Abstract
Dual injection is recommended for nearly all chronic total occlusion (CTO) percutaneous coronary intervention (PCI) to determine the optimal crossing strategy and guide wire advancement into the distal true lumen. Strategies that provide enhanced guide catheter support (such as long sheaths, large-bore guiding catheters, use of guide catheter extensions, and anchor techniques) are important for maximising the success rate and efficiency of CTO PCI. Use of a microcatheter or over-the-wire balloon is strongly recommended in CTO PCI for enhancing the penetrating power of the guidewire, enabling change in tip shape and allowing guidewire change (stiff CTO guidewires are not optimal for crossing non-occluded coronary segments). Adherence to a procedural strategy that standardises CTO technique and facilitates procedural success is recommended. Such a strategy would permit stepwise decision-making for antegrade and retrograde methods; inform guidewire selection; and incorporate alternative approaches for instances of initial failure. Given the paucity of long-term outcomes with use of novel crossing techniques (antegrade dissection/re-entry and retrograde), antegrade wire escalation is the preferred CTO crossing technique, if technically feasible. Using measures to minimise radiation exposure (including but not limited to use of 7.5 frames per second fluoroscopy and use of low magnification) and contrast administration is recommended. CTO PCI is best performed at centres with dedicated CTO PCI experience and expertise. Use of crossing difficulty prediction tools, such as the J-CTO score, can facilitate the selection of cases with a high likelihood of quick crossing that can be attempted at less experienced centres.
Keywords: Percutaneous coronary intervention, chronic total occlusions, complications, outcomes
Section A
Techniques for Chronic Total Occlusions Revascularisation
Access Route, Guiding Catheter Selection and Contralateral Injection
The femoral approach is the preferred access route by most operators. However, the radial approach might be chosen because of severe peripheral vascular disease, operator’s preference or for contralateral injection. The guiding principle of access selection is that operators should use access routes that support their typical and optimal technique.[1] The relative merits of potentially larger sheath/guide sizes (femoral) can be weighed against the reduction in vascular complications and improved patient comfort (radial).[2,3] Both are acceptable. When femoral access is used, long (45 cm) sheaths can overcome iliac tortuosity and increase guide catheter support.
Guiding catheter size is usually limited to six French (occasionally seven French) from the radial approach, compared with standard eight or seven French sheaths and guides used in transfemoral CTO PCI. Good passive support with coaxial alignment is crucial, especially in complex CTO procedures. Although the choice of the guiding catheter shape is generally dictated by personal experience, it is important to be willing to search for a guide with optimal back-up support rather than accepting one with merely satisfactory support. The radial operator should be familiar with active guide manipulation to augment the support, and all operators should be versed in balloon anchoring and mother-and-child techniques to improve support when needed.[4]
When the distal vessel is filled by retrograde collaterals, the ipsilateral collaterals can have their flow impaired after wire and catheter advancement, resulting in a collateral or preferential collateral shift to the retrograde collaterals during the procedure. Therefore, in order to achieve the best diagnostic angiography (i.e. to fill the entire collateral bed), contralateral injection should be performed at the start of the procedure, if any visible contralateral collaterals are present. Operators from the EuroCTO Club have used contralateral injection in 62 % of cases in their personal series,[5] whereas dual injection was used in 78 % of cases in a more recent North American series.[6]
Dual injection is best performed using low-magnification, so that the entire coronary tree is visualised without panning. Careful study of the collaterals not only provides important information in choosing the most appropriate collateral, but will also alert the operator to the risk for ischaemia and haemodynamic or electrical instability if the wired collateral becomes occluded. Careful and detailed review of the angiogram is critical for creating a primary and alternative CTO treatment strategies to optimise the efficacy, efficiency and safety of the procedure.[7]
Anticoagulation during CTO PCI is best achieved with unfractionated heparin because it can be reversed with protamine in case of perforation and also allows titration of the anticoagulant effect (an activated clotting time of >350 seconds is recommended by many operators during retrograde CTO PCI to minimise the risk of donor vessel and guide thrombosis).[8] As with all PCI, preloading with a P2Y12 ADP receptor inhibitor is important to reduce the risk of acute stent thrombosis and peri-procedural myocardial infarction.
Prediction of CTO Crossing Difficulty
Predicting the difficulty of CTO crossing with a guidewire is important for case selection and procedural planning. The Multicentre CTO Registry of Japan (J-CTO) score is determined by assigning one point to each of five variables (previously failed lesion, blunt type of entry, calcification, bending, and occlusion length). Patients are classified into four difficulty groups: easy (J-CTO score of 0), intermediate (score of one), difficult (score of two), and very difficult (score of ≥3). The J-CTO score correlated well with the probability of successful guidewire crossing within 30 min (87.7 %, 67.1 %, 42.4 %, and 10.0 %, respectively)[9] and was recently validated in an independent single-centre Canadian cohort.[10] In a large retrograde CTO PCI series the only predictors of procedural failure were corkscrew tortuosity of the collateral channel and nonvisibility of the collateral connection with the recipient vessel.[11]
Antegrade Approach
Antegrade recanalisation of CTOs has been and remains the most common approach worldwide.[12–14]
Single-wire Techniques (Modern Step-up/step-down)
Very soft tapered polymeric wires are currently the initial wire choice in most CTO procedures. In approximately 40 % of cases, these wires will cross the occlusion by taking advantage of small, invisible channels or by tracking relatively soft tissue.[5] The current trend in antegrade recanalisation is to rapidly step-up to stiff, spring-coil, tapered wires to overcome any hard, calcified or fibrotic segments of the occlusion and then quickly change to soft polymer/hydrophilic wires to continue tracking along the occluded segment and complete the crossing of the CTO.[7,15] Balloons or catheters should never be advanced over a wire unless it is certain that the wire is in the structure of the vessel, as wire perforations at the body of CTO are usually benign but can be catastrophic if larger equipment is advanced outside the vessel. Moreover, the CTO crossing wires should be exchanged once they access the distal true lumen for workhorse safer wires, as distal perforation of small vessels can cause delayed tamponade.[16]
Parallel Wire Technique
This technique is applied when the first wire enters the false lumen. The first wire is left in place, and a second wire is passed parallel to the first wire aiming for the distal true lumen. Following this approach, the first wire keeps the dissection channel closed and serves as a marker for advancing and redirecting a second wire, which is selected for greater stiffness and control to overcome lesion resistance.[17]
Occasionally, three or more wires are used. Use of the parallel wire technique can reduce the need for multiple antegrade contrast injections, given that the first wire serves as a marker. Improved guidewires and modern re-entry techniques have reduced the need for parallel wiring in many programs.
Techniques with Subintimal Tracking
The original Subintimal tracking and re-entry (STAR) technique introduced by Colombo et al.[18] involves fashioning an “umbrella-handle-shaped” bend at the tip of a hydrophilic wire once the wire is within the dissection flap. Force is then applied to this tip and evenly distributed over a large surface area and along the length of the umbrella-handle in order to break through the sub-endothelial layer, thereby creating a communication between the false lumen and the true lumen. In theory, the looped “knuckle wire” follows the subintimal path to a point where the dissection can no longer be propagated, thereby achieving re-entry. The limitations of this method include unpredictability of the re-entry location and inability to prospectively control the major side branches proximal to the re-entry site, which can result in poor outflow and high re-occlusion rates, often >50 %.[19,20]
In order to simplify the original STAR technique and to make it more widely applicable, Carlino et al. introduced the contrast-guided STAR technique,[21] which consists of gently injecting contrast into the subintimal space via an over-the-wire balloon or microcatheter or by employing the “microchannel technique”[22] where contrast is injected with the aim of enlarging and connecting the microchannels that already exist within the occluded vessels. Galassi et al.[23] further refined the STAR technique by proposing the “Mini-STAR” variant that uses very soft polymeric guidewires, the Fielder family of wires, Asahi Intecc (Nagoya, Japan). By forcing this type of wire with support from a microcatheter, a J-tip shape is automatically created within the occlusion, allowing for “mini subintimal tracking” and the creation of significantly smaller subintimal spaces. Finally, the limited antegrade subintimal tracking (LAST) technique, introduced by Dr Thompson and Dr Lombardi, is a similar technique designed for refractory anatomies. It uses a stiff, polymer-jacketed wire or a stiff, non-jacketed, penetration wire to redirect to the distal true lumen after facilitating device advancement with the knuckle wire technique.[24] Wire-based subintimal tracking techniques, irrespective of the method chosen, are best applied only as a bail-out to refractory antegrade and retrograde procedures in well-selected patients.
Device-Assisted Antegrade Dissection/Re-entry
The primary mode of failure when recanalising a chronically occluded vessel is entrapment of the guidewire and support equipment within the subintimal/subadventitial space and the inability to return into the distal true lumen. Recent technologies, “CrossBoss™” catheter, Stingray™ balloon and Stingray™ re-entry guidewire, Boston Scientific, (Natik, MA) have addressed this limitation and have provided a reproducible and systematic method for successfully gaining re-entry into the coronary lumen. The CrossBoss catheter is a metal-braided, over-the-wire, support catheter with a 1-mm rounded distal tip, which can be used to support standard guidewire manipulation or can be advanced using rapid rotation, with or without the wire lead. Without the wire lead, this catheter can cross into the distal true lumen of approximately 40 % of lesions.[25] Alternatively it can enter into a side branch (which is important to recognise to avoid perforation) or cross within the subintimal space. This device’s relative safety and minimal perforation risk and the change in technique to a wire-skill independent model make it attractive for CTO crossing.[25]
If the CrossBoss catheter reaches a subintimal position, or if a standard wire strategy leads to subintimal wire entrapment, coronary re-entry can be systematically achieved with the Stingray coronary re-entry technologies (Boston Scientific). The Stingray balloon is a 1 mm thick, over-the-wire, balloon catheter with three exit ports (one distal and two 180°, diametrically opposed, side ports). When the balloon is inflated, it effectively wraps the artery with an exit port that is always directed towards the adventitia and an exit port that is always directed towards the lumen. Using fluoroscopy, operators can select the lumen port with the dedicated Stingray re-entry wire and achieve distal lumen control. Occasionally, subintimal wire entry can cause subintimal haematoma that can compress the distal true lumen, requiring aspiration through an over-the-wire balloon or microcatheter for decompression to enable distal true lumen re-entry (subintimal transcatheter withdrawal [STRAW] technique). For operators with access to these technologies and techniques, the need for the aforementioned parallel wire or wire-based re-entry methods is low. These technologies have been highly successful and have had low complication rates, even in early experiences and in refractory cases.[25,26]
The Retrograde Approach
In 2005, Katoh et al. pioneered the modern era of retrograde CTO recanalisation,[27] by introducing the following new techniques – targeted septal or epicardial collateral crossing, retrograde lesion crossing and management of the subintimal space through the use of balloon dilation for connecting antegrade and retrograde channels.[8] Currently, retrograde procedures account for 15–34 % of all CTO PCI procedures in European and USA registries.[5,28–30] These methods require access to the distal CTO vessel from a collateral (or occasionally bypass graft) vessel with successful placement of a support catheter.[31]
Retrograde Wire Crossing
Retrograde wire crossing indicates lesion crossing in the distal to proximal cap direction with successful true lumen access to the proximal vessel.[8] The standard approach after successful retrograde wire manipulation includes placing the wire and then the microcatheter into the antegrade guide catheter and then exchanging for a long wire to be externalised from the antegrade guide.[32] The externalised wire, such as the ViperWire™ Advance (CSI, St Paul Minnesota), R350, Vascular Solutions (Minneapolis, Minnesota), RG3 (Asahi Intecc) is then used as the interventional platform to complete the PCI procedure.[33] It is important for the retrograde guidewire to remain covered by a microcatheter to protect the collateral vessel from injury and to pay careful attention to guide movement to prevent guide-induced donor vessel injury.
Kissing Wire Technique
This technique combines the antegrade and retrograde approaches,[34] although CTO penetration is achieved from the antegrade route. If the CTO lesion is relatively soft, the retrograde wire can be advanced easily, with the operator stopping the wire half way through the lesion. If the tip nears the CTO proximal cap, the operator can aim the tip towards the antegrade guide wire. Eventually, the antegrade and retrograde guide wires meet or “kiss”. This technique is generally used to reduce the use of contrast and to eliminate any potential ambiguity regarding the course of the vessel, thus making advancement of the antegrade wire safer. After crossing with the antegrade wire, the balloon catheter is advanced into the occlusion and dilatation is performed. The kissing wire technique is rarely performed by experienced retrograde operators, as the reverse controlled antegrade and retrograde tracking and dissection [CART], as described below) technique provides a more consistent method for connecting the channels in refractory cases.
Knuckle Technique[35]
In this technique a dissection of the subintimal space is created by forming a loop in the retrograde wire, which is then advanced into the occluded segment. For this technique, soft hydrophilic wires are preferred, especially if there is good support from the retrograde microcatheter, for example the Corsair channel dilator (Asahi Intecc). However, an antegrade stiff wire is generally required in order to pass through the dissected lumen created by the knuckle wire. After crossing with the antegrade wire, PCI is performed as per usual fashion.[36]
CART Technique[37]
The principle of this technique is to create a subintimal dissection with limited extension only at the CTO site, thereby facilitating antegrade wire crossing. In practice, this involves exchanging the microcatheter for a balloon catheter after septal collateral dilation with a Corsair catheter or a small balloon (however, epicardial collaterals should never be dilated). This balloon catheter is advanced retrograde into the lesion and overlapped with the antegrade equipment. Dilation with an appropriately sized balloon will typically create a connection between the antegrade and retrograde spaces, which can be subsequently wired. The main disadvantages of this method include the need to pass the wire into an often small and diffusely diseased distal lumen and the inability to effectively use intravascular ultrasound to optimise the strategy.
Reverse CART Technique
The principle behind this technique is the same as that of the CART technique, except that the connected space is created with antegrade balloon dilation (after overlapping with the retrograde catheter), thereby facilitating the crossing of the occlusion with the retrograde wire. Currently, this is the dominant technique in the retrograde CTO approach.[29] The optimal wire position for reverse CART is when both the antegrade and retrograde guidewire are located within the subintimal space. The most common reason for failure of this technique is use of undersized balloons, that can be prevented by using intravascular ultrasound for optimal balloon sizing and positioning.[38]
The most common current methods for successfully completing a retrograde CTO PCI procedure are the “true” wire crossing with externalisation and the reverse CART with wire externalisation. The kissing wire technique, the knuckle technique (without adjunctive reverse CART) and the classic CART technique are included for review completeness but are rarely performed at present.[29]
Hybrid Strategy for CTO PCI
Contemporary antegrade, retrograde and dissection and re-entry techniques are complementary and necessary for the full spectrum of CTO PCI. Exploring sequential CTO crossing options can increase success, shorten procedural time and reduce radiation exposure. The CTO expert operator needs broad skillsets, versatility and flexibility to accommodate the wide range of anatomic scenarios for chronic occlusion that will be present in patients with strong indications for revascularisation.[39]
While significant variability exists with many operators with respect to procedural approaches, the “hybrid method” for CTO PCI represents an effort to standardise initial and provisional technique selection based on patient anatomy (see Figure 1).[7] The implementation of the hybrid method requires skillset development in optimal wire manipulation, dissection/re-entry strategies and retrograde techniques. The development and adoption of only one or two of these skillsets will ultimately limit the experienced operator who wishes to approach all patients with appropriate indications for revascularisation. Failure to develop all three skillsets will likely lead to the dilemma of underutilisation of revascularisation in subgroups of patients who may derive the greatest benefit from these techniques.
Figure 1: Hybrid Algorithm for Chronic Total Occlusion Percutaneous Coronary Intervention.
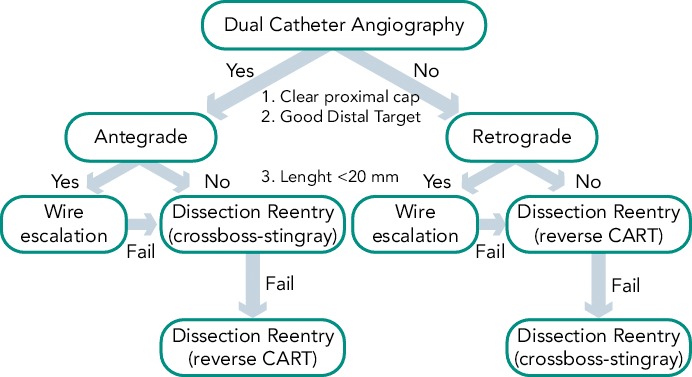
CART = controlled antegrade and retrograde tracking and dissection
Development of a CTO PCI Program
Successfully developing a CTO PCI program requires several steps; developing consensus among cardiologists (interventional and non-invasive), cardiac surgeons and hospital administrators regarding the rationale for CTO revascularisation; ensuring appropriate patient selection with individualised consideration of the risk/benefit; obtaining CTO-specific didactic and hands-on training by experienced CTO operators; and establishing a quality assurance program with accountability of procedural results and acute and long-term clinical outcomes.[40] The importance of the “heart team” that enables collaborative work between cardiac surgeons and cardiologists in making coronary revascularisation decisions for patients with complex coronary artery disease including CTOs, cannot be overemphasised.[41]
Section B
Treatment of Lesions Resistant to Dilation
Although less common than failure to cross a CTO lesion with a guidewire, occasionally a coronary lesion cannot be crossed with a balloon or cannot be dilated with a balloon. It is important to confirm that the guidewire is in the vessel architecture or in the distal true lumen before proceeding with any over-the-wire catheter or before applying various dilation strategies. The vessel architecture can usually be determined by observing calcium deposits or other signs of the vessel outline moving or “dancing” in sync with the interventional guidewire. The distal true lumen can be determined by contralateral angiography.
For cases in which a balloon cannot cross the lesion, the initial step is to advance a small (1.2–1.5 mm in diameter) balloon as deep as possible into the lesion to modify the proximal cap. When using small balloons, it is important to use longer length balloons (15–20 mm) because the largest profile of these balloons is at the mid shaft marker and the balloon tip will often penetrate the occlusion and stop at the marker. At this point, the balloon can be inflated to high pressure (14–16 atm) to determine if the lesion can be crossed from proximal to distal. If this fails, the next maneuver is to intentionally rupture the small balloon so as to modify the morphology of the proximal vessel/cap. If this fails to enable crossing then either a Corsair catheter, Asahi Intecc, (Nagoya, Japan) for more tortuous or less calcified lesions, or the Tornus catheter (Asahi Intecc) for more calcified lesions with shorter proximal stumps can be used.[42] This is followed by maneuvers that can increase guide catheter support, such as use of guide catheter extensions[43] or anchor techniques.[4] Occasionally, a FineCross (Terumo, Somerset, New Jersey) or Valet (Volcano, Rancho Cordova, California) catheter may be able to cross lesions that the Corsair and Tornus catheters could not. The catheters can be rotated to facilitate their passage, as this will reduce friction within the vessel.
If these maneuvers fail then more aggressive techniques, such as the use of coronary laser,[44] rotational atherectomy,[45] and the Crosser catheter, FlowCardia Inc. (Bard, Peripheral Vascular, Tempte, AZ)[46] can be employed. Rotational atherectomy and the Crosser catheter can be used even when the wire is not in the distal lumen but still in the vessel architecture. Small burrs (usually 1.25 mm and no larger than 1.5 mm) can be used to modify the proximal vessel architecture, which may allow the base of operations (i.e. the over-the-wire catheter) to be moved to a more advantageous location. However, rotational atherectomy requires exchanging the guidewire, which can be accomplished by exchanging through an over-the-wire balloon or microcatheter. In situations where the distal true lumen has not been reached, the last 2 cm of the radiopaque wire tip can be removed prior to placement in the artery, thereby providing further reach with the burr (part of the radiopaque portion of the wire should be preserved to prevent the burr from going off the guidewire). Alternatively, the wire can be looped further down the vessel prior to atherectomy. Rotational atherectomy in the subintimal space should only be attempted by very advanced CTO operators.
Similarly, there are several strategies for lesions resistant to balloon dilation, such as high pressure inflation of non-compliant balloons (with or without buddy wires), the use of cutting balloons or the AngioSculpt (AngioScore, Fremont, CA), the use of the Tornus catheter (Asahi Intecc), rotational atherectomy, and laser.[47]
If all of these techniques fail to achieve balloon crossing and/or balloon dilation of the lesion then success can often be achieved by recrossing the lesion within the subintimal space either in the antegrade direction using a prolapsed guide wire or CrossBoss catheter with re-entry of the true lumen prior to major branches,[48] or using a retrograde approach with retrograde dissection and re-entry to go around the non-crossable or non-dilatable lesion.
Section C
Use of Adjunctive Devices
Multislice Computed Tomography and Intravascular Ultrasonography
Intravascular ultrasonography can facilitate the recanalisation of stumpless CTO lesions by revealing the entry point of the occlusion when the occlusion site is angiographically unrecognisable due to its smooth entrance into the adjacent side branch. Moreover, intravascular ultrasonography can facilitate the repositioning of a guidewire in the event of an inadvertent subintimal passage and enable the selection of an optimal balloon size when reverse CART is performed.[38]
Multislice computed tomography can provide a detailed characterisation of the amount of calcification, tortuosity and length of the occluded segment, which can facilitate the procedural planning and estimation of the probability of successful PCI. Moreover, 3D reconstruction of the coronary anatomy and its integration with 2D fluoroscopy images during CTO PCI may help identify the best angiographic projection and provide a directional guide for the “missing segment” in the angiography.[49]
Section D
CTO PCI Complications
Complications during CTO PCI can be either acute or occur during long-term follow-up. Acute CTO PCI complications can be classified as coronary artery-related, (such as coronary occlusion, coronary perforation, and equipment loss or entrapment); cardiac non-coronary (such as periprocedural myocardial infarction, arrhythmias, and tamponade); and non-cardiac (such as vascular access complications, systemic embolisation, contrast-induced nephropathy, allergic reactions, and radiation-induced injury).[16] A number of complications (such as donor vessel dissection or thrombosis) are specific to CTO interventions.[16]
A meta-analysis of 65 studies with 18,061 patients undergoing CTO PCI reported a low incidence of acute complications. These were death 0.2 % (95 % CI: 0.1 % to 0.3 %); emergent coronary artery bypass grafting 0.1 % (95 % CI: 0.0 % to 0.2 %); stroke <0.01 % (95 % CI: 0.0 % to 0.1 %); MI 2.5 % (95 % CI: 1.9 % to 3.0 %); Q-wave MI 0.2 % (95 % CI: 0.1 % to 0.3 %); coronary perforation 2.9 % (95 % CI: 2.2 % to 3.6 %); tamponade 0.3 % (95 % CI: 0.2 % to 0.5 %); and contrast nephropathy 3.8 % (95 % CI: 2.4 % to 5.3 %).[50] Given the low frequency of emergency coronary artery bypass grafting, the authors believe that surgical backup is not essential for CTO PCI programs, provided there is a tested plan for urgent transfer to a facility with cardiac surgery in case of complications. However, the performance of CTO PCI at facilities without on-site surgery is discouraged unless performed by an operator with considerable experience in CTO PCI and at a laboratory that has immediately available all interventional equipment needed for CTO PCI and for the management of potential complications.
Although coronary perforations are common in CTO PCI (27.6 % in one series[15]), most perforations are related to localised wire exit sites from the vessel architecture and limited to angiographic evidence of contrast staining. In the above meta-analysis of CTO complications the risk of perforation was 2.9 % but the risk of tamponade only 0.3 %.[50] There are three main perforation types. The first is main vessel perforation, which may require implantation of a covered stent. The second is distal wire perforation and the third is collateral vessel perforation, which may require coil embolisation.[16] The availability of equipment for perforation management (covered stents and coils) and familiarity with their use is important for every CTO PCI program.
Contrast induced nephropathy (CIN), which was recently termed contrast induced acute renal injury (CI-ARI), is a well-recognised complication of invasive procedures using iodinated contrast agents. Patients at highest risk are those with baseline impaired renal function and diabetes. Mehran et al. developed a risk score for development of contrast nephropathy that includes eight variables (hypotension, intra-aortic balloon pump, congestive heart failure, chronic kidney disease, diabetes, age >75 years, anaemia and volume of contrast).[51] CIN/CI-ARI is a contrast-dose dependent phenomenon.[52] Laskey et al. determined that a contrast volume to creatinine clearance ratio >3.7 significantly increased the risk of CIN/CI-ARI in PCI patients.[53] The use of this ratio to identify patients undergoing PCI who will benefit from less contrast and closer follow-up may improve care. Table 1 lists the recent 2011 ACC/AHA/ SCAI PCI guidelines for CIN/CI-ARI.[41]
Table 1: Percutaneous Coronary Intervention Guideline Recommendations for Contrast-Induced Acute Kidney Injury.
| Class I |
|---|
|
|
|
| Class III: No Benefit |
| Administration of N-acetyl-L-cysteine is not useful for the prevention of contrast-induced acute kidney injury. |
Taken from 2011 ACC/AHA/SCAI guidelines. ACC = American College of Cardiology; AHA = American heart association; SCAI = Society for Cardiovascular Angiography and Interventions
Over the long-term, CTO interventions can be complicated by in-stent restenosis, stent thrombosis and coronary aneurysm formation. The use of drug-eluting stents significantly reduces the risk of restenosis (as described in part 1) without increasing the risk of stent thrombosis.[54] The risk of coronary aneurysm formation and their management has been poorly studied. Among 560 patients undergoing CTO PCI in Japan, aneurysms were observed in 7.3 % of those where retrograde intervention was performed vs 2.6 % of those where antegrade intervention was performed.[55] There is limited information on the long-term outcomes of the novel dissection/re-entry and retrograde techniques.[20] Target lesion revascularisation was required at five months for 52 % of 31 patients treated with the STAR technique,[18] whereas target vessel revascularisation was required in 47.9 % of 74 patients treated with the contrast-guided STAR technique.[56] In a single-centre study of 170 patients undergoing CTO PCI patients in whom the CrossBoss and Stingray devices were used (n=60) had similar long-term outcomes with 110 patients treated with other crossing strategies, in spite of its use in higher complexity cases.[57] There are no published, long-term, outcome data on the use of the mini-STAR and LAST techniques (20). Similarly, only one of the 20 studies that examined the outcomes of patients treated with the retrograde approach provided long-term outcomes.[20] The incidence of major adverse cardiac events in 24 patients during a median follow-up of 10.3 months was 18 %.[58] Obtaining additional long-term data will be critical for assessing the comparative effectiveness of the various CTO crossing strategies.
Given that CTO PCI may expose patients to significant amounts of radiation, those patient should undergo careful follow-up to diagnose radiation-induced skin injury.[59] The fluoroscopy time should not be used as the sole method for measuring radiation exposure because fluoroscopy time does not include cine imaging. Therefore, fluoroscopy time in and of itself is not a useful descriptor of the patient radiation dose. Total air kerma at the interventional reference point (Ka,r, Gy) is the procedural cumulative air kerma (X-ray energy delivered to a volume of air) at the interventional reference point and is used to monitor deterministic skin effects. No observable effects are present with Ka,r, <2 Gy while effects may occur at >5 Gy, with significant skin injury possible at >15 Gy. In clinical practice, much higher doses could be tolerated before radiation skin injury occurs if multiple imaging angles are used, as this would produce smaller, single-site, peak skin doses than would occur if the X-ray source were stationary. Table 2 outlines a comprehensive approach to patient dose management that should be standard practice in all interventional laboratories, especially those performing CTO PCI.[59]
Table 2: Radiation Dose Management in Percutaneous Coronary Intervention.
| I. Pre-Procedure |
|---|
|
|
|
|
| II. Procedure |
| A. Limit fluoroscopy – step on pedal only when looking at screen |
| B. Limit cine – use “fluoroscopy store” function when high quality imaging is not required, for example to document balloon and stent inflation |
| C. Limit magnification, frame rate (7.5 frames per second is preferred), and steep angles |
| D. Use collimation and filters to fullest extent possible |
| E. Vary tube angle when possible to change skin area exposed |
| F. Position table and image receptor appropriately: the patient should be placed as far possible from the X-ray tube and the image intensifier as close as possible to the patient |
| G. Keep patient and operator body parts out of field of view |
| H. Maximise shielding and distance from x-ray source for all personnel |
| I. Manage and monitor dose in real time from the beginning of the case |
| III. Post Procedure |
| A. Document radiation dose in records (fluoroscopy time, Ka,r, PKA) |
B. Patient and referring physician notification for high doses
|
| C. Adverse skin effects should be referred to appropriate consultant |
Section E
Summary and Recommendations
Specific recommendations regarding CTO PCI technique are as follows:
Dual injection is recommended for nearly all CTO PCI to determine the optimal crossing strategy and guide wire advancement into the distal true lumen.
Strategies that provide enhanced guide catheter support (such as long sheaths, large-bore guiding catheters, use of guide catheter extensions, and anchor techniques) are important for maximising the success rate and efficiency of CTO PCI.
Use of an over-the-wire system is strongly recommended in CTO PCI for enhancing the penetrating power of the guidewire, enabling change in tip shape and allowing guidewire change (stiff CTO guidewires are not optimal for crossing non-occluded coronary segments).
Adherence to a procedural strategy (Figure 1) that standardises CTO technique and facilitates procedural success is recommended. Such a strategy would permit stepwise decision making for antegrade and retrograde methods, inform guidewire selection and incorporate alternative approaches for instances of initial failure.
Given the paucity of long-term outcomes with use of novel crossing techniques (antegrade dissection/re-entry and retrograde), antegrade wire escalation is the preferred frontline CTO crossing technique, if technically feasible.
Using measures to minimise radiation exposure (including but not limited to use of 7.5 frames per second fluoroscopy and use of low magnification) and contrast administration is recommended.
CTO PCI is best performed at centres with dedicated CTO PCI experience and expertise. Use of crossing difficulty prediction tools, such as the J-CTO score,[9] can facilitate the selection of cases with a high likelihood of quick crossing that can be attempted at less experienced centres.
Acknowledgments
The authors would like to thank Ms Sheila Agyeman for her invaluable effort in coordinating the manuscript creation process.
References
- 1.Rinfret S, Joyal D, Nguyen CM et al. Retrograde recanalization of chronic total occlusions from the transradial approach; early Canadian experience. Catheter Cardiovasc Interv. 2011;78:366–74. doi: 10.1002/ccd.23140. [DOI] [PubMed] [Google Scholar]
- 2.Hamon M, Mehta S, Steg PG et al. Impact of transradial and transfemoral coronary interventions on bleeding and net adverse clinical events in acute coronary syndromes. EuroIntervention. 2011;7:91–7. doi: 10.4244/EIJV7I1A16. [DOI] [PubMed] [Google Scholar]
- 3.Cantor WJ, Mahaffey KW, Huang Z et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Interv. 2007;69:73–83. doi: 10.1002/ccd.20897. [DOI] [PubMed] [Google Scholar]
- 4.Di Mario C, Ramasami N. Techniques to enhance guide catheter support. Catheter Cardiovasc Interv. 2008;72:505–12. doi: 10.1002/ccd.21670. [DOI] [PubMed] [Google Scholar]
- 5.Galassi AR, Tomasello SD, Reifart N et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention. 2011;7:472–9. doi: 10.4244/EIJV7I4A77. [DOI] [PubMed] [Google Scholar]
- 6.Michael TT, Mogabgab O, Fuh E et al. Application of the “hybrid approach” to chronic total occlusion interventions: a detailed procedural analysis. J Interv Cardiol. 2014;27:36–43. doi: 10.1111/joic.12083. [DOI] [PubMed] [Google Scholar]
- 7.Brilakis ES, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2012;5:367–79. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Brilakis ES, Grantham JA, Thompson CA et al. The retrograde approach to coronary artery chronic total occlusions: a practical approach. Catheter Cardiovasc Interv. 2012;79:3–19. doi: 10.1002/ccd.23004. [DOI] [PubMed] [Google Scholar]
- 9.Morino Y, Abe M, Morimoto T et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–21. doi: 10.1016/j.jcin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Nombela-Franco L, Urena M, Jerez-Valero M et al. Validation of the J-chronic total occlusion score for chronic total occlusion percutaneous coronary intervention in an independent contemporary cohort. Circ Cardiovasc Interv. 2013;6:635–43. doi: 10.1161/CIRCINTERVENTIONS.113.000447. [DOI] [PubMed] [Google Scholar]
- 11.Rathore S, Katoh O, Matsuo H et al. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries: procedural outcomes and predictors of success in contemporary practice. Circ Cardiovasc Interv. 2009;2:124–32. doi: 10.1161/CIRCINTERVENTIONS.108.838862. [DOI] [PubMed] [Google Scholar]
- 12.Grantham JA, Marso SP, Spertus J et al. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2:479–86. doi: 10.1016/j.jcin.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Morino Y, Kimura T, Hayashi Y In-hospital outcomes of contemporary percutaneous coronary intervention in patients with chronic total occlusion insights from the J-CTO Registry (Multicenter CTO Registry in Japan). JACC Cardiovasc Interv. [DOI] [PubMed]
- 14.Sianos G, Werner GS, Galassi AR et al. Recanalisation of chronic total coronary occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention. 2012;8:139–45. doi: 10.4244/EIJV8I1A21. [DOI] [PubMed] [Google Scholar]
- 15.Rathore S, Matsuo H, Terashima M et al. Procedural and in-hospital outcomes after percutaneous coronary intervention for chronic total occlusions of coronary arteries 2002 to 2008: impact of novel guidewire techniques. JACC Cardiovasc Interv. 2009;2:489–97. doi: 10.1016/j.jcin.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Brilakis ES, Karmpaliotis D, Patel V, Banerjee S. Complications of chronic total occlusion angioplasty. Interventional Cardiology Clinics. 2012;1:373–89. doi: 10.1016/j.iccl.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Mitsudo K, Yamashita T, Asakura Y et al. Recanalization strategy for chronic total occlusions with tapered and stiff-tip guidewire. The results of CTO new techniQUE for STandard procedure (CONQUEST) trial. J Invasive Cardiol. 2008;20:571–7. [PubMed] [Google Scholar]
- 18.Colombo A, Mikhail GW, Michev I et al. Treating chronic total occlusions using subintimal tracking and reentry: the STAR technique. Catheter Cardiovasc Interv. 2005;64:407–11. 412. doi: 10.1002/ccd.20307. discussion. [DOI] [PubMed] [Google Scholar]
- 19.Valenti R, Vergara R, Migliorini A et al. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol. 2013;61:545–50. doi: 10.1016/j.jacc.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Michael TT, Papayannis AC, Banerjee S, Brilakis ES. Subintimal dissection/reentry strategies in coronary chronic total occlusion interventions. Circ Cardiovasc Interv. 2012;5:729–38. doi: 10.1161/CIRCINTERVENTIONS.112.969808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlino M, Godino C, Latib A et al. Subintimal tracking and re-entry technique with contrast guidance: a safer approach. Catheter Cardiovasc Interv. 2008;72:790–6. doi: 10.1002/ccd.21699. [DOI] [PubMed] [Google Scholar]
- 22.Carlino M, Latib A, Godino C et al. CTO recanalization by intraocclusion injection of contrast: the microchannel technique. Catheter Cardiovasc Interv. 2008;71:20–6. doi: 10.1002/ccd.21396. [DOI] [PubMed] [Google Scholar]
- 23.Galassi AR, Tomasello SD, Costanzo L et al. Mini-STAR as bail-out strategy for percutaneous coronary intervention of chronic total occlusion. Catheter Cardiovasc Interv. 2012;79:30–40. doi: 10.1002/ccd.22998. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi WL. Retrograde PCI: what will they think of next? J Invasive Cardiol. 2009;21:543. [PubMed] [Google Scholar]
- 25.Whitlow PL, Burke MN, Lombardi WL et al. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs (Facilitated Antegrade Steering Technique in Chronic Total Occlusions) trial. JACC Cardiovasc Interv. 2012;5:393–401. doi: 10.1016/j.jcin.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Werner GS, Schofer J, Sievert H et al. Multicentre experience with the BridgePoint devices to facilitate recanalisation of chronic total coronary occlusions through controlled subintimal re-entry. EuroIntervention. 2011;7:192–200. doi: 10.4244/EIJV7I2A33. [DOI] [PubMed] [Google Scholar]
- 27.Surmely JF, Tsuchikane E, Katoh O et al. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique. J Invasive Cardiol. 2006;18:334–8. [PubMed] [Google Scholar]
- 28.Thompson CA, Jayne JE, Robb JF et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv. 2009;2:834–42. doi: 10.1016/j.jcin.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Karmpaliotis D, Michael TT, Brilakis ES et al. Retrograde coronary chronic total occlusion revascularization: procedural and in-hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc Interv. 2012;5:1273–9. doi: 10.1016/j.jcin.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Michael TT, Karmpaliotis D, Brilakis ES et al. Procedural Outcomes of Revascularization of Chronic Total Occlusion of Native Coronary Arteries (from a Multicenter United States Registry). Am J Cardiol. 2013;112:488–92. doi: 10.1016/j.amjcard.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 31.El Sabbagh A, Patel VG, Jeroudi OM et al. Angiographic success and procedural complications in patients undergoing retrograde percutaneous coronary chronic total occlusion interventions: A weighted meta-analysis of 3482 patients from 26 studies. Int J Cardiol. 2014;174:243–8. doi: 10.1016/j.ijcard.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchikane E, Katoh O, Kimura M et al. The first clinical experience with a novel catheter for collateral channel tracking in retrograde approach for chronic coronary total occlusions. JACC Cardiovasc Interv. 2010;3:165–71. doi: 10.1016/j.jcin.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Galassi AR, Tomasello SD, Costanzo L, Tamburino C. Retrograde approach for chronic total occlusion percutaneous revascularization. Interventional Cardiology. 2010;2:391–403. [Google Scholar]
- 34.Hsu JT, Tamai H, Kyo E et al. Traditional antegrade approach versus combined antegrade and retrograde approach in the percutaneous treatment of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2009;74:555–63. doi: 10.1002/ccd.22035. [DOI] [PubMed] [Google Scholar]
- 35.Galassi AR. Coronary Interventions for Chronic Total Occlusions; Galassi’s Tips and Tricks: Alpha. 2010.
- 36.Kimura M, Katoh O, Tsuchikane E et al. The efficacy of a bilateral approach for treating lesions with chronic total occlusions the CART (controlled antegrade and retrograde subintimal tracking) registry. JACC Cardiovasc Interv. 2009;2:1135–41. doi: 10.1016/j.jcin.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Sianos G, Barlis P, Di Mario C et al. European experience with the retrograde approach for the recanalisation of coronary artery chronic total occlusions. A report on behalf of the euroCTO club. EuroIntervention. 2008;4:84–92. doi: 10.4244/eijv4i1a15. [DOI] [PubMed] [Google Scholar]
- 38.Rathore S, Katoh O, Tuschikane E et al. A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc Interv. 2010;3:155–64. doi: 10.1016/j.jcin.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Thompson CA. Percutaneous revascularization of coronary chronic total occlusions: the new era begins. JACC Cardiovasc Interv. 2010;3:152–4. doi: 10.1016/j.jcin.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Karmpaliotis D, Lembo NJ, Brilakis ES, Kandzari DE. Percutaneous Chronic Total Occlusion Revascularization Program Development, Resource Utilization, and Economic Outcomes. Intervent Cardiol Clin. 2012;1:391–5. doi: 10.1016/j.iccl.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/ SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012;79:453–95. doi: 10.1002/ccd.23438. [DOI] [PubMed] [Google Scholar]
- 42.Fang HY, Lee CH, Fang CY et al. Application of penetration device (Tornus) for percutaneous coronary intervention in balloon uncrossable chronic total occlusion-procedure outcomes, complications, and predictors of device success. Catheter Cardiovasc Interv. 2011;78:356–62. doi: 10.1002/ccd.22862. [DOI] [PubMed] [Google Scholar]
- 43.Luna M, Papayannis A, Holper EM et al. Transfemoral use of the GuideLiner catheter in complex coronary and bypass graft interventions. Catheter Cardiovasc Interv. 2012;80:437–46. doi: 10.1002/ccd.23232. [DOI] [PubMed] [Google Scholar]
- 44.Shen ZJ, Garcia-Garcia HM, Schultz C et al. Crossing of a calcified “balloon uncrossable” coronary chronic total occlusion facilitated by a laser catheter: a case report and review recent four years’ experience at the Thoraxcenter. Int J Cardiol. 2010;145:251–4. doi: 10.1016/j.ijcard.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 45.Pagnotta P, Briguori C, Mango R et al. Rotational atherectomy in resistant chronic total occlusions. Catheter Cardiovasc Interv. 2010;76:366–71. doi: 10.1002/ccd.22504. [DOI] [PubMed] [Google Scholar]
- 46.Galassi AR, Tomasello SD, Costanzo L et al. Recanalization of complex coronary chronic total occlusions using high-frequency vibrational energy CROSSER catheter as first-line therapy: a single center experience. J Interv Cardiol. 2010;23:130–8. doi: 10.1111/j.1540-8183.2010.00526.x. [DOI] [PubMed] [Google Scholar]
- 47.Lindsey JB, Banerjee S, Brilakis ES. Two “buddies” may be better than one: use of two buddy wires to expand an underexpanded left main coronary stent. J Invasive Cardiol. 2007;19:E355–8. [PubMed] [Google Scholar]
- 48.Michael TT, Banerjee S, Brilakis ES. Subintimal distal anchor technique for “balloon-uncrossable” chronic total occlusions. J Invasive Cardiol. 2013;25:552–4. [PubMed] [Google Scholar]
- 49.Magro M, Schultz C, Simsek C et al. Computed tomography as a tool for percutaneous coronary intervention of chronic total occlusions. EuroIntervention. 2010;6(Suppl G):G123–31. [PubMed] [Google Scholar]
- 50.Patel VG, Brayton KM, Tamayo A et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128–36. doi: 10.1016/j.jcin.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Mehran R, Aymong ED, Nikolsky E et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 52.Dangas G, Iakovou I, Nikolsky E et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–9. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 53.Laskey WK, Jenkins C, Selzer F et al. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:584–90. doi: 10.1016/j.jacc.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 54.Saeed B, Kandzari DE, Agostoni P et al. Use of drug-eluting stents for chronic total occlusions: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2011;77:315–32. doi: 10.1002/ccd.22690. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka H, Kadota K, Hosogi S et al. Mid-term angiographic and clinical outcomes from antegrade versus retrograde recanalization for chronic total occlusions. J Am Coll Cardiol. 2011;57:E1628. [Google Scholar]
- 56.Godino C, Latib A, Economou FI et al. Coronary chronic total occlusions: mid-term comparison of clinical outcome following the use of the guided-STAR technique and conventional anterograde approaches. Catheter Cardiovasc Interv. 2012;79:20–7. doi: 10.1002/ccd.23058. [DOI] [PubMed] [Google Scholar]
- 57.Mogabgab O, Patel VG, Michael TT et al. Long-Term Outcomes With Use of the CrossBoss and Stingray Coronary CTO Crossing and Re-Entry Devices. J Invasive Cardiol. 2013;25:579–85. [PubMed] [Google Scholar]
- 58.Lee NH, Seo HS, Choi JH et al. Recanalization strategy of retrograde angioplasty in patients with coronary chronic total occlusion -analysis of 24 cases, focusing on technical aspects and complications. Int J Cardiol. 2010;144:219–29. doi: 10.1016/j.ijcard.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Chambers CE, Fetterly KA, Holzer R et al. Radiation safety program for the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2011;77:546–56. doi: 10.1002/ccd.22867. [DOI] [PubMed] [Google Scholar]


