Abstract
Objectives
The current laboratory study was to investigate the effect of different sterilization treatments on surface characteristics of zirconia, and biofilm formation on zirconia surface after exposure to these sterilization treatments.
Methods
Commercially available zirconia discs (Cerconbase, Degu-Dent, Hanau, Germany) were prepared and polished to the same value of surface roughness. The discs were treated with one of the following sterilization methods: steam autoclave sterilization, dry heat sterilization, ultraviolet C (UVC) irradiation, and gamma (γ) ray irradiation. The characteristics of zirconia surfaces were evaluated by scanning electron microscopy (SEM), surface roughness, surface free energy (SFE), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD) measurements. Then, Staphylococcus aureus (S.a.) and Porphyromonas gingivalis (P.g.) bacteria were used and cultured on the respective sterilized zirconia surfaces. The amount of biofilm formation on zirconia surface was quantified by colony forming unit (CFU) counts.
Results
Significant modifications were detected on the colour and SFE of zirconia. The colour of zirconia samples after UVC irradiation became light yellow whilst dark brown colour was observed after gamma ray irradiation. Moreover, UVC and gamma ray irradiation increased the hydrophilicity of zirconia surface. Overall, dry heat sterilized samples showed the significantly lowest amount of bacteria growth on zirconia, while UVC and gamma ray irradiation resulted in the highest.
Significance
It is evident that various sterilization methods could change the surface which contribute to different biofilm formation and colour on zirconia.
Keywords: zirconia, sterilization methods, bacteria, colour, gamma ray, UV
1. Introduction
Zirconia ceramic is an attractive material for dentistry and considered a material of choice. For example, metal implants may reveal a bluish discoloration of the overlying gingiva when apical bone loss and gingival recession occurred. However, a full zirconia implant avoids this aesthetic problem because of its more tooth-like colour [1]. Moreover, zirconia is also popular due to its high flexural strength and toughness [2].
Conclusions from different studies about zirconia implants application and performance are controversial. A retrospective clinical study by Roehling et al. investigated the clinical performance of zirconia implants up to and after 7 years of loading, and concluded that first-generation zirconia implants showed low overall survival (77.3%) and considered all surviving implants, the success rate was 77.6% [3]. Another study by Hashim et al. concluded that although the overall survival rate was 92 % for zirconia implants after 1 year of function, further clinical studies are required to establish long-term results, and to determine the risk of technical and biological complications [4]. However, some studies have reported that zirconia implants have similar osseointegration capability compared to conventional titanium implants [5–7], and they could potentially be the alternative to titanium implants for a non-metallic implant solution. Therefore, zirconia has been increasingly used in implant dentistry due to its aesthetic performance [8], as well as good mechanical properties [9] and biocompatibility [10].
Implants, as surgical components that have intimate contact with bone, need to be properly sterilized prior to the implantation - or during the storage. Sterilization is considered as the final finishing procedure during the manufacturing process, because it may affect the implant surface and its modification, i.e., the physico-chemical properties of implant surface might be changed and thus have an important clinical impact [11, 12]. Moreover, sterilization is also applied as an essential step before in vitro biological tests, because it is a process enabling the device to be free from viable microorganisms, preventing the proliferation and accumulation of unrelated microorganism that we do not want to focus on [13].
There are many issues to be considered when selecting a sterilization method for a particular application condition, e.g., cost and effectiveness. The ultimate goal is to sterilize implant materials and devices properly without compromising their key surface characteristics that may influence their interaction with surrounding tissue [14]. Various sterilization methods have been used in dentistry, depending on the desired application and material properties [15, 16], such as steam autoclaving and γ-irradiation are the most commonly used sterilization processes for implant materials storage since they safe with respect to chemical contamination of the surface [15]. Dry heat and UV sterilization has been for dental implements, e.g. reamer, drill [17–19]. In addition, enhanced osteoblast function has been confirmed on ultraviolet light-irradiated zirconia [20].
The sterilization techniques can be categorized by physical and chemical methods. Physical methods include dry heat sterilization, steam autoclave sterilization, UV radiation, and gamma ray irradiation. Treatments with chemicals such as ethylene oxide (EO), ozone, formaldehyde and phenols belong to chemical sterilization methods [21]. However, cold chemicals for routine sterilization of instruments are not recommend by the American Dental Association, since monitoring the solution can be difficult, and their efficaciousness can be limited by the inability to wrap the instruments in a sterile package [22]. Therefore, physical methods deemed to be the most suitable for sterilizing implant.
Steam autoclaving is a sterilization method commonly used in the dental field, due to its convenience, low cost and reliable sterilization effect [13]. Dry-heat sterilization can be used when the moisture in steam autoclaving would cause corrosion and deterioration of specific material [22]. Indeed, steam autoclave sterilization and dry heat sterilization offer both cost effectiveness and efficacy, but some materials cannot withstand invasive moisture or temperatures above 100 °C [23]. Therefore, other sterilization techniques, such as gamma (γ) irradiation and ultraviolet (UV) irradiation, are also available. Gamma irradiation from a cobalt-60 (Co-60) source is lethal to all forms of microorganism and it has the advantage of sterilizing without high temperature and pressure, chemicals or gases [13]. UV irradiation is divided into four distinct spectral areas according to wavelength, namely: vacuum-UV (100–200 μnm), UVC (200–280 μnm), UVB (280–315 μnm), and UVA (315–400 μnm), such that UVC is found to possess a high antimicrobial capability. UV sources, e.g., light-emitting diodes, lasers, and microwave-generated UV plasma, are available for biomedical applications [24].
Cell and bacteria adhesion is sensitive to the surface properties of implant materials [25, 26] and different sterilization treatments may influence the surface chemistry and wettability, consequently affecting cellular behavior [14]. A study by Vezeau et al. had explored the effects of sterilization on titanium surface characteristics and fibroblasts attachment in vitro. They reported that titanium surface characteristics can be altered by steam autoclave sterilization, and less murine fibroblasts attachment compared to that after UV irradiation [27]. However, there is still no published study investigating the effect of different sterilization treatments on zirconia surface characteristics, as well as the biological responses of biofilm formation.
The aims of this study are twofold: to examine the effects of sterilization methods, i.e., steam autoclave sterilization, dry heat sterilization, UVC irradiation, and gamma ray irradiation, on the surface characteristics of zirconia. Furthermore, after the four different sterilization treatments, the in vitro biofilm formation on zirconia surfaces was compared.
2. Materials and methods
2.1 Zirconia sample preparation
Commercially available cylindrical pre-sintered Y-TZP zirconia blocks (Cerconbase, DeguDent, Hanau, Germany) were used in this study. The zirconia blocks were cut into quadrant-shaped specimen (12.5 mm in radius and 1 mm in thickness) using a diamond precision saw (IsoMet™ 5000, Buehler, USA) under cold running water. After being polished with 4000-grit SiC abrasive paper, the samples were sintered according to the manufacturer instructions. Then, all the fully-sintered zirconia specimens were ultrasonically cleansed in 70% ethanol solution for 15 min, rinsed with de-ionized water, and allowed to dry in clean ambient air for 30 s. A polished only specimen was used as control.
2.2 Sterilization treatment
The zirconia samples were randomly divided into five study groups and treated with one of the following surface modification protocols (Table 1). The dose of γ irradiation in Group γ was set as 25 kGy, since it was recommended as standard dose for medical products in the European Union (EU) [28, 29].
Table 1.
Sterilization treatment condition of different groups.
| Group | Treatment | Equipment | Treatment condition/protocol |
|---|---|---|---|
|
| |||
| Control | Nil | Nil | No further treatment |
| Group SA | Steam autoclave sterilization | Autoclave ASB300BT, ASTELL, UK | 121°C for 15 min [15] |
| Group DH | Dry heat sterilization | Universal Oven U, MEMMERT, Germany | 160°C for 2 h [30] |
| Group UVC | UVC irradiation | UV fluorescence cabinet CL-150, SPECTROLINE ,USA | 254 nm wavelength and 490 μW/cm2 at the distance of 25cm for 30 min on both sides [31] |
| Group γ | Γ ray irradiation | Co-60 gamma irradiator,BFT-3, BINE, China | 25 kGy at room temperature [28, 29]. |
2.3 Surface characteristics of zirconia surface
(a) Visual sample observation
The colour of specimens before and after treatment was observed and assessed visually.
(b) Scanning electron microscopy (SEM)
A scanning electron microscope (SU-1510, HITACHI, Japan) was used to observe the surface morphology of different zirconia groups. The samples were gold sputtered and the analyzing procedures were carried out at 1000× magnification [32].
(c) Surface roughness
Three zirconia samples of each group were measured for Ra values using a profilometer (Surtronic3+, Taylor-Hobson, UK). The stylus tip radius of the diamond probe is 5μm. The cut-off value was set at 0.8 mm. Each zirconia sample was tested for three times and the average value of each group was calculated.
(d) Surface free energy (SFE)
Contact angle was determined using the sessile drop method (Drop shape analyzer DSA100, KRÜSS, Germany). Purified water and diiodomethane were used as probe-liquids. Three zirconia samples of each group were tested. Surface free energy (SFE) was calculated in accordance with the Owens-Wendt-Rabel-Kaelble (OWRK) method [33].
(e) X-ray photoelectron spectroscopy (XPS)
The surface chemical composition of the samples was analyzed with the ultra-high vacuum chamber of an X-ray photoelectron spectrometer (X-ray Photoelectron Spectroscopy/ESCA, Thermo Fisher Scientific, USA). The sample was irradiated with a monochromatic X-ray source Al Kα (1486.6 eV) with an accelerating voltage of 15 kV. The working vacuum under X-ray irradiation was 2×10−9 mbar. Energy calibration was performed based on the Ag 3d5/2 peak standard. Wide survey scans (with 100 eV pass energy) were recorded to identify the chemical elements of the surface. Then, high resolution narrow scans (20 eV pass energy) were applied on the main peaks to determine the elemental binding states. All spectra were aligned on the binding energy scale to the C 1s peak (284.8 eV). The quantitative evaluation of the chemical composition of the surface was performed with Al Thermo1 Library [34, 35].
(f) X-ray diffractionn analysis (XRD)
X-ray diffraction (Empyrean, PANalytical, The Netherlands) examination was used to analyze the changes of surface crystalline structure. The scanning was carried out within the 2θ range between 5° and 80° at a speed of 10°/min with a voltage of 40 kV and a current of 40 mA [36].
2.4 Bacteriological study
(a) Biofilm formation
To grow Staphylococcus aureus (S. a.), a solution with 107 bacteria/ml in brain-heart infusion (BHI) medium was prepared and dispensed into a 24-well microtitre plates (Corning, USA) containing a zirconia disc in each well (1 ml/well). The growth medium was refreshed every 24 h. The biofilm formation was evaluated 2 days after inoculation.
For Porphyromonas gingivalis (P. g.), 108 bacteria/ml in P. g. broth (composed of 30 g TSB, 5 g yeast extract, 1 L distilled water, and 10 mL hemin/vitamin K stock solution) was prepared and dispensed into a 24-well microtitre plates (Corning, USA) containing a zirconia disc in each well (1 ml/well). The growth medium was refreshed every 3 days. The adhesion of bacterial cells or the biofilm formation was evaluated 7 days after inoculation.
In each test, all test groups contained three samples, and the experiment was repeated 3 times.
(b) Quantification of biofilm formation
At a designated biofilm collection time point, the zirconia discs with biofilms were rinsed once in phosphate buffered saline (PBS) solution and transferred into 1 ml of growth medium (BHI for S. a. and P. g. broth for P. g.). The biofilms were removed from the discs and dispersed by vortexing for 30 s. Serially diluted samples were plated onto blood agar plates and incubated anaerobically at 37°C to allow for colonies to grow. The plates were taken out from the incubator and individual colonies were counted. This method for quantifying bacteria on substrates was adapted from Seil et al., [37], which concluded that vortexing was an effective way to remove bacteria from surfaces.
2.5 Statistical analysis
The data were analyzed by Statistical Package for Social Science (SPSS®, Version 23, IBM, USA). The statistical analysis was performed using one-way analysis of variance (ANOVA) at a significance level of 5%.
3. Results
Visual sample observation
The colour of the white zirconia samples did not have obvious change in Group SA and Group DH compared to the Control group. However, UVC and γ ray irradiation caused discoloration of zirconia samples. The colour of the zirconia changed to light yellow in Group UVC and dark brown in Group γ (Fig. 1).
Fig. 1.
Zirconia samples sterilized with different methods including (left to right) Groups Control, SA, DH, UVC, and γ. NB. UVC becomes yellow and γ becomes brown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Scanning electron microscopy (SEM)
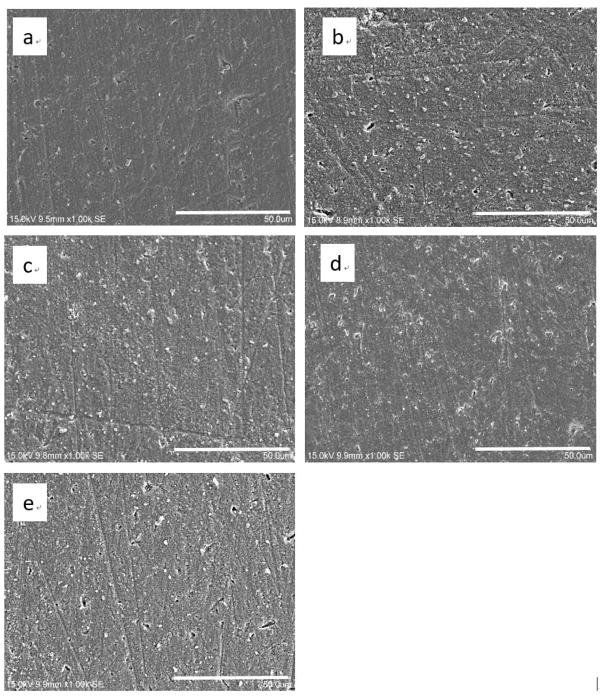
Fig. 2 shows the morphological appearance of zirconia surface before and after being sterilized, respectively. Zirconia appeared to have similar surface overall in all the groups after different sterilization treatments. Small pits and shallow grooves generated in the polishing procedures could be observed. However, there seemed to be more debris on the zirconia surface after sterilization treatments compared to the zirconia in the control group.
Fig. 2.
Results of SEM examination (original magnification 1000×) on zirconia surfaces sterilized with different methods: (a) control, (b) steam autoclave sterilization, (c) dry heat sterilization, (d) UVC irradiation, and (e) γ ray irradiation.
Surface roughness
The average surface roughness (Ra) values in the five groups of zirconia after different sterilization treatments are shown in Table 2. There was no significant difference in Ra values between the five groups.
Table 2.
Surface roughness of different groups (Ra).
| Group | Mean (μm) ± Standard deviation |
|---|---|
| Control | 0.24 ± 0.08 |
| Group SA | 0.22 ± 0.08 |
| Group DH | 0.22 ± 0.08 |
| Group UVC | 0.18 ± 0.03 |
| Group γ | 0.22 ± 0.03 |
Surface free energy (SFE)
The changes in contact angles and SFE values in different groups after sterilization treatments are shown in Table 3. The hydrophilicity of zirconia surfaces increased after all the four types of sterilization treatments, compared to the control group. The SFE values of different groups from the lowest to the highest were 30.41 (Control), 33.97 (Group SA), 34.81 (Group DH), 37.38 (Group UVC), and 43.28 (Group γ).
Table 3.
Surface free energy of different groups.
| Group | Water contact angle (°) | Diiodomethane contact angle (°) | Surface free energy (mN/m) |
|---|---|---|---|
| Control | 100.24 ± 2.48 | 57.47 ± 5.25 | 30.41 |
| Group SA | 92.03 ± 4.52 | 53.03 ± 3.84 | 33.97 |
| Group DH | 96.14 ± 3.08 | 50.47 ± 1.90 | 34.81 |
| Group UVC | 81.58 ± 1.14 | 52.21 ± 4.00 | 37.38 |
| Group γ | 69.89 ± 0.17 | 50.33 ± 1.43 | 43.28 |
X-ray photoelectron spectroscopy (XPS)
To investigate whether the chemical composition changes after sterilization, X-ray photoelectron spectroscopy (XPS) was used to analyze the atomic top layers of all five groups. The XPS analysis confirmed that surface chemistry was altered by sterilization treatments as shown in Table 4. It was shown that carbon decreased and oxygen increased obviously by atomic% in Group UVC and Group γ. The Zr/O ratio in Group SA (0.3348) and Group DH (0.338) increased compared to the control group (0.3259), whereas the Zr/O ratio decreased in Group UVC (0.3131) and Group γ (0.2653) (Table 3). The lowest Zr/O ratio value was found in Group γ (0.2653) while the highest value was found in Group DH (0.338). In addition, XPS spectra showed Zr (3d) ionization for all the zirconia samples after different sterilization treatments, which is the same with the zirconia sample in the control group (Fig. 3).
Table 4.
Variation of surface composition of the carbon, oxygen, zirconium element and the ratio in different grous.
| Group | C1sAtomic % | O1sAtomic % | Zr3dAtomic % | C1s/O1s ratio | Zr3d/O1sratio |
|---|---|---|---|---|---|
| Control | 26.08 | 49.39 | 16.1 | 0.528 | 0.3259 |
| Group SA | 29.49 | 46.67 | 15.64 | 0.632 | 0.3348 |
| Group DH | 26.34 | 49.52 | 16.75 | 0.532 | 0.3380 |
| Group UVC | 19.68 | 55.07 | 18.36 | 0.357 | 0.3131 |
| Group γ | 18.98 | 58.71 | 15.58 | 0.323 | 0.2653 |
Fig. 3.
XPS spectra of Zr(3d) ionization for zirconia samples sterilized with different methods: (a) control, (b) steam autoclave sterilization, (c) dry heat sterilization, (d) UVC irradiation, and (e) γ ray irradiation.
Crystallographic structure analysis (XRD)
In Figure 4, it is shown that only tetragonal (T) phase structure could be detected on the zirconia surfaces of all the four groups. No monoclinic (M) phase was detected in zirconia samples after the four sterilization treatments.
Fig. 4.
Results of XRD analysis on zirconia surface after different sterilization treatments (T represents tetragonal phase zirconia).
Biofilm formation as function of time
The amount of bacterial cells adhered to the zirconia surfaces was counted by the colony forming unit (CFU) method. Figure 5 demonstrates the log transformed CFU counts of S. aureus on zirconia surfaces sterilized with different methods after cultured for 2 days and P. gingivalis on zirconia surfaces sterilized with different methods after cultured for 7 days. Significant difference of biofilm formation of S. aureus was found on zirconia surfaces between Group DH and Group γ (p<0.05). Moreover, our study also found that there was significant difference of P. gingivalis biofilm growth between Group DH and Group UVC (p<0.05). There was no significant difference of the bacteria between Group SA and the other three groups both in the S. aureus and P. gingivalis biofilm formation.
Fig. 5.
Results of CFU counts of S. aureus on zirconia surfaces sterilized with different methods after cultured for 2 days and P. gingivalis on zirconia surfaces sterilized with different methods after cultured for 7 days. * denotes statistically significant (p < 0.05) difference between different groups.
4. Discussion
This laboratory study investigated the effects of sterilization treatments on the surface characteristics of zirconia and bacteria adhesion on sterilized zirconia. Steam autoclave sterilization, dry heat sterilization, UVC irradiation and gamma ray irradiation were chosen as sterilization treatments in our study.
One of the clear differences after sterilization treatments which was observed was the discoloration seen in Group UVC and Group γ, based on visual observation and assessment. The colour of the zirconia samples changed to light yellow in Group UVC and brown in Group γ, while there was no obvious discoloration in Group SA and Group DH compared to the white colour of zirconia in Group Control. The effect of high-energy UVC or γ radiation in zirconia may lead to the generation of free electrons with a range of energies through absorption of the radiation energy and subsequent ionization [38]. Such a process can produce colour centers in zirconia, which may result in discoloration in surface and deeper layers of the material. This may also be the underlying reason for the discoloration phenomenon of zirconia-containing resin composites after UVC irradiation [39]. As a result, the discoloration phenomena indicated zirconia cannot be sterilized by γ irradiation if it is used as crown, since the discoloration will affect the aesthetic performance of zirconia, and additionally the aesthetic influence of UVC on the zirconia implant should be further investigated.
As determined by the SEM evaluations, the overall morphology of zirconia surface did not seem to be affected by sterilization treatments used in this study. This result was consistent with the finding by previous studies [13, 27]. A study by Vezeau et al. investigated the effect of sterilization treatments on pure titanium surfaces and concluded the sterilization treatments failed to alter the morphology of the samples [27]. Another previous study revealed the dentin surface morphology was not affected by gamma rays and no dentin structural change was detected [13].
For surface roughness, the zirconia samples did not show significant difference between different sterilization treatments in our study. A study by Park et al. also found that sterilization does not alter the surface roughness of pretreated titanium (PT, Ra = 0.4 μm) [40]. In fact, the limitation of our study is that we did not use the 3D-surface height, spatial, and hybrid roughness parameters (e.g. Sa, Sz, Ssk, Sku, Sal, Str and Sdr ) to assess the surface characteristics, since certain studies suggested to choose a set of roughness parameters for the purpose of properly characterizing an implant surface [41]. However, it still remains to be explored, which roughness parameters are the most appropriate for characterizing an implant surface concerning with biological effect, such that Matinlinna et al. [42] has explicitly mentioned various study used different forms of roughness that is lack of unity. Therefore, while we might need to explore the effects of different sterilization methods on three-dimensional surface roughness of zirconia in further study, another focus could be the standardization of the roughness units or test methodology.
For surface free energy, the zirconia samples increased after sterilization treatments in all groups in our study. The result is consistent with a study by Wittenburg also revealed that steam sterilized glass exhibited significantly lower contact angle values than untreated glass samples [43]. A study by Kummer et al. which found that UV irradiation on Ti could increase the formation of Ti-OH and result in the increase of surface wettability and surface energy [14]. Another reason illustrated by previous studies was that titanium and zirconia constantly adsorb organic impurities such as hydrocarbons from the environment, which could lead to an increase in hydrophobicity. UV irradiation could remarkably decrease carbon content and increase the amount of hydroxyl groups on titanium and zirconia surface [20, 44]. The underlying reason was that UV light irradiation could create surface O2 vacancies at bridging O2 sites [45]. This study was consistent with previous studies and we observed a decreased carbon contamination and increased oxide element on zirconia surface after UVC irradiation from XPS result. Our study demonstrated that gamma ray could also increase the hydrophilicity of zirconia surface as detected by the surface free energy test. Indeed, Ueno et. al found that, although gamma ray treatment on titanium did not change the surface topography, it could reduce contact angle. The mechanism possibly due to the gamma ray irradiation could decompose organic molecules adsorbed on titanium dioxide surfaces and generate activated oxygen species come from O2 in air, e.g. O2−, O−, and O (atom). These processes effectively contributed to the surface hydrophilicity [46]. Even so, exact mechanism on zirconia is still need to be further explored.
According to the XRD results, zirconia samples in our study only contained tetragonal (T) phase structure in all the groups. This result was consistent with a previous study which revealed that no evidence of zirconia phase transformation was observed in any zirconia/alumina composites after treated by 25 kGy γ irradiation sterilization and 50 kGy γ irradiation sterilization [47]. Although previous study found steam autoclave sterilization methods may cause a phase change and surface roughening of yttria-stabilized zirconia femoral head components [48], no tetragonal (T) to monoclinic (M) phase transformation could be observed in all the zirconia samples in our study. The underlying reason may be the difference of sterilization condition between different studies. Y2O3-ZrO2 tetragonal polycrystal (Y-TZP) materials, containing 2–3 mol% Y2O3, are constituted predominantly of tetragonal zirconia grains. A previous study on hydrothermal degradation behavior of a CAD/CAM-machined dental zirconia (3 mol% Y-TZP, IPS e.max ZirCAD, Ivoclar-Vivadent, Schaan, Liechtenstein) using identical steam autoclave temperature/cycles revealed that a much longer sterilization time and more sterilization cycles are required for the phase transformation of zirconia [49]. In addition, another study investigated the low temperature degradation behavior of a medical grade zirconia (3 mol% Y-TZP, Prozyr Y-TZP, Norton, East Granby, CT) in a universal oven at 200°C in air [50]. It was found significant tetragonal to monoclinic phase transformation occurred after a prolonged 12 hours holding time.
The other aim of this study was to evaluate the effects of different sterilization treatments on biofilm formation on zirconia. Previous studies showed various factors may have influence on the bacteria adhesion on material surfaces, e.g., surface roughness [51, 52], surface free energy [34, 53], surface chemistry [54]. In this study, we choose the bacteria of S. aureus and P. gingivalis as the target bacteria. Recently, a clinical study was conducted to examine the prevalence and levels of six bacterial pathogens within the subgingival/submucosal microbiota at teeth versus implants with various clinical conditions A gram-positive cariogenic bacterial species, S. aureus was revealed to be the most commonly detected bacteria species in both periodontal and peri-implant sites, irrespective of their health status [55]. Antimicrobial chemotherapy treatment which aimed to reduce the number of S. aureus enabled a better quality of bone repair of tibial surgical bone defects in rats [56]. P. gingivalis is considered to be one of the most important microflora of dental peri-implantitis [57]. It has been a focus for peri-implantitis aetiology studies for a long time [58].
The results showed S. aureus biofilm formation on zirconia surface treated by gamma irradiation sterilization was higher than that on zirconia surface treated by dry heat sterilization treatment after 2 days. The difference was significant (p<0.05). However, no significant difference of S. aureus biofilm formation on zirconia surfaces was found between the other groups. For P. gingivalis, significant difference of P.gingivalis biofilm growth was found between zirconia surface after sterilized by dry heat and UVC irradiation (p<0.05). More P. gingivalis bacteria were found on zirconia surface after sterilized by UVC irradiation compared to that by dry heat. Given this, it can be concluded that dry heat sterilization treatment may induce less biofilm formation on zirconia compared to UVC and gamma irradiation sterilization treatment.
Now, dry heat treatment of zirconia samples was shown to exhibit the lowest biofilm formation on zirconia samples between different groups. The underlying reason is not quite clear. Dry heat sterilization simply means raising the temperature of an item to 160°C under normal air pressure, during which the sterilization process is conducted in dry condition without steam and high pressure compared to steam autoclave sterilization. Since water dissociation can produce OH− and is affected by pressure, we speculate that zirconia surface of Group DH contained lower amount of OH− compared to Group SA, which requires further study to confirm. It was found that OH− ions existing at the topmost surface layer are beneficial to the adsorption of cell adhesion proteins. This said, the selective adsorption of cell adhesion proteins dominates cell adhesion onto a surface [59]. Therefore, the lowest amount of bacteria may adhere on the zirconia surface after dry heat sterilization treatment among all the sterilized study groups.
As for the UVC and γ irradiation sterilized samples, they showed a significantly higher P. gingivalis bacteria amount on zirconia surface after UVC irradiation and higher S. aureus bacteria on zirconia surface after gamma irradiation respectively compared to dry heat sterilized samples. This can be explained by the increased surface free energy of zirconia surface after UVC and γ irradiation. Our result is consistent with the previous study by Zhao et al., who reported that UV sterilization gives rise to higher surface free energy of titania surfaces [31]. It has been reported that the hydrophilicity of the samples can have drastic effects on cell adhesion [60]. Moreover, increased hydrophilicity can alter the adsorption of specific proteins onto a biomaterial surface which can further influence interactions with cells [61]. In addition, some bacteria species were more sensitive to surface chemistry whereas others to surface roughness [54]. Therefore, the underlying mechanism of P. gingivalis and S. aureus adhesion on zirconia surfaces after different sterilization treatments are complicated and need further investigation.
5. Conclusion
In conclusion, the surface free energy and surface chemistry of zirconia changed after sterilization treatments. The selection of sterilization method used for zirconia plays an important role in the degree of biofilm formation onto the zirconia surface. In addition, it is evident that the sterilization treatment, surface chemistry, and hydrophilicity are playing some role in the biofilm formation on zirconia material. Moreover, the zirconia samples treated by dry heat sterilization showed decreased biofilm formation, while the zirconia samples treated by UVC irradiation and γ ray irradiation showed higher bacteria formation on zirconia surface compared to that treated by dry heat sterilization.
Acknowledgments
This work was done in partial fulfilment of the requirements of the degree of Doctor of Philosophy for AH at the Faculty of Dentistry, The University of Hong Kong. Part of the results of this paper has been presented at the 2016 IADR General Session, Seoul, South Korea. YZ would like to thank the United States National Institutes of Health, National Institute of Dental and Craniofacial Research (Grant No. R01DE017925 and Grant No. R01DE026772) and the International Congress of Oral Implantologists (Implant Dentistry Research and Education Foundation Grant) for their support.
References
- 1.Ozkurt Z, Kazazoglu E. Zirconia dental implants: a literature review. J Oral Implantol. 2011;37:367–76. doi: 10.1563/AAID-JOI-D-09-00079. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JR, Denry I. Stabilized zirconia as a structural ceramic: An overview. Dental Materials. 2008;24:289–98. doi: 10.1016/j.dental.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Roehling S, Woelfler H, Hicklin S, Kniha H, Gahlert M. A retrospective clinical study with regard to survival and success rates of zirconia implants up to and after 7 years of loading. Clinical implant dentistry and related research. 2016;18:545–58. doi: 10.1111/cid.12323. [DOI] [PubMed] [Google Scholar]
- 4.Hashim D, Cionca N, Courvoisier DS, Mombelli A. A systematic review of the clinical survival of zirconia implants. Clinical oral investigations. 2016;20:1403–17. doi: 10.1007/s00784-016-1853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzano G, Herrero LR, Montero J. Comparison of clinical performance of zirconia implants and titanium implants in animal models: a systematic review. Int J Oral Maxillofac Implants. 2014;29:311–20. doi: 10.11607/jomi.2817. [DOI] [PubMed] [Google Scholar]
- 6.Andreiotelli M, Wenz HJ, Kohal RJ. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clinical oral implants research. 2009;20(Suppl 4):32–47. doi: 10.1111/j.1600-0501.2009.01785.x. [DOI] [PubMed] [Google Scholar]
- 7.Hisbergues M, Vendeville S, Vendeville P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J Biomed Mater Res B Appl Biomater. 2009;88:519–29. doi: 10.1002/jbm.b.31147. [DOI] [PubMed] [Google Scholar]
- 8.Ananth H, Kundapur V, Mohammed HS, Anand M, Amarnath GS, Mankar S. A Review on Biomaterials in Dental Implantology. Int J Biomed Sci. 2015;11:113–20. [PMC free article] [PubMed] [Google Scholar]
- 9.Zarone F, Russo S, Sorrentino R. From porcelain-fused-to-metal to zirconia: clinical and experimental considerations. Dental materials. 2011;27:83–96. doi: 10.1016/j.dental.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Apratim A, Eachempati P, Krishnappa Salian KK, Singh V, Chhabra S, Shah S. Zirconia in dental implantology: A review. J Int Soc Prev Community Dent. 2015;5:147–56. doi: 10.4103/2231-0762.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi X, Xu L, Violin KB, Lu S. Improved osseointegration of long-term stored SLA implant by hydrothermal sterilization. J Mech Behav Biomed Mater. 2016;53:312–9. doi: 10.1016/j.jmbbm.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed M, Punshon G, Darbyshire A, Seifalian AM. Effects of sterilization treatments on bulk and surface properties of nanocomposite biomaterials. J Biomed Mater Res B Appl Biomater. 2013;101:1182–90. doi: 10.1002/jbm.b.32928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho FG, Goncalves LS, Carlo HL, Soares CJ, Correr-Sobrinho L, Puppin-Rontani RM. Influence of sterilization method on the bond strength of caries-affected dentin. Braz Oral Res. 2009;23:11–6. doi: 10.1590/s1806-83242009000100003. [DOI] [PubMed] [Google Scholar]
- 14.Kummer KM, Taylor EN, Durmas NG, Tarquinio KM, Ercan B, Webster TJ. Effects of different sterilization techniques and varying anodized TiO2 nanotube dimensions on bacteria growth. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2013;101B:677–88. doi: 10.1002/jbm.b.32870. [DOI] [PubMed] [Google Scholar]
- 15.Serro A, Saramago B. Influence of sterilization on the mineralization of titanium implants induced by incubation in various biological model fluids. Biomaterials. 2003;24:4749–60. doi: 10.1016/s0142-9612(03)00372-7. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho FG, Gonçalves LS, Carlo HL, Soares CJ, Correr-Sobrinho L, Puppin-Rontani RM. Influence of sterilization method on the bond strength of caries-affected dentin. Brazilian oral research. 2009;23:11–6. doi: 10.1590/s1806-83242009000100003. [DOI] [PubMed] [Google Scholar]
- 17.Thompson S. An overview of nickel–titanium alloys used in dentistry. International endodontic journal. 2000;33:297–310. doi: 10.1046/j.1365-2591.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- 18.Vendrell RJ, Hayden CL, Taloumis LJ. Effect of steam versus dry-heat sterilization on the wear of orthodontic ligature-cutting pliers. American journal of orthodontics and dentofacial orthopedics. 2002;121:467–71. doi: 10.1067/mod.2002.122175. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai M, Shigeno A, Noguchi Y. UV-sterilizer for a dental implement such as a reamer and drill. Google Patents. 1988 [Google Scholar]
- 20.Att W, Takeuchi M, Suzuki T, Kubo K, Anpo M, Ogawa T. Enhanced osteoblast function on ultraviolet light-treated zirconia. Biomaterials. 2009;30:1273–80. doi: 10.1016/j.biomaterials.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YZ, Bjursten LM, Freij-Larsson C, Kober M, Wesslen B. Tissue response to commercial silicone and polyurethane elastomers after different sterilization procedures. Biomaterials. 1996;17:2265–72. doi: 10.1016/0142-9612(96)00055-5. [DOI] [PubMed] [Google Scholar]
- 22.Vendrell RJ, Hayden CL, Taloumis LJ. Effect of steam versus dry-heat sterilization on the wear of orthodontic ligature-cutting pliers. Am J Orthod Dentofacial Orthop. 2002;121:467–71. doi: 10.1067/mod.2002.122175. [DOI] [PubMed] [Google Scholar]
- 23.Lin JJ, Hsu PY. Gamma-ray sterilization effects in silica nanoparticles/gamma-APTES nanocomposite-based pH-sensitive polysilicon wire sensors. Sensors (Basel) 2011;11:8769–81. doi: 10.3390/s110908769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A, Avci P, Dai T, Huang YY, Hamblin MR. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Adv Wound Care (New Rochelle) 2013;2:422–37. doi: 10.1089/wound.2012.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Zhou J, Yang Y, Zheng M, Yang J, Tan J. Surface modification of zirconia with polydopamine to enhance fibroblast response and decrease bacterial activity in vitro: A potential technique for soft tissue engineering applications. Colloids Surf B Biointerfaces. 2015;136:74–83. doi: 10.1016/j.colsurfb.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Han A, Tsoi JKH, Rodrigues FP, Leprince JG, Palin WM. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. International Journal of Adhesion and Adhesives. 2016;69:58–71. [Google Scholar]
- 27.Vezeau PJ, Koorbusch GF, Draughn RA, Keller JC. Effects of multiple sterilization on surface characteristics and in vitro biologic responses to titanium. J Oral Maxillofac Surg. 1996;54:738–46. doi: 10.1016/s0278-2391(96)90694-1. [DOI] [PubMed] [Google Scholar]
- 28.Ohan MP, Dunn MG. Glucose stabilizes collagen sterilized with gamma irradiation. J Biomed Mater Res A. 2003;67:1188–95. doi: 10.1002/jbm.a.20018. [DOI] [PubMed] [Google Scholar]
- 29.Humenyuk I, Temple-Boyer P, Sarrabayrouse G. The effect of gamma-sterilization on the pH-ChemFET behaviour. Sensors and Actuators a-Physical. 2008;147:165–8. [Google Scholar]
- 30.Alavi S, Sinaee N. Effect of dry heat and steam sterilization on load-deflection characteristics of β-titanium wires: An in vitro study. Dental research journal. 2012;9:541. doi: 10.4103/1735-3327.104871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Mei S, Wang W, Chu PK, Wu Z, Zhang Y. The role of sterilization in the cytocompatibility of titania nanotubes. Biomaterials. 2010;31:2055–63. doi: 10.1016/j.biomaterials.2009.11.103. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Pow EH, Tsoi JK, Matinlinna JP. Evaluation of four surface coating treatments for resin to zirconia bonding. J Mech Behav Biomed Mater. 2014;32:300–9. doi: 10.1016/j.jmbbm.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Owens DK, Wendt R. Estimation of the surface free energy of polymers. Journal of applied polymer science. 1969;13:1741–7. [Google Scholar]
- 34.Al-Radha ASD, Dymock D, Younes C, O’Sullivan D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. Journal of Dentistry. 2012;40:146–53. doi: 10.1016/j.jdent.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Zinelis S, Thomas A, Syres K, Silikas N, Eliades G. Surface characterization of zirconia dental implants. Dental Materials. 2010;26:295–305. doi: 10.1016/j.dental.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Matinlinna JP, Tsoi JK, Pow EH, Miyazaki T, Shibata Y, et al. A new modified laser pretreatment for porcelain zirconia bonding. Dent Mater. 2013;29:559–65. doi: 10.1016/j.dental.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Seil JT, Rubien NM, Webster TJ, Tarquinio KM. Comparison of quantification methods illustrates reduced Pseudomonas aeruginosa activity on nanorough polyvinyl chloride. J Biomed Mater Res B Appl Biomater. 2011;98:1–7. doi: 10.1002/jbm.b.31821. [DOI] [PubMed] [Google Scholar]
- 38.Kreidl N, Hensler J. Formation of color centers in glasses exposed to gamma radiation. Journal of the American Ceramic Society. 1955;38:423–32. [Google Scholar]
- 39.Catelan A, Briso AL, Sundfeld RH, Goiato MC, dos Santos PH. Color stability of sealed composite resin restorative materials after ultraviolet artificial aging and immersion in staining solutions. J Prosthet Dent. 2011;105:236–41. doi: 10.1016/S0022-3913(11)60038-3. [DOI] [PubMed] [Google Scholar]
- 40.Park JH, Olivares-Navarrete R, Baier RE, Meyer AE, Tannenbaum R, Boyan BD, et al. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta biomaterialia. 2012;8:1966–75. doi: 10.1016/j.actbio.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kournetas N, Spintzyk S, Schweizer E, Sawada T, Said F, Schmid P, et al. Comparative evaluation of topographical data of dental implant surfaces applying optical interferometry and scanning electron microscopy. Dent Mater. 2017 doi: 10.1016/j.dental.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Matinlinna JP, Tsoi JK, de Vries J, Busscher HJ. Characterization of novel silane coatings on titanium implant surfaces. Clinical oral implants research. 2013;24:688–97. doi: 10.1111/j.1600-0501.2012.02504.x. [DOI] [PubMed] [Google Scholar]
- 43.Wittenburg G, Lauer G, Oswald S, Labudde D, Franz CM. Nanoscale topographic changes on sterilized glass surfaces affect cell adhesion and spreading. J Biomed Mater Res A. 2014;102:2755–66. doi: 10.1002/jbm.a.34943. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe H, Saito K, Kokubun K, Sasaki H, Yoshinari M. Change in surface properties of zirconia and initial attachment of osteoblastlike cells with hydrophilic treatment. Dental materials journal. 2012;31:806–14. doi: 10.4012/dmj.2012-069. [DOI] [PubMed] [Google Scholar]
- 45.Noro A, Kaneko M, Murata I, Yoshinari M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal) Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2013;101:355–63. doi: 10.1002/jbm.b.32846. [DOI] [PubMed] [Google Scholar]
- 46.Ueno T, Takeuchi M, Hori N, Iwasa F, Minamikawa H, Igarashi Y, et al. Gamma ray treatment enhances bioactivity and osseointegration capability of titanium. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:2279–87. doi: 10.1002/jbm.b.32799. [DOI] [PubMed] [Google Scholar]
- 47.Nam KW, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Optimal sterilization method for the zirconia/alumina composites used for total hip replacements. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;90:962–6. doi: 10.1002/jbm.b.31358. [DOI] [PubMed] [Google Scholar]
- 48.Roy ME, Whiteside LA, Katerberg BJ, Steiger JA. Phase transformation, roughness, and microhardness of artificially aged yttria- and magnesia-stabilized zirconia femoral heads. J Biomed Mater Res A. 2007;83:1096–102. doi: 10.1002/jbm.a.31438. [DOI] [PubMed] [Google Scholar]
- 49.Kim J-W, Covel N, Guess P, Rekow E, Zhang Y. Concerns of hydrothermal degradation in CAD/CAM zirconia. Journal of dental research. 2010;89:91–5. doi: 10.1177/0022034509354193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Pajares A, Lawn BR. Fatigue and damage tolerance of Y-TZP ceramics in layered biomechanical systems. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2004;71:166–71. doi: 10.1002/jbm.b.30083. [DOI] [PubMed] [Google Scholar]
- 51.Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clinical oral implants research. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 52.Han A, Li X, Huang B, Tsoi JK-H, Matinlinna JP, Chen Z, et al. The effect of titanium implant surface modification on the dynamic process of initial microbial adhesion and biofilm formation. International Journal of Adhesion and Adhesives. 2016;69:125–32. [Google Scholar]
- 53.Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, Schwartz Z, et al. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014;10:2907–18. doi: 10.1016/j.actbio.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almaguer-Flores A, Ximenez-Fyvie LA, Rodil SE. Oral bacterial adhesion on amorphous carbon and titanium films: effect of surface roughness and culture media. J Biomed Mater Res B Appl Biomater. 2010;92:196–204. doi: 10.1002/jbm.b.31506. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang LF, Watt RM, Mattheos N, Si MS, Lai HC, Lang NP. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clinical oral implants research. 2016;27:13–21. doi: 10.1111/clr.12508. [DOI] [PubMed] [Google Scholar]
- 56.Dos Reis JA, Jr, Dos Santos JN, Barreto BS, de Assis PN, Almeida PF, Pinheiro AL. Photodynamic Antimicrobial Chemotherapy (PACT) in osteomyelitis induced by Staphylococcus aureus: Microbiological and histological study. J Photochem Photobiol B. 2015;149:235–42. doi: 10.1016/j.jphotobiol.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Pye AD, Lockhart DE, Dawson MP, Murray CA, Smith AJ. A review of dental implants and infection. J Hosp Infect. 2009;72:104–10. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Lin HY, Liu Y, Wismeijer D, Crielaard W, Deng DM. Effects of oral implant surface roughness on bacterial biofilm formation and treatment efficacy. Int J Oral Maxillofac Implants. 2013;28:1226–31. doi: 10.11607/jomi.3099. [DOI] [PubMed] [Google Scholar]
- 59.Hirano M, Kozuka T, Asano Y, Kakuchi Y, Arai H, Ohtsu N. Effect of sterilization and water rinsing on cell adhesion to titanium surfaces. Applied Surface Science. 2014;311:498–502. [Google Scholar]
- 60.Yun KD, Yang YZ, Lim HP, Oh GJ, Koh JT, Bae IH, et al. Effect of nanotubular-micro-roughened titanium surface on cell response in vitro and osseointegration in vivo. Materials Science & Engineering C-Materials for Biological Applications. 2010;30:27–33. [Google Scholar]
- 61.Rosengren A, Pavlovic E, Oscarsson S, Krajewski A, Ravaglioli A, Piancastelli A. Plasma protein adsorption pattern on characterized ceramic biomaterials. Biomaterials. 2002;23:1237–47. doi: 10.1016/s0142-9612(01)00244-7. [DOI] [PubMed] [Google Scholar]