Abstract
The recent surge in opioid-related overdoses and related fatalities underscores the need for assertive mechanisms for linking individuals with opioid use disorders (OUD) to medication-assisted treatment (MAT). This pilot study investigated the feasibility of an intervention that used peer outreach workers to identify out-of-treatment individuals with OUD combined with a modified version of the Recovery Management Checkup to link individuals to methadone treatment. The study was conducted in high-risk communities in Chicago over 8 weeks; peer outreach workers identified 88 active opioid/heroin users; 72 were screened as eligible, and 70 showed to the study intake/initial linkage meeting. Most participants were male (73%) and African American (94%), with an average age of 52.0 (sd=7.6). Nearly all (67/70, 96%) were admitted to methadone treatment; median time from initial linkage meeting to treatment admission was 2.6 days. Most were still in treatment at 30 and 60 days post-intake (69% and 70%, respectively). A high-risk sub-group was identified that had ever received naloxone for an opioid overdose; they had one third of the odds of being in treatment at 30 days post-intake compared with others. The intervention model holds promise as an assertive method for identifying and engaging individuals with OUD into treatment.
Keywords: opioids, peer outreach, recovery management checkup, treatment linkage and engagement, methadone treatment
1. Introduction
The recent surge in misuse of prescription opioids and, relatedly, increase in heroin use associated with its relatively lower cost and wide availability, has been extensively documented within the U.S. (Cicero, Ellis, Surratt, & Kurtz, 2014; Compton, Jones, & Baldwin, 2016). Data from the National Survey on Drug Use and Health (NSDUH) shows that although the rate of nonmedical use of prescription opioids declined from 2003–05 to 2012–14, following the imposition of stricter prescribing practices that limited supply and raised prices, the prevalence of past-year opioid use disorders (OUD) actually increased over this time (Jones, 2017). Concurrently, there were dramatic increases in drug-related deaths due to prescription opioid overdose (Han et al., 2015). The most recent data from the Centers for Disease Control and Prevention (CDC) show that between 2013 – 2014 the death rate from the most commonly prescribed opioid pain relievers (natural and semisynthetic opioids) increased by 9%, the death rate from heroin increased 26%, and the death rate from synthetic opioids, which includes illicitly manufactured fentanyl and synthetic opioid pain relievers other than methadone, increased 80% (CDC, 2016).
The state of Illinois has not been immune to these increases. In Illinois, 2,135 drug-related overdose deaths were reported from January 1, 2014 through October 31, 2015. Heroin (including when combined with fentanyl) use accounted for 59.3% (1,266) of these drug overdose deaths and other opiates accounted for an additional 36.9% (788) of these fatalities. This represents a 60% increase in the number of opiate-related overdose deaths among Illinois residents between 2010 and 2015. Cook County, which includes the City of Chicago, accounted for nearly half (n=607, 47.9%) of the statewide heroin overdose deaths. In 2011, the Chicago Metropolitan Area ranked first nationally for both emergency department (ED) mentions (24,627) for heroin (Substance Abuse and Mental Health Services Administration [SAMHSA], Drug Abuse Warning Network, 2011) and percentage (18.6%) of individuals who were arrested and tested positive for heroin (Arrestee Drug Abuse Monitoring Program, 2012). Chicago also reported the highest number of heroin ED mentions (13,178) among African Americans in the country. These data are consistent with a recent study analyzing trends in prescription opioid and heroin-related overdose hospitalizations showing that the highest rates of increase among African Americans for heroin-related overdose hospitalizations are in the East North Central census region, which is inclusive of Illinois (Unick & Ciccarone, 2017).
1.1. Challenges of Linking Individuals with OUD to Treatment
Despite the increasing numbers and visibility of individuals using opioids and surge in opioid-related overdoses, there is a dearth of mechanisms for helping people with OUD to access and stay in treatment, particularly following an overdose. The efficacy of medication-assisted treatment (MAT) for OUD is well-established for reducing opioid use and associated adverse health outcomes, including death, and positive outcomes are enhanced with longer duration in treatment (Ball & Ross, 1991; Mattick, Breen, Kimber, & Davoli, 2014). However, numerous barriers to accessing MAT (i.e., methadone, buprenorphine) for OUD have been identified. These include (1) limited treatment capacity due to lack of qualified physicians/treatment programs (Jones, Campopiano, Baldwin, & McCance-Katz, 2015; Knudsen, 2015); (2) financial barriers, such as lack of insurance coverage of MAT or inability to self-pay (Burns et al., 2016); (3) regulatory barriers stemming from restrictions on number of patients that qualified individual physicians who dispense these medications can treat or on the licensing of programs (Knudsen, Abraham, & Oser, 2011); (4) geographic barriers, including limited availability of treatment providers or programs in some areas, particularly in rural areas (Rosenblatt, Andrilla, Catlin, & Larson, 2015); (5) attitudinal barriers, including negative attitudes regarding the use of medications to treat opioid use disorders (Alanis-Hirsch et al., 2016) as well as persistent stigma regarding these disorders (Schwartz et al., 2008); and (6) logistical barriers, such as lack of transportation to treatment programs and limited hours of operation (Sharma et al., 2017). One study of over 900 injection drug users in Baltimore showed that a minority (26.2%) had sought drug treatment in the 30 days following their last overdose; the odds of entering any kind of treatment were significantly higher among individuals who had spoken with someone (i.e., spouse or partner, crisis counselor or hospital staff) about drug treatment after the overdose. This suggests that more assertive mechanisms are needed to facilitate treatment entry, particularly following an overdose.
Most efforts to link individuals with OUD to treatment following an overdose have focused on screening patients in hospital ED or inpatient settings, which have shown preliminary evidence of successful engagement. Yet many individuals who overdose and receive emergency intervention from first responders either refuse to go to an ED or, if admitted, leave prior to any effective intervention or referral to treatment. In the absence of treatment following an overdose rescue, there remains a high likelihood that individuals will resume heroin/opioid use and the risk of death increases with successive overdose experiences (Mueller, Walley, Calcaterra, Glanz, & Binswanger, 2015; Stoové, Dietze, & Jolley, 2009). Moreover, Individuals who are at risk for multiple overdoses are most in need of assistance, as they typically have co-occurring mental health problems, lack resources or family/social support, and report polysubstance use, particularly involving benzodiazepines (Boscarino et al., 2016; Yarborough et al., 2016).
1.2. Peer Outreach Workers to Identify Opioid Users in the Community
Use of peer outreach workers is a promising way to identify individuals with OUD in the community who are not currently in treatment and at risk of overdose, and may be receptive to efforts to help them access it. Dating to the 1980s, NIDA funded a large portfolio of research on developing interventions to identify individuals at risk of HIV, such as injection drug users, engage them in brief informational interventions, and, if receptive, refer them into treatment or other health and social services (Needle et al., 1998). Peer outreach workers commonly work with treatment and other service providers in the target community, make contact with individuals in areas identified as high-risk, and distribute flyers or brochures, thus increasing access to the target population through referrals from peer networks within the community.
Typically, peer outreach workers are individuals who have experienced the same challenges as the target population; in this case, individuals who have a history of heroin/opioid use, but who have a demonstrated history of treatment participation and are currently in recovery and stably functioning in the community. Their own knowledge of the community, including the venues targeted for recruiting opioid users at risk of relapse, and the local treatment/service system, is instrumental in establishing rapport with prospective participants based on common knowledge and understanding (Marshall, Dechman, Minichiello, Alcock, & Harris, 2015). This approach has been validated by a systematic review of over 40 studies using the peer outreach worker model, which concluded that “the evidence for the effectiveness of a community-based outreach strategy is strong” (Needle, Burrows, Friedman, & Latkin, 2005, pg. 45).
1.3. Recovery Management Checkup Intervention
An existing evidence-based intervention, the Recovery Management Checkup (RMC; Scott & Dennis, 2003), holds potential for linking out-of-treatment individuals with OUD to MAT. The RMC was developed to engage and link individuals with substance use disorders (SUD) to treatment and support their treatment engagement and recovery. The conceptual framework is based on the public health theory that long-term monitoring through regular checkups and early (re)intervention will facilitate early detection of relapse, reduce the time to treatment re-entry, and, consequently, improve long-term outcomes (Scott & Dennis, 2009, 2010; Scott, Dennis, Laudet, Funk, & Simeone, 2011). This approach does not rely on participants having to initiate help-seeking. Using standard motivational interviewing techniques (Apodaca & Longabaugh, 2009), the Linkage Manager (LM) contacts participants by phone and discusses with them the benefits of going to treatment, engages in problem solving about their expressed barriers to treatment, and provides assertive linkage (e.g., making appointments, providing transportation, and negotiating access). For individuals who initially refuse the referral to treatment, the LM explores the benefits, consequences, and/or inconveniences of the person’s current substance use as well as explores the person’s motivation for treatment. Using open-ended questions, the LM explores not only reasons the participant may opt out of the treatment referral but also the potential benefits of treatment. The LM seeks to develop discrepancy between how the participant currently perceives his/her situation and stated goals, and uses the technique of “rolling with resistance” to enhance treatment motivation. The LM assures the participant that the decision is up to him/her regarding treatment, thereby empowering the patient in the decision process and encouraging “change talk.”
The RMC model has been evaluated and shown to be effective in two randomized trials in which individuals were recruited from SUD treatment and received quarterly checkups for 2 to 4 years (Dennis & Scott, 2012; Dennis, Scott, & Funk, 2003; Scott & Dennis, 2009; Scott, Dennis, & Foss, 2005), one randomized trial in which individuals were recruited at discharge from jail and received quarterly checkups from 3 years (Scott & Dennis, 2012; Scott, Dennis, & Lurigio, 2017), and one quasi-experiment with patients recruited from Federally Qualified Health Centers (FQHC), with RMC focused on the initial referral to treatment, rather than treatment re-entry (Scott et al., 2017). Across these four studies, which included participants with a range of types of SUDs, RMC was used to provide ongoing monitoring, early re-intervention and, when indicated, linkage back to SUD treatment for over 1,300 individuals. In the longest trial, which included quarterly checkups for 4 years, individuals assigned to RMC were significantly more likely (p<.05) than those assigned to a control group to enter SUD treatment sooner (13 vs. 45 months d=−0.61), enter treatment at any time (70% vs. 51% any admissions, d=.50), and stay in treatment longer (112 vs. 79 days, d=0.23; Dennis & Scott, 2012). The latter is important because process analyses show that only those who stayed in treatment 10 or more days significantly reduced their substance use. Moreover, the size of these effects increased over time with repeated quarterly exposures to RMC, and RMC participants also reported significantly more total days of abstinence (1,026 vs. 932 days, d=+0.24) and fewer past-month SUD symptoms (89 vs. 126 symptom-months, d=−0.27) relative to the comparison sample that received usual care.
This paper reports findings from a pilot study of a combined intervention using peer outreach workers for contacting and identifying out-of-treatment individuals with OUD and a modified version of the RMC intervention that focused only on the initial linkage to treatment and engagement. This pilot study was conducted in order to develop and refine these strategies within the context of both federal and state policy initiatives to address the current opioid crisis (SAMHSA, 2016). Hence, because this pilot study was focused on identifying and recruiting out-of-treatment individuals with OUD in the community, the longer-term “management” component of the RMC was not implemented. Further, because of limited resources for this small-scale pilot study as well as the limited availability of the full range of MAT options, treatment linkage was focused solely on methadone treatment. Lastly, because we were particularly interested in identifying individuals who are at high risk of opioid-related overdose and linking them to treatment, we examined overdose history, including receipt of opioid-reversal medication (i.e., naloxone), and whether these characteristics are related to treatment entry and engagement.
2. Material and Methods
In January – March 2017, the pilot study tested a model for using peer outreach workers to go to communities within Chicago that have high concentrations of individuals with OUD and opioid-related overdose and to engage and refer them to project Linkage Managers, who then used a modified version of the RMC model that focused on the initial treatment linkage and engagement process (and re-linkage as needed) for an intervention period of 60 days (vs. 12 months in prior clinical trials). Below is a description of the intervention, study sample, assessments, and analysis.
2.1. Peer Outreach and RMC Procedures
Peer outreach workers were individuals who had a history of opioid use disorders and stable participation in methadone treatment for at least one year, based on recommendations from local treatment providers. Five individuals received extensive training on the procedures for recruiting potential study participants, including reviewing recruitment scripts, observing study investigators enact sample scenarios, and participating in role-playing of various scenarios for approaching individuals and referring them for study participation. They worked closely with the Study Project Director, who debriefed with them following field trips in order to continuously refine the recruiting method. Based on discussions with the peer outreach workers, as well as knowledge of the study investigators, several community “hot spots” with high concentrations of opioid users and high rates of overdose were identified, including specific neighborhoods and locations for outreach, such as fast-food restaurants, parks, bus stations, and shelters.
The outreach encounters generally consisted of a brief introduction by the peer outreach worker, who initiated conversation by referring to the problem of heroin use in the community. The outreach workers then explained that they were looking to find individuals who were actively using heroin, but not currently in treatment, for a study that aimed to help such individuals get into treatment. If the individual self-identified as meeting those criteria and expressed interest in the study, the peer outreach worker then called the study staff who screened the prospective participant for eligibility over the phone. Those who were eligible and agreed to schedule a study intake/linkage appointment were provided with transportation to the study office. Time from the outreach referral to the initial linkage meeting ranged from 0 to 39 days (median = 1; mean = 2 [SD=6] days). For individuals that indicated they personally were not eligible for the study, but knew others who might be, the peer outreach workers gave them fliers with the study information and encouraged them to pass them on to others.
At the study intake/linkage meeting, a research interviewer first completed the informed consent and a baseline interview. Next, a linkage manager met with the participant and, using motivational interviewing techniques, discussed with them the benefits of going to treatment, engaged in problem solving about their expressed barriers to treatment, and provided assertive linkage (e.g., making appointments, providing transportation, and negotiating access) to treatment. For participants who were interested in treatment, a treatment intake appointment was made and transportation was arranged. Linkage Managers stayed in weekly contact (approximately) with participants who entered treatment to check in on their status and attempted to re-engage those who had dropped out back into treatment. For those participants who did not initially want a treatment referral, the linkage manager maintained weekly contact for 30 days. During this time, for participants who changed their mind and decided to accept a treatment referral, an intake appointment was made and transportation was scheduled. All study procedures were approved by the Chestnut Health Systems Institutional Review Board.
2.2. Study Sample
Study inclusion criteria included: heroin/opioid use in past 30 days, not currently in SUD treatment, able to speak/write English, plan to live in Chicago area within next 4 weeks, non-incarcerated, at least 18 years of age, and no evidence of serious mental health problems or cognitive impairment or other disability that would preclude participation or ability to provide informed consent. Over the course of 8 weeks, peer outreach workers identified 88 active opioid/heroin users in areas identified as high-risk for continued opioid use and overdose. Of these, 72 were screened as eligible, and 70 showed to the study intake/initial linkage meeting, which constitutes the study sample.
2.3. Assessment
At study intake participants were administered the GAIN-Q3 (Titus et al., 2003), which is a 25- minute brief assessment used to identify and address a wide range of problems in clinical and indicated populations. An index of problem severity was developed by summing the number of areas in which the participant indicated they had experienced problems in the past year (range 0 – 8); these included problems pertaining to work, physical health, stress, high-risk behaviors, internalizing disorder, externalizing disorders, substance use, and crime and violence. Additionally, participants completed a brief interview developed by the study investigators that asked for more in-depth information on their history of heroin/opioid use, treatment participation, overdose experiences, and whether they had ever received overdose reversal medication (i.e., naloxone/”Narcan”), and if so, how long ago and how many times. Participants also provided locator information for follow-up contact.
At 30 and 60 days post-intake, a brief 5-minute assessment was conducted by research staff regarding participants’ substance use and treatment participation with reference to the past 30 days. These were usually conducted in face-to-face sessions at the research office, although a few were conducted by telephone for individuals who were unable to travel due to illness. Treatment records were used to verify dosing at 30 and 60 days post-intake. Participants were compensated $35 for completing the intake interview and $20 for completing the 30- and the 60-day interviews.
2.4. Analyses
Demographic and background characteristics were examined for the total sample, including their past overdose experiences. Treatment entry and status at 30 and 60 days post-intake were examined with simple descriptive statistics. Time from the initial linkage meeting to treatment admission was examined using survival analysis. Lastly, post hoc comparisons on treatment entry and treatment status were conducted by comparing the sub-group that had ever received naloxone with others, using crosstabs and chi square statistics; differences in time to admission were examined for the two groups using survival analyses.
3. Results
3.1. Characteristics of Study Sample
Most participants were male (73%) and African American (94%), and 6% were Hispanic. The average age was 52.0 (sd=7.6) years, with few under the age of 36. Consistent with the study inclusion criteria, all but one reported using heroin at least weekly in the prior 90 days and one reported misuse of prescription opioids. About 20% reported other substance use at least weekly, including tobacco (91%), alcohol (17%), crack/cocaine (16%), and marijuana (11%). Nearly all were rated with “high” severity on the SUD screener; most (61%) had a history of opioid-related overdose, ranging from one to eight times, and over half of these (23/43, 53%) had received naloxone at least once. Over half (54%) of the sample indicated a high level of problem severity in the past year. Insurance status was obtained from treatment records; 52 participants were covered by Managed Care Organizations (MCOs), 9 were covered directly through Medicaid, and 6 did not have insurance and were covered through the state block grant. This information was not available for 3 participants who did not enter treatment.
3.2. Treatment Linkage, Entry and Status
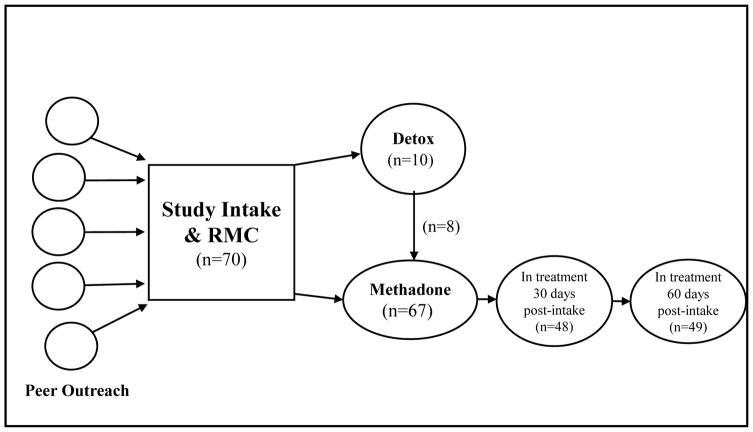
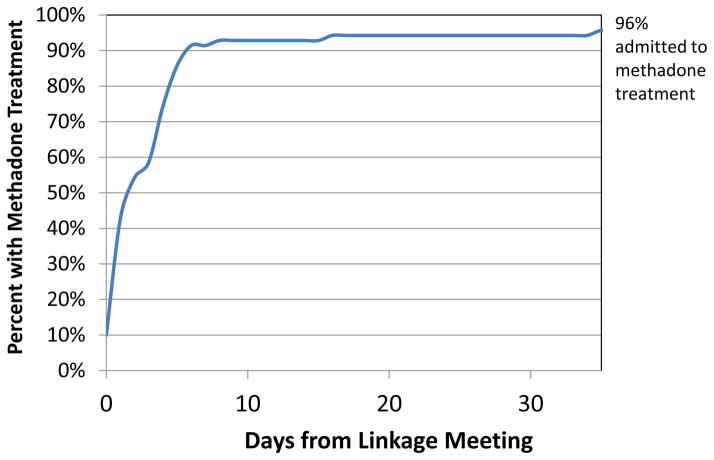
Figure 1 depicts the flow of participants from study recruitment by peer outreach to RMC linkage, treatment entry and status at 30 and 60 days post-intake. Of the 70 individuals who attended the RMC linkage meeting, 14% (10/70) accepted a referral to detox and all showed up to the program; 80% (8/10) of these were subsequently admitted to methadone treatment. Of the total sample, all accepted a referral to treatment and 96% (67/70) showed up and were admitted for methadone maintenance (including some who entered following completion of detox). In addition, one individual chose not to go to treatment but agreed to undertake a recovery work plan, which included recovery activities like attending self-help meetings, attending church, and/or reconnecting with non-substance using friends and family, instead of treatment. The median time from initial linkage meeting to treatment admission was 2.63 days, with 91% of the sample entering treatment within 6 days of the initial linkage meeting (see Figure 2).
Figure 1.
Treatment Linkage, Entry, and Status at 30 and 60 Days Post-Intake
Figure 2.
Days from Linkage to Admission to Methadone Treatment (n = 70)
As shown in Figure 1, at 30 and 60 days post-intake, 69% (48/70) and 70% (49/70) were in methadone treatment, respectively. Note this includes a few people who left and were readmitted (hence the higher number at 6 months). Overall the rate of dosing received was very high, with half (35/70) of the sample receiving methadone doses on at least 57 out of 60 days of the follow-up period.
3.3. Post hoc Comparison Based on Naloxone History
Because this pilot study was undertaken as formative work for a larger project that entails targeting individuals for treatment linkage immediately following an overdose reversal with naloxone, we performed post-hoc exploratory analyses to compare individuals who had a history of overdose and receipt of naloxone with others. About one third (33%, 23/70) reported that they had ever received naloxone to reverse an overdose, with 61% (14/23) of these having received naloxone on two or more occasions. Moreover, 70% (16/23) reported that they had an overdose reversal within the past 12 months, with the remainder having received naloxone over a year ago.
Individuals who had received naloxone were younger on average compared to the others who had not received it (49.1 vs. 53.5 years; Mann-Whitney rank test: U = 345.0, p = .014). There were disproportionately more males than females in the naloxone group compared with others, but the difference did not reach statistical significance in this small pilot study (83% vs. 68%; OR = 2.23, 95% CI: 0.64, 7.70).
There was no difference between groups in their rate of treatment entry or time to treatment admission. However, a smaller proportion of individuals with a history of naloxone were in treatment at 30 days post-intake compared with others (52% vs. 77%, OR = 0.33; 95% CI 0.12, 0.96). Similarly, the naloxone group was less likely to be in treatment at 60 days post-intake, but this difference was non-significant (61% vs 74%, OR = 0.53; 95% CI 0.18, 1.55).
4. Discussion
Given the urgency of the current wave of opioid use disorders and associated high rates of overdose, policy efforts have focused on developing mechanisms to engage individuals into medication-assisted treatment. The findings from this pilot study demonstrate that a combined peer outreach – treatment linkage intervention targeting out-of-treatment individuals with OUD in the community is a promising strategy to achieve this goal. Peer outreach workers, who were familiar and comfortable with the communities targeted for recruitment, were able to successfully identify and enlist the target population, as shown by the high proportion of individuals who presented for the initial treatment linkage meeting. Further, the modified treatment linkage and engagement intervention, which builds on a platform of successful implementation of the Recovery Management Checkup intervention and has demonstrated efficacy with individuals in treatment or criminal justice settings, was able to facilitate linkage to methadone for nearly all individuals in the pilot study. It is noteworthy that most of these individuals were successfully retained in treatment and dosed on a majority of days up to 60 days post-intake, demonstrating that an assertive linkage and engagement mechanism can help overcome the obstacles that many individuals with OUD face to entering treatment.
The study also identified a sub-group of individuals who had received naloxone to reverse an opioid overdose, most often occurring in the past year, who were younger and more often male. These characteristics are similar to those found in a recent review of emergency medical services (EMS) responses to opioid-related overdoses, with younger males using heroin more likely to receive naloxone compared to those whose overdose was related to prescription opioid use (Banta-Green et al., 2017). In the current study, these individuals were equally likely to show to treatment, but they were less likely to have dosed at 30 or 60 days post-intake. Although these findings are exploratory, and the small sample size precludes adjustments for covariates, they suggest that this group may require more intensive support to sustain treatment engagement. More research is warranted to understand this sub-group of individuals who have recent naloxone exposure due to overdose.
The recency of these experiences may stem from the broader dissemination of naloxone that has occurred as a result of policies aimed at curbing opioid-related overdoses (Compton et al., 2013; Davis, 2015; Unick, Rosenblum, Mars, & Ciccarone, 2013). Recent federal funding through the 21st Century CURES Act supports further training and dissemination efforts related to use of naloxone and expansion of MAT capacity; hence, identifying mechanisms to engage individuals into MAT following an overdose and support their continued treatment retention will be critical to averting the high rates of relapse and recurrent overdose that have been observed.
5. Conclusion
In sum, the study findings suggest that a peer outreach – assertive linkage and engagement intervention is a promising approach for identifying and engaging out-of-treatment opioid users into medication-assisted treatment. Further research is needed to refine the intervention, particularly as it applies to those most at risk of treatment attrition and relapse, and to test its effectiveness in a larger, fully powered experimental trial. Moreover, as the current study is limited by its focus on linkage to one methadone provider, future studies should include linkage to multiple programs and providers that offer a wider range of MAT options within the community.
Highlights.
Peer outreach workers referred 70 people with opioid use disorders for linkage
The assertive linkage intervention enrolled 96% into methadone, 91% within 6 days
69% and 70% were in treatment at 30 and 60 days post-intake, respectively
Individuals who had ever received naloxone were less likely to stay in treatment
Acknowledgments
The authors acknowledge the peer outreach workers and treatment providers for their contributions to this study, as well as Lilia Hristova, Rodney Funk, and Brittany Moody for their assistance with preparing the manuscript. The opinions here are those of the authors and do not reflect official positions of Chestnut or the funder.
Funding source
This pilot was internally funded by Chestnut Health System’s Lighthouse Institute. This paper was partially produced with funds from NIDA grant no. DA045774.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alanis-Hirsch K, Croff R, Ford JH, 2nd, Johnson K, Chalk M, Schmidt L, et al. Extended-release naltrexone: a qualitative analysis of barriers to routine use. Journal of Substance Abuse Treatment. 2016;62:68–73. doi: 10.1016/j.jsat.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca TR, Longabaugh R. Mechanisms of change in motivational interviewing: A review and preliminary evaluation of the evidence. Addiction. 2009;104:705–715. doi: 10.1111/j.1360-0443.2009.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrestee Drug Abuse Monitoring Program (ADAM) II 2011 Annual Report. Office of National Drug Control Policy Executive Office of the President; 2012. May, Retrieved from https://www.whitehouse.gov/sites/default/files/emailfiles/adam_ii_2011_annual_rpt_web_version_corrected.pdf. [Google Scholar]
- Ball JC, Ross A. The effectiveness of methadone maintenance treatment. New York: Springer-Verlag; 1991. [Google Scholar]
- Banta-Green CJ, Coffin PO, Schoeppe JA, Merrill JO, Whiteside LK, Ebersol AK. Heroin and pharmaceutical opioid overdose events: Emergency medical response characteristics. Drug and Alcohol Dependence. 2017;178:1–6. doi: 10.1016/j.drugalcdep.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Kirchner HL, Pircavage JM, Nadipelli VR, Ronquest NA, Fitzpatrick MH, Han JJ. Factors associated with opioid overdose: A 10-year retrospective study of patients in a large integrated health care system. Substance Abuse and Rehabilitation. 2016;7:131–141. doi: 10.2147/SAR.S108302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RM, Pacula RL, Bauhoff S, Gordon AJ, Hendrikson H, Leslie DL, et al. Policies related to opioid agonist therapy for opioid use disorders: the evolution of state policies from 2004 to 2013. Substance Abuse. 2016;37(1):63–69. doi: 10.1080/08897077.2015.1080208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. Morbidity and Mortality Weekly Report. 2016 Jan 1;64(50):137882. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: A retrospective analysis of the past fifty years. JAMA Psychiatry. 2014;71:821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. New England Journal of Medicine. 2016;374:154–163. doi: 10.1056/NEJMra1508490. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND, Throckmorton DC, Lurie P. Expanded across to opioid overdose intervention: Research, practice, and policy needs. Annals of Internal Medicine. 2013;158:65–66. doi: 10.7326/0003-4819-158-1-201301010-00013. [DOI] [PubMed] [Google Scholar]
- Davis C. Naloxone for Community Opioid Overdose Reversal. Public Health Law Research. 2015 Jun; Available at: http://prescribetoprevent.org/wp2015/wp-content/uploads/PHLRKnowledgeAsset_Naloxone_FINALfull_8June15.pdf.
- Dennis ML, Scott CK. Four-year outcomes from the Early Re-Intervention Experiment (ERI) with recovery management checkups (RMC) Drug and Alcohol Dependence. 2012;121(1):10–17. doi: 10.1016/j.drugalcdep.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Scott CK, Funk R. An experimental evaluation of recovery management checkups (RMC) for people with chronic substance use disorders. Evaluation and Program Planning. 2003;26(3):339–352. doi: 10.1016/S0149-7189(03)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;314(14):1468–1478. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- Jones CM. The paradox of decreasing nonmedical opioid analgesic use and increasing abuse or dependence - An assessment of demographic and substance use trends, United States 2003–2014. Addictive Behaviors. 2017;65:229–235. doi: 10.1016/j.addbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health. 2015;105(8):e55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK. The supply of physicians waivered to prescribe buprenorphine for opioid use disorders in the United States: a state-level analysis. Journal of Studies of Alcohol and Drugs. 2015;76(4):644–654. doi: 10.15288/jsad.2015.76.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Oser CB. Barriers to the implementation of medication-assisted treatment for substance use disorders: The importance of funding policies and medical infrastructure. Evaluation and Program Planning. 2011;34(4):375–381. doi: 10.1016/j.evalprogplan.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall Z, Dechman MK, Minichiello A, Alcock L, Harris GE. Peering into the literature: A systematic review of the roles of people who inject drugs in harm reduction initiatives. Drug and Alcohol Dependence. 2015;151:1–14. doi: 10.1016/j.drugalcdep.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews, 2014. 2014;(2) doi: 10.1002/14651858.CD002207.pub43. Art. No.: CD002207. [DOI] [PubMed] [Google Scholar]
- Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and nalozone prescribing: Implications for translating community programming into clinical practice. Substance Abuse. 2015;36(2):240–253. doi: 10.1080/08897077.2015.1010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needle RH, Burrows D, Friedman SR, Latkin C. Effectiveness of community-based outreach in preventing HIV/AIDS among injecting drug users. International Journal of Drug Policy. 2005;16(Sup 1):45–57. doi: 10.1016/j.drugpo.2005.02.009. [DOI] [Google Scholar]
- Needle RH, Coyle SL, Normand J, Lambert E, Cesari H. HIV prevention with drug-using populations: current status and future prospects. Public Health Reports. 1998;113(Sup 1):4–18. [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Annals of Family Medicine. 2015;13(1):23–26. doi: 10.1370/afm.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Peterson JA, Reisinger HS, et al. Attitudes toward buprenorphine and methadone among opioid-dependent individuals. American Journal on Addiction. 2008;17(5):396–401. doi: 10.1080/10550490802268835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML. The first 90 days following release from jail: Findings from recovery management checkups for women offenders (RMCWO) experiment. Drug and Alcohol Dependence. 2012;125(1):110–118. doi: 10.1016/j.drugalcdep.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML. Recovery management check-ups: An early reintervention model. Chicago, IL: Lighthouse Institute; 2003. [Google Scholar]
- Scott CK, Dennis ML. Results from two randomized clinical trials evaluating the impact of quarterly recovery management checkups with adult chronic substance users. Addiction. 2009;104(6):959–971. doi: 10.1111/j.1360-0443.2009.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML. Recovery management checkups with adult chronic substance users. In: Kelly JF, White WL, editors. Addiction Recovery Management: Theory, Research, and Practice. New York, NY: Springer; 2010. pp. 87–102. [Google Scholar]
- Scott CK, Dennis ML, Foss MA. Utilizing recovery management checkups to shorten the cycle of relapse, treatment reentry, and recovery. Drug and Alcohol Dependence. 2005;78(3):325–338. doi: 10.1016/j.drugalcdep.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Laudet A, Funk RR, Simeone RS. Surviving drug addiction: The effect of treatment and abstinence on mortality. American Journal of Public Health. 2011;101(4):737–744. doi: 10.2105/AJPH.2010.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Lurigio AJ. The effects of specialized probation and Recovery Management Check-Ups (RMCs) on treatment participation, substance use, HIV-risk behaviors, and recidivism among female offenders: Main findings of a three-year experiment using subject by intervention interaction analysis. Journal of Experimental Criminology. 2017;13(1):53–77. doi: 10.1007/s11292-016-9281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Grella CE, Dennis ML, Nicholson L. Linking individuals with substance use disorders (SUD) in primary care to SUD treatment: The recovery management checkups - primary care (RMC-PC) pilot study. Journal of Behavioral Health Services & Research. 2017 doi: 10.1007/s11414-017-9576-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, Swartz RP. Update on barriers to pharmacotherapy for opioid use disorders. Current Psychiatry Reports. 2017;19(6):35. doi: 10.1007/s11920-017-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoové MA, Dietze PM, Jolley D. Overdose deaths following previous non-fatal heroin overdose: Record linkage of ambulance attendance and death registry data. Drug and Alcohol Review. 2009;28(4):347–352. doi: 10.1111/j.1465-3362.2009.00057.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Funding opportunity announcement TI-17-014: State Targeted Response to the Opioid Crisis Grants. Rockville, MD: Author; 2016. Available at https://www.samhsa.gov/grants/grant-announcements/ti-17-014. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Drug Abuse Warning Network. 2011 National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Retrieved from: https://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. [Google Scholar]
- Titus JC, Feeney T, Smith DC, Rivers TL, Kelly LL, Dennis ML. Global Appraisal of Individual Needs–Q3 3.2 (GAIN-Q3): Administration, clinical interpretation and brief intervention. Normal, IL: Chestnut Health Systems; 2003. Available at http://www.gaincc.org/instruments. [Google Scholar]
- Unick GJ, Ciccarone D. US regional and demographic differences in prescription opioid and heroin-related overdose hospitalizations. International Journal of Drug Policy. 2017;46:112–119. doi: 10.1016/j.drugpo.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. PLoS One. 2013;8(2):e54496. doi: 10.1371/journal.pone.0054496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarborough BJ, Stumbo SP, Janoff SL, Yarborough MT, McCarty D, Chilcoat HD, … Green CA. Understanding opioid overdose characteristics involving prescription and illicit opioids: A mixed methods analysis. Drug and Alcohol Dependence. 2016;167:49–56. doi: 10.1016/j.drugalcdep.2016.07.024. [DOI] [PubMed] [Google Scholar]