Abstract
Intestinal infection by the zoonotic protozoan, Cryptosporidium parvum, causes significant alterations in the gene expression profile in host epithelial cells. The molecular mechanisms of how C. parvum may modulate host cell gene transcription and the pathological significance of such alterations are largely unclear. Previous studies demonstrate that a panel of parasite RNA transcripts are delivered into infected host cells and may modulate host gene transcription. Using in vitro models of intestinal cryptosporidiosis, in this study, we analyzed the impact of host delivery of C. parvum Cdg2_FLc_0220 RNA transcript on host gene expression profile. We found that alterations in host gene expression profile following C. parvum infection were partially associated with the nuclear delivery of Cdg2_FLc_0220. Specifically, we identified a total of 46 overlapping upregulated genes and 8 overlapping downregulated genes in infected cells and cells transfected with Full-Cdg2_FLc_0220. Trans-suppression of the DAZ interacting zinc finger protein 1 like (DZIP1L) gene, the top overlapping downregulated gene in host cells following C. parvum infection and cells transfected with Full-Cdg2_FLc_0220, was mediated by G9a, independent of PRDM1. Cdg2_FLc_0220-mediated trans-suppression of the DZIP1L gene was independent of H3K9 and H3K27 methylation. Data from this study provide additional evidence that delivery of C. parvum Cdg2_FLc_0220 RNA transcript in infected epithelial cells modulates the transcription of host genes, contributing to the alterations in the gene expression profile in host epithelial cells during C. parvum infection.
Keywords: Cryptosporidium, Intestinal epithelium, DZIP1L, Gene transcription, Epithelial homeostasis
1. Introduction
Cryptosporidium is an important protozoan diarrheal pathogen in animals and humans (Checkley et al., 2015, Certad et al., 2017). Persistent watery diarrhea caused by Cryptosporidium infection has been reported as potentially fetal in youth, the in immunocompromised (e.g., HIV/AIDS), and transplant recipients (Borad and Ward, 2010; Fishman et al., 2011; Acikgoz et al., 2012). Investigations on diarrheal etiologies in children also showed that Cryptosporidium is responsible for 15–25% of diarrheal cases (Chen et al., 2002; Checkley et al., 2015). Although asymptomatic infection of Cryptosporidium was observed in the majority of cases in humans and animals, colonization of this parasite can damage the intestinal barriers, affecting nutrition absorption, possibly causing persistent retardation of growth and impairing the immune response of host (Guerrant et al., 1999; Mondal et al., 2009; Squire and Ryan, 2017). More importantly, uneliminated oocysts excreted from these ignored hosts into environment could spread the infection to other hosts and would be an important source of waterborne outbreak of cryptosporidiosis (Checkley et al., 2015). At least 163 outbreaks of waterborne diseases were caused by Cryptosporidium infection (Karanis et al., 2007), and this protist has been listed as an indicator for water quality in the USA, UK, Australia and China. However, only one drug, nitazoxanide, has been approved by the American Food and Drug Administration (FDA) to treat cryptosporidiosis, and is efficacious in only 56–96% of immunocompetent hosts, but lacks efficacy in cryptosporidiosis patients with advanced AIDS (Rossignol et al., 2001; Amadi et al., 2002, 2009; Checkley et al., 2015).
Considering the close relationship between the severity of cryptosporidiosis and host status (e.g., immunity, nutrition, and age), exploring the mechanism of interaction between Cryptosporidium and host is key to developing resolution strategies for controlling Cryptosporidium. After internalization and residence in mature parasitophorous vacuoles at the apex of the host cells (Marcial and Madara, 1986), a direct connection (the feeder organelle) is formed between Cryptosporidium and host cell cytoplasm at the host cell-parasite interfaces, and is an important structure for regulating transportation of nutrition, molecular effectors and drugs (Marcial and Madara, 1986; Tzipori and Griffiths, 1998; Perkins et al., 1999; Huang et al., 2004; O’Hara and Chen, 2011; Wang et al., 2017a, b). Genomic and transcriptomic analyses showed that various protein-coding genes encoded in the Cryptosporidium parvum genome were released and involved in host-parasite interaction, and parasite intracellular development (Abrahamsen et al., 2004; Huang et al., 2004; Puiu et al., 2004; Wastling et al., 2009; Certad et al., 2017). A comprehensive transcriptomic analysis of the intracellular stages of C. parvum revealed a cascade of gene expression consistent with unique biologies for each developmental stage following parasitization of intestinal epithelial cells; many of these putative developmental stage-specific genes are of unknown function (Mauzy et al., 2012). In 2011, one hundred eighteen “orphan” RNA transcripts were identified in the sporozoites of C. parvum (Yamagishi et al., 2011). Our previous study indicated that several of them could be selectively delivered into the nuclei of infected host epithelial cells (Wang et al., 2017a). Further study revealed that nuclear delivery of parasite Cdg7_FLc_0990 RNA (GeneBank ID: FX115678.1) (Yamagishi et al., 2011) into infected intestinal epithelial cells suppresses transcription of the LRP5, SLC7A8, and IL33 genes through histone modification-mediated epigenetic mechanisms (Wang et al., 2017b). The parasite Cdg2_FLc_0220 RNA, a transcript from a hypothetical protein gene (GeneBank ID: FX115592.1) located at the Chromosome 2 (Yamagishi et al., 2011), is delivered into the nuclei of infected host epithelial cells (Wang et al., 2017a). In the present study, the distinct role of nuclear delivery of Cdg2_FLc_0220 RNA in modulating transcription of host genes, such as the DAZ interacting zinc finger protein 1-like (DZIP1L), in intestinal epithelial cells infected with C. parvum was addressed.
2. Materials and methods
2.1 Parasites and in vitro infection model
C. parvum oocysts used in this study were the Iowa isolate purchased from Bunch Grass Farm. Oocysts were firstly treated with 20% sodium hypochlorite at 4°C for 20 min, and then washed twice with Phosphate Buffered Saline (PBS) and RPMI-1640. Viable oocysts were resuspended in RPMI-1640, and respectively used to infect human carcinoma intestinal epithelial HCT-8 cells (ATCC) and non-carcinoma small intestinal epithelial FHs 74 Int cells (INT) (ATCC) with a ratio of oocysts to host cells at 5:1 to 10:1 in the serum free mediums to establish in vitro infection models. Stable HCT-8-G9a−/− cells were generated through transfection of cells with the G9a-CRISPR/Cas9 KO(h) and G9a-HDR plasmids (Santa Cruz), as previously reported (Ming et al., 2017). Cells were washed with PBS to remove oocyst walls, oocysts and free sporozoites, and changed with complete culture medium at 4 h post infection.
2.2 Overexpression of C. parvum Cdg2_FLc_0220
Total RNA was isolated from C. parvum oocyst using Tri-reagent solution (invitrogen) and chloroform/isopropanol. The genomic DNA was removed from total RNA samples by DNase Treatment & Removal (ambion), and treated RNA was reverse transcribed into cDNA with M-MLV (invitrogen). C. parvum Cdg2_FLc_0220 was amplified using primers listed in Table 1, sub-cloned into the pcDNA3.1 vector (invitrogen) and transformed into Escherichia coli DH5α to construct the overexpression plasmid of Cdg2_FLc_0220 (named as Full-Cdg2_FLc_0220). INT (1×105) and HCT-8 (2×106) cells were respectively seeded into 24-well plates. After cultured for 24 h at 37°C and 5% CO2, each well with cells was transfected with 1 μg Full-Cdg2_FLc_0220 or empty vector pcDNA3.1 using the Lipofectamine 2000 Reagent (Invitrogen) and Opti-MEM (Gibco). Cells were changed with complete mediums and collected at 24 h and 48 h after transfection for further study.
2.3 Microarray analysis
The Agilent SurePrint G3 Human Gene Expression Microarray and service to process the samples were applied to genome-wide analysis. Briefly, INT cells were grown to 80% confluence and exposed to C. parvum infection or Full-Cdg2_FLc_0220transfection, respectively. Total RNA of harvested cells was isolated with the RNeasy Mini kit (Qiagen). A mixture of equal amounts of total RNAs from each group was used as the reference pool. A total of 2 μg RNA from each sample was then labeled with the Agilent Gene Expression Hybridization Kit (Agilent). After hybridization, the slides were scanned with the Agilent Microarray Scanner (Agilent). The Feature Extraction software (version10.7.1.1, Agilent Technologies) was used to analyze array images to get raw data and Genesrping software was employed to finish the basic analysis with the raw data. Quantified positive signals were then extracted and analyzed by the LC Sciences, in accordance with MIAME guidelines. Protein coding genes differentially expressed after C. parvum infection and overexpression of Cdg2_FLc_0220 were screened by fold change > 1.3 and P < 0.05. The heatmaps for genes differentially expressed in both arrays after infection and overexpression were depicted by using MeV 4.9.0.
2.4 Total RNA extraction from whole cells and nuclear extracts and cDNA synthesis
Cells were directly cleaved by using Tri-reagent solution (invitrogen) to extract total RNA from whole cells, and cell pellets were collected by using trypsin-EDTA (Gemini Bio Products) and washed with PBS for isolation nuclear extracts. Cell pellets were then treated by 500 μl nuclear buffer A (10 mM Tris-HCl pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.5% NP-40) for 10 min at 4°C, centrifuged at 1000 rpm for 2 min at 4°C, and resuspended in 500 μl nuclear buffer B (10 mM Tris-HCl pH 7.4, 10 mM NaCl, 3 mM MgCl2). Nuclear extracts were collected by centrifugation at 2300 rpm for 2 min at 4°C, and treated by Tri-reagent solution (invitrogen). Both total RNA samples of whole cells and nuclear extracts were extracted and reversely transcribed into cDNA as 2.2.
2.5 Real-time PCR
Realtime PCR was performed using the SYBR Green polymerase chain reaction master mix (Applied Biosystems) in ABI-Prism 7900HT (Applied Biosystems, Foster City, CA, USA) with primers listed in Table S1. Experiments were performed in triplicate and the values were normalized to GAPDH (for whole cell analysis) or U2 (for nuclear delivery analysis) and expressed as 2−ΔΔCt.
2.6 Western Blot
Whole cell protein lysates were prepared using M-PER® Mammalian Protein Extraction Reagent (Thermo scientific) in the presence of protease inhibitors and measured using Bio-Rad DC Protein Assay Reagent (Bio-Rad). Proteins (20 μg) were loaded into 10% SDS–polyacrylamide electrophoresis gels and transferred to nitrocellulose membrane. The following antibodies were used for blotting: anti-DZIP1L (Santa Cruz Biotechnology), anti-H3 (Cell Signaling), anti-Cyclophilin A (Cell Signaling), and anti-GAPDH (Santa Cruz Biotechnology).
2.7 Formaldehyde cross-linking RNA Immunoprecipitation (RIP)
Cell pellets were collected for HCT-8 cells infected with C. parvum oocysts at 24 h post infection (pi) and washed with PBS. The cross-linking reaction was performed with 0.3% of formaldehyde at 37°C for 10 min and quenched by 0.25 M glycine at room temperature for 5 min. Nuclear extracts from resultant cell pellets were isolated by using 500 μl nuclear buffer A and 500 μl nuclear buffer B as above in the presence of protease inhibitors. Pellets were resuspended in 100 μl nuclear buffer C (10 mM Tris-HCl pH 7.4, 400 mM NaCl, 1 mM EDTA, 1 mM DTT) and 400 μl WCE buffer (20 mM HEPES, pH 7.4, 0.2 M NaCl, 0.5% Triton X-100, 10% glycerol, 1 mM EDTA, 1 mM EGTA and 1 mM DTT) with protease and RNA inhibitors, sonicated and measured for nuclear protein levels. A total of 500 μg protein for each sample was immunoprecipitated with 2 μg normal mouse or rabbit IgG (Santa Cruz Biotechnology), and polyclonal antibodies of mouse PRDM1 (Santa Cruz Biotechnology) and rabbit G9α (Millipore) by using Magna Protein A+G Magnetic Beads (Millipore). Formaldehyde cross-linking RIP binding complex were reversed by incubation at 65 C for 4 h with rotation and the RNA level of Cdg2_FLc_0220 was determined by using Realtime PCR in the CFX Connect Real-time detection system (Bio-Rad) and normalized to the control IgG and the U2 gene of the input (10% of the starting sample).
2.8 Chromatin Immunoprecipitation (ChIP)
Cell pellets were collected for HCT-8 cells infected with C. parvum and transfected with the Full-Cdg2_FLc_0220 after 24 h, washed with PBS, cross-linked with 1% formaldehyde at 37°C for 10 min and quenched by 0.25 M glycine at room temperature for 5 min. Cell protein isolation and genomic DNA fragmentation were performed by using SDS lysis buffer (1% SDS, 10 mM EDTA and 50 mM Tris-HCl pH 8.1) and sonication. 500 μg protein for each sample was used for ChIP analysis with Salmon Sperm DNA/Protein A Agarose (Millipore) and 2 μg normal mouse or rabbit IgG (Santa Cruz Biotechnology), rabbit polyclonal to Histone H3 (tri methyl K9) (Abcam), mouse monoclonal to Histone H3 (tri methyl K27) (Abcam), mouse polyclonal antibody to PRDM1 (Santa Cruz Biotechnology), and rabbit polyclonal antibody to G9α (Millipore) at 4°C overnight. Formaldehyde cross-linking complex was reversed at 65°C overnight. The methylation level for each selective DNA site was quantified by using Realtime PCR with primers listed in Table S1, and the value was normalized to the input (1% of the starting chromatin).
2.9 Statistical analysis
Data was analyzed using the program Graphpad Prism 5.0 and expressed as mean ± standard error of the mean (SEM). The student t test was used to compare differences between three independent experiments, and differences were considered statistically significant when p value < 0.05.
3. Results
3.1 Nuclear delivery of parasite Cdg2_FLc_0220 RNA during C. parvum infection
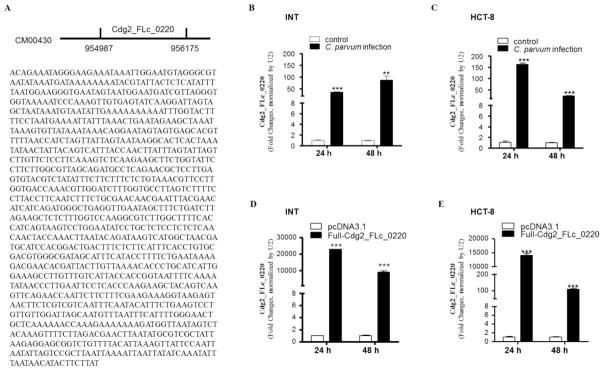
In our previous studies, we identified that several C. parvum RNA transcripts were selectively delivered into the nuclei of infected host epithelial cells (Wang et al., 2017a). One of such transcripts is Cdg2_FLc_0220, a transcript with a sequence of 1189 nt from a hypothetical protein gene (GeneBank ID: FX115592.1) located at the Chromosome 2 (Yamagishi et al., 2011) (Fig. 1A). After exposure to C. parvum infection for 24 h and 48 h, INT and HCT-8 cells were collected and nuclear extracts isolated. A significant level of Cdg2_FLc_0220 was detected in the nuclear extracts of INT cells (Fig. 1B) and HCT-8 cells (Fig. 1C) after exposure to C. parvum. We used the nuclear extracts because it is not technically feasible to separate the internalized intracellular parasites from the cytoplasmic components of infected cells. The purity of our nuclear extract preparation was confirmed by Western blot for the nuclear protein marker (H3) and a cytoplasmic protein marker (Cyclophilin A); nuclear extracts from cells exposed to heat-inactive C. parvum oocysts or parasite lysis showed no detectable level of Cdg2_FLc_0220 (data not shown). Moreover, we further measured its nuclear content in cells expressing Cdg2_FLc_0220. The full-length of Cdg2_FLc_0220 was amplified by PCR using primers listed in Table S1, sequenced and cloned into the plasmid pcDNA3.1. We then transfected INT and HCT-8 cells with the Full-Cdg2_FLc_0220 construct for 24 h and 48 h. A significantly higher level of Cdg2_FLc_0220 was detected in the nuclear extracts of INT and HCT-8 cells expressing Cdg2_FLc_0220, compared with that in cells transfected with the empty vector (Fig. 1D and 1F).
Fig. 1.
Delivery of parasite Cdg2_FLc_0220 RNA transcript into the nuclei of intestinal epithelial cells following C. parvum infection. (A) Genomic location and sequence of the Cdg2_FLc_0220 gene (GeneBank ID: FX115592.1) in C. parvum. (B) and (C) Delivery of Cdg2_FLc_0220 RNA transcript into the nuclei of cultured human intestinal epithelial cells following C. parvum infection in vitro. INT and HCT-8 cell lines were cultured and exposed to C. parvum oocyst infection for 24 h and 48 h. Nuclear extracts of INT (B) and HCT-8 (C) cell cultures were isolated and contents of Cdg2_FLc_0220 were quantified by real-time PCR, normalized by the expression level of U2 RNA. (D) and (E) Overexpression of Cdg2_FLc_0220 in INT and HCT-8 cells resulted in nuclear delivery of Cdg2_FLc_0220 in the cells. Cells were transfected with the Full-Cdg2_FLc_0220 vector for 24 h and 48 h. Nuclear extracts of INT (D) and HCT-8 (E) cell cultures were isolated and contents of Cdg2_FLc_0220 were quantified by real-time PCR, normalized by the expression level of U2 RNA. Cells transfected with the empty vector were used as the control. Data represent three independent experiments. ** P<0.01 and ***P<0.001, ANOVA versus non-infected or empty vector controls.
3.2 Alterations in host gene expression profile following C. parvum infection is partially associated with the nuclear delivery of Cdg2_FLc_0220
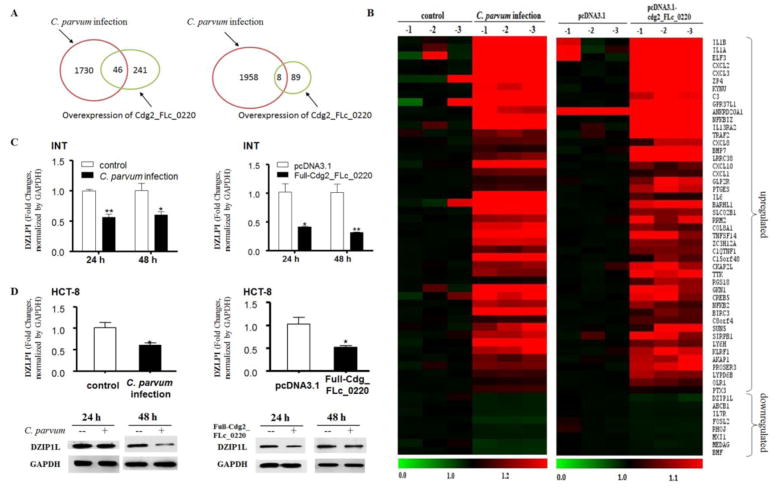
Since C. parvum Cdg2_FLc_0220 could be delivered into host cell nuclei, we examined the potential influence of Cdg2_FLc_0220 on the host gene expression profile. INT cells exposed to C. parvum for infection for 48 h or transfected with the Full-Cdg2_FLc_0220 for 48 h were collected and genome-wide transcriptome analysis was performed using the Agilent SurePrint G3 Human Gene Expression Microarray (G4851B). Non-infected cells and cells transfected with the empty vector pcDNA3.1 for 48 h were used as the controls. All array data were deposited at GEO database (accession number: GSE94128; for reviewer access: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=cxybmaqajfajhyf&acc=GSE94128). Given the fact that the impact of C. parvum infection on host cell gene transcription is generally very mild compared to other pathogens (Deng et al., 2004; Yang et al., 2009; Zhou et al., 2009), we chose fold changes >1.3 combined with a P < 0.05 as the threshold for data analysis. We identified that 1776 genes were upregulated and 1966 genes were downregulated in infected cells compared with the non-infected cells (Fig. 2A, Table S2 and S3). Compared with cells transfected with the empty vector, 287 genes were upregulated and 97 genes were downregulated in cells transfected with the Full-Cdg2_FLc_0220 (Fig. 2A, Table S4 and S5). There were 46 overlapping upregulated genes and 8 overlapping downregulated genes in infected cells and cells transfected with Full-Cdg2_FLc_0220 (Fig. 2A and B, Table S6). Heatmaps represent these overlapping genes either upregulated or downregulated (Fig. 2B).
Fig. 2.
Alterations in host gene expression profiles in cells following C. parvum infection and overexpressing Cdg2_FLc_0220. (A) Genome-wide transcriptome analysis in INT cells following C. parvum infection and cells after transfection of the Full-Cdg2_FLc_0220 for 48 h, taking the fold changes >1.3 combined with a p < 0.05 as the threshold. The total numbers of genes whose expression is significantly altered after infection or transfection are shown. (B) Heatmaps representing the overlapping genes either upregulated or downregulated in cells following Full-Cdg2_FLc_0220 transfection and C. parvum infection. Expression levels of RNAs were presented as the fold changes normalized to the mean value of the non-infected control. (C) Downregulation of the DZLP1L gene in INT cells following C. parvum infection or in cells following Full-Cdg2_FLc_0220 transfection as measured by using real-time PCR. INT cells following C. parvum infection and cells after transfection of the Full-Cdg2_FLc_0220 for 48 h, followed by real-time PCR analysis of DZLP1L. (D) Downregulation of the DZLP1L gene at the mRNA and protein levels in cells following C. parvum infection or Full-Cdg2_FLc_0220 transfection was further validated in HCT-8 cells. HCT-8 cells following C. parvum infection and cells after transfection of the Full-Cdg2_FLc_0220 for 48 h were collected, followed by real-time PCR and Western blot for DZLP1L. Data represent three independent experiments. *P<0.05 and ** P<0.01, ANOVA versus non-infected or empty vector controls.
These overlapping upregulated genes include interleukins (e, g., IL6, IL1B, IL1A) and their receptors (e. g., IL13RA2), chemokine ligands (e. g., CXCL10, CXCL2, CXCL3, CXCL1, CXCL8), complement components (e, g., C3, C1QTNF1) and other inflammatory mediators (e, g., TNFSF14, TRAF2, GKN1, ELF3, NFKB2, NFKBIZ), indicating a similar inflammatory response in cells following C. parvum infection or transfection with the parasite Cdg2_FLc_0220 RNA. Of the eight overlapping downregulated genes, the top one is the DZIP1L gene. Downregulation of DZIP1L at the RNA level in INT cells following C. parvum infection or transfection with Full-Cdg2_FLc_0220 was confirmed by real-time PCR (Fig. 2C). Furthermore, downregulation of DZIP1L at both the RNA and protein levels was also validated in HCT-8 cells infected with C. parvum and transfected with Full-Cdg2_FLc_0220 (Fig. 2D).
3.3 Trans-suppression of the DZIP1L gene in host cells following C. parvum infection is mediated by G9a, independent of PRDM1
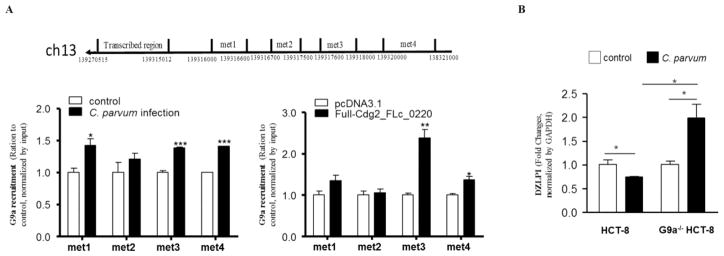
We then focused on the downregulation of the DZIP1L gene to explore the molecular mechanisms underlying Cdg2_FLc_0220-medicated trans-suppression of host genes in infected cells. Our previous studies demonstrated that G9a, an important histone methyltransferase mediating transcriptional repression of genes (Artal-Martinez de Narvajas et al., 2013; Tong et al., 2013; Fan et al., 2015), is involved in parasite RNA-mediated trans-suppression of several host genes during C. parvum infection (Ming et al., 2017; Wang et al., 2017a, b). We then questioned whether G9a is recruited to the DZIP1L gene locus in HCT-8 cells after C. parvum infection or overexpression of Cdg2_FLc_0220. We designed PCR primer sets (Set 1 to 4) covering the different promoter regions of the DZIP1L gene locus for ChIP analysis (Fig. 3A). Of four selected regions within the promoter of DZIP1L gene locus, three (Met1, Met3, Met4) regions showed an increase in G9a recruitment in cells following infection for 24 h (Fig. 3A). Interestingly, two regions (Met3 and Met4) also showed an increase in recruitment of G9a in cells after transfection of Full- Cdg2_FLc_0220 (Fig. 3A). To further explore the role of G9a for C. parvum infection, we generated the stable G9a knockout (G9a−/−) HCT-8 cells using the G9a-CRISPR/Cas9 KO(h) and G9a-HDR plasmids (Santa Cruz). In contrast to the downregulation of the DZIP1L gene in HCT-8 cells following infection, we detected a significant increase of DZIP1L RNA level in the G9a−/− HCT-8 cells following C. parvum infection (Fig. 3B). These results suggested that the downregulated expression of DZIP1L was associated with G9a. To test the physical relationship between G9a and Cdg2_FLc_0220 in the infected cells, we performed the RIP analysis. Anti-G9a antibody was used to pull down the G9a complex in the nuclear extracts of HCT-8 cells infected with C. parvum for 24 h. A significant amount of Cdg2_FLc_0220 was detected in the immunoprecipitated G9a complex from the nuclear extracts of infected HCT-8 cells (Fig. 4A), indicating the assembly of Cdg2_FLc_0220 in the G9a complex in the nuclei of infected cells.
Fig. 3.
Trans-suppression of DZLP1L gene in HCT-8 cells following C. parvum infection is associated with promoter recruitment of G9a. (A) Schematic map of the transcribed region and promoter of the DZIP1L gene (upper), and recruitment of the G9a protein into the promoter regions of the DZIP1L gene by the CHIP technology after C. parvum infection or overexpression of Cdg2_FLc_0220 in HCT-8 cells. (B) mRNA level of DZIP1L gene in wild and G9a−/− HCT-8 cells following C. parvum infection. *P<0.05, ** P<0.01, and ***P<0.001, t test versus non-infected cells or cells transfected with the empty vector.
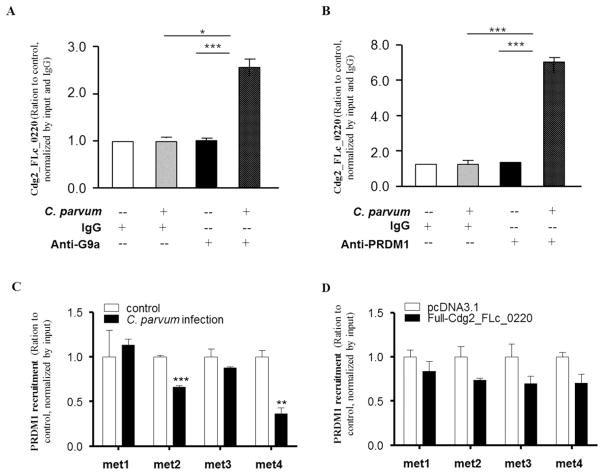
Fig. 4.
Assembly of Cdg2_FLc_0220 to the G9a- and PRDM1-complexes, but not the enrichment of PRDM1 within the promoter of the DZIP1L gene, in HCT-8 cells following C. parvum infection or transfection of the Full-Cdg2_FLc_0220. (A and B) HCT-8 cells were exposed to C. parvum infection for 24 h, followed by RNA immunoprecipitation analysis using anti-G9a (A) and anti-PRDM1 (B), respectively, followed by real-time PCR analysis of Cdg2_FLc_0220. Non-specific IgG was used as the control. Data represent means ± SEM from three independent experiments. *P<0.05 and ***P<0.001, ANOVA versus non-infected controls. (C and D) Recruitment of the PRDM1 within the promoter of the DZIP1L gene in HCT-8 cells after C. parvum infection and transfection of the Full-Cdg2_FLc_0220. HCT-8 cells were exposed to C. parvum infection for 24 h or transfected with the Full-Cdg2_FLc_0220 for 24 h. Non-infected cells or cells transfected with the empty vector were used as the control. The recruitment of the PRDM1 within promoter of the DZIP1L gene in cells after C. parvum infection (C) and transfection of the Full-Cdg2_FLc_0220 (D) were measured by ChIP analysis, using anti-PRDM1 and the PCR primer sets as designed. Data represent means ± SEM from three independent experiments. ** P<0.01, and ***P<0.001, ANOVA versus non-infected.
The PR domain zinc finger protein 1 (PRDM1, also known as BLIMP-1), a G9a-interacting protein (John and Garrett-Sinha, 2009) and an RNA binding protein using the PR zinc finger domain to interact with RNA molecule (John and Garrett-Sinha, 2009), has been shown to be involved in the transcriptional repression of genes through recruitment of G9a (Gyory et al., 2004). We then determined whether PRDM1 is involved in Cdg2_FLc_0220-mediated suppression of DZIP1L. We first measured the physical relationship between PRDM1 and Cdg2_FLc_0220 in the infected cells using the RIP analysis. A significant amount of Cdg2_FLc_0220 was detected in the immunoprecipitated PRDM1 complex in the nuclear extracts from HCT-8 cells following infection for 24 h (Fig. 4B). However, ChIP analysis failed to detect any recruitment of the PRDM1 complex to the promoter region of the DZIP1L gene in HCT-8 cells following C. parvum infection (Fig. 4C) or transfection with the Full-Cdg2_FLc_0220 (Fig. 4D), suggesting that PRDM1 would not be directly involved in Cdg2_FLc_0220-mediated transcriptional repression of DZIP1L in cells following infection.
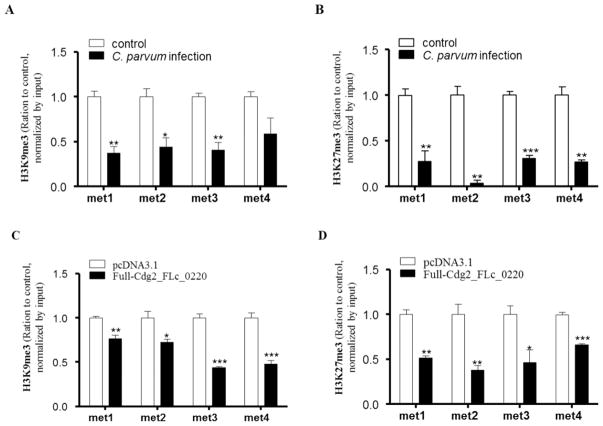
3.4 Cdg2_FLc_0220-mediated trans-suppression of the DZIP1L gene during C. parvum infection is independent of H3K9 and H3K27 methylations
Given the importance of G9a as a histone methyltransferase to enhance histone methylation to induce epigenetic gene suppression, coupled with the recruitment of G9a to the DZIP1L gene promoter in infected cells, we speculated that H3K9 and H3K27 methylations associated with the DZIP1L gene locus may occur in cells following C. parvum infection. HCT-8 cells infected with C. parvum and transfected with Cdg2_FLc_0220 for 24 h were collected, followed by ChIP analysis using anti-H3K9me3 and anti-H3K27me3, respectively. In contrast to our speculation, de-enrichment of H3K9 and H3K27 methylations was detected in the DZIP1L gene promoter in cells following C. parvum infection (Figs. 5A, B) or overexpression of Cdg2_FLc_0220 (Figs. 5C, D), indicating no association of DZIP1L repression with the methylation of H3K9 and H3K27.
Fig. 5.
Enrichments of H3K9me3 and H3K27me3 within the promoter of the DZIP1L gene in HCT-8 cells after C. parvum infection and transfection of the Full-Cdg2_FLc_0220. HCT-8 cells were exposed to C. parvum infection for 24 h or transfected with the Full-Cdg2_FLc_0220 for 24 h. Non-infected cells or cells transfected with the empty vector were used as the control. The enrichments of H3K9me3 (A and C) and H3K27me3 (B and D) within promoter of the DZIP1L gene in cells after C. parvum infection (A and B) and transfection of the Full-Cdg2_FLc_0220 (C and D) were measured by ChIP analysis, using anti-H3K9me3 or anti-H3K27me3 and the PCR primer sets as designed. Data represent means ± SEM from three independent experiments. *P<0.05, ** P<0.01, and ***P<0.001, ANOVA versus non-infected or empty vector controls.
4. Discussion
Intestinal infection by Cryptosporidium causes significant alterations in the gene expression profile in host epithelial cells. Whereas alterations in the expression levels of many genes are due to host responses to infection, such as inflammatory and defense genes, increasing evidence suggests that Cryptosporidium may modulate host gene transcription to its own benefit (Deng, et al., 2004; Ming et al., 2017; Wang et al., 2017a and 2017b). A well-known example is the suppression of host DEFB1 gene in intestinal epithelial cells following infection, which may help the parasite to evade host defense against infection (Zaalouk et al., 2004). Neverthenless, the molecular mechanisms of how C. parvum may modulate host cell gene transcription and the pathological significance of such alterations are largely unclear. Previous studies demonstrated that a panel of C. parvum RNA transcripts are delivered into infected host cells and may modulate host gene transcription (Wang et al., 2017a and 2017b). Data from this study provide additional evidence that Cdg2_FLc_0220, one of the parasite RNAs that are selectively delivered into the nuclei of infected cells, may modulate the transcription of host genes, contributing to the alterations in the gene expression profile in host epithelial cells during C. parvum infection.
Consistent with findings from previous studies (Ming et al., 2017; Wang et al., 2017a, b), nuclear delivery of Cdg2_FLc_0220 may only account for the alterations for a small fraction of host genes induced by C. parvum infection. Specifically, we identified a total of 46 overlapping upregulated genes and 8 overlapping downregulated genes in infected cells and cells transfected with Full-Cdg2_FLc_0220, whereas a total of 1776 genes were upregulated and 1966 genes were downregulated in infected cells compared with the non-infected cells. Of note, most of these overlapping upregulated genes are inflammatory mediators, indicating a general inflammatory response in cells following C. parvum infection or transfection with the parasite Cdg2_FLc_0220 RNA. In contrast, we speculate that these overlapping downregulated genes, such as the DZIP1L gene, may be modulated by parasite Cdg2_FLc_0220 RNA.
How nuclear delivery of Cdg2_FLc_0220 RNA may cause trans-suppression of the DZIP1L gene is still unclear. Previous studies demonstrated that promoter recruitment of G9a (a key methyltransferase for H3K9) (Shinkai and Tachibana, 2011) and PRDM1 (a G9a-interacting protein) (John and Garrett-Sinha, 2009; Gyory et al., 2004) is involved in the trans-suppression of the host CDH3 gene by C. parvum Cdg7_FLc_1000 RNA in infected cells (Ming et al., 2017). Trans-suppression of host LRP5, SLC7A8, and interleukin 33 genes mediated by nuclear delivery of Cdg_FLc_0990 RNA in infected cells also requires the promoter recruitment of G9a/PRDM1 complex (Wang et al., 2017a, b). Interestingly, an increased recruitment of G9a, but not PRDM1, was detected in the promoter region of the DZIP1L gene locus in cells following infection or transfection of the Full-Cdg2_FLc_0220. This suggests to us a G9a-dependent mechanism for Cdg2_FLc_0220-mediated trans-suppression of the DZIP1L gene in host cells. This is further evident by the completely restoration of DZIP1L expression in the G9a−/− cells following C. parvum or transfection with the Full-Cdg2_FLc_0220.
As a key methyltransferase for H3K9, G9a has been reported to suppress gene transcription through induction of H3K9 methylation (Shinkai and Tachibana, 2011). In contrast with our speculation, a decrease of both H3K9me3 and H3K27me3 was detected within the promoter region of the DZIP1L gene locus in cells following infection or transfection of the Full-Cdg2_FLc_0220. Therefore, G9a may mediate trans-suppression of DZIP1L associated with the nuclear delivery of Cdg2_FLc_0220 through other mechanisms, rather than induction of H3K9 methylation. The DZIP1L gene encodes the DZIP1L protein, which localizes to centrioles and to the distal ends of basal bodies, and interacts with septin2, a protein implicated in maintenance of the periciliary diffusion barrier at the ciliary transition zone (Glazer et al., 2010). Differing from the ciliated columnar epithelium in the upper respiratory tract, intestinal epithelium is non-ciliated and appears to lack primary cilia (single cilia) (Saqui-Salces et al., 2012). Therefore, the significance of ZDIP1L downregulation in the pathogenesis of intestinal cryptosporidiosis is unclear and merits further investigation.
Supplementary Material
Highlights.
The parasite Cdg2_FLc_0220 RNA transcript is delivered into the nuclei of infected host epithelial cells following C. parvum infection.
Alterations in host gene expression profile following C. parvum infection are partially associated with the nuclear delivery of Cdg2_FLc_0220.
Trans-suppression of the DAZ interacting zinc finger protein 1 like (DZIP1L) gene, the top overlapping downregulated gene in host cells following C. parvum infection, is mediated by G9a, independent of PRDM1.
Cdg2_FLc_0220-mediated trans-suppression of the DZIP1L gene is independent of H3K9 and H3K27 methylation.
Acknowledgments
We thank Dr. Zhenping Ming (Wuhan University, China) for helpful and stimulating discussions, and Barbara L. Bittner (Creighton University) for her assistance in writing the manuscript. This work was supported by funding from the National Institutes of Health (AI116323 and AI136877) and by revenue from Nebraska’s excise tax on cigarettes awarded to Creighton University through the Nebraska Department of Health & Human Services (DHHS) (LB595). Dr. Guang-Hui Zhao was a visiting scholar supported by the China Scholarship Council. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the State of Nebraska, or DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, Buck GA, Xu P, Bankier AT, Dear PH, Konfortov BA, Spriggs HF, Iyer L, Anantharaman V, Aravind L, Kapur V. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304(5669):441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- Acikgoz Y, Ozkaya O, Bek K, Genc G, Sensoy SG, Hokelek M. Cryptosporidiosis: a rare and severe infection in a pediatric renal transplant recipient. Pediatr Transplant. 2012;16(4):e115–119. doi: 10.1111/j.1399-3046.2011.01473.x. [DOI] [PubMed] [Google Scholar]
- Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360(9343):1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- Amadi B, Mwiya M, Sianongo S, Payne L, Watuka A, Katubulushi M, Kelly P. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis. 2009;9:195. doi: 10.1186/1471-2334-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artal-Martinez de Narvajas A, Gomez TS, Zhang JS, Mann AO, Taoda Y, Gorman JA, Herreros-Villanueva M, Gress TM, Ellenrieder V, Bujanda L, Kim DH, Kozikowski AP, Koenig A, Billadeau DD. Epigenetic regulation of autophagy by the methyltransferase G9a. Mol Cell Biol. 2013;33(20):3983–3993. doi: 10.1128/MCB.00813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borad A, Ward H. Human immune responses in cryptosporidiosis. Future Microbiol. 2010;5(3):507–519. doi: 10.2217/fmb.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmen JC, Hardi L, Sinai AP. Toxoplasma gondii inhibits ultraviolet light-induced apoptosis through multiple interactions with the mitochondrion-dependent programmed cell death pathway. Cell Microbiol. 2006;8(2):301–315. doi: 10.1111/j.1462-5822.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- Carmen JC, Sinai AP. Suicide prevention: disruption of apoptotic pathways by protozoan parasites. Mol Microbiol. 2007;64(4):904–916. doi: 10.1111/j.1365-2958.2007.05714.x. [DOI] [PubMed] [Google Scholar]
- Certad G, Viscogliosi E, Chabé M, Cacciò SM. Pathogenic Mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017;33(7):561–576. doi: 10.1016/j.pt.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Chen XM, Keithly JS, Paya CV, LaRusso NF. Cryptosporidiosis. N Engl J Med. 2002;346(22):1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- Chen XM, LaRusso NF. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology. 2000;118(2):368–379. doi: 10.1016/s0016-5085(00)70219-8. [DOI] [PubMed] [Google Scholar]
- Chen XM, Levine SA, Splinter PL, Tietz PS, Ganong AL, Jobin C, Gores GJ, Paya CV, LaRusso NF. Cryptosporidium parvum activates nuclear factor kappaB in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology. 2001;120(7):1774–1783. doi: 10.1053/gast.2001.24850. [DOI] [PubMed] [Google Scholar]
- Chen XM, O’Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS, Limper AH, LaRusso NF. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005;175(11):7447–7456. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- Checkley W, White AC, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA, Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis. 2015;15(1):85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Lancto CA, Abrahamsen MS. Cryptosporidium parvum regulation of human epithelial cell gene expression. Int J Parasitol. 2004;34(1):73–82. doi: 10.1016/j.ijpara.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Fan J, Xing Y, Wen X, Jia R, Ni H, He J, Ding X, Pan H, Qian G, Ge S, Hoffman AR, Zhang H, Fan X. Long non-coding RNA ROR decoys gene-specific histone methylation to promote tumorigenesis. Genome Biol. 2015;14(16):139. doi: 10.1186/s13059-015-0705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. 2011;17(Suppl 3):S34–37. doi: 10.1002/lt.22378. [DOI] [PubMed] [Google Scholar]
- Glazer AM, Wilkinson AW, Backer CB, Lapan SW, Gutzman JH, Cheeseman IM, Reddien PW. The Zn finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Dev Biol. 2010;337(1):148–156. doi: 10.1016/j.ydbio.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61(5):707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- Gyory I, Wu J, Fejér G, Seto E, Wright KL. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol. 2004;5(3):299–308. doi: 10.1038/ni1046. [DOI] [PubMed] [Google Scholar]
- Huang BQ, Chen XM, LaRusso NF. Cryptosporidium parvum attachment to and internalization by human biliary epithelia in vitro: a morphologic study. J Parasitol. 2004;90(2):212–221. doi: 10.1645/GE-3204. [DOI] [PubMed] [Google Scholar]
- Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen XM. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013;9(4):e1003261. doi: 10.1371/journal.ppat.1003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Garrett-Sinha LA. Blimp1: a conserved transcriptional repressor critical for differentiation of many tissues. Exp Cell Res. 2009;315(7):1077–1184. doi: 10.1016/j.yexcr.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Jarczak J, Kościuczuk EM, Lisowski P, Strzałkowska N, JóŸwik A, Horbańczuk J, Krzyżewski J, Zwierzchowski L, Bagnicka E. Defensins: natural component of human innate immunity. Hum Immunol. 2013;74(9):1069–1079. doi: 10.1016/j.humimm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health. 2007;5(1):1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review) Int J Mol Med. 2006;18(6):1019–1123. [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Lantier L, Lacroix-Lamandé S, Potiron L, Metton C, Drouet F, Guesdon W, Gnahoui-David A, Le Vern Y, Deriaud E, Fenis A, Rabot S, Descamps A, Werts C, Laurent F. Intestinal CD103+ dendritic cells are key players in the innate immune control ofCryptosporidium parvum infection in neonatal mice. PLoS Pathog. 2013;9(12):e1003801. doi: 10.1371/journal.ppat.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent F, Kagnoff MF, Savidge TC, Naciri M, Eckmann L. Human intestinal epithelial cells respond to Cryptosporidium parvum infection with increased prostaglandin H synthase 2 expression and prostaglandin E2 and F2alpha production. Infect Immun. 1998;66(4):1787–1790. doi: 10.1128/iai.66.4.1787-1790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q, Shen J, Liu J, Sun X, Zhao G, Chang Y, Xu L, Li X, Zhao Y, Zheng H, Zhao Y, Wu Z. Genome-wide identification and functional annotation of Plasmodium falciparum long noncoding RNAs from RNA-seq data. Parasitol Res. 2014;113(4):1269–1281. doi: 10.1007/s00436-014-3765-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Deng M, Lancto CA, Abrahamsen MS, Rutherford MS, Enomoto S. Biphasic Modulation of Apoptotic Pathways inCryptosporidium parvum -Infected Human Intestinal Epithelial Cells. Infect Immun. 2009;77(2):837–849. doi: 10.1128/IAI.00955-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcial MA, Madara JL. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986;90(3):583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- Mauzy MJ, Enomoto S, Lancto CA, Abrahamsen MS, Rutherford MS. The Cryptosporidium parvumtranscriptome during in vitro development. PLoS ONE. 2012;7:e31715. doi: 10.1371/journal.pone.0031715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald V, Korbel DS, Barakat FM, Choudhry N, Petry F. Innate immune responses againstCryptosporidium parvum infection. Parasite Immunol. 2013;35(2):55–64. doi: 10.1111/pim.12020. [DOI] [PubMed] [Google Scholar]
- Ming ZP, Gong AY, Wang Y, Zhang XT, Li M, Mathy NW, Strauss-Soukup JK, Chen XM. Involvement of Cryptosporidium parvum Cdg7_FLc_1000 RNA in the attenuation of intestinal epithelial cell migration via trans-suppression of host cell SMPD3 gene. J Infect Dis. 2017 doi: 10.1093/infdis/jix392. https://doi.org/10.1093/infdis/jix392. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Mondal D, Haque R, Sack RB, Kirkpatrick BD, Petri WA., Jr Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am J Trop Med Hyg. 2009;80(5):824–826. [PMC free article] [PubMed] [Google Scholar]
- O’Connor RM, Wanyiri JW, Wojczyk BS, Kim K, Ward H. Stable expression of Cryptosporidium parvum glycoprotein gp40/15 in Toxoplasma gondii. Mol Biochem Parasitol. 2007;152(2):149–158. doi: 10.1016/j.molbiopara.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue PJ. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25(2):139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- O’Hara SP, Chen XM. The cell biology of cryptosporidium infection. Microbes Infect. 2011;13(8–9):721–730. doi: 10.1016/j.micinf.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ME, Riojas YA, Wu TW, Le Blancq SM. CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host-parasite boundary in intracellular stages. Proc Natl Acad Sci U S A. 1999;96(10):5734–5739. doi: 10.1073/pnas.96.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CA, Krumholz KA, Carmen J, Sinai AP, Burleigh BA. Trypanosoma cruzi infection and nuclear factor kappa B activation prevent apoptosis in cardiac cells. Infect Immun. 2006;74(3):1580–1587. doi: 10.1128/IAI.74.3.1580-1587.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth KV, Spector DL. Eukaryotic regulatory RNAs: an answer to the ‘genome complexity’ conundrum. Genes Dev. 2007;21(1):11–42. doi: 10.1101/gad.1484207. [DOI] [PubMed] [Google Scholar]
- Puiu D, Enomoto S, Buck GA, Abrahamsen MS, Kissinger JC. CryptoDB: the Cryptosporidium genome resource. Nucleic Acids Res. 2004;32(Database issue):D329–331. doi: 10.1093/nar/gkh050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KA, Rogers AB, Leav BA, Sanchez A, Vannier E, Uematsu S, Akira S, Golenbock D, Ward HD. MyD88-dependent pathways mediate resistance to Cryptosporidium parvum infection in mice. Infect Immun. 2006;74(1):549–556. doi: 10.1128/IAI.74.1.549-556.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis. 2001;184(1):103–106. doi: 10.1086/321008. [DOI] [PubMed] [Google Scholar]
- Saqui-Salces M, Dowdle WE, Reiter JF, Merchant JL. A high-fat diet regulates gastrin and acid secretion through primary cilia. FASEB J. 2012;26(8):3127–3139. doi: 10.1096/fj.11-197426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Wahnschaffe U, Schäfer M, Zippel T, Arvand M, Meyerhans A, Riecken EO, Ullrich R. Rapid increase of mucosal CD4 T cells followed by clearance of intestinal cryptosporidiosis in an AIDS patient receiving highly active antiretroviral therapy. Gastroenterology. 2001;120(4):984–987. doi: 10.1053/gast.2001.22557. [DOI] [PubMed] [Google Scholar]
- Semple F, Dorin JR. β-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4(4):337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 2011;25(8):781–788. doi: 10.1101/gad.2027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304(5668):248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- Squire SA, Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasit Vectors. 2017;10(1):195. doi: 10.1186/s13071-017-2111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepen B. Parasitic infections: Time to tackle cryptosporidiosis. Nature. 2013;503(7475):189–191. doi: 10.1038/503189a. [DOI] [PubMed] [Google Scholar]
- Tong X, Zhang D, Buelow K, Guha A, Arthurs B, Brady HJ, Yin L. Recruitment of histone methyltransferase G9a mediates transcriptional repression of Fgf21 gene by E4BP4 protein. J Biol Chem. 2013;288(8):5417–5425. doi: 10.1074/jbc.M112.433482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Griffiths JK. Natural history and biology of Cryptosporidium parvum. Adv Parasitol. 1998;40:5–36. doi: 10.1016/s0065-308x(08)60116-5. [DOI] [PubMed] [Google Scholar]
- Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembar SS, Scherf A, Siegel TN. Noncoding RNAs as emerging regulators of Plasmodium falciparum virulence gene expression. Curr Opin Microbiol. 2014;20:153–161. doi: 10.1016/j.mib.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayak S, Pawlowic MC, Sateriale A, Brooks CF, Studstill CJ, Bar-Peled Y, Cipriano MJ, Striepen B. Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature. 2015;523(7561):477–480. doi: 10.1038/nature14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gong AY, Ma S, Chen X, Li Y, Su CJ, Norall D, Chen J, Strauss-Soukup JK, Chen XM. Delivery of Parasite RNA Transcripts Into Infected Epithelial Cells During Cryptosporidium Infection and Its Potential Impact on Host Gene Transcription. J Infect Dis. 2017a;215(4):636–643. doi: 10.1093/infdis/jiw607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gong AY, Ma S, Chen X, Strauss-Soukup JK, Chen XM. Delivery of parasite Cdg7_Flc_0990 RNA transcript into intestinal epithelial cells during Cryptosporidium parvum infection suppresses host cell gene transcription through epigenetic mechanisms. Cell Microbiol. 2017b;19(11):1–11. doi: 10.1111/cmi.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastling JM, Xia D, Sohal A, Chaussepied M, Pain A, Langsley G. Proteomes and transcriptomes of the Apicomplexa--where’s the message? Int J Parasitol. 2009;39(2):135–143. doi: 10.1016/j.ijpara.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Yamagishi J, Wakaguri H, Sugano S, Kawano S, Fujisaki K, Sugimoto C, Watanabe J, Suzuki Y, Kimata I, Xuan X. Construction and analysis of full-length cDNA library of Cryptosporidium parvum. Parasitol Int. 2011;60(2):199–202. doi: 10.1016/j.parint.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Yang YL, Serrano MG, Sheoran AS, Manque PA, Buck GA, Widmer G. Over-expression and localization of a host protein on the membrane of Cryptosporidium parvum infected epithelial cells. Mol Biochem Parasitol. 2009;168(1):95–101. doi: 10.1016/j.molbiopara.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaalouk TK, Bajaj-Elliott M, George JT, McDonald V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect Immun. 2004;72(5):2772–2779. doi: 10.1128/IAI.72.5.2772-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Gong AY, Eischeid AN, Chen XM. miR-27b targets KSRP to coordinate TLR4-mediated epithelial defense against Cryptosporidium parvum infection. PLoS Pathog. 2012;8(5):e1002702. doi: 10.1371/journal.ppat.1002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Hu G, Liu J, Gong AY, Drescher KM, Chen XM. NF-kappaB p65-dependent transactivation of miRNA genes following Cryptosporidium parvum infection stimulates epithelial cell immune responses. PLoS Pathog. 2009;5(12):e1000681. doi: 10.1371/journal.ppat.1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.