzie DB et al. How should I treat severe calcific coronary artery disease. EuroIntervention. 2011;7:400–7. doi: 10.4244/EIJV7I3A65. 10.4244/EIJV7I3A65 [DOI] [PubMed] [Google Scholar]
- 38.Lam SC, Bertog S, Sievert H. Excimer laser in management of underexpansion of a newly deployed coronary stent. Catheter Cardiovasc Interv. 2014;83:E64–8. doi: 10.1002/ccd.25030. 10.1002/ccd.25030 [DOI] [PubMed] [Google Scholar]
- 39.Egred M. A novel approach for under-expanded stent: excimer laser in contrast medium. J Invasive Cardiol. 2012;24:E161–E163. [PubMed] [Google Scholar]
- 40.Latib A, Takagi K, Chizzola G et al. Excimer Laser LEsion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15:8–12. doi: 10.1016/j.carrev.2013.10.005. 10.1016/j.carrev.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 41.Lowe H, Oesterle S, Khachigian LM. Coronary in-stent restenosis: current status and future strategies. J Am Coll Cardiol. 2002;39:183–93. doi: 10.1016/s0735-1097(01)01742-9. [DOI] [PubMed] [Google Scholar]
- 42.Dangas G, Claessen B, Caixeta A et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–907. doi: 10.1016/j.jacc.2010.07.028. 10.1016/j.jacc.2010.07.028 [DOI] [PubMed] [Google Scholar]
- 43.Mehran R, Mintz GS, Satler LF et al. Treatment of in-stent restenosis with excimer laser coronary angioplasty: mechanisms and results compared with PTCA alone. Circulation. 1997;96:2183–9. doi: 10.1161/01.cir.96.7.2183. [DOI] [PubMed] [Google Scholar]
- 44.Rawlins J, Talwar S, Green M. O’Kane P. Optical Coherance Tomography follwoing percutaneous coronary intervention with Excimer Laser coronary atherectomy. Cardiovasc Revasc Med. 2014;15:29–34. doi: 10.1016/j.carrev.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Webb JG, Carere RG, Virmani R et al. Retrieval and analysis of particulate debris after saphenous vein graft intervention. J Am Coll Cardiol. 1999;34:468–75. doi: 10.1016/s0735-1097(99)00196-5. [DOI] [PubMed] [Google Scholar]
- 46.Baim DS, Wahr D, George B et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002;105:1285–90. [PubMed] [Google Scholar]
- 47.Bittl JA, Sanborn TA, Yardley DE et al. Predictors of outcome of percutaneous excimer laser coronary angioplasty of saphenous vein bypass graft lesions. The Percutaneous Excimer Laser Coronary Angioplasty Registry. Am J Cardiol. 1994;74:144–8. doi: 10.1016/0002-9149(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 48.Giugliano GR, Falcone MW, Mego D et al. A prospective multicenter registry of laser therapy for degenerated saphenous vein graft stenosis: the COronary graft Results following Atherectomy with Laser (CORAL) trial. Cardiovasc Revasc Med. 2012;13:84–9. doi: 10.1016/j.carrev.2012.01.004. 10.1016/j.carrev.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 49.Ebersole D, Dahm JB, Das T et al. Excimer laser revascularization of saphenous vein grafts in acute myocardial infarction. J Invasive Cardiol. 2004;16:177–80. [PubMed] [Google Scholar]