Abstract
Placental insufficiency is a primary cause of intrauterine growth restriction (IUGR). IUGR increases the risk of developing type 2 diabetes mellitus (T2DM) throughout life, which indicates that insults from placental insufficiency impair β-cell development during the perinatal period because β-cells have a central role in the regulation of glucose tolerance. The severely IUGR fetal pancreas is characterized by smaller islets, less β-cells, and lower insulin secretion. Because of the important associations among impaired islet growth, β-cell dysfunction, impaired fetal growth, and the propensity for T2DM, significant progress has been made in understanding the pathophysiology of IUGR and programing events in the fetal endocrine pancreas. Animal models of IUGR replicate many of the observations in severe cases of human IUGR and allow us to refine our understanding of the pathophysiology of developmental and functional defects in islet from IUGR fetuses. Almost all models demonstrate a phenotype of progressive loss of β-cell mass and impaired β-cell function. This review will first provide evidence of impaired human islet development and β-cell function associated with IUGR and the impact on glucose homeostasis including the development of glucose intolerance and diabetes in adulthood. We then discuss evidence for the mechanisms regulating β-cell mass and insulin secretion in the IUGR fetus, including the role of hypoxia, catecholamines, nutrients, growth factors, and pancreatic vascularity. We focus on recent evidence from experimental interventions in established models of IUGR to understand better the pathophysiological mechanisms linking placental insufficiency with impaired islet development and β-cell function.
Keywords: IUGR, pancreas, islet, β-cell
Introduction
Intrauterine growth restriction (IUGR) is defined as the failure of a fetus to achieve its genetic potential for size. Placental insufficiency is a leading cause of IUGR in humans and complicates 4–8% of pregnancies (Hendrix and Berghella 2008; Platz and Newman 2008). The definition of IUGR contrasts with that for small-for-gestational-age infants (SGA), who are typically defined as having birth weights below the 10th percentile, though other definitions have been employed. Essentially, the definition of IUGR differs from SGA in that it refers to a pathophysiological process as opposed to a statistically derived definition. The differences in terminology and definitions has introduced heterogeneity into human studies (Crovetto, et al. 2016), but most studies have identified a higher risk for perinatal mortality and short and long term morbidities in IUGR and SGA newborns (von Beckerath, et al. 2013). This includes an increased risk of developing type 2 diabetes mellitus as they age (T2DM; Hales and Barker 2001).
Reduced nutrient and oxygen transport to the fetus are hallmarks of placental insufficiency and are caused by lower placental mass (Marconi, et al. 2006; Regnault, et al. 2002b), abnormal placental structure (Mayhew, et al. 2003; Sibley, et al. 2005), and decreased glucose and amino acid transporter abundance and activity (Cetin 2003; Cetin, et al. 1990; Economides, et al. 1989; Jansson, et al. 1998; Paolini, et al. 2001; Platz and Newman 2008). These placental defects lead to hypoxemic and hypoglycemic fetuses with resulting higher circulating catecholamine concentrations and lower circulating insulin concentrations (Greenough, et al. 1990; Nicolini, et al. 1990). IUGR fetuses also are characterized by impaired glucose stimulated insulin secretion (GSIS; Nicolini et al. 1990). In cases of severe IUGR, fetuses have smaller and less vascularized pancreatic islets with fewer β-cells (Van Assche, et al. 1977). This contrasts to cases of less severe IUGR in which structural pancreatic defects are less pronounced (Béringue, et al. 2002). These data indicate that the degree of impaired islet development may correlate with the severity of placental insufficiency, a phenomenon observed in animal models of placental insufficiency (Gatford, et al. 2008; Limesand, et al. 2005; Limesand, et al. 2006; Rozance, et al. 2009b). These findings also show that reduced insulin secretion in IUGR fetuses is not solely due to smaller islets and less β-cells.
The relationship between fetal plasma insulin concentrations and fetal growth is critical. In addition to regulating glucose metabolism (Hay, et al. 1988), insulin produced by the fetuses also promotes their systemic growth (Fowden, et al. 1989). Thus, the fetal β-cell links fetal nutrient supply with fetal nutrient metabolism and anabolic signals for growth. The β-cell defects in IUGR are even more important when one realizes that T2DM is characterized by the combination of tissue insulin resistance and insufficient insulin secretion (Barker, et al. 1993; Hales and Barker 2001; Kasuga 2006). Interestingly, children and adults that were formerly SGA fetuses develop insulin resistance (Jensen, et al. 2002; Mathai, et al. 2012; Mericq, et al. 2005; Milovanovic, et al. 2014). Insulin sensitivity is lower in adults born SGA and experiencing catch up growth compared to adults born appropriate for gestational age (AGA) or SGA without compensatory growth (Fabricius-Bjerre, et al. 2011; Kajantie, et al. 2015; T. Forsén, et al. 2000). Several studies also report impaired β-cell function, which persists throughout life (Bazaes, et al. 2003; Cook, et al. 1993; Crowther, et al. 2000; Jensen et al. 2002; Setia, et al. 2006). Therefore, the greater risk for developing T2DM in formerly IUGR and SGA individuals is due to a combination of both an increased risk of insulin resistance and an increased risk of impaired β-cell development and function. The mechanisms by which IUGR impair β-cell development and function is the focus of this review.
Due to ethical considerations, evaluation of pancreatic islet development and function in human IUGR other than the basic observations noted above are limited. As a result, animal models are used to investigate the mechanistic link between placental insufficiency and the endocrine pancreas phenotype (Green, et al. 2010). Similar to IUGR humans, animal models of placental insufficiency and IUGR are characterized by progressive islet and β-cell dysfunction, impaired glucose tolerance, and a propensity to develop T2DM (Green et al. 2010). In this review, we focus on recent studies with animal models of IUGR in which interventional experiments have manipulated the fetal and/or neonatal environment following established placental insufficiency and/or IUGR to further our understanding of the mechanisms linking placental insufficiency with β-cell dysfunction and impaired islet development and function.
Animal models of IUGR with β-cell dysfunction
Rodent models
Rodent models of placental insufficiency and IUGR are valuable for determining the adulthood pathogenesis of diabetes and the intergenerational impacts due to their short lifespan and generation interval. Three well-defined rat models of IUGR show impaired pancreatic islet development, establish lasting effects in adults, and have been subjected to experimental perinatal interventions to better define the mechanisms leading to diminished islet development and function. Uterine artery ligation (UAL) at embryonic day (E) 18–19 causes an acute decrease in fetal nutrients and oxygen near term (E22), and results in a 10 to 20% reduction in body weight (Delghingaro-Augusto, et al. 2014; Ikeda, et al. 2016; Ogata, et al. 1986; Siebel, et al. 2010; Wigglesworth 1964). Maternal dietary restrictions also are widely utilized in rodents to induced IUGR. Specifically, IUGR is produced when dams are fed an isocaloric diet with low protein (LP; generally 40–50% relative to controls) throughout gestation (Boujendar, et al. 2002; Snoeck, et al. 1990; Zhang, et al. 2016). Reductions in fetal weight are observed as early as E14 (Zhang et al. 2016). IUGR also is produced when maternal caloric intake is reduced by 50% during the last week of gestation and results in a 12–20% reduction in offspring birth weight (CR; Garofano, et al. 1997, 1998; Woodall, et al. 1996). Observations in these models demonstrate a strong connection between in utero fetal undernutrition with impaired pancreatic islet development and progressive β-cell dysfunction which can be evident at or before birth and persist into maturity leading to glucose intolerance and T2DM.
Sheep Models
In fetal sheep, β-cell responsiveness to glucose develops during the second half of gestation, paralleling the fetal glucose responsiveness observed in human pregnancies (Aldoretta and Hay 1999; Molina, et al. 1993; Nicolini et al. 1990). Due to the accessibility of the fetal vasculature for chronic catheterization, sheep models of placental insufficiency and IUGR allow for testing mechanisms that link in utero conditions of IUGR, impaired islet development and β-cell dysfunction. Placental insufficiency occurs when pregnant sheep are exposed to elevated ambient temperatures in mid to late gestation. Experimental replication of this naturally occurring phenomenon in sheep (PI-IUGR) results in a progressive reduction in the capacity for nutrient and oxygen transport to the fetus resulting in IUGR (Bell, et al. 1987; Regnault, et al. 2002a). Near term (~ 90% of gestation to term), placental and fetal weights are reduced by ~40% (de Vrijer, et al. 2004; Galan, et al. 1999; Limesand et al. 2006). Alternatively, the cotyledonary placenta, unique to ruminants, can be experimentally manipulated by the surgical removal of most of the uterine caruncles prior to pregnancy to induce placental restriction and IUGR (UC-PI; Robinson, et al. 1979). Similar to the PI-IUGR model, the placental restriction resulting from carunclectomy lowers the supply of nutrients and oxygen to the fetus, resulting in a 20% decrease in body weight near term compared with controls (Mellor, et al. 1977; Owens, et al. 2007; Robinson et al. 1979). Comparing data from both of these models provides the opportunity to see a spectrum of fetal outcomes based perhaps on the severity of placental insufficiency and IUGR. The sheep models of IUGR are advantageous in that decrements in placental nutrient and oxygen supply to the fetus can be quantified and in vivo fetal experimental interventions can be performed. Following these interventions fetal glucose tolerance tests and in vitro islet functional assays can be combined with structural analysis of the pancreas to determine which specific nutrients or hormones are limiting for islet development and function.
Pancreatic development
Relationships between IUGR and pancreatic β-cell function must be interpreted within the context of pancreatic development. Although fetal sheep more closely mimic humans in the overlapping developmental transitions of the pancreas and islets, the distinct developmental transitions in rodent models allow investigators to elucidate the specificity of the developmental stages in their response to insults or interventions. Important transitional periods and developmental adaptations in the pancreas of IUGR animals have been recently reviewed (Conrad, et al. 2014; Green et al. 2010; Jennings, et al. 2015; Willmann, et al. 2016). Briefly, the primary transition of the endoderm begins at embryonic day (E) 11 in rats, before 24 days gestational age (dGA) in sheep, and 25–26 dGA in humans (term gestation is E22, 147 dGA, and 40 weeks of gestational age for the rat, sheep, and human, respectively). Initiation of the secondary transition in the fetal rat occurs at E15-18 with the identification of mature secretory products and is followed by isletogenesis (E17-21) and endocrine cell proliferation (E21.5-22). The secondary transition, isletogenesis, and endocrine cell proliferation in sheep and humans occurs over a broad period during gestation (approximately 20% of gestation to term).
Reduced insulin secretion and pancreatic dysfunction in the IUGR fetus
Lower insulin secretion, smaller islets, lower islet density, less β-cells, and decreased pancreatic weight and insulin content are hallmarks of the IUGR fetal pancreas in the various animal models (Abuzgaia, et al. 2015; Green et al. 2010). Defects in β-cell replication and expression of genes regulating β-cell proliferation, apoptosis, and neogenesis all contribute to reduced β-cell mass to varying extents in the different models. (Dumortier, et al. 2007; Kelly, et al. 2017; Limesand et al. 2005; Petrik, et al. 1999). In PI-IUGR fetal sheep, basal insulin concentrations and glucose-stimulated insulin secretion (GSIS) were lower than controls at both 70% and 90% of gestation (Limesand, et al. 2013; Limesand et al. 2006). Additionally, the insulin content of the islets isolated from near term IUGR fetuses was ~80% lower than control islets. Despite this, the IUGR islets secreted a higher proportion of their insulin in response to glucose and other secretagogues, which likely relates to increased expression of genes responsible for much of the stimulus-secretion coupling in the islets (Kelly et al. 2017; Limesand et al. 2006). In contrast to the severe insulin secretion defects observed in the PI-IUGR model, insulin secretion was not reduced in fetuses following uterine carunclectomy when compared to controls. However, insulin secretion was inversely correlated with factors associated with placental insufficiency like fetal weight and blood oxygen concentrations (Owens et al. 2007). Importantly, the subtle fetal insulin secretion defects in the carunclectomy model of placental insufficiency were progressive as insulin secretion was reduced in one month old post carunclectomy lambs (De Blasio, et al. 2007). Although a compensatory increase in β-cell mass occurred into adulthood, insulin secretion of UC-PI sheep remained insufficient relative to progressive declines in insulin sensitivity (Gatford et al. 2008), findings which are similar to formerly SGA humans (Cook et al. 1993). Progressive insulin secretory defects are also identified in CR, LP, and UAL rat models (Dumortier et al. 2007; Fernandez-Twinn, et al. 2005; Garofano et al. 1997; Garofano, et al. 1999; Simmons, et al. 2001).
Perinatal interventions to determine the mechanisms of pancreatic islet dysfunction in IUGR
Nutrient regulation of β-cells
Glucose
The β-cell functions to link carbohydrate, amino acid, and lipid metabolism to insulin secretion (Newsholme, et al. 2014). In the fetus, this results in a situation in which the β-cell acts as a sensor of the fetal nutrient supply from the placenta and can link this supply to appropriate production of fetal growth factors (Brown, et al. 2011; Gleason, et al. 2007; Newsholme et al. 2014). The ability of nutrients to stimulate insulin secretion largely depends on the ability of the β-cell to utilize the nutrient as a fuel source (Malaisse, et al. 1979). Glucose, the primary insulin secretagogue, is metabolized through glycolysis and the tricarboxylic acid (TCA) cycle stimulating ATP production, elevating the cytosolic ATP/ADP ratio causing β-cell membrane depolarization, increased cytosolic calcium concentrations and insulin secretion (Henquin 2000; Wiederkehr and Wollheim 2012). Furthermore, metabolism of glucose also leads to the generation of many different secondary messengers that also stimulate insulin secretion (Andersson, et al. 2015; Cline, et al. 2011; German 1993; Gleason et al. 2007; Göhring, et al. 2014; Huang and Joseph 2012; Patel, et al. 2014; Spégel, et al. 2011). Given the role of β-cell nutrient stimulation-insulin secretion coupling in the fetus for the regulation of growth, multiple studies of the IUGR fetal β-cell and pancreatic function have focused on manipulation of fetal nutrient concentrations and supply.
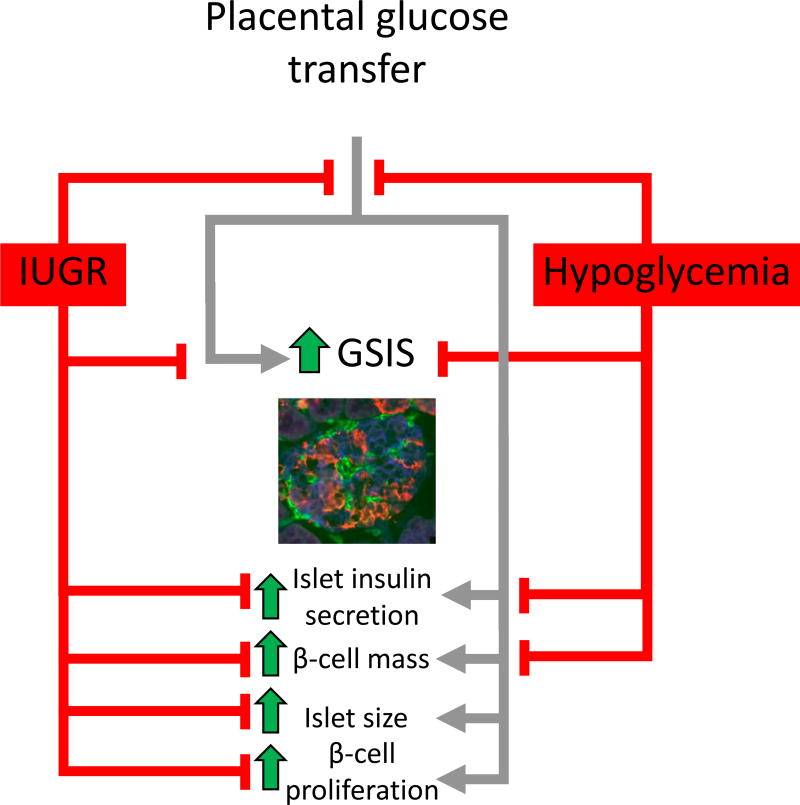
We have utilized chronic maternal insulin infusions to test the effects of reduced fetal glucose supply on β-cell function independent of lower placental capacity to transport amino acids or oxygen to the fetus (Figure 1). Maternal insulin infusions markedly decrease transfer of glucose to the fetus, fetal plasma glucose concentrations, and fetal weight. Because insulin does not cross the placenta, fetal insulin concentrations also are markedly reduced (17%; DiGiacomo and Hay 1990; Limesand and Hay 2003; Rozance, et al. 2006). Additionally, the insulin secretory response of hypoglycemic fetuses and their isolated islets to glucose and amino acids is diminished despite similar numbers of pancreatic β-cells in control and hypoglycemic fetuses following two weeks of maternal insulin treatment near term (Limesand and Hay 2003; Rozance and Hay 2006; Rozance et al. 2006; Rozance, et al. 2007). Importantly, when glucose concentrations were returned to normal for five days, fetal insulin concentrations also returned to normal but insulin secretory defects did not fully recover showing programming of fetal β-cell function with even short duration nutrient deprivation (Limesand and Hay 2003). When we extended this experimental paradigm from two weeks to eight weeks, beginning at approximately 50% of gestation, the hypoglycemic fetuses show a similar attenuation of GSIS (Lavezzi, et al. 2013). β-cell mass, however, was significantly lower in these hypoglycemic fetuses compared to controls, thus showing that chronic experimental fetal hypoglycemia can mimic the two main pancreatic islet defects seen in IUGR fetuses, attenuated GSIS and lower β-cell mass (Lavezzi et al. 2013; Nicolini et al. 1990; Van Assche et al. 1977).
Figure 1. A schematic representation of fetal pancreatic and β-cell dysfunction in models of IUGR and potential mechanisms of glucose regulation for β-cell dysfunction.
The fetal pancreas response to glucose is represented by the gray lines. Pancreatic dysfunctions associated with models of IUGR are depicted by red lines. A fetal sheep islet is at 90% of gestation is depicted in the micrograph and has been immunostained for insulin (β-cell; blue), glucagon+somatostatin+pancreatic polypeptide (red), and vasculature (GS1; green).
Having demonstrated that experimental fetal hypoglycemia replicates the major pancreatic islet defects in IUGR, we sought to determine if restoring glucose concentrations to control concentrations would restore fetal insulin secretion and β-cell mass to normal. Following direct infusions of glucose into PI-IUGR sheep fetuses which were adjusted to increase fetal glucose concentrations to control levels for two weeks, fetuses developed worse hypoxemia, acidosis, and increased norepinephrine concentrations compared to saline infused PI-IUGR fetuses by the end of the study (Rozance et al. 2009b). Not surprisingly, fetal GSIS was also lower than the saline infused PI-IUGR fetuses. Furthermore, there was no impact of the glucose infusion on pancreatic β-cell mass (Rozance et al. 2009b).
Amino acids
Many amino acids stimulate insulin secretion through the above mentioned pathways for glucose metabolism and other, complimentary metabolic pathways (Cline et al. 2011; Doliba, et al. 2007; Gadhia, et al. 2013; Gao, et al. 2003; Gleason et al. 2007). Branched chain amino acids (BCAAs) are especially important for stimulation of β-cells (MacDonald, et al. 1991; Milner 1969; Newsholme, et al. 2006). BCAAs are metabolized by branched-chain aminotransferases (e.g. leucine to α-ketoisocaproate [KIC]) and the mitochondrial branched-chain α-keto acid dehydrogenase complexes (BCKAD) to produce glutamate, C3-acylcarnitine, succinyl-CoA, acetyl-CoA, and acetoacetate, thus providing TCA cycle intermediates which increase the β-cell ATP/ADP ratio and stimulate insulin secretion (Newgard 2012). In addition, leucine stimulates metabolism of other nutrients by allosterically activating glutamate dehydrogenase to enhance glutaminolysis and anapleurosis (Gao et al. 2003).
The acute stimulatory effect of amino acids on β-cell insulin secretion has been demonstrated in fetal sheep, both in vivo and in isolated fetal sheep islets in vitro (Brown, et al. 2009; Molina et al. 1993; Rozance et al. 2006). Amino acids not only stimulate fetal insulin secretion, but they also potentiate fetal GSIS (Gadhia et al. 2013). In human cases of placental insufficiency and IUGR, amino acid transfer to the fetus is lower than in normally growing fetuses (Brown, et al. 2012; Cetin et al. 1990; Galan, et al. 2009; Jansson et al. 1998). Lower placental transfer of the BCAA leucine to the fetus is one of the most consistent features of placental insufficiency and IUGR. In fact, one study showed that the size of the decrement in leucine transfer to the IUGR fetus was directly associated with the severity of IUGR in human pregnancies complicated by placental insufficiency (Marconi, et al. 1999).
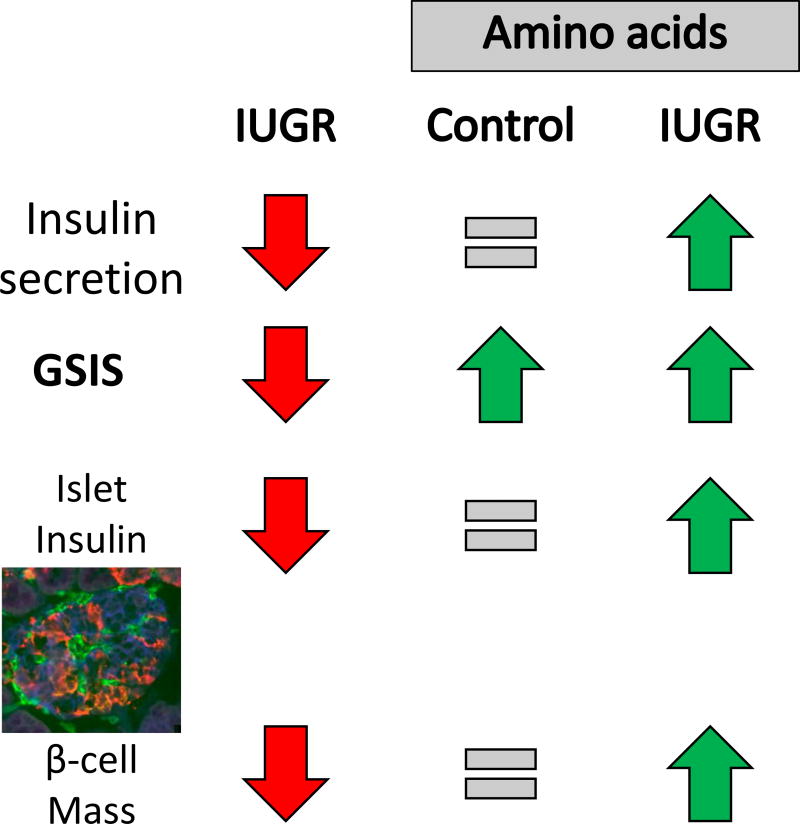
Because of these relationships among placental insufficiency, amino acid transfer to the fetus, and β-cell function; we performed a series of studies to quantify the impact of amino acid supplementation on fetal insulin secretion, islet structure, and islet function in normally grown and IUGR fetal sheep (Figure 2). Following a direct fetal infusion of a complete mixture of amino acids enriched in the essential and BCAA, targeting a 25–50% increase in BCAA concentrations for ten to 12 days, fetal insulin concentrations were not different between amino acid- and saline-infused fetuses (Gadhia et al. 2013; Maliszewski, et al. 2012). Fetal GSIS, however, almost doubled with amino acid supplementation despite equivalent islet size, numbers of β-cells, and pancreatic insulin content (Gadhia et al. 2013). In PI-IUGR sheep fetuses, where GSIS and amino acid stimulated insulin secretion were significantly attenuated in vivo, GSIS was enhanced almost to that of control fetuses after eleven days of amino acid supplementation (Brown, et al. 2016). We then tested the impact of amino acids on pancreatic structure and insulin secretion from isolated islets from PI-IUGR sheep fetuses. In contrast to pancreatic islets isolated from control fetal sheep, islets from PI-IUGR fetal sheep were not responsive to supplemental amino acids. Importantly, and unlike observations in the amino acid infused control fetuses, pancreatic insulin content, islet size, and β-cell mass in the amino acid infused PI-IUGR fetuses were significantly greater than in saline infused PI-IUGR fetuses (Brown et al. 2016). The enhanced insulin secretion and pancreatic morphology were independent of any differences in glucose, norepinephrine or oxygen concentrations between the groups of IUGR fetuses (Brown et al. 2016).
Figure 2. Pancreatic and β-cell dysfunction in PI-IUGR and the response to increased exogenous amino acid concentrations.
A fetal sheep islet is at 90% of gestation is depicted in the micrograph and has been immunostained for insulin (β-cell; blue), glucagon+somatostatin+pancreatic polypeptide (red), and vasculature (GS1; green).
A consistent finding with both acute and chronic amino acid infusion studies has been increased fetal plasma glucagon concentrations and/or pancreatic glucagon content (Brown et al. 2016; Gadhia et al. 2013; Rozance, et al. 2009a). This has led to speculation that in addition to directly increasing β-cell metabolism and generation of secondary messengers, amino acids may also be potentiating β-cell function via glucagon dependent mechanisms which include the generation of secondary messengers (Huypens, et al. 2000; Pipeleers, et al. 1985; Schuit and Pipeleers 1985). Although generation of secondary messengers in the IUGR β-cell has not been investigated specifically, there is some evidence suggesting their role in impaired β-cell function in IUGR fetuses. For example, suppression of Wnt signaling and differential expression of potassium channels and mediators of calcium signaling were observed in the transcriptome of IUGR fetal sheep islets compared with controls (Kelly et al. 2017). It is important to note that second messaging compounds may influence either nutrient sensing or insulin exocytosis mechanisms and also can link nutrient stimulated insulin secretion to β-cell proliferation. Thus impaired generation of these secondary messengers could impact both of the main islet phenotypes found in IUGR, decreased insulin secretion and decreased β-cell mass (Cline et al. 2011; Doliba et al. 2007; Erion, et al. 2015; Fridlyand and Philipson 2016; Gleason et al. 2007).
Hypoxemia, catecholamines and β-cell dysfunction
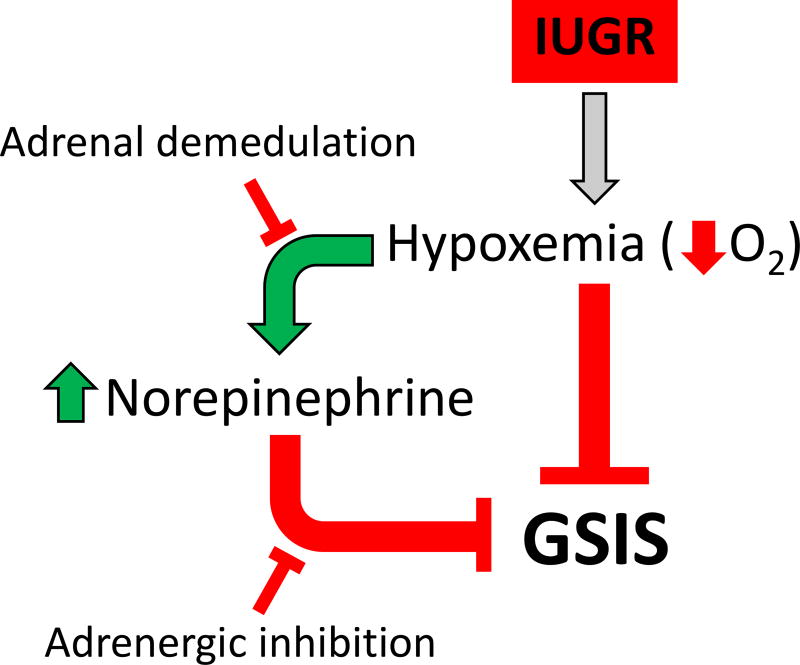
Fetal hypoxemia is a common feature in human and animal models of placental insufficiency that is proposed to have direct and indirect actions that inhibit insulin secretion and is also implicated in the developmental programming of pancreatic islets. Direct effects of oxygen on insulin secretion has been demonstrated in isolated adult rat and canine pancreatic islets (Dionne, et al. 1993), but until recently the effects have not been isolated in IUGR fetuses because of the parallel increase in circulating catecholamines seen with hypoxemia (Yates, et al. 2012). Surgical procedures to ablate the adrenal medullae of control and PI-IUGR fetal sheep at 65% and 85% of gestation were used to separate the effects of hypercatecolinemia and hypoxemia (Macko, et al. 2016; Yates et al. 2012). In these studies, we demonstrated that acutely decreased blood oxygen concentrations inhibit GSIS only in presence of an intact adrenal gland, presumably, due to catecholamine secretion. In PI-IUGR fetuses with chronic hypoxemia, GSIS was significantly improved, despite similar blood oxygen content, following ablation of the adrenal medullae compared to the intact, sham operated PI-IUGR fetuses. However, the GSIS was not fully restored to control fetal sheep levels. Acutely increasing fetal oxygen concentrations by maternal tracheal insufflation of humidified oxygen to 100%, significantly increased GSIS in both intact, sham operated and adrenal demedullated IUGR fetuses. The increased GSIS these fetuses were independent of changes in circulating fetal norepinephrine concentrations, demonstrating some inhibition of GSIS by hypoxemia in PI-IUGR fetuses. These studies show that catecholamines and hypoxemia have both dependent and independent roles in suppressing fetal insulin secretion in PI-IUGR (Figure 3). This conclusion is supported by experiments demonstrating lower GSIS following nine days of fetal anemic hypoxemia (Benjamin, et al. 2017).
Figure 3. A schematic representation of hypoxic and adrenergic regulation of insulin secretion in PI-IUGR fetuses.
Hypoxemia is induced in PI-IUGR and plasma catecholamine concentrations increase to inhibit fetal GSIS. Experimental interventions to disrupt catecholamine signaling are depicted.
Pharmacological adrenergic receptor blockade enhanced GSIS in PI-IUGR fetuses at 70% and 90% gestation, providing even more evidence that catecholamines persistently suppress insulin secretion during the second half of gestation (Leos, et al. 2010; Macko, et al. 2013). In contrast to the adrenal demedullated PI-IUGR fetuses, a compensatory enhancement in GSIS was observed in PI-IUGR fetuses with acute pharmacological inhibition of adrenergic receptors to levels even greater than control fetuses treated with pharmacological adrenergic inhibitor. This was independent of any changes in oxygen content (Leos et al. 2010; Macko et al. 2013). The improved insulin secretion also occurs in fetuses despite lower β-cell mass, which was shown at both gestation ages (Limesand et al. 2005; Limesand et al. 2013). Further evidence for fetal β-cell hyper-responsiveness to glucose stimulation following inhibition of chronic adrenergic suppression was provided by experiments in near term control fetal sheep following 7–11 days of a chronic fetal insulin infusion where fetal hypoxemia and hypercatecholinemia developed and GSIS was completely attenuated. However, GSIS was completely restored to control levels during acute pharmacological adrenergic inhibition, (Andrews, et al. 2015) similar to observations in PI-IUGR fetuses. Recently, we confirmed a direct role for chronically elevated norepinephrine concentrations in the development of β-cell hyper-responsiveness to glucose stimulation following acute discontinuation of adrenergic signaling. After a seven day infusion of norepinephrine into near term control fetuses was discontinued, a compensatory and persistent increase in GSIS secretion was observed compared to vehicle infused fetuses (Chen, et al. 2014; Chen, et al. 2017). These experiments are the first to show fetal β-cell hyper-responsiveness to glucose stimulation following inhibition of chronic adrenergic suppression. Consistent with these in vivo data, IUGR fetal sheep islets at 90% gestation have higher expression of α-adrenergic receptors compared to controls, which likely explains the persistent adrenergic suppression of insulin secretion despite chronic exposure of the islet to high circulating catecholamine concentrations. This may also explain why β-cells remain defective postnatally because increased expression of α-adrenergic receptors can lead to indiscriminant adrenergic signaling in islets (Leos et al. 2010; Macko et al. 2013; Rosengren, et al. 2010). Together, these studies show that catecholamines not only have inhibitory effects on insulin secretion in placental insufficiency, but also that as an adaptation to chronic elevations in fetal catecholamine concentrations, the pancreatic β-cells become hyper-responsive to glucose when the adrenergic inhibition is removed. This phenomenon explains the observation that some previously IUGR fetuses develop features of hyperinsulinism shortly after birth (Arya, et al. 2013; Camacho, et al. 2017; Collins and Leonard 1984).
Pancreatic vascularity and paracrine signaling
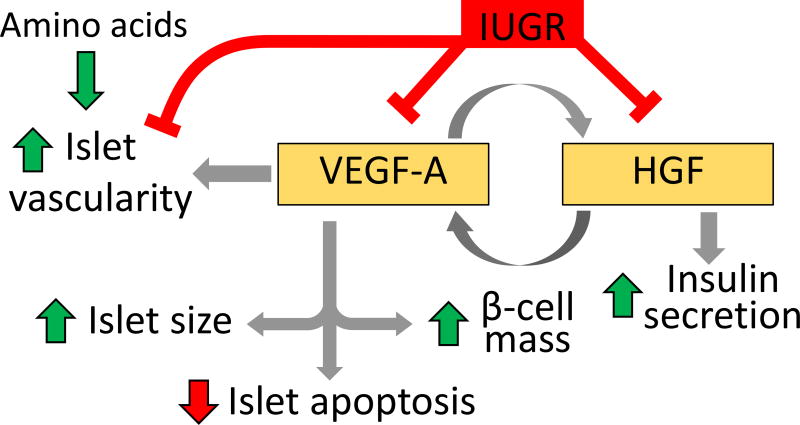
Pancreatic vascularity and paracrine signaling between endothelial cells and β-cells represent another possible mechanism linking placental insufficiency and impaired β-cell function in IUGR fetuses as depicted in Figure 4. Lower pancreatic islet vascularity has been observed in severely growth restricted humans at approximately 80% of gestation (Van Assche et al. 1977). Similar reductions in pancreatic islet vascularity occur in animal models of IUGR as early as 70% of gestation (Boujendar, et al. 2003; Ham, et al. 2009; Rozance, et al. 2015). By 90% of gestation the decrement between control and PI-IUGR fetuses in islet size is greater than at 70% of gestation, but the vessel density is equivalent (Rozance et al. 2015), suggesting that islet size was limited by vascular supply. Islet expression of the potent angiogenic factor vascular endothelial growth factor A (VEGFA) was decreased at 90% of gestation, in LP rats (Boujendar et al. 2002; Dumortier et al. 2007; Petrik et al. 1999; Snoeck et al. 1990), UAL rats (Ham et al. 2009), and PI-IUGR sheep. Although VEGFA expression is critical in in islet development, VEGFA inactivation in adult mice islets results in decreased islet vascularity but only slightly impaired glucose metabolism and insulin secretion (Reinert, et al. 2013).
Figure 4. A schematic representation of paracrine signaling between vascular endothelial growth factor A (VEGFA) and hepatocyte growth factor (HGF) in the fetal pancreas.
Increased VEGFA enhances endothelial cell HGF, islet size, islet vascularity, and β-cell mass, and decreases islet apoptosis. Increased HGF increases islet VEGFA and enhances islet insulin secretion. As depicted by the red lines, IUGR decreases expression of islet vascularity and VEFGA, as well as endothelial cell HGF. VEFGA expression is increased with exogenous amino acid supplementation.
Decreased islet vascularity has important implications for β-cell function because there are multiple mechanisms of action by which endothelial cells stimulate β-cells (Beattie, et al. 1996; Brissova, et al. 2006; Lammert, et al. 2003; Nikolova, et al. 2006). One of these pathways implicated in IUGR is the hepatocyte growth factor (HGF) pathway, as pancreatic islet endothelial cells isolated from PI-IUGR fetuses produced less HGF compared to islet endothelial cells isolated from control fetal sheep (Rozance et al. 2015). HGF increases the insulin content of islets isolated from both PI-IUGR and control fetal sheep (Brown et al. 2016; Rozance et al. 2015). In adult islets, HGF also increases VEGFA secretion (Johansson, et al. 2006). Finally, a complete mixture of amino acids, leucine individually, and VEGFA can all increase endothelial cell expression and/or production of HGF (Brown et al. 2016; Johansson et al. 2006). Importantly, when PI-IUGR fetuses were infused chronically with a complete mixture of amino acids enriched in the BCAAs, not only did islet size increase, but islet vascularity increased in proportion to the increase in islet size (Brown et al. 2016). Similar results occurred in the LP fetal rat, where gestational taurine supplementation prevented the decrease in fetal islet vascularity and VEGFA expression, and the increase in islet cell apoptosis (Abuzgaia et al. 2015). These were associated with higher β-cell mass in taurine supplemented LP fetal rats compared to non-supplemented LP fetal rats although insulin secretion was not enhanced (Boujendar et al. 2003; Boujendar et al. 2002). Together these findings suggest an impaired paracrine signaling loop between pancreatic endothelial and β-cells in cases of IUGR which may, in fact, be nutrient sensitive (reviewed in Rozance and Hay 2016).
Glucagon like peptide and inflammatory responses
Glucagon like peptide-1 and its receptor agonist Exendin-4 (Ex-4) increase β-cell mass and enhance insulin secretion in UAL rats (De León, et al. 2006; Stoffers 2004; Xu, et al. 1999). A more subtle increase in insulin secretion occurred in Ex-4 treated UC-PI neonatal sheep (Liu, et al. 2015), which may be due to the relative maturity of the neonatal sheep pancreas compared to the rat. In addition to preventing the deleterious effects of placental insufficiency on pancreatic islet dysfunction and the later development of diabetes, neonatal Ex-4 attenuated the decrease in pancreatic vascularity and islet VEGFA in UAL rats (Ham et al. 2009). The effect of Ex-4 occurs, in part, by reversing histone deacetylation and DNA methylation in the proximal promoter of Pdx1 in IUGR islets (Pinney, et al. 2011). UAL rat fetuses are characterized by pancreatic islet lymphocyte and macrophage invasion with an associated transient inflammatory response which precedes the decrease in β-cell mass, GSIS, and islet vascularity. The local fetal islet immune and inflammatory response was inhibited with administration of a neutralizing interleukin-4 (IL-4) antibody during the neonatal period. The neutralizing IL-4 antibody also restored islet vascularity and β-cell function into adulthood, mechanistically linking inflammation with progressive islet failure in this model of IUGR. These results further underscore the importance of blood vessel and endothelial cell interactions with the β-cell in the pathogenesis of impaired islet development and function following placental insufficiency.
Summary
Further studies are needed to better characterize impaired islet and β-cell development and function throughout the lifespan of IUGR humans. In the meantime, results from animal models have provided substantial insights in the etiology of β-cell dysfunction in IUGR. Considerable evidence from the interventional studies discussed in this review indicates islet vascularity is an important regulator of β-cell development and function and that signaling between the endothelial cell and β-cell is likely to be involved in the pathogenesis of impaired islet function following placental insufficiency. Studies in both rat and sheep models have shown depressed islet vascularity and VEGFA in the IUGR fetal islets, which are improved by a number of interventions, including neutralizing IL-4 antibodies, Ex-4, and amino acids (Brown et al. 2016; Ham et al. 2009; Lammert et al. 2003; Liu et al. 2015; Rozance et al. 2015). Reduced vascularity in the IUGR islets may result in either reduced nutrient transfer to the β-cell or attenuate its ability to release insulin (Brissova et al. 2006; Richards, et al. 2010). Additionally, the timing and duration of nutritional insults are critical in determining the severity of impaired islet function in IUGR. Although decreased β-cell mass, hypoinsulinemia, and lower insulin secretion are observed by 70% of gestation in IUGR fetal sheep, the magnitude of the decrement in these features of IUGR sheep becomes more pronounced as gestation progresses (Limesand et al. 2005; Limesand et al. 2013; Limesand et al. 2006). Likewise, earlier restriction in UAL fetal rats (E17 vs. E18) results in a greater reduction of β-cell mass at one day of age (De Prins and Van Assche 1982; Simmons et al. 2001). Similarly, the negative impact of nutrient restriction is enhanced when these restrictions are continued through lactation (Dumortier et al. 2007; Garofano et al. 1999).
Future directions
Recently, emergent –omic technologies have been adopted for measurement of various molecules in SGA infants and animal models of IUGR. They provide a new opportunity for understanding the links between placental insufficiency and the later development of T2DM (Atzori, et al. 2009; Dessì, et al. 2013). Metabolic profiles of SGA children experiencing delayed “catch up” growth suggest reduced insulin and IGF-I phospho-kinase mediated signaling (PI3K, AKT, and ERK). This likely reflects changes in cellular metabolism associated with insulin sensitivity and growth failure observed in these children (Murray, et al. 2016). Differences in the metabolic profiles of IUGR or SGA neonates support the concept that defects in glucose and amino acid metabolism, insulin signaling, and angiogenesis contribute to the etiology of IUGR (Conde-Agudelo, et al. 2013; Dessì, et al. 2012). Another emergent technology, noninvasive, in vivo β-cell imaging, is being developed for clinical and investigational applications (Souza, et al. 2006). Fluorescence imaging combined with several microscopy techniques have been successfully utilized to verify responsiveness to glucose and vascular networking in islet transplants or excised pancreases (Nyman, et al. 2008; Speier, et al. 2008). Adaptations of clinical imaging techniques including MRI, PET, and SPECT have been used to distinguish the exocrine and endocrine pancreas and increased resolution of these techniques may be enhanced by the continued evaluation of β-cell specific contrast agents and radio-ligands (Antkowiak, et al. 2009; Moore 2009; Steyn, et al. 2015; Willekens, et al. 2015). Further development of in vivo imaging techniques will allow for detailed investigation of complex cellular processes regulating β-cell proliferation, vascularization, and insulin secretion in pathophysiological conditions. These studies will provide valuable insights to the etiology of impaired β-cell function following IUGR and how the β-cell mass changes over the lifespan of these individuals.
Current reviews suggest maternal or neonatal nutrient supplementation has little impact in mitigating IUGR in humans (Brown et al. 2011; Devaskar and Chu 2016; Hay 2008). Furthermore, most nutritional interventions are associated with increased adiposity and not increased linear growth. More promising are interventions which increase uterine blood flow (Carr, et al. 2016; David, et al. 2008; Mehta, et al. 2014; Oyston, et al. 2016; Satterfield, et al. 2010; von Dadelszen, et al. 2011). Despite this progress, animal studies are required to evaluate the effect of these interventions on the fetal pathophysiological processes described in this review and to refine further the therapies to specific complications of placental insufficiency. Given the length of time between birth and the development of T2DM in humans, animal models are also required to test the impact of perinatal interventions on the development of insulin resistance, β-cell failure and T2DM throughout the lifespan. In addition to improving the healthy growth of the fetus and prolonging pregnancy, interventions that restore normal islet development and β-cell function are needed to diminish the onset of obesity, insulin resistance, and T2DM as these individuals reach adulthood.
Acknowledgments
Funding
This work was supported by NIH Grants R01DK088139 (PJR, PI), T32007186 (BHB, Fellow), and R01DK084842 (SWL, PI). This content is solely the responsibility of the authors and does not represent the views of NICHD or NIDDK.
Footnotes
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- Abuzgaia AM, Hardy DB, Arany E. Regulation of postnatal pancreatic Pdx1 and downstream target genes after gestational exposure to protein restriction in rats. Reproduction. 2015;149:293–303. doi: 10.1530/REP-14-0245. [DOI] [PubMed] [Google Scholar]
- Aldoretta PW, Hay WW. Effect of glucose supply on ovine uteroplacental glucose metabolism. Am J Physiol Regul Integr Comp Physiol. 1999;277:R947–R958. doi: 10.1152/ajpregu.1999.277.4.R947. [DOI] [PubMed] [Google Scholar]
- Andersson LE, Valtat B, Bagge A, Sharoyko VV, Nicholls DG, Ravassard P, Scharfmann R, Spégel P, Mulder H. Characterization of stimulus-secretion coupling in the human pancreatic endoc-βh1 beta cell line. PLoS ONE. 2015;10:e0120879. doi: 10.1371/journal.pone.0120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SE, Brown LD, Thorn SR, Limesand SW, Davis M, Hay WW, Rozance PJ. Increased adrenergic signaling is responsible for decreased glucose-stimulated insulin secretion in the chronically hyperinsulinemic ovine fetus. Endocrinology. 2015;156:367–376. doi: 10.1210/en.2014-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkowiak PF, Tersey SA, Carter JD, Vandsburger MH, Nadler JL, Epstein FH, Mirmira RG. Noninvasive assessment of pancreatic β-cell function in vivo with manganese-enhanced magnetic resonance imaging. Am J Physiol Endocrinol Metab. 2009;296:E573–E578. doi: 10.1152/ajpendo.90336.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya VB, Flanagan SE, Kumaran A, Shield JP, Ellard S, Hussain K, Kapoor RR. Clinical and molecular characterisation of hyperinsulinaemic hypoglycaemia in infants born small-for-gestational age. Arch Dis Child Fetal Neonatal Ed. 2013;98:F356–F358. doi: 10.1136/archdischild-2012-302880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori L, Antonucci R, Barberini L, Griffin JL, Fanos V. Metabolomics: A new tool for the neonatologist. J Matern Fetal Neonatal Med. 2009;22:50–53. doi: 10.1080/14767050903181500. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Hales CN, Fall CHD, Osmond C, Phipps K, Clark PMS. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Bazaes RA, Salazar TE, Pittaluga E, Peña V, Alegría A, Íñiguez G, Ong KK, Dunger DB, Mericq MV. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics. 2003;111:804–809. doi: 10.1542/peds.111.4.804. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Rubin JS, Mally MI, Otonkoski T, Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes. 1996;45:1223–1228. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]
- Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol. 1987;9:17–29. [PubMed] [Google Scholar]
- Benjamin JS, Culpepper CB, Brown LD, Wesolowski SR, Jonker SS, Davis MA, Limesand SW, Wilkening RB, Hay WW, Rozance PJ. Chronic anemic hypoxemia attenuates glucose-stimulated insulin aecretion in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2017 doi: 10.1152/ajpregu.00484.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béringue F, Blondeau B, Castellotti MC, Bréant B, Czernichow P, Polak M. Endocrine pancreas development in growth-retarded human fetuses. Diabetes. 2002;51:385–391. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- Boujendar S, Arany E, Hill D, Remacle C, Reusens B. Taurine supplementation of a low protein diet fed to rat dams normalizes the vascularization of the fetal endocrine pancreas. J Nutr. 2003;133:2820–2825. doi: 10.1093/jn/133.9.2820. [DOI] [PubMed] [Google Scholar]
- Boujendar S, Reusens B, Merezak S, Ahn M-T, Arany E, Hill D, Remacle C. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia. 2002;45:856–866. doi: 10.1007/s00125-002-0833-6. [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, et al. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- Brown LD, Davis M, Wai S, Wesolowski SR, Hay WW, Jr, Limesand SW, Rozance PJ. Chronically increased amino acids improve insulin secretion, pancreatic vascularity, and islet size in growth-restricted fetal sheep. Endocrinology. 2016;157:3788–3799. doi: 10.1210/en.2016-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 2011;3:428–444. doi: 10.2741/s162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW. Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab. 2009;296:E56–E63. doi: 10.1152/ajpendo.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW. Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab. 2012;303:E352–E364. doi: 10.1152/ajpendo.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho LE, Chen X, Hay WW, Limesand SW. Enhanced insulin secretion and insulin sensitivity in young lambs with placental insufficiency-induced intrauterine growth restriction. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2017 doi: 10.1152/ajpregu.00068.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Wallace JM, Aitken RP, Milne JS, Martin JF, Zachary IC, Peebles DM, David AL. Peri- and postnatal effects of prenatal adenoviral VEGF gene therapy in growth-restricted sheep. Biol Reprod. 2016 doi: 10.1095/biolreprod.115.133744. In Press. [DOI] [PubMed] [Google Scholar]
- Cetin I. Placental transport of amino acids in normal and growth-restricted pregnancies. Eur J Obstet Gynecol Reprod Biol. 2003;110(Supplement):S50–S54. doi: 10.1016/s0301-2115(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol. 1990;162:253–261. doi: 10.1016/0002-9378(90)90860-a. [DOI] [PubMed] [Google Scholar]
- Chen X, Green AS, Macko AR, Yates DT, Kelly AC, Limesand SW. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am J Physiol Endocrinol Metab. 2014;306:E58–E64. doi: 10.1152/ajpendo.00517.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kelly AC, Yates DT, Macko AR, Lynch RM, Limesand SW. Islet adaptations in fetal sheep persist following chronic exposure to high norepinephrine. J Endocrinol. 2017;232:285–295. doi: 10.1530/JOE-16-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline GW, Pongratz RL, Zhao X, Papas KK. Rates of insulin secretion in INS-1 cells are enhanced by coupling to anaplerosis and Kreb’s cycle flux independent of ATP synthesis. Biochem Biophys Res Commun. 2011;415:30–35. doi: 10.1016/j.bbrc.2011.09.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JE, Leonard JV. Hyperinsulinism in asphyxiated and small-for-dates infants with hypoglycaemia. Lancet. 1984;324:311–313. doi: 10.1016/s0140-6736(84)92685-0. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: A systematic review and meta-analysis. BJOG. 2013;120:681–694. doi: 10.1111/1471-0528.12172. [DOI] [PubMed] [Google Scholar]
- Conrad E, Stein R, Hunter CS. Revealing transcription factors during human pancreatic β cell development. Trends Endocrinol Metab. 2014;25:407–414. doi: 10.1016/j.tem.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JT, Levy JC, Page RC, Shaw JA, Hattersley AT, Turner RC. Association of low birth weight with beta cell function in the adult first degree relatives of non-insulin dependent diabetic subjects. BMJ. 1993;306:302–306. doi: 10.1136/bmj.306.6873.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovetto F, Triunfo S, Crispi F, Rodriguez-Sureda V, Dominguez C, Figueras F, Gratacos E. Differential performance of first trimester screening in predicting small for gestational age neonates or fetal growth restriction. Ultrasound Obstet Gynecol. 2016;49:349–356. doi: 10.1002/uog.15919. [DOI] [PubMed] [Google Scholar]
- Crowther NJ, Trusler J, Cameron N, Toman M, Gray PI. Relation between weight gain and beta-cell secretory activity and non-esterified fatty acid production in 7-year-old African children: results from the Birth to Ten study. Diabetologia. 2000;43:978–985. doi: 10.1007/s001250051479. [DOI] [PubMed] [Google Scholar]
- David AL, Torondel B, Zachary I, Wigley V, Nader KA, Mehta V, Buckley SMK, Cook T, Boyd M, Rodeck CH, et al. Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep. Gene Ther. 2008;15:1344–1350. doi: 10.1038/gt.2008.102. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology. 2007;148:1350–1358. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- De León DD, Crutchlow MF, Ham J-YN, Stoffers DA. Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int J Biochem Cell Biol. 2006;38:845–859. doi: 10.1016/j.biocel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- De Prins F, Van Assche F. Intrauterine growth retardation and development of endocrine pancreas in the experimental rat. Biol Neonate. 1982;41:16–21. doi: 10.1159/000241511. [DOI] [PubMed] [Google Scholar]
- de Vrijer B, Regnault TRH, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab. 2004;287:E1114–E1124. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]
- Delghingaro-Augusto V, Madad L, Chandra A, Simeonovic CJ, Dahlstrom JE, Nolan CJ. Islet inflammation, hemosiderosis, and fibrosis in intrauterine growth-restricted and high fat-fed Sprague-Dawley rats. Am J Pathol. 2014;184:1446–1457. doi: 10.1016/j.ajpath.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Dessì A, Ottonello G, Fanos V. Physiopathology of intrauterine growth retardation: from classic data to metabolomics. J Matern Fetal Neonatal Med. 2012;25:13–18. doi: 10.3109/14767058.2012.714639. [DOI] [PubMed] [Google Scholar]
- Dessì A, Puddu M, Ottonello G, Fanos V. Metabolomics and fetal-neonatal nutrition: Between"not enough” and"too much”. Molecules. 2013;18:11724–11732. doi: 10.3390/molecules181011724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaskar SU, Chu A. Intrauterine growth restriction: Hungry for an answer. Physiology (Bethesda) 2016;31:131–146. doi: 10.1152/physiol.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiacomo JE, Hay WW. Fetal glucose metabolism and oxygen consumption during sustained hypoglycemia. Metabolism. 1990;39:193–202. doi: 10.1016/0026-0495(90)90075-n. [DOI] [PubMed] [Google Scholar]
- Dionne KE, Colton CK, Lyarmush M. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- Doliba NM, Wehrli SL, Vatamaniuk MZ, Qin W, Buettger CW, Collins HW, Matschinsky FM. Metabolic and ionic coupling factors in amino acid-stimulated insulin release in pancreatic β-HC9 cells. Am J Physiol Endocrinol Metab. 2007;292:E1507–E1519. doi: 10.1152/ajpendo.00282.2006. [DOI] [PubMed] [Google Scholar]
- Dumortier O, Blondeau B, Duvillie B, Reusens B, Bréant B, Remacle C. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia. 2007;50:2495–2503. doi: 10.1007/s00125-007-0811-0. [DOI] [PubMed] [Google Scholar]
- Economides D, Proudler A, Nicolaides K. Plasma insulin in appropriate-and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;160:1091–1094. doi: 10.1016/0002-9378(89)90167-1. [DOI] [PubMed] [Google Scholar]
- Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Chronic exposure to excess nutrients left-shifts the concentration dependence of glucose-stimulated insulin secretion in pancreatic β-cells. J Biol Chem. 2015;290:16191–16201. doi: 10.1074/jbc.M114.620351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius-Bjerre S, Jensen RB, Færch K, Larsen T, Mølgaard C, Michaelsen KF, Vaag A, Greisen G. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS ONE. 2011;6:e20595. doi: 10.1371/journal.pone.0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Hughes P, Comline RS. The effects of insulin on the growth rate of the sheep fetus during late gestation. Q J Exp Physiol. 1989;74:703–714. doi: 10.1113/expphysiol.1989.sp003322. [DOI] [PubMed] [Google Scholar]
- Fridlyand LE, Philipson LH. Pancreatic beta cell G-protein coupled receptors and second messenger interactions: A systems biology computational analysis. PLoS ONE. 2016;11:e0152869. doi: 10.1371/journal.pone.0152869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadhia MM, Maliszewski AM, O'Meara MC, Thorn SR, Lavezzi JR, Limesand SW, Hay WW, Jr, Brown LD, Rozance PJ. Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase β-cell mass in fetal sheep. Am J Physiol Endocrinol Metab. 2013;304:E352–E362. doi: 10.1152/ajpendo.00377.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, Battaglia FC. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol. 1999;180:1278–1282. doi: 10.1016/s0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- Galan HL, Marconi AM, Paolini CL, Cheung A, Battaglia FC. The transplacental transport of essential amino acids in uncomplicated human pregnancies. Am J Obstet Gynecol. 2009;200:91.e91–91.e97. doi: 10.1016/j.ajog.2008.06.054. [DOI] [PubMed] [Google Scholar]
- Gao Z, Young RA, Li G, Najafi H, Buettger C, Sukumvanich SS, Wong RK, Wolf BA, Matschinsky FM. Distinguishing features of leucine and α-ketoisocaproate sensing in pancreatic β-cells. Endocrinology. 2003;144:1949–1957. doi: 10.1210/en.2002-0072. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat beta-cell development. Diabetologia. 1997;40:1231–1234. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. Beta-cell mass and proliferation following late fetal and early postnatal malnutrition in the rat. Diabetologia. 1998;41:1114–1120. doi: 10.1007/s001250051038. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. Effect of ageing on beta-cell mass and function in rats malnourished during the perinatal period. Diabetologia. 1999;42:711–718. doi: 10.1007/s001250051219. [DOI] [PubMed] [Google Scholar]
- Gatford KL, Mohammad SNB, Harland ML, De Blasio MJ, Fowden AL, Robinson JS, Owens JA. Impaired β-cell function and inadequate compensatory increases in β-cell mass after intrauterine growth restriction in sheep. Endocrinology. 2008;149:5118–5127. doi: 10.1210/en.2008-0233. [DOI] [PubMed] [Google Scholar]
- German MS. Glucose sensing in pancreatic islet beta cells: the key role of glucokinase and the glycolytic intermediates. Proc Natl Acad Sci U S A. 1993;90:1781–1785. doi: 10.1073/pnas.90.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic β-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- Göhring I, Sharoyko VV, Malmgren S, Andersson LE, Spégel P, Nicholls DG, Mulder H. Chronic high glucose and pyruvate levels differentially affect mitochondrial bioenergetics and fuel-stimulated insulin secretion from clonal INS-1 832/13 cells. J Biol Chem. 2014;289:3786–3798. doi: 10.1074/jbc.M113.507335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010;205:211–224. doi: 10.1677/JOE-09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A, Nicolaides KH, Lagercrantz H. Human fetal sympathoadrenal responsiveness. Early Human Development. 1990;23:9–13. doi: 10.1016/0378-3782(90)90124-2. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Ham JN, Crutchlow MF, Desai BM, Simmons RA, Stoffers DA. Exendin-4 normalizes islet vascularity in intrauterine growth restricted rats: Potential role of VEGF. Pediatr Res. 2009;66:42–46. doi: 10.1203/PDR.0b013e3181a282a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay WW. Strategies for feeding the preterm infant. Neonatology. 2008;94:245–254. doi: 10.1159/000151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay WW, Meznarich HK, Digiacomo JE, Hirst K, Zerbe G. Effects of insulin and glucose concentrations on glucose utilization in fetal sheep. Pediatr Res. 1988;23:381–387. doi: 10.1203/00006450-198804000-00008. [DOI] [PubMed] [Google Scholar]
- Hendrix N, Berghella V. Non-placental causes of intrauterine growth restriction. Semin Perinatol. 2008;32:161–165. doi: 10.1053/j.semperi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- Huang M, Joseph JW. Metabolomic analysis of pancreatic β-cell insulin release in response to glucose. Islets. 2012;4:210–222. doi: 10.4161/isl.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–1019. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Shoji H, Suganuma H, Ohkawa N, Kantake M, Murano Y, Sakuraya K, Shimizu T. Effect of insulin-like growth factor-I during the early postnatal period in intrauterine growth-restricted rats. Pediatr Int. 2016;58:353–358. doi: 10.1111/ped.12855. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jennings RE, Berry AA, Strutt JP, Gerrard DT, Hanley NA. Human pancreas development. Development. 2015;142:3126–3137. doi: 10.1242/dev.120063. [DOI] [PubMed] [Google Scholar]
- Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA. Early differential defects of insulin secretion and action in 19-year-old Caucasian men who had low birth weight. Diabetes. 2002;51:1271–1280. doi: 10.2337/diabetes.51.4.1271. [DOI] [PubMed] [Google Scholar]
- Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson P-O. Islet endothelial cells and pancreatic β-cell proliferation: Studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Strang-Karlsson S, Hovi P, Wehkalampi K, Lahti J, Kaseva N, Järvenpää A-L, Räikkönen K, Eriksson JG, Andersson S. Insulin sensitivity and secretory response in adults born preterm: The Helsinki Study of very low birth weight adults. J Clin Endocrinol Metab. 2015;100:244–250. doi: 10.1210/jc.2014-3184. [DOI] [PubMed] [Google Scholar]
- Kasuga M. Insulin resistance and pancreatic β cell failure. J Clin Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AC, Bidwell CA, McCarthy FM, Taska DJ, Andersen MJ, Camacho LE, Limesand SW. RNA sequencing exposes novel adaptive and immune responses to intrauterine growth restriction in fetal sheep islets. Endocrinology. 2017;158:743–755. doi: 10.1210/en.2016-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber H-P, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Lavezzi JR, Thorn SR, O'Meara MC, LoTurco D, Brown LD, Hay WW, Rozance PJ. Increased fetal insulin concentrations for one week fail to improve insulin secretion or β-cell mass in fetal sheep with chronically reduced glucose supply. Am J Physiol Regul Integr Comp Physiol. 2013;304:R50–R58. doi: 10.1152/ajpregu.00413.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298:E770–E778. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand SW, Hay WW. Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol. 2003;547:95–105. doi: 10.1113/jphysiol.2002.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand SW, Jensen J, Hutton JC, Hay WW. Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–R1305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW. Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab. 2013;304:E516–E523. doi: 10.1152/ajpendo.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147:1488–1497. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- Liu H, Schultz CG, De Blasio MJ, Peura AM, Heinemann GK, Harryanto H, Hunter DS, Wooldridge AL, Kind KL, Giles LC, et al. Effect of placental restriction and neonatal exendin-4 treatment on postnatal growth, adult body composition, and in vivo glucose metabolism in the sheep. Am J Physiol Endocrinol Metab. 2015;309:E589–E600. doi: 10.1152/ajpendo.00487.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MJ, McKenzie DI, Kaysen JH, Walker TM, Moran SM, Fahien LA, Towle HC. Glucose regulates leucine-induced insulin release and the expression of the branched chain ketoacid dehydrogenase E1 alpha subunit gene in pancreatic islets. Journal of Biological Chemistry. 1991;266:1335–1340. [PubMed] [Google Scholar]
- Macko AR, Yates DT, Chen X, Green AS, Kelly AC, Brown LD, Limesand SW. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced β-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. J Dev Orig Health Dis. 2013;4:402–410. doi: 10.1017/S2040174413000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko AR, Yates DT, Chen X, Shelton LA, Kelly AC, Davis MA, Camacho LE, Anderson MJ, Limesand SW. Adrenal demedullation and oxygen supplementation independently increase glucose-stimulated insulin concentrations in fetal sheep with intrauterine growth restriction. Endocrinology. 2016;157:2104–2115. doi: 10.1210/en.2015-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W, Sener A, Herchuelz A, Hutton J. Insulin release: The fuel hypothesis. Metabolism. 1979;28:373–386. doi: 10.1016/0026-0495(79)90111-2. [DOI] [PubMed] [Google Scholar]
- Maliszewski AM, Gadhia MM, O'Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab. 2012;302:E1483–E1492. doi: 10.1152/ajpendo.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi AM, Paolini CL, Stramare L, Cetin I, Fennessey PV, Pardi G, Battaglia FC. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res. 1999;46:114–119. doi: 10.1203/00006450-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Marconi AM, Paolini CL, Zerbe G, Battaglia FC. Lactacidemia in intrauterine growth restricted (IUGR) pregnancies: Relationship to clinical severity, oxygenation and placental weight. Pediatr Res. 2006;59:570–574. doi: 10.1203/01.pdr.0000205477.70391.3e. [DOI] [PubMed] [Google Scholar]
- Mathai S, Cutfield WS, Derraik JGB, Dalziel SR, Harding JE, Robinson E, Biggs J, Jefferies C, Hofman PL. Insulin sensitivity and β-cell function in adults born preterm and their children. Diabetes. 2012;61:2479–2483. doi: 10.2337/db11-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24:219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- Mehta V, Abi-Nader KN, Shangaris P, Shaw SW, Filippi E, Benjamin E, Boyd M, Peebles DM, Martin J, Zachary I, et al. Local over-expression of VEGF-DΔNΔC in the uterine arteries of pregnant sheep results in long-term changes in uterine artery contractility and angiogenesis. PLoS ONE. 2014;9:e100021. doi: 10.1371/journal.pone.0100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor DJ, Mitchell B, Matheson IC. Reductions in lamb weight caused by pre-mating carunclectomy and mid-pregnancy placental ablation. J Comp Pathol. 1977;87:629–633. doi: 10.1016/0021-9975(77)90070-6. [DOI] [PubMed] [Google Scholar]
- Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small-and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- Milner RDG. Stimulation of insulin secretion in vitro by essential amino acids. The Lancet. 1969;293:1075–1076. doi: 10.1016/s0140-6736(69)91709-7. [DOI] [PubMed] [Google Scholar]
- Milovanovic I, Njuieyon F, Deghmoun S, Chevenne D, Levy-Marchal C, Beltrand J. SGA children with moderate catch-up growth are showing the impaired insulin secretion at the age of 4. PLoS ONE. 2014;9:e100337. doi: 10.1371/journal.pone.0100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina RD, Carver TD, Hay WW. Ontogeny of insulin effect in fetal sheep. Pediatr Res. 1993;34:654–660. doi: 10.1203/00006450-199311000-00018. [DOI] [PubMed] [Google Scholar]
- Moore A. Advances in beta-cell imaging. Eur J Radiol. 2009;70:254–257. doi: 10.1016/j.ejrad.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PG, Butcher I, Dunn WB, Stevens A, Perchard R, Hanson D, Whatmore A, Westwood M, Clayton PE. Metabolites involved in glycolysis and amino acid metabolism are altered in short children born small for gestational age. Pediatr Res. 2016 doi: 10.1038/pr.2016.72. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard Christopher B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, Brennan L, Bender K. Amino acid metabolism, β-cell function, and diabetes. Diabetes. 2006;55:S39–S47. [Google Scholar]
- Newsholme P, Cruzat V, Arfuso F, Keane K. Nutrient regulation of insulin secretion and action. J Endocrinol. 2014;221:R105–R120. doi: 10.1530/JOE-13-0616. [DOI] [PubMed] [Google Scholar]
- Nicolini U, Hubinont C, Santolaya J, Fisk N, Rodeck C. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–430. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber H-P, Ferrara N, et al. The Vascular Basement Membrane: A Niche for Insulin Gene Expression and β Cell Proliferation. Developmental Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. The Journal of Clinical Investigation. 2008;118:3790–3797. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism. 1986;35:970–977. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- Owens JA, Gatford KL, De Blasio MJ, Edwards LJ, McMillen IC, Fowden AL. Restriction of placental growth in sheep impairs insulin secretion but not sensitivity before birth. J Physiol. 2007;584:935–949. doi: 10.1113/jphysiol.2007.142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston C, Stanley JL, Oliver MH, Bloomfield FH, Baker PN. Maternal administration of sildenafil citrate alters fetal and placental growth and fetal–placental vascular resistance in the growth-restricted ovine fetus. Hypertension. 2016;68:760–767. doi: 10.1161/HYPERTENSIONAHA.116.07662. [DOI] [PubMed] [Google Scholar]
- Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- Patel MS, Srinivasan M, Strutt B, Mahmood S, Hill DJ. Featured Article: Beta cell specific pyruvate dehydrogenase alpha gene deletion results in a reduced islet number and β-cell mass postnatally. Exp Biol Med (Maywood) 2014;239:975–985. doi: 10.1177/1535370214531895. [DOI] [PubMed] [Google Scholar]
- Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology. 1999;140:4861–4873. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- Pinney SE, Jaeckle Santos LJ, Han Y, Stoffers DA, Simmons RA. Exendin-4 increases histone acetylase activity and reverses epigenetic modifications that silence Pdx1 in the intrauterine growth retarded rat. Diabetologia. 2011;54:2606–2614. doi: 10.1007/s00125-011-2250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers DG, Schuit FC, In’t Veld PA, Maes E, Hooghe-Peters EL, Van De Winkel M, Gepts W. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology. 1985;117:824–833. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- Platz E, Newman R. Diagnosis of IUGR: Traditional biometry. Semin Perinatol. 2008;32:140–147. doi: 10.1053/j.semperi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Regnault TRH, de Vrijer B, Battaglia FC. Transport and metabolism of amino acids in placenta. Endocrine. 2002a;19:23–41. doi: 10.1385/ENDO:19:1:23. [DOI] [PubMed] [Google Scholar]
- Regnault TRH, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies - A review. Placenta. 2002b;23:S119–S129. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C, et al. Vascular endothelial growth factor-A and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62:4154–4164. doi: 10.2337/db13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31:343–363. doi: 10.1210/er.2009-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J Dev Physiol. 1979;1:379–398. [PubMed] [Google Scholar]
- Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li D-Q, Nagaraj V, Reinbothe TM, Tuncel J, Eliasson L, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327:217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- Rozance PJ, Anderson M, Martinez M, Fahy A, Macko AR, Kailey J, Seedorf GJ, Abman SH, Hay WW, Limesand SW. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes. 2015;64:555–564. doi: 10.2337/db14-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozance PJ, Crispo MM, Barry JS, O'Meara MC, Frost MS, Hansen KC, Hay WW, Brown LD. Prolonged maternal amino acid infusion in late-gestation pregnant sheep increases fetal amino acid oxidation. Am J Physiol Endocrinol Metab. 2009a;297:E638–E646. doi: 10.1152/ajpendo.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozance PJ, Hay WW. Hypoglycemia in newborn infants: features associated with adverse outcomes. Neonatology. 2006;90:74–86. doi: 10.1159/000091948. [DOI] [PubMed] [Google Scholar]
- Rozance PJ, Hay WW. Pancreatic islet hepatocyte growth factor and vascular endothelial growth factor A signaling in growth restricted fetuses. Mol Cell Endocrinol. 2016 doi: 10.1016/j.mce.2016.01.025. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW. Glucose replacement to euglycemia causes hypoxia, acidosis, and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatr Res. 2009b;65:72–78. doi: 10.1203/PDR.0b013e318189358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozance PJ, Limesand SW, Hay WW. Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab. 2006;291:E404–E411. doi: 10.1152/ajpendo.00643.2005. [DOI] [PubMed] [Google Scholar]
- Rozance PJ, Limesand SW, Zerbe GO, Hay WW. Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic β-cells. Am J Physiol Endocrinol Metab. 2007;292:E1256–E1264. doi: 10.1152/ajpendo.00265.2006. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Bazer FW, Spencer TE, Wu G. Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr. 2010;140:251–258. doi: 10.3945/jn.109.114678. [DOI] [PubMed] [Google Scholar]
- Schuit FC, Pipeleers DG. Regulation of adenosine 3′,5′-monophosphate levels in the pancreatic β cell. Endocrinology. 1985;117:834–840. doi: 10.1210/endo-117-3-834. [DOI] [PubMed] [Google Scholar]
- Setia S, Sridhar MG, Bhat V, Chaturvedula L, Vinayagamoorti R, John M. Insulin sensitivity and insulin secretion at birth in intrauterine growth retarded infants. Pathology. 2006;38:236–238. doi: 10.1080/00313020600696256. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CAR, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58:827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- Siebel AL, Gallo LA, Guan TC, Owens JA, Wlodek ME. Cross-fostering and improved lactation ameliorates deficits in endocrine pancreatic morphology in growth-restricted adult male rat offspring. J Dev Orig Health Dis. 2010;1:234–244. doi: 10.1017/S2040174410000383. [DOI] [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of Type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Neonatology. 1990;57:107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- Souza F, Freeby M, Hultman K, Simpson N, Herron A, Witkowsky P, Liu E, Maffei A, Harris PE. Current progress in non-invasive imaging of beta cell mass of the endocrine pancreas. Curr Med Chem. 2006;13:2761–2773. doi: 10.2174/092986706778521940. [DOI] [PubMed] [Google Scholar]
- Spégel P, Malmgren S, Sharoyko Vladimir V, Newsholme P, Koeck T, Mulder H. Metabolomic analyses reveal profound differences in glycolytic and tricarboxylic acid cycle metabolism in glucose-responsive and -unresponsive clonal β-cell lines. Biochem J. 2011;435:277–284. doi: 10.1042/BJ20100655. [DOI] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn LV, Ananthakrishnan K, Anderson MJ, Patek R, Kelly A, Vagner J, Lynch RM, Limesand SW. A synthetic heterobivalent ligand composed of glucagon-like peptide 1 and yohimbine specifically targets β cells within the pancreas. Mol Imaging Biol. 2015;17:461–470. doi: 10.1007/s11307-014-0817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA. The development of beta-cell mass: Recent progress and potential role of GLP-1. Horm Metab Res. 2004;36:811–821. doi: 10.1055/s-2004-826168. [DOI] [PubMed] [Google Scholar]
- T Forsén T, Eriksson J, Tuomilehto J, Reunanen ACO, Barker D. SIze at birth, growth in childhood, and the risk for type 2 diabetes. Ann Intern Med. 2000;133:176–182. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- Van Assche FA, Prins FD, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. BJOG. 1977;84:751–753. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- von Beckerath A-K, Kollmann M, Rotky-Fast C, Karpf E, Lang U, Klaritsch P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol. 2013;208:130.e131–130.e136. doi: 10.1016/j.ajog.2012.11.014. [DOI] [PubMed] [Google Scholar]
- von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D, et al. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG. 2011;118:624–628. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol Cell Endocrinol. 2012;353:128–137. doi: 10.1016/j.mce.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Wigglesworth JS. Experimental growth retardation in the foetal rat. J Pathol Bacteriol. 1964;88:1–13. [PubMed] [Google Scholar]
- Willekens S, van der Kroon I, Bos D, Joosten L, Frielink C, Brom M, Gotthardt M. Quantitative imaging of transplanted pancreatic islets with SPECT using I-123 labeled benzamide. J Nucl Med. 2015;56:592. [Google Scholar]
- Willmann SJ, Mueller NS, Engert S, Sterr M, Burtscher I, Raducanu A, Irmler M, Beckers J, Sass S, Theis FJ, et al. The global gene expression profile of the secondary transition during pancreatic development. Mech Dev. 2016;139:51–64. doi: 10.1016/j.mod.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Breier BH, Johnston BM, Gluckman PD. A model of intra uterine growth retardation caused by chronic maternal undernutrition in the rat: Effect of the somatotropic axis and postnatal growth. J Endocrinol. 1996;150:231–242. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- Yates DT, Macko AR, Chen X, Green AS, Kelly AC, Anderson MJ, Fowden AL, Limesand SW. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J Physiol. 2012;590:5439–5447. doi: 10.1113/jphysiol.2012.237347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen W, Dai Y, Zhu Z, Liu Q. Detection of expressional changes induced by intrauterine growth restriction in the developing rat pancreas. Exp Biol Med (Maywood) 2016;241:1446–1456. doi: 10.1177/1535370216638771. [DOI] [PMC free article] [PubMed] [Google Scholar]