Abstract
Many learned responses depend on the coordinated activation and inhibition of synaptic pathways in the striatum. Local dopamine neurotransmission acts in concert with a variety of neurotransmitters to regulate cortical, thalamic, and limbic excitatory inputs to drive the direct and indirect striatal spiny projection neuron outputs that determine the activity, sequence and timing of learned behaviors. We review recent advances in the characterization of stereotyped neuronal and operant responses that predict and then obtain rewards. These depend on local release of dopamine at discrete times during behavioral sequences, that acting with glutamate, provides a presynaptic filter to select which excitatory synapses are inhibited and which signals pass to indirect pathway circuits. This is followed by dopamine-dependent activation of specific direct pathway circuits to procure a reward. These steps may provide a means by which higher organisms learn behaviors in response to feedback from the environment.
Introduction
For over 60 years (Olds and Milner, 1954), scientists have sought to unravel the brain structures, circuits, and neurotransmitters that govern reinforcement-associated behaviors, including reward seeking and retrieval (Wise, 2002). Using a variety of approaches including local electrical and optical stimuli and drug microinfusion, considerable progress has been made in identifying the brain regions that collectively form the brain “reward” pathway (Fields et al., 2007; Ikemoto, 2010). These interconnected structures are activated by both natural rewards and drugs of abuse (Sulzer, 2011). A central player in this circuitry is the ventral striatum, also known as the nucleus accumbens (NAc). More than 90% of the neuronal cell bodies in the NAc are spiny projection neurons (SPNs), also known as medium spiny neurons, that receive excitatory glutamatergic inputs from the medial prefrontal cortex, basal lateral amygdala, thalamus, and hippocampus (Ikemoto and Bonci, 2014; Wolf, 2016). SPN activity is modified by dopaminergic inputs from the ventral tegmental area (Ikemoto and Bonci, 2014; Wolf, 2016) that occurs with reinforcement-associated behaviors, and cholinergic inputs from the basal forebrain, as well as by GABAergic, peptidergic, and cholinergic local interneurons, and from axon collaterals of other SPNs. Here, we describe advances in the understanding of this circuitry and describe how dopamine in the striatum appears to regulate rapid neuronal responses during reward behavior.
Unraveling reward circuits
The discovery by James Olds and Peter Milner that rats would press a lever repeatedly to deliver an electrical pulse train to electrodes in their brain demonstrated direct evidence for the existence of a brain reward system (Olds and Milner, 1954). Their approach, now termed intracranial self-stimulation (ICSS), has been particularly important because it is rapidly learned by many species, and animals do not become satiated, enabling multiple repetitions.
Stimulation of many regions of the brain supports the responses to ICSS. After considerable experimentation, Olds and colleagues determined a specific site that was particularly effective at supporting this behavior. This site, the medial forebrain bundle, contains axons that project from the midbrain to higher order structures including the basal ganglia and cortex. Soon after, the same lab introduced “intracranial self-administration” in which a lever press provided injection of drugs directly into the brain, and comparison of injections sites confirmed that this bundle was integral to the learned reward behavior (Olds and Olds, 1958).
Shortly after the discovery of ICSS, the catecholamine dopamine, a molecule that plays many roles in plant and animal biology, was established as a neurotransmitter in the striatum (Carlsson et al., 1957). Prior to this recognition, experiments had shown that ICSS could be blocked by certain pharmacological agents. These agents either inhibited catecholamine storage by synaptic vesicles (reserpine) (Carlsson et al., 1957) or blocked dopamine receptors (Olds et al., 1956). Further mapping of the position for the stimulating electrode in the midbrain revealed that proximity to sites that activate dopamine neurons also produced optimal results (Corbett and Wise, 1980). Once the role and site of action of these drugs were recognized, dopamine was established as a key player in brain reward circuitry, particularly for drug self-administration, as virtually all self-administered drugs enhance dopamine neurotransmission (Sulzer, 2011). As Olds had surmised, a very broad array of primary reinforcers, and cues for primary reinforcers, activate this pathway, including in his words, “hunger, sex, and drugs” (Olds and Olds, 1958).
The initial insights into the temporal role of dopamine signaling in brain reward circuitry came from single-unit recordings of the firing of dopamine neurons. In this type of measurement, a microelectrode (a metal, carbon, or fluid-filled pipet) is inserted into the brain. When the microelectrode is proximal to a neuronal soma, voltage transients, termed single units, can be measured. Single units arise from action potentials in nearby neurons (Lemon and Prochazka, 1984), and thus the frequency of their occurrence is a measure of the firing rate. This approach was used in another pioneering study by Olds (Phillips and Olds, 1969), which showed that activity of the cell bodies of the neurons located in the ventral tegmental area of the midbrain increased following a sound cue that was paired with a lever press for food or water, depending on whether a rat was hungry or thirsty. These observations led to a conclusion that essentially remains a contemporary understanding of the system: that the activity of these neurons following a cue was dependent on motivation.
A body of subsequent literature by Wolfram Schultz and coworkers working with nonhuman primates (Mirenowicz and Schultz, 1996) demonstrated the firing patterns of dopaminergic neurons. Normally they fire at a relatively low frequency (~5Hz), referred to as tonic firing, and a burst of single units, referred to as phasic firing, occur in response to unexpected reward in the form of an appetitive liquid. Furthermore, when the subject learned that a cue predicted the delivery of the reward, the burst of unit activity transferred from the reward to the preceding cue. Thus, the dopamine system responded not only to primary reinforcers, but also learned secondary and even higher-level reinforcers. This pattern of cell firing is that predicted by temporal difference models of reward and prediction (Schultz et al., 1997). This mode of learning is suggested by some, including Ann Graybiel (Graybiel, 1998), to provide the basis for learning complex tasks through a process of learning higher level relationships of reinforcers known as “chunking” (Miller, 1956).
During the decades that measurements of reward-related unit activity were developed, parallel research developed a method to measure dopamine and other easily oxidized neurotransmitters deep within the brain at a sub-second time scale (Adams, 1976). These measurements were made with fast-scan cyclic voltammetry (FSCV) (Garris et al., 1997; Robinson et al., 2008) using carbon-fiber microelectrodes (Gonon et al., 1978). Early work showed that dopamine exhibited periodic concentration transients in the NAc in awake rats (Robinson et al., 2002), and these transients were shown to be a direct consequence of burst firing of dopamine neurons (Sombers et al., 2009), which punctuated a background level that occurred during tonic firing. These dopamine transients achieved concentrations higher that 100 nM and had a duration of approximately a second (Robinson et al., 2003), in part due to saturation of the dopamine uptake transporter (Schmitz et al., 2003; Sulzer et al., 2016).
The combined contributions from phasic and tonic firing sets the basal concentration of extracellular dopamine, although as reviewed (Watson et al., 2006), establishing this precise value has proven technically difficult. Using slow electrochemical recording techniques, estimates of the extracellular striatal dopamine concentration were 25 nM (Gonon and Buda, 1985). FSCV has a large background that must be removed and thus only provides a differential concentration. Basal concentrations of striatal dopamine evaluated by no net flux microdialysis sampling are in the low nanomolar range (Shou et al., 2006). These values, however, may provide an underestimate due to tissue damage caused by probe insertion (Yang et al., 1998). In summary, while there is variability in reports of the average extracellular level, data have coalesced in the low nanomolar range (Dreyer et al., 2010).
Thus, as predicted from the behavioral studies by Olds and others, in well-trained rats, dopamine is released in response to cues that predict reward, and this release can be detected by cyclic voltammetry.
Striatal spiny projection neurons in the brain reward circuitry
Within both the dorsal and the ventral striatum, information is distributed via SPNs that release the inhibitory neurotransmitter GABA in response to activation. These cells receive a dense innervation from dopaminergic neurons that project from the midbrain. In rodents, SPN cell bodies comprise more than 90% of the neuronal somata in this region (Gerfen and Surmeier, 2011) and tend to fire at about 1 Hz in behaving adults unless they receive convergent and temporally coincident excitation from cortical and/or thalamic afferents (Wilson and Groves, 1981). The remaining neurons are cholinergic and GABAergic interneurons that are distinguished in single-unit recordings by their high firing rate (Berke et al., 2004).
The foundational studies showing that dorsal striatum SPNs are active during movement were by Mahlon DeLong using single unit recordings in primates who were trained to push or pull objects. He initially showed that the globus pallidus externa (GPe) and globus pallidus interna (GPi: the typical term in primates, often called the entopeduncular nucleus in rodents) displayed changes in activity during the movements (DeLong, 1971), and soon after that increased firing in phasic discharge patterns in both GPe and GPi were associated with the firing of SPN output neurons during these behaviors (DeLong, 1972).
The central importance of dopamine in this circuitry was revealed in early work (Filion, 1979; Miller and DeLong, 1987; Tremblay et al., 1989) and the concepts were extended in a review by Albin and colleagues that modeled motor disorders due to the balance of SPN outputs to the GPe/SNr or the GPi (Albin et al., 1989). The names direct and indirect pathway were introduced by DeLong (DeLong, 1990) when referring to another review by Alexander and Crutcher (Alexander and Crutcher, 1990), members of the DeLong lab who demonstrated the centrality of dopamine innervation to basal ganglia output by examining primates treated with the dopamine neurotoxin MPTP.
In the dorsal striatum, the SPNs that comprise the direct pathway project to the substantia nigra reticulata (SNr) and the GPi, which are output nuclei of the basal ganglia. Excitation of these direct pathway SPNs by glutamate released from cortical and likely thalamic inputs leads to GABA release and a monosynaptic inhibition of SNr and GPi neurons. By decreasing SNr and GPi GABAergic output to the thalamus, activation of the direct pathway allows thalamocortical excitation to proceed and drive movement: this is often called the “go” pathway.
In contrast, the indirect pathway acts via a disynaptic pathway, in which GABA release first inhibits activity of the GPe and subthalamic nuclei that then project to the SNr and GPi, thus disinhibiting their GABAergic output, which inhibits thalamocortical excitation: this is often called the “no-go pathway”, although as discussed below, for operant and motor behaviors a good term for the indirect projections may be the “ready/steady pathway”. For habit based learning, the indirect projections may mediate cued anticipation and “urge” to prepare for a behavior.
Activation of this pathway exerts effects opposite of activation of the direct pathway, in that it increases inhibition of the outputs from the basal ganglia (Hikosaka et al., 2014). Adding to the complexity is that many direct and some indirect pathway neurons arborize to give off branches to both pallidal segments (Kawaguchi et al., 1990; Wu et al., 2000; Cazorla et al., 2014).
The ventral striatum also contains direct- and indirect-pathway SPNs (Kupchik et al., 2015). In this region, the direct pathway projects to the ventral tegmental area, while the indirect pathway is a more complex, multi-synaptic pathway (Soares-Cunha et al., 2016a).
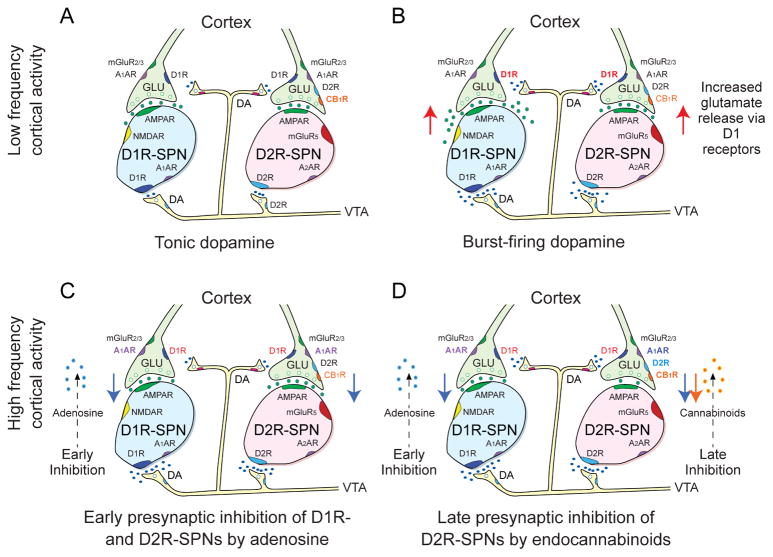
Mature SPNs possess extensive potassium conductances that inhibit them from exhibiting spontaneous activity (Wilson and Kawaguchi, 1996), and much work has evaluated the roles of the excitatory inputs that are required to activate them. Glutamate release from cortical, thalamic, hippocampal, and amygdala projections to the NAc can interact with AMPA, NMDA, and metabotropic glutamate receptors (mGLURs) (Wang et al., 2012; Soares-Cunha et al., 2016a). AMPA and NMDA receptors are mostly located on the dendrites of SPNs, whereas mGLURs are located postsynaptically and extrasynaptically on SPNs and presynaptically on the glutamate terminals (Testa et al., 1994; Lovinger and McCool, 1995; Testa et al., 1998; Sesack and Grace, 2010) (Figure 1A).
Figure 1.
Proposed model for how coincident cortical and dopaminergic inputs control rapid signal processing during operant behaviors in the NAc. Each panel shows D1-containing and D2-containing SPNs. D1-SPNs express AMPA, NMDA and A1A receptors, while D2-SPNs express AMPA, mGluR5 and A2A receptors. Cortical terminals to both types of SPNs present D1, A1A and mGluR2/3 receptors. Only cortical terminals to D2-SPNs present D2 and CB1 receptors. Dopamine projections from the VTA form synapses at both D1- and D2-containing SPNs. A. Low activity cortical and dopamine inputs. When dopamine neurons are tonically active, they produce low levels of striatal dopamine that inhibits the indirect corticoaccumbal pathway by binding to a fraction of pre-and postsynaptic high affinity D2 receptors. B. Low activity cortical and highly active dopamine inputs. High levels of dopamine reached during burst firing (due in part to saturation of dopamine reuptake) enhance corticoaccumbal activity through D1Rs that strengthen glutamate release from presynaptic terminals to both direct and indirect pathway SPNs. C. Highly activity cortical and dopamine input effects on the direct pathway. When corticoaccumbal stimulation of NMDA and AMPA receptors on direct SPNs are concurrent with high levels of dopamine that activate D1Rs, adenylate cyclase is activated and adenosine is released from direct pathway SPNs. The adenosine retrogradely inhibits presynaptic activity to both D1R and D2R-presenting SPNs via A1Rs. D. Highly activity cortical and dopamine input effects on the indirect pathway. High corticoaccumbal stimulation produces extrasynaptic overflow of glutamate, which activates group 1 mGLU receptors on indirect SPNs. When this is concurrent with high levels of dopamine that activate additional D2 receptors on indirect SPNs, the SPNs release endocannabinoids, which retrogradely inhibits presynaptic inputs to indirect SPNs via presynaptic CB1Rs. The most active corticoaccumbens release sites appear to escape presynaptic inhibition, although the means by which that occurs are unclear. Reproduced with permission from Wang et.al., J Physiol. 590:3743–69 (2012).
SPNs and the synapses that innervate them feature a variety of additional modulatory receptors. The most studied have been dopamine receptors, which were classically considered as pharmacologically distinct classes of D1-like receptors (D1Rs) and D2-like receptors (D2Rs). The discovery of the modulation of SPN activation of basal ganglia circuits by dopamine was again due to research by DeLong and colleagues and their results following dopamine depletion with MPTP (DeLong, 1990). Research by Gerfen and colleagues led to the concept that D2 receptors are found on SPNs of the indirect pathway, whereas D1 receptors are found on SPNs of the direct pathway (Gerfen et al., 1990), a concept reinforced by studies from multiple laboratories (Le Moine et al., 1990; Meador Woodruff et al., 1991; Yung et al., 1995).
Initially there was a controversy as to whether or not SPNs contained only one or multiple types of dopamine receptors (Gerfen and Surmeier, 2011). This issue was clarified in transgenic mice carrying bacterial artificial chromosomes that were used to express reporter genes driven by specific promoters for different types of dopamine receptors tagged with fluorescent markers (Valjent et al., 2009). This approach demonstrated that SPNs contain either D1Rs or D2Rs, but few contain both (Gangarossa et al., 2013). D2Rs were mostly found in the indirect pathway, although both types of receptors were found in the SPNs of the indirect pathway of the ventral striatum (Smith et al., 2013).
Electrophysiological and gene expression analysis using single-cell rtPCR have found both D1- and D2-like receptors on cholinergic interneurons (Yan et al., 1997) and D2Rs on GABA interneurons (Ibanez-Sandoval et al., 2010), where they are thought to participate in gating of corticostriatal information (Koos and Tepper, 2002) and regulation of dopamine transmission (Exley et al., 2012).
D1Rs and D2Rs are also found on axonal and presynaptic sites in the dorsal striatum and the NAc (see recent review, (Burke et al., 2017). D1Rs are found on presynaptic corticoaccumbal projections, but less so in the dorsal striatum (Dumartin et al., 2007). In the dorsal and ventral striatum, D2Rs are both present presynaptically (Sesack et al., 1994). D2Rs located presynaptically on dopamine axons serve as inhibitory autoreceptors (Limberger et al., 1991). D2Rs are also found presynaptically on the terminals of corticostriatal projections (Wang and Pickel, 2002).
A variety of other receptors play important roles at these synapses. GABAergic receptors are important regulators of activity, both by interneurons and SPN collaterals (Tepper et al., 2004). Adenosine can presynaptically inhibit D1 and D2-type SPNs via A1 adenosine receptors and also acts postsynaptically at A2 receptors on D2-type SPNs (Harvey and Lacey, 1997; Wang et al., 2012). Other substances modulating this synapse include endocannabinoids, which inhibit D2-type SPNs presynaptically at CB1 receptors (Yin and Lovinger, 2006; Wang et al., 2012), and acetylcholine, which acts through nicotinic receptors on dopaminergic terminals (Rice and Cragg, 2004; Zhang and Sulzer, 2004; Exley et al., 2012), as well as excitatory nicotinic and inhibitory muscarinic receptors on cortical terminals (Calabresi et al., 2000; Threlfell et al., 2012; Wang et al., 2013; Yorgason et al., 2017).
How do D1 and D2-containing SPNs modulate behavior?
The hypothesis that the timing of direct and indirect pathway SPNs is responsible for appropriate behavior extends from DeLong’s model, that stated “it would appear that under normal conditions the direct pathway effectively provides positive feedback to the precentral motor fields, from which much of the movement-related activity within the circuit is thought to arise… In contrast, activity conducted along the indirect pathway appears to provide negative feedback to the precentral motor fields” (DeLong, 1990).
DeLong’s collaborators Alexander and Crutcher in a companion article (Alexander and Crutcher, 1990) expanded this to define a still current central issue, writing that “the indirect pathway might be seen as either ‘braking’ or ‘smoothing’ the same cortically initiated motor pattern that was being reinforced by the direct pathway. Alternatively, the direct and indirect inputs associated with a particular motor pattern could be directed to separate sets of GPi/SNr neurons. In this configuration, the motor circuit might be seen as playing a dual role in the modulation of motor patterns initiated at cortical levels by both reinforcing the currently selected pattern via the direct pathway and suppressing potentially conflicting patterns via the indirect pathway. Overall, this could result in the focusing of neural activity underlying each tonically initiated movement in a fashion analogous to the ‘inhibitory surround’ seen in various sensory systems.”
The distinction, if there is one, between the indirect pathway modulating the direct pathway’s behavioral drive via “smoothing” the pattern or by suppressing conflicting patterns continues to be examined in multiple ways as outlined here.
Driving D1 and D2-containing SPNs with channelrhodopsin
One way to distinguish the roles of D1R and D2R-containing SPN populations has been to use optogenetics, in which targeted neurons are genetically altered to express a photoactive protein that activates ion conductances (Deisseroth, 2015), such as channelrhodopsin-2 (ChR2), the light-sensitive pigment-associated cation channel of the eye spot of the pond scum Chlamydomonas. ChR2 can be expressed in the dopaminergic neurons projecting from the midbrain or in the SPNs themselves. ChR2 expression in VTA dopaminergic neurons established that their selective stimulation was sufficient to drive behavioral conditioning in mice (Tsai et al., 2009). Subsequent studies in rats established that the responses could be blocked by D1 and D2 receptor antagonists (Steinberg et al., 2014), and that it was subject to the expectations of theories of reward prediction error, namely blocking and extinction experiments (Keiflin and Janak, 2015).
In concordance with the electrophysiological studies of DeLong and colleagues in earlier decades, a study employing ChR2 expressed in D2R-containing SPNs in the dorsal striatum of mice, bilateral optical activation induced a parkinsonian state with increased freezing and decreased locomotor initiation, whereas an opposite effect was obtained in mice that expressed ChR2 in direct pathway SPNs (Kravitz et al., 2010). Similar activation of D1-containing SPNs (D1R-SPNs) in mice that had dopaminergic neurons lesioned with the neurotoxin 6-OHDA eliminated their parkinsonian-like symptoms. This was consistent with DeLong’s earlier results with the neurotoxin MPTP, and the hypothesis that dopamine increases glutamatergic excitation in D1R-SPNs and decreases it in D2R-containing SPNs is now widely accepted (Tritsch and Sabatini, 2012).
Mice with ChR2 expressed in D1R-containing SPNs were more likely to depress a lever to deliver light stimuli to their brain, while the opposite was true for D2R-containing SPNs (Kravitz et al., 2012). These data suggest that activation of the direct pathway is appetitive whereas activation of the indirect pathway is aversive: note however that during operant behavior recordings discussed in subsequent sections (i.e., in genetically unaltered animals), both pathways are activated, including in classical studies that indicated that both direct and indirect pathway pathways are activated during the same behaviors (Alexander and Crutcher, 1990). Thus, the activity in operant behavior is fundamentally different than that seen with experimenter-driven stimuli.
The precise rules that govern optogenetically-driven behaviors may, as with the operant behaviors, differ between the NAc and dorsal striatum. Soares-Cunha and coworkers showed that optogenetic activation of D1R- or D2R-containing SPNs in dorsal striatum both enhance motivation in mice (Soares-Cunha et al., 2016b). Consistent with this, optogenetic inhibition of D2R-containing neurons decreases motivation. This study, in agreement with the results obtained with microiontophoresis, suggest that D2R-containing SPNs play a more prominent role in promoting motivation than originally anticipated.
While activation or inhibition of neurons can be achieved by direct stimulation, the major advantage of the ChR2 approach over electrical stimulation is that it is selectively expressed in specific sets of neurons. There are, however, limitations to the use of ChR2 noted in the literature. Photoactivated ChR2 requires seconds for recovery to its dark state (Schneider et al., 2015), and this can limit the upper range of stimulus frequencies that can be assessed by optical activation (Gunaydin et al., 2010). Indeed, most studies of SPNs stimulation using ChR2 stimulation have been performed at lower frequencies than used with electrical stimulation. Furthermore, there is a potential for ‘ectopic’ expression of TH transcript and Cre expression in neurons that contain no TH protein (Lammel et al., 2015). Most importantly for understanding synaptic networks underlying operant behaviors, experimenter-triggered activation of a set of neurons and correlating this with behavioral response may be very different from self-initiated neuronal activation that mediates an animal’s interactions with its environment.
SPN firing during behavior
Optogenetic approaches provide information on responses to experimenter-driven neuronal firing, but not how the pathways are activated by behaving animals. Early attempts to probe animal-driven SPN activity produced very complex results, as reviewed (Nicola et al., 2000). However, some clear patterns of SPN firing rates were obtained. For example, Regina Carelli and Samuel Deadwyler trained water-deprived rats to depress a lever to obtain water reinforcement (Carelli and Deadwyler, 1994). In this experiment, a schedule of differential reinforcement of low rates of responding was employed so that after each lever press, a timeout period of 15 s occurred with the interval signaled by a cue comprised of a simultaneous tone and houselight. Well-trained animals did not approach the lever until tone-houselight termination, whereupon they executed the water-reinforced lever press. The SPNs that were phasic around the lever press showed three different firing patterns. One group showed an anticipatory response with an increase in firing rate immediately before (~2 s) the lever press. A second group exhibited an excitation immediately after (within ~2 s) the lever press. A third group showed an inhibition within 2 s of the lever press. Thus, individual NAc SPNs in water-deprived rats are either non-phasic around the lever press or have differential patterns of phasic activity during the operant behavior. These unique firing patterns were found in other reward-related behaviors as well such as during cocaine self-administration (Carelli and Deadwyler, 1994). At the time, however, there were no means available to differentiate the activity of direct and indirect pathway SPNs.
Another form of analysis of behavior-driven SPN activity involves the expression of calcium selective fluorophores that can be used with fiber optics to monitor calcium entry into cells during behavior (Cui et al., 2013; Calipari et al., 2016; Kupferschmidt et al., 2017; Natsubori et al., 2017). This approach was used to selectively examine D1R- or D2R-containing SPNs, and the overall findings were that activation of D2R’s decreases SPN firing, whereas the opposite result was obtained in D1R-containing cells (Calipari et al., 2016), consistent with responses to dopamine agonists in brain slices (Gerfen and Surmeier, 2011). These approaches thus confirmed the conclusions of the earlier electrophysiological studies from DeLong and colleagues by indicating both that dopamine activated the direct pathway and inhibited the indirect pathway, but that both pathways were active during behavior (DeLong, 1990), likely with the direct pathway promoting a response and the indirect pathway inhibiting competing responses (Alexander and Crutcher, 1990; Cui et al., 2013).
Dopamine release in the NAc during reward based behaviors
Wightman and Carelli designed experiments to probe dopamine release in the NAc during reward-based behaviors using voltammetric techniques (Carelli and Wightman, 2004).. Akin to the unit activity changes described above, dopamine transients occurred immediately before a lever press for cocaine, which is considered an anticipatory response, as well as immediately after the lever press (Phillips et al., 2003). Importantly, these were not direct, drug-induced responses since both occurred before the cocaine reached the brain. Rather, the dopamine transients after the lever press were associated with the accompanying tone and houselight cues, as a cue alone evoked a dopamine release response in well-trained animals. During extinction, when a lever press did not result in cocaine self-administration, the dopamine transients diminished in amplitude with each extinguished trial (Stuber et al., 2005).
Dopamine release was also examined in rats during ICSS using FSCV. In initial experiments from Wightman’s group, each lever press delivered a brief (0.4 s, 60 Hz) electrical stimulus to midbrain dopamine neurons via a chronically implanted stimulating electrode. When the rat pressed the lever repeatedly, the dopamine release evoked by each stimulus decreased (Garris et al., 1999). This suggested that dopamine was not playing a direct reward-based role, as the rats continued vigorous lever pressing despite the diminished release of dopamine.
To allow the dopamine signals to be time correlated to specific events during ICSS, Carelli and Wightman introduced a protocol in which a cue (a simultaneous light and tone) signaled impending (2 s later) lever availability. As before, depression of the lever elicited brain electrical stimulation (Owesson-White et al., 2008). With the time between events extended, biphasic dopamine transients were observed. Dopamine release occurred immediately after the cue and then again following depression of the lever with its associated stimulus delivery. The dopamine surge at the cue, long prior to the electrical stimulus, further indicated that dopamine is an anticipatory or preparatory signal in reward acquisition. The prolonged pause between cue presentation and lever acquisition (more than 8 s for the entire sequence, including a 1.7 s latency from the lever extension to lever press) allowed a means to resolve dopamine’s concentration fluctuations with discrete events during the trial.
Naïve rats quickly learned the behavioral sequence. Initially, with trials repeated at random intervals (5 to 25 s), dopamine release only occurred after the lever press in the NAc (Owesson-White et al., 2008). Following 250 trials, however, a stable pattern of dopamine release in response to the cue emerged that was followed by a dopamine release transient evoked by the stimulus. The evoked dopamine transient was of similar amplitude to the cue evoked release and resembled that evoked by non-contingent delivery of the electrical stimulus. During ICSS extinction, where the stimulus was turned off, the cue responsive dopamine transient diminished in amplitude and disappeared after only 5 lever presses. As expected, the dopamine transient following the lever press did not occur during the extinction protocol.
The Carelli and Wightman groups also found that dopamine transients occurred during self-administration of sucrose (Roitman et al., 2004). Animals were trained using a similar operant task as in the ICSS experiments in which a cue predicted that a lever would be extended 2 s later. Depression of the lever provided delivery of a sucrose pellet that the rat consumed. In well-trained animals, a dopamine transient occurred in the NAc core in response to the cue, but there was no dopamine released in response to the food pellet (Cacciapaglia et al., 2011; Cacciapaglia et al., 2012). Dopamine in the NAc shell behaved similarly, although there was a small dopamine transient following the lever press. Note that these results are like those from the Schultz laboratory (Schultz, 2007), where primates were trained that a cue was a predictor of an unconditioned reward in the form of a sweetened solution: in those electrophysiological experiments, dopamine neurons were found to increase in firing rate in response to reward predicting cues.
Another approach to monitor the activity of dopamine axons during behavior is to express a calcium selective fluorophore in cells containing the dopamine transporter (Howe and Dombeck, 2016). Signaling in dopamine axons in the striatum was found to be rapid and phasic. This is consistent with the view that dopamine extracellular concentrations are set by the rate of dopamine transients (Owesson-White et al., 2012). Further, Howe and Dombeck found that there were two populations of dopaminergic release sites: one involved in locomotion and another that responded to unexpected rewards.
Simultaneous single-unit and voltammetric recordings
To examine whether changes in SPN firing rate accompany dopamine release, the Wightman and Carelli groups developed a technology to record single units and FSCV with the same carbon-fiber electrode within the NAc of rats executing reward based behaviors. In these experiments, the time between cyclic voltammograms was 200 ms, and the activity of SPNs in the NAc were recorded during the intervals between them (Takmakov et al., 2011). Recordings were made around the behavioral event of interest (typically ± 10 s), and results from multiple trials were averaged to generate a perievent histogram of the unit activity. The time course of the dopamine concentration transients was superimposed on these histograms, providing a readout of neurochemical changes and the accompanying changes in firing rate.
With this combined electrophysiology/electrochemical technique, the cocaine self-administration experiments were repeated (Owesson-White et al., 2009). Of the recorded cells, all of which had low firing rates consistent with expectations for SPNs, 40 % showed phasic activity. Interestingly, those sites in the NAc in which cells were non-phasic with respect to the behavior also did not have presynaptic sites that released dopamine.
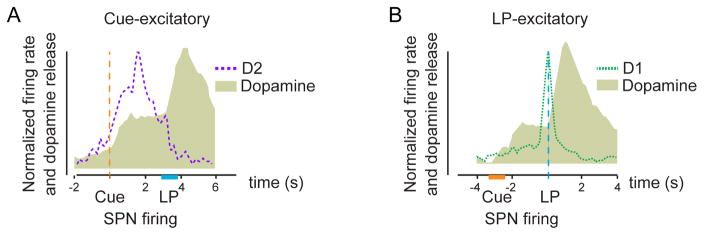
This combined technique was also used to measure events within the NAc during ICSS (Owesson-White et al., 2016). In this case, 80% of the recorded cells showed phasic activity around the lever press, and these were accompanied by the biphasic dopamine concentration transients described above. The phasic cells in these experiments exhibited three types of firing patterns. Some cells showed an increase in firing rate after the cue presentation but before the lever press. Additionally, there were two types of responses immediately before the time of lever extension—either a transient inhibition or a transient excitation. Thus, unlike the dopamine changes that were always biphasic, the phasic changes in unit activity during the ICSS experiment were monophasic, varied by location, and could be categorized into 3 different types (Figure 2).
Figure 2.
Dopamine release and SPN firing rate measured in the rat NAc during ICSS. Both responses are measured at the same carbon fiber electrode. Receptor subtype identified with controlled iontophoresis (see text). A. Responses at Cue-excitatory SPNs. All were determined to be D2R-containing cells. Dopamine release occurs immediately after cue initiation and rises further after the lever press (LP) due to electrical stimulus of the VTA/SN region. D2R-SPNs adjacent to the region of DA release fire following the cue. Their activity diminishes around lever extension (occurs at 2 s) and returns to baseline after the LP. B. Responses at LP-excitatory SPNs. Dopamine release follows an identical pattern as in A. LP-excitatory cells, that were predominantly D1R-containing SPNs, increase in firing around the time of the LP. D2R-responsive cells that responded around the LP predominantly displayed an inhibition (data not shown). Note that the large spike of dopamine release that reaches maximum values ~ 1s after lever press during ICSS is due to direct activation of dopamine axons by the stimulating electrode and is not an intrinsic CNS step in operant conditioning. Adapted from Owesson-White et.al., J Neurosci. 36:6011–21 (2016).
Carelli and coworkers observed similar patterns in the NAc of rats pressing for sugar pellets for both single-unit firing and dopamine transients (Cacciapaglia et al., 2011). In this case, 33% of SPNs did not show phasic activity around the cue or lever press, and dopamine transients were not observed at these sites. The single-unit activity exhibited identical patterns as during ICSS, including increase in firing at cue, increase in firing around lever-press, and decrease in firing around lever press. In these experiments, there were also additional patterns including cells that displayed multiphasic patterns, and cells that were inhibited following the cue.
In an additional set of experiments using this paradigm, NMDA antagonists were infused into the ventral tegmental area: such infusions have been shown to inhibit the generation of dopamine transients in the NAc (Sombers et al., 2009). During the pressing for food pellets, the excitatory events that occurred at the cue and lever press were suppressed, but the other phasic patterns were not (Cacciapaglia et al., 2011).
Together these experiments show that with sucrose reward, ICSS, and cocaine self-administration, dopamine transients are associated with changes in SPN unit activity. Indeed, changes in SPN activity were not observed if the region recorded did not show dopamine (Kirkpatrick et al., 2016)transients around the time of cue and lever extension, indicating a central role for dopamine neurotransmission in these behaviors. The data also indicate that for SPNs that show enhanced excitation associated with cue and lever press, these changes require excitatory glutamatergic inputs. Therefore, coincident dopaminergic and cortical and/or thalamic input may be required for the operation of neural circuits that underlie these behaviors.
In vivo identification of D1R and D2R-containing SPNs
The new approaches discussed above demonstrate that a confluence of dopamine and NMDA receptor-activated synaptic excitation is associated with altered activity of SPNs during behavior. While the results are consistent with dopamine release mediating these changes in activity, the above data do not test that hypothesis. The remainder of this review discusses research that attempts to define the mechanisms by which dopamine might mediate SPN activity during behavior.
To reveal the roles for the subtypes of dopamine receptors that regulate SPN activity during ICSS, the Wightman laboratory adapted a multimodal sensor that in addition to simultaneous FSCV and single-unit recordings, allows for microiontophoretic ejection of three different drugs, including receptor agonists and antagonists that enable receptor identification. This type of probe was developed by Julian Millar and coworkers in the 1980’s (Millar et al., 1981), although it had not been previously used in awake animals. As the carbon-fiber electrode is adjacent to the iontophoretic barrels, cyclic voltammetry can measure the concentrations of drugs ejected (Herr et al., 2008). If the pharmacological agent is not electroactive, an electroactive marker such as acetaminophen can be added to the barrel to follow the ejections, allowing estimation of the concentration of the drug, a procedure they termed controlled microiontophoresis (Belle et al., 2013; Kirkpatrick et al., 2016). Additionally, monitoring of chemical and electrical activity with the carbon fiber allows identification of other events during such measurements.
Preliminary experiments in awake rats characterized the effectiveness of microiontophoretic introduction of pharmacological agents to alter stimulated release of dopamine in the NAc. The clearance rate of dopamine release was lowered following iontophoretic introduction of nomifensine (30 s ejection), a dopamine-transporter inhibitor (Herr et al., 2010). With careful calibration, the approach was used to characterize the dose response curve of the D2 receptor agonist, quinpirole, during its inhibition of evoked dopamine release (Kirkpatrick et al., 2016). Remarkably, iontophoresis of dopamine itself at extracellular concentrations measured to be 16 μM, far above the binding constant at its receptors, did not affect stimulated release of dopamine (Belle et al., 2013): this was due to its rapid uptake before it could access autoreceptors and additional striatal D2 receptors that regulate dopamine release (Anzalone et al., 2012; Kharkwal et al., 2016), as ejected dopamine did inhibit evoked dopamine release if nomifensine was also present. Thus, studies with iontophoretic dopamine should typically include reuptake blockade.
The Carelli/Wightman groups investigated the effects of dopamine receptor antagonists on the firing rate of SPNs in the NAc of freely moving rats. Within rats resting but awake, SPNs in the NAc exhibit a firing rate of 1 Hz. This firing does not occur in slices (Kirkpatrick et al., 2016) and is attenuated under anesthesia (White and Wang, 1986). The enhanced firing of SPNs in awake animals likely arises from glutamate release from excitatory afferents originating from the cerebral cortex, thalamus and other regions (Wilson, 1993; Cowan and Wilson, 1994).
To evaluate the effect of dopamine receptors on the ongoing firing of SPNs, SPN firing was measured during iontophoretic applications (30 replicates, 15 s applications) of dopaminergic antagonists. After each drug ejection or baseline trial, the animal was allowed to execute an ICSS sequence with the ejected drug present (Kirkpatrick et al., 2014; Owesson-White et al., 2016). The experimental design allowed evaluation of the type of dopamine receptor-expressing SPN (D1 or D2) adjacent to the electrode via the antagonist response, as well as the modulation of its firing rate during the ICSS sequence. The extent of drug spread from the microiontophoresis site is very circumscribed (Kirkpatrick et al., 2014), so that effects on collaterals are negligible.
Using this approach, 96 % of phasic cells responded to either D1 or D2 antagonists but not both, consistent with separate populations of D1R and D2R-containing cells.
These patterns obtained in vivo were entirely consistent with the well characterized effects of dopamine receptors obtained in brain slices as well as the classical single unit recordings (Alexander and Crutcher, 1990; DeLong, 1990): activation of D1 receptors enhanced excitation of cells, whereas activation of D2R’s reduced their excitation. In these experiments, only 4% of the cells tested responded to both types of antagonists, consistent with the lack of complete segregation of D1R- and D2R-containing SPNs in the NAc (Smith et al., 2013).
Dopaminergic modulation of SPN activity during ICSS
Having classified the type of receptor on SPNs adjacent to the sensor, the Wightman/Carelli groups next analyzed the modulation of firing rate changes and their temporal pattern during the ICSS sequence. Importantly, the small amounts of locally applied antagonists did not affect the latency to press (i.e., the animal’s behaviors), but the local firing rates during ICSS were modified.
All SPNs that exhibited increased activity after cue presentation were identified as D2R-containing as administration of a D2 antagonist enhanced firing, whereas a D2 agonist reduced it. Notably, the doses delivered with microiontophoresis, while sufficient to affect cell firing, did not cause an effect on autoreceptors as dopamine release was not significantly changed. Whether the effects on cell firing were due to pre- or post-synaptic sites could not be distinguished. However, work discussed below suggests that the dopamine acts mostly at D2R on SPNs, but the subsequent reduction in D2R-SPN neuronal activity arise from activation of a retrograde signal from the SPNs that activates presynaptic cannabinoid receptors and inhibits glutamate release from cortical inputs (Wang et al., 2012; Wong et al., 2015).
Most of the cells that showed excitation immediately before the lever extension (n = 10) were classified as D1R-containing. The excitation was suppressed by a D1 receptor antagonist and enhanced by the D1 receptor agonist SKF38393. The cells with inhibited firing immediately before the press were both D1R-containing and D2R-containing cells. However, insufficient cells were encountered to further characterize the inhibitory firing around the lever press.
Thus, during ICSS that is predicted by a cue, the initial event at the corticostriatal synapse in the NAc is an excitation in response to sensory input that is dependent on activation of D2 receptors: note that evidence in previous sections suggests that this input may be mediated by NMDA receptors. This event is followed by a second excitatory response of a different group of SPNs that dependent on activation of D1 receptors, which also appears to be NMDA-dependent. A third group of neurons is inhibited immediately before the press that can contain either D1 or D2 receptors, but for which the effects of the receptors are still unclear.
Dopamine can alter SPN activity by presynaptic and retrograde effects
To decode these dopamine-dependent phasic changes during reward-based behaviors, an understanding of dopamine’s actions at SPNs is required. Because dopamine is a neuromodulator, the study of its actions requires that the predominant activity it modulates (i.e., glutamatergic synaptic input to SPNs) should be present, and most studies have examined these effects in cortical-striatal brain slice preparations. As discussed below, the studies indicate that dopamine impedes glutamate evoked increase in firing at D2R-containing SPNs and increases it at D1-containing SPNs (Surmeier et al., 2007; Gerfen and Surmeier, 2011; Tritsch and Sabatini, 2012). The mechanisms by which this occur, however, are complex and involve second and retrograde messengers and their modulation of ion channels (Beaulieu and Gainetdinov, 2011).
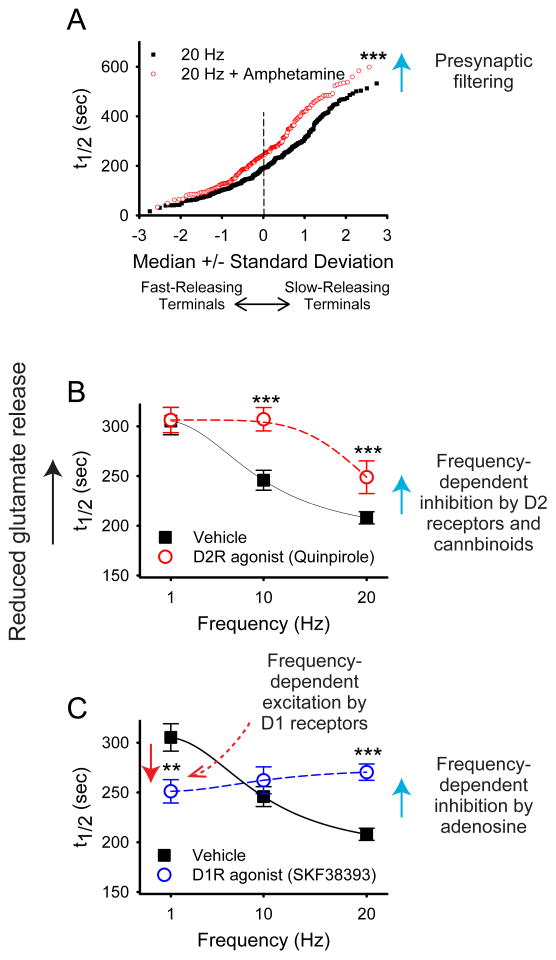
To investigate the presynaptic effects of dopamine on the cortical inputs to SPNs, glutamate release from cortical terminals was indirectly monitored optically using the endocytic fluorophore, FM1-43 (Bamford et al., 2004). FM1-43 can be preloaded into recycling glutamatergic synaptic vesicles, and the destaining of sites can be monitored during cortical stimulation. In the untreated acute slice containing both cortex and striatum, the half-life of evoked destaining at large populations of cortical presynaptic sites followed a normal distribution. In the presence of synaptic dopamine release, the dopamine-releasing drug amphetamine, or the dopamine reuptake inhibitor cocaine, the destaining of the most rapidly releasing presynaptic sites was unaffected, but the destaining of slower presynaptic sites was inhibited (Bamford et al., 2004). This dopamine-dependent effect was far more powerful at high (20 Hz) physiologically relevant stimulation frequencies of the cortex than at lower frequencies (1 Hz) (Figure 3).
Figure 3.
Modulation of stimulated release from glutamatergic terminals in mouse brain slices containing the NAc. Corticoaccumbal terminals in the NAc were loaded with FM1-43 during a pre-measurement interval, and then the destaining rate during a subsequent stimulation period at individual nerve terminals was monitored as t1/2 values (the time required for florescence to decay to half of its original value). A. Normal probability plot comparison of individual half-times of release in slices shows that the dopamine releaser amphetamine filters cortical activity by reducing exocytosis of FM1-43 from terminals with lowest probability of release (i.e. those with the highest t1/2). At 20 Hz, t1/2 = 207 s; n = 330. At 20 Hz with amphetamine, t1/2 = 264 s; n = 193; ***P < 0.001, Mann-Whitney. B. Distribution of mean t1/2 of release for slices containing the NAc as a function of frequency. The release of FM1-43 was reduced at higher frequencies (20 Hz) when the slice was exposed the D2R agonist quinpirole. This plot demonstrates that activation of D2 receptors inhibits glutamate release at higher frequencies (n = 80 – 96 terminals; ***P < 0.001 Mann-Whitney). C. A D1R agonist increased release at low frequencies (1 Hz). At higher frequencies of cortical stimulation (20 Hz), the D1R agonist reduced release by promoting presynaptic inhibition by adenosine (not shown). (n = 97 – 260 terminals; ***P < 0.001 Mann-Whitney). Reproduced with permission from Wang et.al., J Physiol. 590:3743–69 (2012).
Thus, dopamine appears to act as a high pass filter, suppressing only the glutamate release sites with less active release rates, while leaving the fast release sites unaffected. The presynaptic inhibition by dopamine was blocked by an antagonist of D2Rs, was absent in a D2 knockout mutant, and was potentiated in dopamine deficient animals (Bamford et al., 2004): thus the D2R role in inhibitory presynaptic filtering of cortical inputs is strongly supported by a variety of data.
Bamford and Sulzer initially proposed that the inhibitory effects were exerted by D2Rs located presynaptically on the corticostriatal projections. Subsequently, modulatory effects by both adenosine and endocannabinoids on SPN activity in the NAc were revealed using a similar approach (Yin and Lovinger, 2006; Wang et al., 2012). When endocannabinoid receptors were inhibited, the dopamine-dependent, high-pass filtering of cortical inputs was blocked. Further investigation showed that the source of endocannabinoid release was likely the indirect pathway SPNs and required activation of both extrasynaptic SPN D2R and group 1 mGLU receptors (Kreitzer and Malenka, 2005; Wang et al., 2012; Wong et al., 2015).
While a role for D2R-dependent endocannabinoid presynaptic effects is supported by multiple lines of evidence, inhibition by indirect pathway D2R was also detected in CB1−/− mice in the absence of stimulation (Wang et al., 2012). This observation suggests that dopamine produces a relatively weak presynaptic inhibition at low frequencies, possibly via presynaptic D2Rs, and elicits a more robust synaptic depression at higher frequencies by enhancing stimulus-dependent endocannabinoid efflux that bind to CB1 or CB1/D2 receptor heteromers on presynaptic terminals.(Kearn et al., 2005; Pickel et al., 2006; Marcellino et al., 2008).
Together, these findings may explain why dopamine’s synaptic filtering occurs when both cortical excitation and dopamine activity are both at high levels. The extrasynaptic metabotropic glutamate receptors on SPNs would be activated only when there is sufficient glutamate release to overflow beyond the glutamate uptake transporters at the synapse (Zhang and Sulzer, 2003). This would explain why dopamine’s presynaptic filtering is more potent at higher cortical firing rates (Bamford et al., 2004). It also suggests a learning mechanism that would operate only when both cortical drive of a behavior and unexpected reward driving dopamine release occur simultaneously.
A central feature of the presynaptic filtering that remains opaque, however, is why the most active release sites are spared. A clue is that presynaptic imaging experiments conducted in dopamine-deficient mice showed that corticostriatal filtering was absent under dopamine-depleted conditions, and that every presynaptic cortical input was inhibited by either dopamine or D2R stimulation (Bamford et al., 2004). This mechanism is supported by similar experiments in parkinsonian mouse models, which display disrupted synaptic filtering, dopamine receptor hypersensitivity and poor abilities on cognitive tests and with motor learning (Zhou and Palmiter, 1995; Bamford et al., 2004; Darvas and Palmiter, 2009, 2011; Wong et al., 2015).
Appropriate presynaptic filtering may be central to understanding how the most effective corticostriatal synapses are selected during learning. The resulting selection of specific synapses may provide which information is subsequently channeled through basal ganglia pathways, which is expected to further underlie appropriate learning.
While presynaptic D2 and endocannabinoid receptors modulate excitatory projections to D2R-expressing SPNs in both the dorsal and ventral striatum, the presynaptic D1Rs to date clearly present only in the NAc, where they boost excitatory signal strength by promoting synaptic filtering of cortical inputs to both D1 and D2R-containing SPNs (Wang et al., 2012). In the dorsal striatum, presynaptic excitation by D1Rs is difficult to observe (Maura et al., 1988; Hsu et al., 1995; Bamford et al., 2004; Dumartin et al., 2007), unless the animals are subjected to dopamine release by intermittent and repeated use of psychostimulants (Wang et al., 2013). Under those conditions, psychostimulant drug withdrawal produced a very long-lasting chronic presynaptic depression (over 140 days in treated mice) in presynaptic activity (Bamford et al., 2008; Wang et al., 2013). In these drug-withdrawn mice, psychostimulant reinstatement promoted a paradoxical excitation of SPNs, apparently through D1R located on cholinergic interneurons (Wang et al., 2013; Storey et al., 2016). Therefore, the presence of presynaptic D1Rs in the NAc, but not in the dorsal striatum, may provide a mechanism for NAc-specific synaptic filtering that is available under normal physiological conditions, while effects in the dorsal striatum only arise after chronic exposure to psychostimulants.
By counteracting the enhanced excitation evoked by presynaptic D1Rs, at both D1R- and D2R-expressing SPNs, adenosine can limit glutamate synaptic input to these same neurons (Wang et al., 2012). Inhibition by adenosine is dependent on NMDAR stimulation and is potentiated by AMPARs, suggesting that D1 (Levine et al., 1996) and AMPA (Cherubini et al., 1988) receptors on SPNs may augment NMDAR-dependent calcium entry into D1R-containing cells (Huang et al., 2011) to promote adenosine production through signaling cascades (Husi et al., 2000; Hardingham et al., 2001).
These data suggest that when postsynaptic D1Rs on NAc SPNs are activated, they facilitate adenosine efflux and drive retrograde presynaptic inhibition by decreasing the probability of glutamate release to both D1R and D2R-containing SPNs (Wang et al., 2012) (Figure 1). Remarkably, stimulation of either D1 or adenosine A1 receptors attenuates exocytosis from ~80% of cortical synapses, concordant with the percentage of adenosine receptors on striatal glutamatergic afferents (Ciruela et al., 2006). Adenosine retrograde transmission appears to select strong synapses on D1R-containing SPNs, and more broadly reduces synaptic transmission to D2R-expressing cells.
With phasically driven levels of dopamine release, cortical information along the direct and indirect pathways would be filtered by cannabinoids and adenosine. Dopamine can exert its actions as a neuromodulator quite rapidly. For example, at D2 autoreceptors the effects are maximal approximately 0.5 s after its release (Phillips et al., 2002; Schmitz et al., 2002; Beckstead et al., 2004). Similarly, activation of D1 receptors modulates presynaptic effects of NMDA-glutamate receptors on a subsecond time scale (Chen et al., 2004). Interestingly, the inhibitory effect of endocannabinoids on D2R-containing SPNs are delayed when compared to the relatively fast actions of adenosine or those arising from direct dopamine receptor stimulation (Wang et al., 2012). By suppressing excitatory inputs with a low probability of release, this delay in endocannabinoid response provides further temporal filtering that persists long after phasic dopamine release. Similar to mechanisms in hippocampal LTD (Chevaleyre and Castillo, 2003), where high levels of cortical activity converge with enhanced dopamine release, glutamate efflux to extrasynaptic mGluRs (Wang et al., 2006) would balance cannabinoid-induced LTD with adenosine-induced long-term potentiation that develops through adenosine A2A receptors on D2R-containing SPNs (Higley and Sabatini, 2010).
Together, these experiments indicate that dopamine produces both frequency-dependent and temporal filtering of specific low-probability release synaptic inputs to SPNs. At low cortical frequencies, D1Rs excite striatonigral SPNs, while D2Rs specifically inhibit striatopallidal SPNs. Under these conditions, dopamine release favors activation of D1R-containing SPNs to promote feed-forward activation of cortical circuitry. At higher frequencies, the dopamine-dependent release of adenosine promotes further filtering of cortical signals entering both output pathways, while endocannabinoids provide late temporal filtering of cortical inputs specifically to the indirect pathway. Through this combination of mechanisms, the convergence of high glutamate and dopamine provides frequency and temporal filtering of cortical information by promoting signals with high probability of release through both the direct and indirect pathways, while inhibiting weak signals.
This model in the NAc is consistent with the classic work in the dorsal striatum by Mahlon Delong’s and Wolfram Schultz’s labs, which as above suggested a “go/no-go” model, where motor activation accompanies a rise in direct pathway activity and movement deactivation is caused by a shift in balance toward the indirect pathway (Apicella et al., 1992; Wichmann and DeLong, 1996). In the dorsal striatum, however, excitatory inputs lack presynaptic D1Rs and filtering is asymmetrically applied to the indirect pathway (Bamford et al., 2004). In contrast, in the NAc, dopamine’s strengthening of high-probability synapses to both pathways by presynaptic D1Rs may initiate behaviors by activating specific signals through the direct pathway (“go” signals) while simultaneously activating signals through the indirect pathway which would suppress competing networks (“no-go” signals). The resulting rise in specific SPN output would be curtailed by subsequent feedback inhibition via adenosine and endocannabinoids.
Longer-term dopamine-dependent modifications of the corticostriatal synapse
While we focus in this review on the rapid effects of dopamine on corticoaccumbal neurotransmission during operant behavior, these effects certainly lead to forms of long-term synaptic plasticity, including the formation and removal of synapses involved in learning (Malenka and Bear, 2004).
A widely used model of such changes are “sensitization” models related to drugs that enhance dopamine neurotransmission, such as amphetamines. Mice with repeated exposure to amphetamines become hypersensitive (sensitized) to them (Bamford et al., 2008), and at the cellular level, this sensitization correlates well with the spine density on SPNs (Li et al., 2004): note that this form of analysis is used because spines are easier to measure than presynaptic structures, but that the findings indicate an increase in corticostriatal synapses. Thus, sensitization studies indicate that an increase in corticostriatal synapses is an outcome of enhanced dopamine signaling associated with behavior or environmental cues.
To address how dopamine might increase SPN spines, Kasai and coworkers used optogenetics to examine the timing of spine growth at SPNs in the NAc core (Yagishita et al., 2014). Dopamine release was evoked by optical stimulation of ChR2 expressed in dopamine neurons, while glutamate was locally generated by two-photon photo-uncaging an inert chemical precursor. An SPN receiving these inputs was monitored with a patch pipette that contained a fluorescent dye that allowed visualization of the spines of the SPN. The combination of glutamate and dopamine transients, along with patch-induced action potentials, caused an increase in spine volume in a manner that was critically dependent on the timing of the dopamine delivery. In the absence of dopamine, or when dopamine was delivered a few seconds before or after the combination of glutamate transients or action potential, changes in spine volume did not occur. These experiments illustrate that the timing of glutamatergic and dopamine neurotransmission in the NAc must occur in a precise and sequential manner to produce long-lasting synaptic modulation.
Summary: Dopamine’s roles in the sequence of events during reward based operant behaviors
The investigation of corticostriatal synapses at SPNs over the last decade has revealed multiple novel aspects of neurotransmitter signaling. Together, the information collected over many years in multiple laboratories demonstrates that a major role of dopamine is to promote transient shifts in the balance between direct and indirect striatal pathways. Optogenetic studies have confirmed that activation of the direct pathway is rewarding, an effect enhanced by D1R activation. As shown by experiments in behaving animals during operant behavior, as well as experiments in brain slices, activation of D2R-containing SPNs is suppressed by dopamine and D2R agonists, an action that may lower the “aversion” associated with their activity (Kravitz et al., 2012). Electrochemistry and electrophysiology approaches have revealed that subpopulations of D1R- or D2R-containing SPNs are involved in reward based behaviors, and that the specific neurons involved are a function of the particular reward that is delivered: for both classes of SPN, it appears that local dopamine transients are required for their reward associated activity.
The striatal microcircuit reacts when both high cortical input and dopamine appear simultaneously, and the baseline equilibrium initially shifts in favor of the direct pathway, which would activate the ventral midbrain dopamine neurons to encourage and maintain dopamine release. In the NAc, temporally directed feedback from adenosine and cannabinoids then act to regulate SPN activity and filter cortical signaling to select signals with a high release probability that may contain behaviorally relevant information. In the setting of high cortical and dopamine neurotransmission signaling during a cue, the synaptic filtering may thus prepare the animal for a subsequent reward. Circuitry in the dorsal striatum may be more straightforward than in the NAc, since dopamine neurotransmission may principally determine whether a movement will occur.
The microiontophoretic studies above suggest that SPNs involved in mediating the coordinated responses during reward-based behavior behaviors are only those in regions with local dopamine release, which are also the regions that have phasically activated SPNs. These results reveal the central importance of the precise timing of neurotransmitter release. While it is well documented that dopamine can exert actions on a broad range of time scales (Greengard, 2001), FSCV studies in behaving animals reveal the prominent occurrence of subsecond dopamine responses during operant behaviors. As guide for further investigation, the synaptic mechanisms underlying NAc SPN modulation that occur in parallel with dopamine release from the VTA projections can be summarized as follows:
Step 1. Time limited excitation of D2R-containing SPNs
1A
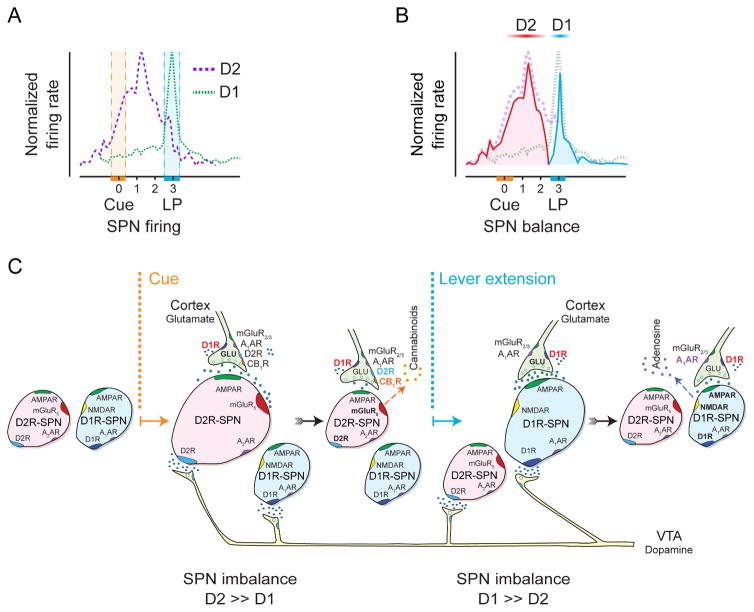
D2R-expressing SPNs (D2R-SPNs) exhibit a low tonic activity (~1 Hz), due to presynaptic inhibition of cortical inputs by tonic eCBs and the prominent potassium currents found in mature SPNs (Wilson and Kawaguchi, 1996). Sensory cues trigger cortical (and likely thalamic) inputs. The more active glutamatergic release sites escape D2R-dependent eCB filtering (Bamford et al., 2004) to drive D2R-SPN firing (to ~6 Hz). A rise in dopamine neurotransmission (from <20 nM to ~100 nM) from the VTA arrives with a short delay (<100 ms) after the cue, and increases excitation via presynaptic D1 receptors (likely by opening calcium channels), driving maximal D2R-SPN activation 1–2 sec after the cue (Figure 4A). During this “ready/steady” period, animals orient to the site of anticipated lever extension and await its appearance.
Figure 4.
Time course of D1 and D2 responses in the rat NAc during an ICSS trial consisting of cue presentation (0) followed two seconds later by lever extension and then a subsequent lever press (LP) to deliver and electrical stimulation to dopamine cell bodies. A. Time course of D2R-containing SPNs that are cue excitatory and D1R-containing SPNs that are LP-excitatory. The hatched areas indicate time variation of either cue or LP, as indicated in Figure 2. B. The differential responses of both sets of SPNs (D2 inhibitory cells were ignored in this representation). Firing rates from D1- and D2-SPNs (dashed) were subtracted to illustrate the imbalance in SPN activity following cue and LP. C. Temporal sequence of chemical activity at SPN synapses. The cue temporarily heightens D2R-SPN activity to create an imbalance in striatal output. The imbalance may alert and orient the animal, perhaps by promoting an urge to move and defining the motor sequence to be activated. The lever extension increases D1R-SPN activity, culminating in the lever press (LP).
1B
Dopamine then provides a time limit to D2R-SPN activation, ~2 sec after the sensory cue. This is due to delayed signal driven by convergent activation of extrasynaptic D2R and to coincident activation of extrasynaptic group 1 mGluR (1/5) glutamate receptors. This combination drives release of more eCB from D2R-SPNs, which acts as a retrograde messenger to inhibit glutamate release via presynaptic CB1 receptors.
The resulting inhibition of D2R-SPNs firing is likely required for Step 2, so that collateral inhibition of D2R to D1R-containing SPNs (D1R-SPNs) is decreased and excitation of appropriate D1R-SPNs can proceed (See a recent review on SPN lateral inhibition (Burke et al., 2017)). Additional mechanisms likely contribute to a return to the tonic low SPN firing rate, including strong presynaptic inhibition driven by mGluR2/3, and possibly presynaptic D2R and cholinergic receptors. Importantly, these responses are only observed in those specific subregions of the NAc that also exhibit cue-evoked dopamine release, indicating that dopamine neurotransmission is required.
Step 2: Timed activation of D1R SPNs
2A
In animals that have learned to associate a sensory cue with a lever press, another series of glutamatergic excitatory inputs, which could include cortical, limbic and possibly thalamic inputs, occurs ~ 2–3 sec following the cue. By this point, D2R-SPNs activity is in a steep decline due to the eCB high pass filtering in Step 1B. Dopamine neurotransmission however remains elevated at this stage, and now enhances inputs to D1R-SPNs by stimulating presynaptic D1Rs. The delay in D1R-SPN activity may be subsequent to D2R-SPN silencing, as these SPNs may inhibit D1R-SPNs via collaterals or interneurons, and so a specific set of D2-SPNs may need to return to low activity rates for activation of specific D1R-SPNs to proceed. Those D1R-SPNs that still receive the few active D2R collateral inputs that remain 2 s after the cue, i.e., the strongest corticostriatal that pass the eCB filter, would remain postsynaptically silenced.
2B
At a time nearly coincident with the lever press, co-activation of post-synaptic D1, NMDA and AMPA receptors increases adenosine efflux, likely released from D1 cells (Dunwiddie and Masino, 2001). The adenosine acts as a retrograde messenger to inhibit glutamate release via presynaptic A1A receptors. Additional potent inhibition is provided by presynaptic mGluR2/3. Importantly, these responses are only observed in NAc regions that exhibit cue-evoked dopamine release, suggesting that dopamine release is required.
A balance of D1R and D2R-SPNs is permissive for many behaviors, including locomotor sensitization (Beutler et al., 2011). The change in firing rates of D2R-SPNs following the cue promotes an imbalance in the NAc output by favoring the indirect pathway (Figure 4B,C). In well-trained animals, this imbalance is followed a short time later by activation of D1R-SPNs in anticipation of the necessary “go pathway” reflexive motor movement required to depress the anticipated lever at its extension. These shifts in the balance of SPN activity are augmented by synaptic filtering, which reduces excitation of less effective excitatory inputs along the corticoaccumbal pathway.
Roughly a third of SPNs exhibit a higher baseline activity (~2 Hz), and these neurons decrease firing rates to ~1 Hz around the LP. This modulation is observed only in NAc regions that exhibit cue-evoked dopamine release, which has already been present for ~2 sec following the cue, and so is apparently a delayed effect that requires DA. This may be due to presynaptic inhibition by A1A receptors from adenosine released from D1 SPNs, which as in Step 2B, is driven by retrograde transmission from D1R-SPNs ~2s after the cue.
If this model provides a useful guide to elucidating the role of dopamine and corticostriatal interactions in operant behaviors, it also presents many gaps to be addressed in future research:
Central to this sequence of events is the release of glutamate, arising from the cortical and other afferents onto SPNs, and dopamine release arising from the activation of hindbrain structures. During the behavioral task, we assume that the excitation of SPNs is mediated by glutamate released from cortical axons, and while corticostriatal axonal calcium currents have recently been recorded by optical methods (Kupferschmidt et al., 2017), this has not been directly measured, and other inputs from thalamus and limbic areas may be involved. The confluence of sequential inputs from a range of regions allows an animal to execute the appropriate response in response to environmental cues. Methods now exist to measure rapid glutamate release (Wassum et al., 2012), and such measurements during ICSS would test current concepts of neurotransmission during reward-based behaviors. It may be that the strongest signals that choose appropriate responses require inputs from multiple brain regions: for example, thalamic inputs to the dendrite shaft may allow cortical signals that are present in spines to “pass” and drive SPN activity.
Optogenetic and other approaches suggest that operant behavior is abolished if the indirect pathway is activated. Nevertheless, the most active inputs appear to escape dopamine’s inhibition of the indirect pathway, and this feature seems to enable effective operant behaviors such as acquisition of sugar pellets in the corridor test (Wong et al., 2015): and as long noted, the indirect pathway is quite active during behavior (DeLong, 1972). If these interpretations are. If this interpretation is correct, how would the selection of a small set of indirect pathway synapses that avoid dopamine inhibition and remain highly activated lead to selection of appropriate behavioral circuits?
Dopamine axons course throughout the striatum, but during operant behaviors, dopamine appears to be released from specific and reproducible hot spots by particular stimuli (Wightman et al., 2007). In the acute slice, most of the regions in dopamine axons with clusters of synaptic vesicles may be silent (Pereira et al., 2016), and there are many means by which dopamine inhibition is regulated presynaptically, including by D2R autoreceptors, metabotropic glutamate, GABAergic, and cholinergic presynaptic receptors (Sulzer et al., 2016), but the apparent local control of dopamine release from specific sites during operant behavior has not been explained.
We note that this review has only focused on the actions of glutamate and dopamine on SPNs. The roles of interneurons and other neurotransmitters, such as GABA and acetylcholine, are important to these steps, and require further investigation. The correlation between dopamine release and the pause in cholinergic interneurons along with powerful inhibition provided by presynaptic GABAB and postsynaptic GABAA receptors suggest that these interneurons promote information gaiting for both novel and learned behaviors (Graybiel et al., 1994; Gangarossa et al., 2013).
A related issue that remains unexamined is whether thalamocortical signals may bypass SPNs and basal ganglia circuits, even in patients who have lost dopamine in Parkinson’s disease or those with pallidotomy procedures or other functional surgical interventions. It appears that some operant behavioral responses, particularly ones following very strong sensory inputs such as a loud noise or onrushing train, may not require the pathways discussed.
We note that results from other paradigms may differ from the sequential events observed with controlled iontophoretic characterization during ICSS and other operant behaviors discussed here. Whether circuit patterns associated with other behaviors requires further investigation.
Finally, in this review we have focused on rapid synaptic activity associated with behavior in the basal ganglia, rather than how these may lead to longer-term changes that influence future behavior. We note that while there are strong clues to longer-term synaptic changes that follow operant behaviors, including an extensive literature on both pre- and postsynaptic changes in the acute slice, the means of transfer of dopamine and excitation history during behavior to affect learning that outlasts the behavioral sequences remains unclear, as recently reviewed (Calabresi et al., 2014). Future studies using improved measurement techniques and behavioral paradigms will be needed to resolve these actions.
IN BRIEF Statement.
Several neurotransmitters regulate striatal spiny projection neurons that synchronize reward-driven behaviors. Bamford et al. review advances in the measurement of functional neurotransmitter dynamics. These approaches reveal that local dopamine release is differentially regulated in adjacent circuits to coordinate these behaviors.
Acknowledgments
Research in basal ganglia physiology by the Bamford lab is supported by NINDS R01060803. The Sulzer lab is supported by NIDA R0107418 and NIMH R01 MH108186, and by the Parkinson’s, JPB, Michael J. Fox, and Simons Foundations. Sulzer is a Brain and Behavior Distinguished Investigator. Research in the Wightman lab is supported by NIDA R01DA010900. Critical discussions with Regina Carelli, Caterina Owesson-White, Thomas Wichmann, and Mahlon DeLong are greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RN. Probing brain chemistry with electroanalytical techniques. Anal Chem. 1976;48:1126A–1138A. doi: 10.1021/ac50008a001. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, Caron MG, Bonci A, Sulzer D, Borrelli E. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32:9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. doi: 10.1152/jn.1992.68.3.945. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu NP, Andre VM, Cohen R, Cepeda C, Levine MS, Harleton E, Sulzer D. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58:89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharm Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Belle AM, Owesson-White C, Herr NR, Carelli RM, Wightman RM. Controlled iontophoresis coupled with fast-scan cyclic voltammetry/electrophysiology in awake, freely moving animals. ACS Chemical Neuroscience. 2013;4:761–771. doi: 10.1021/cn400031v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. Proc Natl Acad Sci U S A. 2011;108:4206–4211. doi: 10.1073/pnas.1101424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DA, Rotstein HG, Alvarez VA. Striatal local circuitry: a new framework for lateral inhibition. Neuron. 2017;96:267–284. doi: 10.1016/j.neuron.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. J Neurosci. 2011;31:13860–13869. doi: 10.1523/JNEUROSCI.1340-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia F, Saddoris MP, Wightman RM, Carelli RM. Differential dopamine release dynamics in the nucleus accumbens core and shell track distinct aspects of goal-directed behavior for sucrose. Neuropharmacology. 2012;62:2050–2056. doi: 10.1016/j.neuropharm.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Nat Acad Sci. 2016;113:2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. JNeurosci. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, Ahmari SE, Moore H, Kellendonk C. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Greengard P, Yan Z. Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:2596–2600. doi: 10.1073/pnas.0308618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Herrling PL, Lanfumey L, Stanzione P. Excitatory amino acids in synaptic excitation of rat striatal neurones in vitro. J Physiol. 1988;400:677–690. doi: 10.1113/jphysiol.1988.sp017143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: a moveable electrode mapping study. Brain Res. 1980;185:1–15. doi: 10.1016/0006-8993(80)90666-6. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc Natl Acad Sci U S A. 2009;106:14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Contributions of striatal dopamine signaling to the modulation of cognitive flexibility. Biol Psychiatry. 2011;69:704–707. doi: 10.1016/j.biopsych.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nature Neuroscience. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of basal ganglia neurons during movement. Brain Res. 1972;40:127–135. doi: 10.1016/0006-8993(72)90118-7. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumartin B, Doudnikoff E, Gonon F, Bloch B. Differences in ultrastructural localization of dopaminergic D1 receptors between dorsal striatum and nucleus accumbens in the rat. Neurosci Lett. 2007;419:273–277. doi: 10.1016/j.neulet.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Exley R, McIntosh JM, Marks MJ, Maskos U, Cragg SJ. Striatal alpha5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J Neurosci. 2012;32:2352–2356. doi: 10.1523/JNEUROSCI.4985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Filion M. Effects of interruption of the nigrostriatal pathway and of dopaminergic agents on the spontaneous activity of globus pallidus neurons in the awake monkey. Brain Res. 1979;178:425–441. doi: 10.1016/0006-8993(79)90704-2. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d’Exaerde A, El Mestikawy S, Gerfen CR, Herve D, Girault JA, Valjent E. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Christensen JR, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997;68:152–161. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gonon F, Cespuglio R, Ponchon JL, Buda M, Jouvet M, Adams RN, Pujol JF. In vivo continuous electrochemical determination of dopamine release in rat neostriatum. C R Acad Sci Hebd Seances Acad Sci D. 1978;286:1203–1206. [PubMed] [Google Scholar]
- Gonon FG, Buda MJ. Regulation of dopamine release by impulse flow and by autoreceptors as studied by in vivo voltammetry in the rat striatum. Neuroscience. 1985;14:765–774. doi: 10.1016/0306-4522(85)90141-1. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;15:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr NR, Kile BM, Carelli RM, Wightman RM. Electroosmotic flow and its contribution to iontophoretic delivery. Anal Chem. 2008;80:8635–8641. doi: 10.1021/ac801547a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr NR, Daniel KB, Belle AM, Carelli RM, Wightman RM. Probing presynaptic regulation of extracellular dopamine with iontophoresis. ACS ChemicalNeuroscience. 2010;1:627–638. doi: 10.1021/cn100056r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Basal ganglia circuits for reward value-guided behavior. Annu Rev Neurosci. 2014;37:289–306. doi: 10.1146/annurev-neuro-071013-013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, Dombeck DA. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 2016;535:505–510. doi: 10.1038/nature18942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Yang CH, Gean PW. Presynaptic D2 dopaminergic receptors mediate inhibition of excitatory synaptic transmission in rat neostriatum. Brain Res. 1995;690:264–268. doi: 10.1016/0006-8993(95)00734-8. [DOI] [PubMed] [Google Scholar]
- Huang YH, Ishikawa M, Lee BR, Nakanishi N, Schluter OM, Dong Y. Searching for presynaptic NMDA receptors in the nucleus accumbens. J Neurosci. 2011;31:18453–18463. doi: 10.1523/JNEUROSCI.3824-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T, Tepper JM. Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci. 2010;30:6999–7016. doi: 10.1523/JNEUROSCI.5996-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neuroscience & Biobehavioral Reviews. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76(Pt B):329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projections subtypes of rat neostriatal matrix cells revealed by intracelllular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Molecular Pharmacology. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Janak PH. Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry. Neuron. 2015;88:247–263. doi: 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE, Nelson AB, Ramos M, Del Barrio D, Sulzer D, Kreitzer AC, Borrelli E. Parkinsonism driven by antipsychotics originates from dopaminergic control of striatal cholinergic Interneurons. Neuron. 2016;91:67–78. doi: 10.1016/j.neuron.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick DC, Edwards MA, Flowers PA, Wightman RM. Characterization of solute distribution following iontophoresis from a micropipet. Anal Chem. 2014;86:9909–9916. doi: 10.1021/ac5026072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick DC, McKinney CJ, Manis PB, Wightman RM. Expanding neurochemical investigations with multi-modal recording: simultaneous fast-scan cyclic voltammetry, iontophoresis, and patch clamp measurements. The Analyst. 2016;141:4902–4911. doi: 10.1039/c6an00933f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Juczewski K, Cui G, Johnson KA, Lovinger DM. Parallel, but Dissociable, Processing in Discrete Corticostriatal Inputs Encodes Skill Learning. Neuron. 2017;96:476–489. e475. doi: 10.1016/j.neuron.2017.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, Malenka RC. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85:429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R, Prochazka A. Methods for neuronal recording in conscious animals. New York: John Wiley & Co; 1984. [Google Scholar]
- Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, Drago J, Sibley DR, Westphal H. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. European Journal of Neuroscience. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Limberger N, Trout SJ, Kruk ZL, Starke K. “Real time” measurement of endogenous dopamine release during short trains of pulses in slices of rat neostriatum and nucleus accumbens: role of autoinhibition. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:623–629. doi: 10.1007/BF00174745. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73:1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Muller CE, Fisone G, Lluis C, Agnati LF, Franco R, Fuxe K. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Maura G, Giardi A, Raiteri M. Release-regulating D-2 dopamine receptors are located on striatal glutamatergic nerve terminals. J Pharmacol Exp Ther. 1988;247:680–684. [PubMed] [Google Scholar]
- Meador Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–242. [PubMed] [Google Scholar]
- Millar J, Armstrong-James M, Kruk ZL. Polarographic assay of iontophoretically applied dopamine and low-noise unit recording using a multibarrel carbon fibre microelectrode. Brain Research. 1981;205:419–424. doi: 10.1016/0006-8993(81)90354-1. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychological review. 1956;63:81–97. [PubMed] [Google Scholar]
- Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: Carpenter MBJA, editor. The Basal Ganglia II. New York: Plenum Press; 1987. pp. 415–427. [Google Scholar]
- Natsubori A, Tsutsui-Kimura I, Nishida H, Bouchekioua Y, Sekiya H, Uchigashima M, Watanabe M, de Kerchove d’Exaerde A, Mimura M, Takata N, Tanaka KF. Ventrolateral Striatal Medium Spiny Neurons Positively Regulate Food-Incentive, Goal-Directed Behavior Independently of D1 and D2 Selectivity. J Neurosci. 2017;37:2723–2733. doi: 10.1523/JNEUROSCI.3377-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olds J, Olds ME. Positive reinforcement produced by stimulating hypothalamus with iproniazid and other compounds. Science. 1958;127:1175–1176. doi: 10.1126/science.127.3307.1175. [DOI] [PubMed] [Google Scholar]
- Olds J, Killam KF, Bach-Y-Rita P. Self-stimulation of the brain used as a screening method for tranquilizing drugs. Science. 1956;124:265–266. doi: 10.1126/science.124.3215.265. [DOI] [PubMed] [Google Scholar]
- Owesson-White C, Belle AM, Herr NR, Peele JL, Gowrishankar P, Carelli RM, Wightman RM. Cue-evoked dopamine release rapidly modulates D2 neurons in the nucleus Aaccumbens during motivated behavior. J Neurosci. 2016;36:6011–6021. doi: 10.1523/JNEUROSCI.0393-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci U S A. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DB, Schmitz Y, Meszaros J, Merchant P, Hu G, Li S, Henke A, Lizardi-Ortiz JE, Karpowicz RJ, Jr, Morgenstern TJ, Sonders MS, Kanter E, Rodriguez PC, Mosharov EV, Sames D, Sulzer D. Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat Neurosci. 2016;19:578–586. doi: 10.1038/nn.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MI, Olds J. Unit activity: motiviation-dependent responses from midbrain neurons. Science. 1969;165:1269–1271. doi: 10.1126/science.165.3899.1269. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake in mice lacking D2 receptors. J Neurosci. 2002;22(18):8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87:273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- Schneider F, Grimm C, Hegemann P. Biophysics of Channelrhodopsin. Annu Rev Biophys. 2015;44:167–186. doi: 10.1146/annurev-biophys-060414-034014. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT. Monitoring dopamine in vivo by microdialysis sampling and on-line CE-laser-induced fluorescence. Anal Chem. 2006;78:6717–6725. doi: 10.1021/ac0608218. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Current Opinion in Neurobiology. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, Sousa N, Rodrigues AJ. Reappraising striatal D1- and D2-neurons in reward and aversion. Neuroscience & Biobehavioral Reviews. 2016a;68:370–386. doi: 10.1016/j.neubiorev.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, Sousa N, Rodrigues AJ. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Com. 2016b;7:11829. doi: 10.1038/ncomms11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One. 2014;9:e94771. doi: 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey GP, Gonzalez-Fernandez G, Bamford IJ, Hur M, McKinley JW, Heimbigner L, Minasyan A, Walwyn WM, Bamford NS. Nicotine modifies corticostriatal plasticity and amphetamine rewarding behaviors in mice. eNeuro. 2016:3. doi: 10.1523/ENEURO.0095-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal ganglia. 2016;6:123–148. doi: 10.1016/j.baga.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Takmakov P, McKinney CJ, Carelli RM, Wightman RM. Instrumentation for fast-scan cyclic voltammetry combined with electrophysiology for behavioral experiments in freely moving animals. Rev Sci Instrum. 2011;82:074302. doi: 10.1063/1.3610651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Friberg IK, Weiss SW, Standaert DG. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Filion M, Bedard PJ. Responses of pallidal neurons to striatal stimulation in monkeys with MPTP-induced parkinsonism. Brain Res. 1989;498:17–33. doi: 10.1016/0006-8993(89)90395-8. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- Wang W, Darvas M, Storey GP, Bamford IJ, Gibbs JT, Palmiter RD, Bamford NS. Acetylcholine encodes long-lasting presynaptic plasticity at glutamatergic synapses in the dorsal striatum after repeated amphetamine exposure. J Neurosci. 2013;33:10405–10426. doi: 10.1523/JNEUROSCI.0014-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Dever D, Lowe J, Storey GP, Bhansali A, Eck EK, Nitulescu I, Weimer J, Bamford NS. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol. 2012;590:3743–3769. doi: 10.1113/jphysiol.2012.235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Tolosa VM, Tseng TC, Balleine BW, Monbouquette HG, Maidment NT. Transient extracellular glutamate events in the basolateral amygdala track reward-seeking actions. J Neurosci. 2012;32:2734–2746. doi: 10.1523/JNEUROSCI.5780-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Anal Chem. 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J Neurosci. 1986;6:274–280. doi: 10.1523/JNEUROSCI.06-01-00274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res. 1981;220:67–80. doi: 10.1016/0006-8993(81)90211-0. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nature Reviews: Neuroscience. 2016;17:351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Borgkvist A, Choi SJ, Mosharov EV, Bamford NS, Sulzer D. Dopamine-dependent corticostriatal synaptic filtering regulates sensorimotor behavior. Neuroscience. 2015;290:594–607. doi: 10.1016/j.neuroscience.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Richard S, Parent A. The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neuroscience Research. 2000;38:49–62. doi: 10.1016/s0168-0102(00)00140-1. [DOI] [PubMed] [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014;345:1616–1620. doi: 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- Yang H, Peters JL, Michael AC. Coupled effects of mass transfer and uptake kinetics on in vivo microdialysis of dopamine. J Neurochem. 1998;71:684–692. doi: 10.1046/j.1471-4159.1998.71020684.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Zeppenfeld DM, Williams JT. Cholinergic interneurons underlie spontaneous dopamine release in nucleus accumbens. J Neurosci. 2017;37:2086–2096. doi: 10.1523/JNEUROSCI.3064-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J Neurosci. 2003;23:10585–10592. doi: 10.1523/JNEUROSCI.23-33-10585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]