Abstract
Objective
To determine the impact of maternal obesity and gestational weight gain across pregnancy on fetal indices of inflammation and iron status.
Study design
Eighty-five healthy term newborns delivered via elective cesarean were categorized by 2 maternal body mass index (BMI) thresholds; above or below 30 kg/m2 or above or below 35 kg/m2. Umbilical cord plasma levels of C-reactive protein, interleukin (IL)-6, tumor necrosis factor (TNF)-α, ferritin, and hepcidin were assayed. Cytokines released by phytohemagglutinin-stimulated umbilical cord mononuclear cells (MNCs) were assayed.
Results
Maternal class II obesity, defined as BMI of 35 kg/m2 and above, predicted higher C-reactive protein and TNF-α in umbilical cord plasma (P< .05 for both), and also proinflammatory cytokines (IL-1β, IL-6, and TNF-α) from stimulated MNC (P < .05 for all). The rise in plasma TNF-α and MNC TNF-α was not linear but occurred when the threshold of BMI 35 kg/m2 was reached (P < .005, P < .06). Poorer umbilical cord iron indices were associated with maternal obesity. When ferritin was low, IL-6 was higher (P < .04), but this relationship was present primarily when maternal BMI exceeded 35 kg/m2 (P < .03). Ferritin was correlated with hepcidin (P < .0001), but hepcidin was unrelated to either maternal BMI or inflammatory indices.
Conclusions
Class II obesity and above during pregnancy is associated with fetal inflammation in a threshold fashion. Although maternal BMI negatively impacted fetal iron status, hepcidin, related to obesity in adults, was related to iron status and not obesity in fetuses. Pediatricians should be aware of these relationships.
Children born to women with prepregnancy obesity are more likely to develop allergic asthma, obesity, and adult hypertension.1 Excess gestational weight gain (GWG) may pose similar risks.2 Women who are obese prepregnancy are twice as likely to exceed GWG guidelines set by the Institute of Medicine.3 Inflammation may rise as pregnancy body mass index (BMI) in kg/m2 and GWG increase.4 C-reactive protein (CRP) levels rise after rapid weight gain in nongravid adults.5,6 Excessive GWG has been linked to higher umbilical cord tumor necrosis factor (TNF)-α,2 and prepregnancy obesity has been linked to higher umbilical cord plasma interleukin (IL)-67 and other inflammatory indicators.8,9 Data specifically defining the threshold when maternal obesity, and GWG impact newborn inflammatory pathways are limited.
Iron-related and inflammatory pathways are linked by the master iron-regulatory peptide, hepcidin. Normal pregnancy lowers maternal and fetal hepcidin, which works to increase bioavailable iron to the fetus. In adults, hepcidin levels fall in anemia, iron deficiency, and hypoxia, but conversely, hepcidin rises in response to inflammation to reduce iron supply and increase iron sequestration. Obesity stimulates the iron pathway cytokines, including IL-1 β and IL-6,10 and, in one study, also upregulated maternal hepcidin levels,8 decreasing both maternal and fetal iron bioavailabity Fetal hepcidin levels 8 are likely regulated in a similar fashion to that in adults, with lower hepcidin reported in rat pups with gestational iron deficiency 11 and higher umbilical cord levels in human newborns after intrauterine infection.12 We previously showed that maternal obesity and excess GWG interfered with fetal iron endowment,13 but it is not known whether fetal hepcidin levels will mount an appropriate response and decline under low fetal iron conditions. Because iron status impacts fetal neurodevelopment and health,14 it is important to understand the physiological regulation of fetal iron in maternal obesity and/or excess GWG.
Maternal obesity and large GWG were previously shown to influence innate immune status of newborn infants,2 but it is unclear what role iron-related pathways played in this outcome. Iron is essential for normal immune development. Iron deficiency lowers circulating T lymphocyte numbers and can increase infection risks.15,16 Maternal iron deficiency during pregnancy has also been linked to a higher likelihood of childhood wheezing.17,18 An additional study found that lower umbilical tissue iron content was linked to childhood wheezing,19 but did not measure immune indices at birth. We previously found poorer umbilical cord blood iron status in the presence of maternal obesity or with GWG exceeding 18 kg13 and also demonstrated that either poor umbilical cord iron status or GWG exceeding 18 kg predicted the development of eosinophilia during the first year of life.20 Eosinophilia is used to predict the development of allergic asthma.21,22 Cytokine responses from umbilical cord blood mononuclear cell (MNC) cultures are also predictive of future allergic disease.2,23 Therefore, it is important for clinicians to understand the roles of maternal obesity, excess GWG, and iron biology in regulating newborn immune function. Our study objective was to measure umbilical cord plasma and MNC cytokines, as well as iron status and hepcidin levels in the same new-borns. We hypothesized that obesity at delivery and excess GWG would be related to iron and inflammatory pathways in umbilical cord blood.
Methods
Between June 2012 and July 2014, umbilical cord blood was collected from healthy term newborns with gestational age ≥37 weeks. All infants were born by scheduled cesarean delivery at a single center (Meriter Hospital, Madison, Wisconsin), either as repeat cesarean delivery or because of unfavorable fetal lie, with none because of fetal complications or anomaly. Cesarean deliveries were selected to limit the possibility of subclinical chorioamnionitis and allow for more consistency in the duration of time before sample processing, and because previous work showed that umbilical cord cytokine responses does not differ based on mode of delivery.24 Based on evidence of seasonal fluctuation in cytokine responses, 24 enrollment was limited to the summer months. Institutional Review Boards approved this study as exempt from maternal consent. Exclusion criteria included maternal HIV infection, cancer, fetal hemolytic disease, neonatal intensive care unit admission, <37 weeks gestation, major fetal anomaly (cardiac, vascular, renal, or central nervous system), or estimated fetal weight below the first percentile. Markedly abnormal inflammatory indices may reflect sample clotting or other activation during processing, thus, samples were excluded for umbilical cord white cell count or MNC levels of interferon (IFN)-γ or TNF-α either less than the first percentile (1 sample) or greater than the 99th percentile (2 samples). In a prospective fashion, demographic and anthropometric data were extracted from the electronic medical records, including maternal prepregnancy and delivery BMI, race/ethnicity, presence of maternal diabetes, other medical conditions, healthcare payment method as a surrogate of socioeconomic status, newborn sex, and whether the newborn was small, large, or appropriate size for gestational age. Smoking was not recorded, but women delivering at Meriter Hospital have a 9.5% smoking rate. Currently, known risks for developing iron deficiency as an infant were tallied: maternal obesity (BMI ≥30 kg/m2), maternal diabetes, minority status, Medicaid insurance, and fetal growth disturbance (small or large for gestational age).13,25 Subjects were selected after electronic medical record evaluation for inclusion and exclusion criteria. All subject data were de-identified.
Laboratory Procedures
After delivery, umbilical cord blood was collected in sodium heparin and processed within 18 hours. Because previous work showed subtle differences in cytokine responses in samples held 12 hours before processing, 4 time before processing was minimized, with median time 2.25 hours before processing, and only 4 samples held more than 12 hours. No maternal blood samples were collected. Complete blood counts were assayed via a Sysmex PocH-100i hematology analyzer (Sysmex, Mulelein, Illinois) to yield white blood cells (WBCs), WBC differentials, hemoglobin (Hb), hematocrit, mean cell volume, and red blood cell (RBC) distribution width (RDW). A zinc protoporphyrin to heme ratio (ZnPP:H) measured iron-deficient erythropoiesis using a hematofluorometer (Aviv Biomedical, Lakewood, New Jersey), with reticulocyte-enriched ZnPP:H improving ZnPP:H sensitivity. A blinded reviewer determined manual reticulocytes (Reticulocyte Stain, Sigma-Aldrich, St. Louis, Missouri) and nucleated RBC and eosinophil counts on Wright-Giemsa stain smears (Astral Diagnostics, West Deptford, New Jersey).
WBCs and lymphocytes counts were corrected if nucleated RBC were >5% of the WBCs. Additional plasma assays included ferritin (Genway Biotech, San Diego, California), transferrin (ICL, Portland, Oregon), unsaturated iron binding capacity and iron (Pointe Scientific, Canton, Michigan), hepcidin (DRG International, Springfield, New Jersey), CRP (Genway Biotech), IL-6 (R&D Systems, Minneapolis, Minnesota), and TNF-α (R&D Systems).
After plasma removal, the blood cellular fraction underwent processing for MNC using lymphocyte separation medium (Lonza, Walkersville, Maryland) with centrifugation at 1200 × g for 22 minutes followed by washing of the pellet by centrifugation at 1250 × g for 12 minutes. The PocH-100i quantified the MNC yield. Cells were cultured at 0.5 to 1 × 106 cells/mL in Roswell Park Memorial Institute-1640 with 10% fetal bovine serum, L-glutamine (2 mM), Pen Strep (100 U/mL), and Hepes (25 mM), because preliminary data showed that cytokine responses at this range of cell concentrations did not differ. Cell suspensions with and without phytohemagglutinin stimulant (200 μg/mL final concentration) were added to a 24-well plate incubated at 37°C for 24 hours. After 24 hours incubation, well contents were harvested and centrifuged. Supernatants were snap-frozen with liquid nitrogen and stored at −80°C, and later assayed for IFN-γ, IL-1β, IL-6, IL-8, IL-12, and TNF-α using a Human Proinflammatory 7-Plex array (Meso Scale Discovery, Rockville, Maryland). Supernatant IL-5 concentrations were assayed separately via a high-sensitivity enzyme-linked immunosorbent assay (R&D Systems). To estimate MNC viability, a thiazolyl blue tetrazolium bromide 0.9 mg/mL solution (Alfa Aesar, Ward Hill, Massachusetts) was incubated at 37° C for at least 4 hours. After incubation, dimethyl sulfide at 200% of the total volume solu-bilized the cells before spectrophotometer analysis at 570 nm, reference at 630 nm.
Statistical Analyses
Data were initially categorized into 2 groups by the nonpregnant definition of obesity; either maternal BMI <30 kg/m2 or by BMI ≥30 kg/m2 at time of delivery. To further distinguish the effects of more extreme obesity (class II obesity and above, defined as BMI ≥35 kg/m2), the women were then subdivided into two groups, either with a BMI <35 kg/m2 or by BMI ≥35 kg/m2 at time of delivery. Relative maternal GWG was also determined by the difference between prepregnancy and delivery BMIs (ie, ΔBMI). Once assays were performed, plasma ferritin data were also characterized as high, >25th percentile (>84 mg/L), or low ferritin (≤25th percentile). 27 Ratios of MNC cytokines were also calculated to assess the relative expression of the targeted cytokines. The Shapiro-Wilk normality test, which uses the null hypothesis principle to determine if the samples originate from a normally distributed population, was used to assess data distribution. If data were normally distributed or if data were normalized after natural log-conversion, a Student t test or 1-way ANOVA with Fisher post hoc test was used. If the distribution was skewed, data were analyzed by Wilcoxon rank-sum test and natural log-converted for linear regression analysis. Simple and stepwise regression analyses examined relationships between variables. BMI categories, presence of diabetes, hepcidin values, ferritin values, and grouping by high or low ferritin were modeled as independent variables.
CRP, plasma cytokines, or MNC cytokine responses were modeled as dependent variables. A P value of <.05 was considered significant.
Result
A total of 85 umbilical cord blood samples from healthy babies were studied. Table I presents demographic data, although these were not available for all subjects. Most mothers identified as Caucasian, and most carried insurance. Fetal sex nearly matched, and 78% were deemed appropriate for gestational age. At delivery, maternal BMI was less than 30 kg/m2 in 34 women, and BMI was greater than or equal to 30 kg/m2 in 51 women. Diabetes was not present in women with a BMI less than 30 kg/m2. Fifty-five percent of neonates had the lowest quartile of umbilical cord plasma ferritin for a healthy newborn population. 25,27
Table I. Maternal and fetal demographic data.
| Ethnic and racial distribution | |
| Caucasian | 73% |
| African American | 5% |
| Latina | 11% |
| Asian/Southeast Asian | 11% |
| Insurance coverage | |
| Medicaid or self-pay | 27% |
| HMO insurance | 73% |
| Sex | |
| Male | 48% |
| Female | 52% |
| Fetal growth measurements | |
| AGA | 78% |
| SGA | 11% |
| LGA | 11% |
| Maternal gestational diabetes | |
| If BMI <30 kg/m2 | 0% |
| If BMI ≥30 kg/m2 | 25% |
| If BMI ≥35 kg/m2 | 37.5% |
| Umbilical cord ferritin* | |
| Lowest quartile | 55% |
| Highest 3 quartiles | 45% |
Maternal BMI and Umbilical Cord Blood Erythropoietic and Iron Indices
The distribution of ferritin values was lower than that of a normal population, 27 with over one-half exhibiting umbilical cord ferritin in the lowest quartile and only 3.5% in the highest quartile. BMI before pregnancy did not impact the umbilical cord erythrocyte iron or other iron indices. However, Figure 1 shows significant relationships between BMI at delivery and iron indices. As maternal BMI at delivery increased, umbilical cord plasma ferritin levels decreased (R2 = −0.07; P < .04). ΔBMI, an indicator of GWG, was associated with ZnPP:H (R2 = 0.21; P < .008). A direct relationship was also found between DBMI and umbilical cord nucleated RBC (R2 = 0.15; P < .02). Nucleated RBCs, median (IQR), were higher in the lowest quartile of umbilical cord ferritin; 193/μL (47-496/μL) vs 100/μL (0-205/μL) for newborns in the normal ferritin group, (P < .05). When delivery BMI ≥35 kg/m2 was considered, the umbilical cord RDW was higher (P < .02), and plasma ferritin trended lower (P < .06) than for neonates with maternal delivery BMI <35 kg/m2 (Table II).
Figure 1.

Relationships between iron status and BMI. Umbilical cord plasma ferritin and ZnPP:H are expressed on log10 scale. A, Umbilical cord plasma ferritin levels (μg/L), a measure of storage iron, were indirectly related to maternal delivery BMI (kg/m2). B, Umbilical cord ZnPP:H (μmol/mol) rise as erythrocyte iron falls and were directly related to ΔBMI (kg/m2), a surrogate of GWG.
Table II. Umbilical cord iron and inflammatory indices by BMI <35 or ≥35 kg/m2.
| BMI <35 kg/m2, n = 47 | BMI ≥35 kg/m2, n = 16 | P value | |
|---|---|---|---|
| Iron measurements | |||
| Hb (g/dL) | 14.6 (1.4) | 14.5 (1.7) | .8 |
| HCT (%) | 44.7 (4.1) | 44.2 (5.7) | .8 |
| RDW (%) | 16.9 (1.2) | 17.8 (1.5) | <.012 |
| MCV (fL) | 107.3 (4.8) | 108.0 (5.3) | .6 |
| Nucleated RBC (cells/μL) | |||
| ZnPP* (μmol/mol) | 102 (90-125) | 101 (86-144) | .6 |
| Plasma iron* (μg/dL) | 239 (147-260) | 192 (76-213) | .11 |
| TSAT* (%) | 58.5 (40.3-79.2) | 64.3 (43.5-76.9) | .8 |
| Plasma Tf† (g/L) | 1.78 (1.58-2.24) | 2.09 (1.73-2.29) | .18 |
| Plasma ferritin* (mg/L) | 76.4 (55.3-128.6) | 64.7 (43.0-80.3) | <.06 |
| Plasma hepcidin (ng/mL) | 13.4 (5.8) | 12.5 (5.4) | .6 |
| Inflammatory measurements | |||
| WBC (cells × 103/μL) | 12.7 (3.4) | 11.9 (2.8) | .4 |
| Eosinophils† (cells/μL) | 378 (207-527) | 301 (165-563) | .4 |
| Lymphocyte percent† (%) | 38.4 (33.5-44.2) | 49.8 (38.4-54.0) | <.004 |
| Lymphocytes† (cells/μL) | 1543 (1476-1610) | 1681 (1531-1831) | <.03 |
| Plasma CRP† (mg/L) | 4.0 (2.1-5.9) | 5.6 (2.9-12.2) | .02 |
| Plasma IL-6† (pg/mL) | 1.37 (1.01-1.99) | 2.00 (1.44-2.96) | <.03 |
| Plasma TNF-α (pg/mL) | 1.56 (0.38) | 1.87 (0.35) | <.007 |
| Cytokines (pg/mL) | |||
| IFN-γ† | 241 (192-419) | 342 (225-450) | .3 |
| IL-1β* | 1268 (419-2724) | 2111 (1149-5058) | <.045 |
| IL-6* | 3330 (3044-2635) | 5110 (3174-6847) | <.015 |
| IL-8* | 3288 (3174-3653) | 4790 (3351-6691) | <.005 |
| IL-12† | 95.6 (82.4-122.6) | 104.1 (85.3-178.4) | <.042 |
| TNF-α* | 2761 (2165-3046) | 3565 (2570-5174) | <.047 |
| IL-10 | 255 (150-444) | 394 (237-529) | .14 |
| IL-5† | 8.44 (4.47-15.3) | 18.13 (3.79-32.93) | .6 |
| Cytokine ratios | |||
| TNF-α:IFN-γ† | 10.3 (6.8-13.8) | 12.2 (8.5-15.9) | .16 |
| IL-5:IFN-γ† | 0.039 (0.022-0.067) | 0.060 (0.013-0.098) | .8 |
HCT, hematocrit; MCV, mean cell volume; TSAT, transferrin saturation; Tf, transferrin.
All others = mean (SD), t test.
Significant differences are indicated by bolded data.
Median (IQR), log-converted, nonparametric test.
Median (IQR), log-converted, t test.
Maternal Delivery BMI and Umbilical Cord Plasma Inflammatory and Iron Status
No significant relationships between pregravid BMI and any umbilical cord inflammatory measurements were found. However, at delivery, maternal BMI was associated with umbilical cord plasma CRP (R2 = 0.15, P < .002), percentage of lymphocytes (R2 = 0.17; P < .0007), and total lymphocyte number (R2 = 0.12; P < .007). When categorized by delivery BMI <30 kg/m2 or ≥30 kg/m2 groups, inflammatory measurements did not differ between groups. However, when considering delivery BMI ≥35 kg/m2, then umbilical cord plasma CRP (P < .02), IL-6 (P < .03), and TNF-α (P < .007), lymphocyte percent (P < .004), and lymphocyte number (P < .03) were all higher than for those with a delivery BMI <35 kg/m2 (Table II).
Most of the iron measures were unrelated to inflammatory measures. However, umbilical cord lymphocyte number was directly related to umbilical cord plasma ferritin (R2 = 0.06, P < .03). Umbilical cord plasma IL-6 levels, median (IQR), were higher, 1.51 pg/mL (1.08-2.13 pg/mL), if umbilical cord ferritin levels were in the lowest quartile compared with 1.12 pg/mL (0.79-1.99 pg/mL), with normal umbilical cord ferritin (P < .04). When umbilical cord plasma IL-6 levels were examined in a multivariate analysis, having a delivery BMI ≥35 kg/m2 was predictive of IL-6 (R2 = 0.075, P < .035), but the relationship with ferritin approached but did not attain significance (R2 = 0.049, P < .085).
Influence of Maternal Delivery BMI and Umbilical Cord Iron Status on Stimulated Cytokine Release
Cell viability was assessed; the MNC mitochondrial activity (thiazolyl blue tetrazolium bromide) postincubation did not differ if grouped by prepregnancy BMI, delivery BMI, or ΔBMI, or by high and low ferritin.
The level of the cytokines, IL-1β, IL-6, IL-8, IL-12, and TNF-α, released by umbilical cord MNC following phytohemagglutinin stimulation, was higher (P < .05 for all) in new-borns with a delivery BMI ≥35 kg/m2 than in the BMI <35 kg/m2 group (Table II). Cytokine responses did not differ by group when the delivery BMI cut-off was set at ≥30 kg/m2 or <30 kg/m2. To determine the incremental influence of maternal adiposity on TNF-α, BMI was categorized into 3 groups; BMI <30 kg/m2, BMI ≥30 but <35 kg/m2, or ≥35 kg/m2 (Figure 2), which illustrates a threshold effect of maternal BMI with the shift in TNF-α evident as BMIs exceeded ≥35 kg/m2. Because lower umbilical cord MNC IFN-γ responses predict asthma, 23 we examined ratios of several cytokines to IFN-γ. The TNF-α:IFN-γ was directly related to delivery BMI (R2 = 0.11, P < .008). When categorized by normal or lowest quartile of plasma ferritin, TNF-α:IFN-γ did not differ, but the ratio of Th2 cytokine IL-5 to IFN-γ, 0.045 (0.014-0.90), trended higher in the low ferritin group (P < .1), 0.028 (0.18-0.051).
Figure 2.
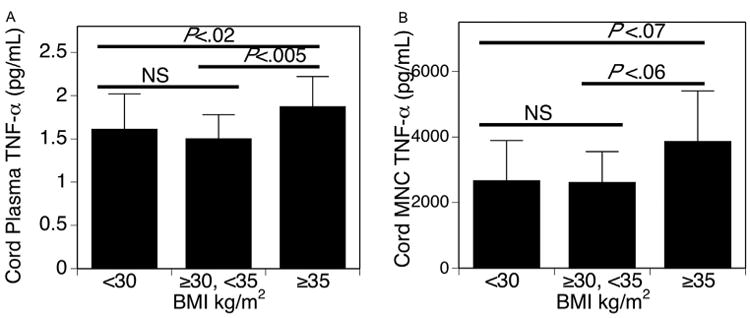
Relationship between obesity and TNF-α by grouping delivery BMI into 3 groups, BMI <30 kg/m2, n = 33; BMI ≥30 and <35 kg/m2, n = 21; and BMI ≥35 kg/m2, n= 16. Bar graph indicates mean (SD), P values listed. A, Umbilical cord plasma TNF-α (pg/mL) is higher in group with BMI ≥35 kg/m2 than other 2 groups. B, PHA-stimulated umbilical cord MNC TNF-α (pg/mL) trends higher in group with BMI ≥35 kg/m2 than other 2 groups. NS, not significant.
Maternal Delivery BMI and Umbilical Cord Plasma Hepcidin
Maternal BMI was not directly associated with umbilical cord plasma hepcidin. However, hepcidin was associated with other indicators of fetal iron status. Umbilical cord hepcidin was lower (P < .0001) in the lowest quartile of umbilical cord plasma ferritin levels and was related to plasma ferritin (R2 = 0.25; P < .0001) (Figure 3). Umbilical cord hepcidin was also inversely related to Hb (R2 = −0.05; P < .05) and to RDW (R2 = −0.13; P < .001). Analyzing multiple factors associated with umbilical cord hepcidin confirmed the relationships with umbilical cord plasma ferritin (R2 = 0.15, P < .0001) and Hb (R2 = −0.12, P < .05), but showed that maternal BMI was only indirectly linked to hepcidin via its influence on fetal iron status.
Figure 3.

Relationships between umbilical cord levels of the master iron regulator, plasma hepcidin (ng/mL), and umbilical cord plasma ferritin (mg/L). Data were categorized byhigh (normal) ferritin ($84 mg/L) vs lowest quartile of umbilical cord plasma ferritin (<84 mg/L) showing that A, umbilical cord plasma hepcidin levels were lower in the low ferritin group. B, A direct relationship between umbilical cord plasma levels of hepcidin (ng/mL) and ferritin (mg/L) was determined.
Influence of Maternal Diabetes
Maternal diabetes was present only in those with a delivery BMI >30 kg/m2, including 37.5% of the BMI ≥35 group, but only 6.7% of the BMI <35 kg/m2 (P < .008). Maternal diabetes increased umbilical cord inflammatory indicators including plasma CRP; 5.00 mg/L (2.7-14.5 mg/L) with diabetes and 4.30 mg/L (0.69-4.41 mg/L) without diabetes (P < .009). Multivariate analysis showed that experiencing both maternal diabetes and a BMI ≥35 kg/m2 was a stronger predictor of plasma CRP (P < .003) than was BMI alone (P < .015), supporting an interaction between diabetes and BMI. However in multivariate examination of the dual impact of BMI ≥35 kg/m2 and diabetes on cytokine responses, only BMI, and not diabetes, predicted umbilical cord plasma levels of IL-6 (P = .051) or TNF-α (P < .017) and MNC levels of IL-6 (P < .025) or TNF-α (P < .045).
Discussion
Expert panels have not yet defined the threshold at which pregnancy BMI or GWG negatively impacts the fetus. The standard definition of obesity in nongravid adults, BMI ≥30 kg/m2, does not account for pregnancy-related increases in body mass. However, over one-half of women in the US begin pregnancy overweight or obese.28 Since the Institute of Medicine established GWG guidelines in 2009, nearly one-half of women still gain more than is recommended for their prepregnancy weight.29 Overweight and obese women are the most likely to exceed these recommendations.30 We previously found that obesity adversely impacted fetal iron status 13 and that fetal iron or GWG predicted altered infant inflammatory pathways.20
Our study shows that an array of fetal inflammatory responses can be predicted by maternal BMI exceeding a threshold of 35 kg/m2 at the time of cesarean delivery.
The standard definition of obesity either prepregnancy or at delivery (BMI ≥30 kg/m2) did not predict fetal inflammation, but class II obesity or greater at delivery (maternal BMI ≥35 kg/m2) impacted iron and increased inflammatory markers, including both plasma and MNC TNF-α. The relationship between delivery BMI and inflammatory markers was not linear but occurred when the threshold of 35 kg/m2 was reached. However, the relationship between obesity and umbilical cord iron was linear, with an incremental decrease in iron stores as BMI rose. Increased GWG also appeared to stimulate erythropoiesis, but there was evidence that iron bioavailability did not keep pace with exaggerated erythropoiesis. Umbilical cord plasma levels of hepcidin, the iron-regulatory peptide that is normally an intermediary between iron and inflammation, solely reflected fetal iron status, but not maternal BMI or plasma indices of newborn inflammation (IL-6, TNF-α, or CRP). This finding supports an appropriate fetal adaptation in an attempt to increase iron availability and not upregulation of fetal hepcidin because of inflammatory processes.
We compared umbilical cord plasma and umbilical cord MNC inflammatory indices based on varying definitions of maternal obesity. Fetal inflammatory measurements did not differ at BMI ≥30 kg/m2 either prepregnancy or at delivery but were higher when categorizing by class II obesity, delivery BMI ≥35 kg/m2. These data are supported by previous published data showing that umbilical cord plasma CRP levels 13 and plasma cytokines 8,9 were unchanged with delivery at BMI ≥30 kg/m2. However, our data contrast with a study reporting higher umbilical cord plasma IL-6 with prepregnancy BMI ≥30 kg/m2,7 and a study reporting higher levels of inflammatory mediators in both maternal and umbilical cord plasma with presence of obesity mid-pregnancy.31 The other published studies 7-9,31 did not report delivery BMI, so our previous and current work may not be in disagreement with these other works, if excessive GWG contributed to a BMI at delivery higher than 35 kg/m2. The current study included a limited number of measured cytokines, but other cytokines responses, including those in the transforming growth factor family, may be altered by maternal obesity during pregnancy and should be studied.
Expression of cytokines, IL-6 and TNF-α can be upregulated in iron deficiency.32 Obesity in nongravid adults is characterized by chronic inflammation that is further propagated by altered adipocyte function, including increased expression of pro-inflammatory TNF-α and IL-6.33,34 We found higher fetal plasma IL-6 in those with poorer fetal iron status, but the relationship was primarily driven by BMI once it exceeded 35 kg/m2. Because diabetes was common in our higher BMI cohort, our data also support work describing that maternal diabetes upregulated umbilical cord inflammatory markers and plasma TNF-α.35,36 Our data in the higher BMI group are consistent with findings that greater maternal GWG predicted higher umbilical cord MNC production of TNF-α.2 This agreement is important because if higher umbilical cord MNC TNF-α persisted into infancy, it predicted asthma.2
Iron is essential for proper function and maintenance of the immune system, affecting both innate and adaptive cell function.37 This mechanistic study was undertaken because we previously found that fetal iron endowment was the link between excessive maternal GWG 13 and eosinophilia at 6-12 months of age.20 Umbilical cord MNC and plasma TNF-α and IL-6 were investigated in the current study because previous work found that GWG was linked to high umbilical cord levels of MNC TNF-α,2 and that gestational iron deficiency upregulated placental expression of IL-6 and TNF-α.38 Published work in children also showed that iron deficiency stimulated T-cell production of IL-6 and TNF-α.18 However, in the current study, no relationship between umbilical cord plasma TNF-α and iron was found, although iron status independently predicted IL-6 until BMI exceeded 35 kg/m2. It is possible that our sample size limited our findings or that diabetes, 39,40 rather than obesity, controlled the interaction between iron and inflammation.
However, when this question was tested specifically in multivariate analysis, BMI, and not diabetes, predicted umbilical cord plasma and MNC levels of IL-6 or TNF-α.
Previously, greater levels of plasma or MNC IL-6 and TNF-α were found in plasma of macrosomic offspring of mothers with diabetes, 36 a population known to be at great risk for tissue iron depletion.39,41 Despite the current study and others reporting low fetal iron status after diabetic gestation, maternal iron status in diabetic pregnancy has been reported as high, not low, consistent with tissue sequestering and diabetic placental dysfunction that results in inadequate transfer of iron to the fetus.42 Because excessive body iron may accompany diabetes in nonpregnant adults, 43 further investigation of both maternal and fetal iron status, as well as the regulation of iron transfer to the fetus during diabetic pregnancy is warranted.
Balance between T-lymphocyte cytokine responses may be important, particularly with excessive T-helper (Th) responses in allergy and asthma.44 We studied the Th2 cytokine IL-545 and Th1 cytokine IFN-γ to determine the MNC Th2:Th1, and saw a trend for a greater ratio in the low ferritin group. However, imbalanced Th2:Th1 cytokine responses may not be seen in umbilical cord MNC of children ultimately developing asthma, and relatively higher umbilical cord MNC levels of either IL-13, a Th2 cytokine, or of IFN-γ, a Th1 cytokine,23 may protect against wheezing in childhood. With recent advent of more sensitive cytokine assays, further work is needed to understand MNC cytokine balance.
Iron status at birth is critical because one-half of the iron needed for infant growth is obtained before birth and impaired iron status may persist into early childhood.14 Maternal obesity is an emerging risk factor for newborn iron deficiency.8,13 The current study reaffirms our prior work showing a direct relationship between maternal obesity and fetal iron status in newborns with risk factors for developing iron deficiency in infancy.13 Fetal iron status in this cohort of elective cesarean deliveries may be less impacted at BMI ≥30 kg/m2 than those with risk factors for developing iron deficiency,13 but iron status was clearly impacted at BMI ≥35 kg/m2. All 3 compartments of iron in fetal blood (storage, erythrocyte, and transport) appeared to adapt to the block in iron delivery, as evidenced by the relationship between iron transporter levels and worsening erythrocyte iron indices. It is unlikely that excess iron contributed to inflammation, as one-half of the fetuses exhibited values in the lowest quartile for umbilical cord ferritin, but none were above the 90th percentile.27 Our cohort consisted entirely of elective scheduled cesarean deliveries and contained a large percentage of women with obesity. Our observations may be important to pediatricians because iron status in infancy can be made poorer by cesarean delivery because of a lack of normal umbilical cord transfusion of iron-rich erythrocytes.46
The current study analyzed inflammatory indices and iron indices with hepcidin levels in umbilical cord blood of neonates born to mothers with obesity. The peptide hepcidin is the master regulator of iron homeostasis, with higher levels inhibiting the iron export channel in enterocytes, trophoblasts or hepatic macrophages.47,48 Elevated hepcidin is both an adaptive response to infection and a propagator of inflammation in older adults.8,48,49 In normal pregnancy, maternal hepcidin falls in response to lower fetal hepcidin in order to maximize maternal-fetal iron transfer.8 However, proinflammatory cytokines in inflammation or obesity upregulate hepcidin,47 an acute phase reactant.49 A small study found that maternal, but not fetal, hepcidin levels were higher with maternal obesity 8 and that maternal hepcidin was unrelated to fetal hepcidin.8 The current study similarly found that the fetal hepcidin-iron axis was unrelated to maternal BMI, but also found that fetal hepcidin was related to iron stores, appropriately falling in response to depleted fetal tissue iron, but not affected by the proinflammatory fetal milieu. These results pinpoint maternal iron delivery in class II obesity as the problem and not impaired fetal signaling of iron needs to the placenta. This is an important distinction because the fetus may be amenable to interventions to improve fetal or neonatal iron status.
Study limitations include the relatively small sample size and the lack of prepregnancy and delivery BMI data for some samples. However, an advantage was the highly sensitive new generation multiplex cytokine analysis, including very few cytokines reading below detectable limits. Current findings may be limited to children delivered without labor, although one study reported that umbilical cord MNC release of IFN-γ, IL-5, IL-13, and IL-10 cytokine responses did not differ between those with vaginal and cesarean deliv-ery.24 Because epidemiologic analyses have suggested cesarean delivery may be associated with greater risk of allergy and asthma, 50 additional data about cytokine activity in umbilical cord blood may set forth putative immune pathways for future investigation.
In summary, obesity decreased fetal iron stores in a linear fashion. If maternal BMI reached 35 kg/m2 by the end of pregnancy, cytokine responses were driven predominantly by BMI and not by iron. At delivery, once the threshold of BMI at 35 kg/m2 was reached, either as a consequence of excessive GWG and/or higher baseline BMI, an inflammatory fetal milieu was observed. Class II obesity at delivery predicted fetal plasma CRP, IL-6, and TNF-α, as well as several MNC cytokines including TNF-α, findings that were not appreciated below this BMI threshold. We speculate that this cascade established a pattern that may predispose a child to an allergic or inflammatory phenotype later in life. Mechanisms behind the epigenetic programming of allergic airway disease are not well established. These altered inflammatory responses may inform potential pathways programmed during fetal life that lead to allergic airway disease. Although the predictions described above do not confirm causality, maternal BMI at delivery might play a role in altering childhood inflammatory patterns and chronic health risks for children.
Acknowledgments
N.D. was supported by the University of Wisconsin Hilldale Undergraduate Research Fellowship. E.G., M.W., and S.M. received fellowship support from the University of Wisconsin Medical Student Shapiro Summer Research and Cardiovascular Research Center Research Fellowships. A.A., C.C., and P.K. are supported by Wisconsin Partnership Collaborative Health Sciences. C.C. is supported by the National Institutes of Health (NIH; HD057064, HD039386, HD080201). P.K. supported by NIH (1 ULRR026011) University of Wisconsin-Madison Clinical and Translational Science Award, Meriter Foundation, and Gerber Foundation.
We thank the participating families, Meriter Hospital Birthing Center Staff, Sharon E. Blohowiak, MS, and the Kling Lab, Anthony P. Auger, PhD, Robert F. Lemanske, Jr, MD (University of Wisconsin, Madison), Daphne Q-D Pham, PhD (University of Wisconsin, Parkside), Marilyn Halonen, PhD, and Anne L. Wright, PhD (University of Arizona).
Glossary
- BMI
Body mass index
- CRP
C-reactive protein
- GWG
Gestational weight gain
- Hb
Hemoglobin
- IFN
Interferon
- IL
Interleukin
- MNC
Mononuclear cell
- RBC
Red blood cell
- RDW
RBC distribution width
- Th
T-helper
- TNF
Tumor necrosis factor
- WBC
White blood cell
- ZnPP:H
Zinc protoporphyrin to heme
Footnotes
The authors declare no conflicts of interest.
References
- 1.Zugna D, Galassi C, Annesi-Maesano I, Baiz N, Barros H, Basterrechea M, et al. Maternal complications in pregnancy and wheezing in early childhood: a pooled analysis of 14 birth cohorts. Int J Epidemiol. 2015;44:199–208. doi: 10.1093/ije/dyu260. [DOI] [PubMed] [Google Scholar]
- 2.Halonen M, Lohman IC, Stern DA, Ellis WL, Rothers J, Wright AL. Perinatal tumor necrosis factor-alpha production, influenced by maternal pregnancy weight gain, predicts childhood asthma. Am J Respir Crit Care Med. 2013;188:35–41. doi: 10.1164/rccm.201207-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaar JL, Crume T, Brinton JT, Bischoff KJ, McDuffie R, Dabelea D. Maternal obesity, gestational weight gain, and offspring adiposity: the exploring perinatal outcomes among children study. J Pediatr. 2014;165:509–15. doi: 10.1016/j.jpeds.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30:441–6. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- 5.Saito I, Yonemasu K, Inami F. Association of body mass index, body fat, and weight gain with inflammation markers among rural residents in Japan. Circ J. 2003;67:323–9. doi: 10.1253/circj.67.323. [DOI] [PubMed] [Google Scholar]
- 6.Fogarty AW, Glancy C, Jones S, Lewis SA, McKeever TM, Britton JR. A prospective study of weight change and systemic inflammation over 9 years. Am J Clin Nutr. 2008;87:30–5. doi: 10.1093/ajcn/87.1.30. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RM, Marshall NE, Jeske DR, Purnell JQ, Thornburg K, Messaoudi I. Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatr Allergy Immunol. 2015;26:344–51. doi: 10.1111/pai.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link? J Perinatol. 2013;33:177–81. doi: 10.1038/jp.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aye IL, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90:129. doi: 10.1095/biolreprod.113.116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102:1906–10. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambling L, Czopek A, Andersen HS, Holtrop G, Srai SK, Krejpcio Z, et al. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1063–70. doi: 10.1152/ajpregu.90793.2008. [DOI] [PubMed] [Google Scholar]
- 12.Cizmeci MN, Kara S, Kanburoglu MK, Simavli S, Duvan CI, Tatli MM. Detection of cord blood hepcidin levels as a biomarker for early-onset neonatal sepsis. Med Hypotheses. 2014;82:310–2. doi: 10.1016/j.mehy.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, et al. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34:513–8. doi: 10.1038/jp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozoff B, Kaciroti N, Walter T. Iron deficiency in infancy: applying a physiologic framework for prediction. Am J Clin Nutr. 2006;84:1412–21. doi: 10.1093/ajcn/84.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jason J, Archibald LK, Nwanyanwu OC, Bell M, Jensen RJ, Gunter E, et al. The effects of iron deficiency on lymphocyte cytokine production and activation: preservation of hepatic iron but not at all cost. Clin Exp Immunol. 2001;126:466–73. doi: 10.1046/j.1365-2249.2001.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61:520–4. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- 17.Triche EW, Lundsberg LS, Wickner PG, Belanger K, Leaderer BP, Bracken MB. Association of maternal anemia with increased wheeze and asthma in children. Ann Allergy Asthma Immunol. 2011;106:131–9.e1. doi: 10.1016/j.anai.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nwaru BI, Hayes H, Gambling L, Craig LC, Allan K, Prabhu N, et al. An exploratory study of the associations between maternal iron status in pregnancy and childhood wheeze and atopy. Br J Nutr. 2014;112:2018–27. doi: 10.1017/S0007114514003122. [DOI] [PubMed] [Google Scholar]
- 19.Shaheen SO, Newson RB, Henderson AJ, Emmett PM, Sherriff A, Cooke M. Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J. 2004;24:292–7. doi: 10.1183/09031936.04.00117803. [DOI] [PubMed] [Google Scholar]
- 20.Weigert R, Dosch NC, Bacsik-Campbell ME, Guilbert TW, Coe CL, Kling PJ. Maternal pregnancy weight gain and cord blood iron are associated with eosinophilia in infancy. J Perinatol. 2015;35:621–6. doi: 10.1038/jp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 22.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–8. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan Dillie KT, Tisler CJ, Dasilva DF, Pappas TE, Roberg KA, Carlson-Dakes KT, et al. The influence of processing factors and non-atopy-related maternal and neonate characteristics on yield and cytokine responses of cord blood mononuclear cells. Clin Exp Allergy. 2008;38:298–304. doi: 10.1111/j.1365-2222.2007.02891.x. [DOI] [PubMed] [Google Scholar]
- 25.McLimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer BA, et al. Impact of multiple prenatal risk factors on newborn iron status at delivery. J Pediatr Hematol Oncol. 2013;35:473–7. doi: 10.1097/MPH.0b013e3182707f2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blohowiak SE, Chen ME, Repyak KS, Baumann-Blackmore NL, Carlton DP, Georgieff MK, et al. Reticulocyte enrichment of zinc proto-porphyrin/heme discriminates impaired iron supply during early development. Pediatr Res. 2008;64:63–7. doi: 10.1203/PDR.0b013e31817328e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff M. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2011. National Center for Health Statistics. Vital Health Stat 10. 2012;254:1–88. [PubMed] [Google Scholar]
- 29.Committee to Reexamine IOM Pregnancy Weight Guide lines. Institute of Medicine, National Research Council. Washington, DC: National Academies Press; 2009. Weight gain during pregnancy: Reexamining the guidelines. [Google Scholar]
- 30.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773–81. doi: 10.1097/AOG.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine. 2009;47:61–4. doi: 10.1016/j.cyto.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–18. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 35.Mestan K, Ouyang F, Matoba N, Pearson C, Ortiz K, Wang X. Maternal obesity, diabetes mellitus and cord blood biomarkers in large-for-gestational age infants. J Pediatr Biochem. 2010;1:217–24. doi: 10.1055/s-0036-1586378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91:4137–43. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 37.Weiss G. Modification of iron regulation by the inflammatory response. Best Pract Res Clin Haematol. 2005;18:183–201. doi: 10.1016/j.beha.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Toblli JE, Cao G, Oliveri L, Angerosa M. Effects of iron deficiency anemia and its treatment with iron polymaltose complex in pregnant rats, their fetuses and placentas: oxidative stress markers and pregnancy outcome. Placenta. 2012;33:81–7. doi: 10.1016/j.placenta.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Lott DG, Zimmerman MB, Labbé RF, Kling PJ, Widness JA. Erythrocyte zinc protoporphyrin ratios are elevated with prematurity and with fetal hypoxia. Pediatrics. 2005;116:414–22. doi: 10.1542/peds.2004-1601. [DOI] [PubMed] [Google Scholar]
- 40.Lesser KB, Schoel SB, Kling PJ. Elevated zinc protoporphyrin/heme ratios in umbilical cord blood after diabetic pregnancy. J Perinatol. 2006;26:671–6. doi: 10.1038/sj.jp.7211600. [DOI] [PubMed] [Google Scholar]
- 41.Chockalingam UM, Murphy E, Ophoven JC, Georgieff MK. The influence of gestational age, size for dates and prenatal steroids on cord transferrin levels in newborn infants. J Pediatr Gastroenterol Nutr. 1987;6:276–80. doi: 10.1097/00005176-198703000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Soubasi V, Petridou S, Sarafidis K, Tsantali C, Diamanti E, Buonocore G, et al. Association of increased maternal ferritin levels with gestational diabetes and intra-uterine growth retardation. Diabetes Metab. 2010;36:58–63. doi: 10.1016/j.diabet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Bao W, Rong Y, Rong S, Liu L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 2012;10:119. doi: 10.1186/1741-7015-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez FD, Stern D, Wright AL, Taussig LM, Halonen M. Differential immune responses to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J Allergy Clin Immunol. 1998;102:915–20. doi: 10.1016/s0091-6749(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 45.Colavita AM, Reinach AJ, Peters SP. Contributing factors to the patho-biology of asthma. The Th1/Th2 paradigm Clin Chest Med. 2000;21:263–77.viii. doi: 10.1016/s0272-5231(05)70265-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhou YB, Li HT, Zhu LP, Liu JM. Impact of cesarean section on placental transfusion and iron-related hematological indices in term neonates: a systematic review and meta-analysis. Placenta. 2014;35:1–8. doi: 10.1016/j.placenta.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Singh R, Singh AK, Leehey DJ. A novel mechanism for angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am J Physiol Renal Physiol. 2005;288:F1183–90. doi: 10.1152/ajprenal.00159.2003. [DOI] [PubMed] [Google Scholar]
- 48.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients. 2014;6:3062–83. doi: 10.3390/nu6083062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanad M, Osman M, Gharib A. Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: a case control study. Ital J Pediatr. 2011;37:34. doi: 10.1186/1824-7288-37-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sevelsted A, Stokholm J, Bonnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135:e92–8. doi: 10.1542/peds.2014-0596. [DOI] [PubMed] [Google Scholar]


