Abstract
Increasing usage of next generation sequencing (NGS) technology will illuminate the extent of RNA modifications, which may affect various pathophysiological conditions. Here, we will highlight one such category of RNA modification called “RNA editing”, which can be detected from RNA sequencing (RNA-seq) data by simply modifying the data analysis pipeline using bioinformatics methods.
Keywords: epitranscriptomics, gene expression, RNA editing, RNA modification
Subject codes: [141] Functional genomics, [142] Gene expression, [143] Gene regulation, [153] Information technology
Introduction
Life on our planet depends on RNA. Despite our early understanding of RNA as an enabling messenger, it is not simply a static reflection of genetic activation. Indeed, the lifespan of RNA is more complex than previously thought. Upon transcription from the DNA template, RNA can be modified by various enzymes, which results in over 100 RNA modifications. Because of the potential involvement of RNA modifications in a variety of pathophysiologies, epitranscriptomics is quickly gaining momentum and our gaps in understanding epitranscriptomics requires further study.1
RNA editing is a post-transcriptional modification to alter the sequence of RNA molecules.2 RNA editing of exons of protein-coding genes may lead to the production of an amino acid sequence that differs from the original DNA sequence. In addition, editing of 3′-untranslated regions (UTRs) may affect binding of RNA binding proteins (RBPs) or microRNAs (miRNAs), thereby modulating RNA stability and/or translation. There are two types of RNA editing: adenosine to inosine (A-to-I) and cytidine to uridine (C-to-U); A-to-I is the more common form. Furthermore, RNA editing can be identified in RNA-seq data without any special treatment prior to the sequencing library construction. A-to-I RNA editing occurs through RNA editing enzymes called “adenosine deaminases acting on RNA (ADARs)”, which convert adenosine in double-stranded RNA into inosine. In humans, there are three ADARs (ADAR1, ADAR2, and ADAR3). In mice, the deletion of Adar1 and Adar2 (also known as Adarb1, “adenosine deaminase, RNA-specific, B1”) are embryonically and postnatally lethal, respectively,3, 4 highlighting the importance of RNA editing in normal physiology. Human ADAR3 (also known as Adarb2, “adenosine deaminase, RNA-specific, B2”) is considered to be catalytically inactive and less important in RNA editing.5 In humans, mutations in ADAR1 have been associated with dyschromatosis symmetrica hereditaria and Aicardi–Goutières syndrome;6 however, whether the expression of ADARs changes in various cardiovascular conditions remains unknown.
RNA editing sites are found mostly in non-protein-coding regions
Although A-to-I editing occurs at the level of RNA, when reverse transcribed to complementary DNA (cDNA), an inosine is converted to guanine (“G”). This “A-to-G conversion” can be identified by comparing to the reference genome as an evidence for RNA editing sites. A number of studies detected RNA editing events from RNA-seq data, including recent study in endothelial cells and atherosclerosis.7 Because of the detection in RNA-seq data, several databases for RNA editing events have been constructed to provide evidence for the frequency of RNA editing in various conditions. When the content of one of such database called “RADAR”8 is examined for humans, ~89% of RNA editing sites are found in introns of both protein-coding genes and non-coding RNAs (ncRNAs) followed by ~5% in intergenic regions that include long intergenic ncRNAs (lincRNAs), ~4% in 3′-UTR, ~1% in exons of ncRNAs, and <1% in 5′-UTR. Surprisingly, only 0.17% of RNA editing sites are found in exons of both protein-coding genes and ncRNAs, which do not code for amino acids. This distribution of RNA editing sites agrees with previous studies that examined RNA editing sites in humans, where they occur mostly in Alu repetitive elements as they form double-stranded RNA, which ADARs bind to.9 Thus, RNA editing sites are found mostly in non-protein-coding regions of the human transcriptome.
RNA editing events in the cardiovascular system are underexplored
There are only three publications that examined RNA editing in diseased hearts. The first study reported increased RNA editing events in MED13 (gene encoding a component of the mediator complex) in cyanotic congenital heart disease patients compared to acyanotic patients.10 Another recent study reported that cathepsin S (CTSS), which encodes a cysteine protease associated with angiogenesis and atherosclerosis, is highly edited.7 Such RNA editing enables the recruitment of stabilizing RBP human antigen R (HuR; encoded by ELAVL1) to the 3′-UTR of CTSS transcript, thereby controlling CTSS mRNA stability and expression. ADAR1 levels and the extent of CTSS RNA editing are associated with changes in CTSS levels in patients with atherosclerotic vascular diseases, including subclinical atherosclerosis, coronary artery disease, aortic aneurysms, and advanced carotid atherosclerotic disease. Above two studies are of mammalian hearts, whose adult cardiomyocytes have a very limited capability of regeneration. Interestingly, in the newt hearts, whose cardiomyocytes can be regenerated upon injury, the increased expression of adar1 mRNA was observed as well as nucleocytoplasmic shuttling of the adar1 protein in the regeneration zone of regenerating newt heart, suggesting that RNA editing might be necessary for cardiac regeneration in newts.11 However, there is no study yet that comprehensively compared the expression of ADARs and RNA editing events in the cardiovascular system under various conditions, including pathological conditions.
Detecting RNA editing events from RNA-seq data
RNA editing can be detected by comparing the sequencing reads from RNA-seq data to the reference genome by examining A-to-G nucleotide changes present only in the read sequence but not in the genomic DNA. Currently, a number of bioinformatics tools can identify RNA editing sites from RNA-seq data (reviewed here12), including our RNAEditor bioinformatics tool.13 Although it sounds rather straightforward to identify A-to-G mismatches to the reference genome, a set of filters must be applied to remove biases arising from the sequencing technique (e.g., sequencing errors, misalignment of reads, PCR duplicates during library preparation leading to misinterpretation of RNA editing sites) and genomic variations (e.g., single nucleotide polymorphisms). In addition, the Short Genetic Variations database (dbSNP), a public-domain archive for sequence variations and the primary source for the filter separating RNA editing sites from known SNPs, contains the sequence variations from both genomic DNAs and cDNAs (generated during RNA-seq, for example).12 Thus, the computational pipeline for the detection of RNA editing sites is complicated by the propensity for errors and extensive hardware time. Furthermore, how to normalize the identified RNA editing sites have not been well established, which makes it difficult to compare the number of RNA editing sites observed in one sample to another.
Most recent comprehensive analysis of various human tissues for RNA editing sites indicates that transcripts in human hearts are edited.14 Given that many cardiac RNA-seq data have been published and several bioinformatics tools are available, it is straightforward to conduct a large-scale bioinformatics analysis of published RNA-seq data to identify condition-dependent RNA editing events. In this context, our RNAEditor tool will be of great help as this tool takes a FASTQ file as input and reports the frequency of RNA editing sites and islands, which is the term we coined to describe the highly edited sites in the given loci. Our previous study indicates that RNA editing islands overlap to ADAR1-bound peaks from RNA immunoprecipitation assay followed by NGS (RIP-seq) data using anti-ADAR1 antibody.15 Because these bioinformatics tools are readily available, there is an urgent need to profile RNA editing events in the cardiovascular system under various conditions.
Concluding remarks
Due to the unprecedented availability of genetic tools, it is now possible to “edit” an individual’s genome. For example, using CRISPR (clustered regularly interspersed short palindromic repeats)/Cas9, permanent change to the human genome is possible; however, there are a number of concerns with, and limitations to, CRISPR/Cas9 technology, which leaves open the prospects for improvement. Given the potential for greater flexibility, improved specificity, and non-genomic DNA sequence editing, application of RNA editing (particularly ADAR-mediated editing with newer Cas varieties)16 technology has the potential to extend further our armamentarium of genetic tools, and such possibilities warrant further study for potential eventual applications in correcting heritable disease traits.
Compared to other fields (e.g., cancer, immunology), cardiovascular research has lagged considerably in its examination of RNA editing events. The first step in understanding the cardiovascular “editome” is to perform a large-scale re-analysis of published RNA-seq data to identify the expression patterns of ADARs and frequencies of RNA editing events in diseased hearts. Because the source of RNA editing events is known (i.e., the enzymatic activity of ADARs to convert adenosine into inosine), the effects of RNA editing events may be investigated by conducting studies in mice deficient in Adar1 and Adar2.3, 4 Furthermore, there are many overexpression constructs readily available for ADARs that have been tested in other fields. As reported, the effect of RNA editing events in the vasculature is prominent;7 however, it is not yet clear whether RNA editing plays a role in cardiomyocytes. Thus, more functional studies are needed to elucidate the pathophysiological roles of ADARs in cardiomyocytes to understand the impact of RNA editing events in the heart and associated disease.
Given that a majority of RNA editing sites are found in non-protein-coding regions, especially in introns, an open question remains: what is the function of RNA editing sites in non-protein-coding regions? One possible answer is that RNA editing may alter alternative splicing patterns via modification of splicing acceptor and donor sites. By modulating splicing, RNA editing may result in diversifying protein isoforms from a single gene. Given that alternative splicing is known to be altered in diseased hearts compared to the healthy ones, by studying RNA editing patterns, it might be possible to dissect splicing patterns further related to cardiovascular disease.
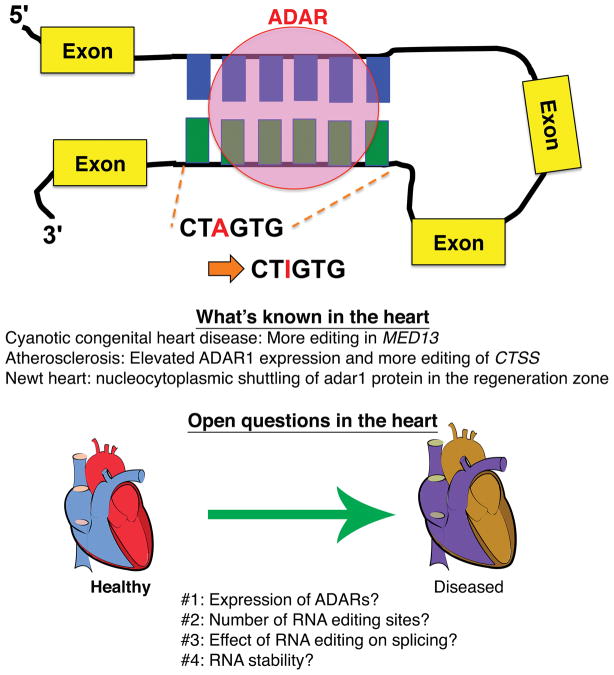
Figure. The effects of RNA editing.
ADARs preferentially bind double-stranded RNA and convert adenosine (A) into iosine (I). Upon transcription to cDNA, inosine is converted to guanine (G) in RNA, which is not present in the corresponding genomic DNA. In diseased human hearts, more RNA editing sites have been recorded for some genes, while in the regenerating newt heart, the adar1 protein is localized more in the nucleus of renerating cardiomyocytes than in the cytoplasm. Due to lack of comprehensive studies of RNA editing in the heart, more systematic research is needed to uncover the roles of ADARs and the effects of RNA editing in cardiovascular disease.
Acknowledgments
The authors would like to thank Dr. Tyler Weirick with the help for the computational analysis of RNA editing events.
Sources of funding
Dr. Uchida has been supported by the V.V. Cooke Foundation (Kentucky, U.S.A.); the University of Louisville School of Medicine; EVPRI Internal Research Grant from the Office of the Executive Vice President for Research and Innovation at the University of Louisville; and the startup funding from the Mansbach Family, the Gheens Foundation and other generous supporters at the University of Louisville. Dr. Jones has been supported by grants from the NIH (R01 HL083320, R01 HL094419, HL131647, P20 GM103492, and P01 HL078825).
Footnotes
Disclosures
None.
References
- 1.Roundtree IA, He C. Rna epigenetics-chemical messages for posttranscriptional gene regulation. Current opinion in chemical biology. 2016;30:46–51. doi: 10.1016/j.cbpa.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishikura K. A-to-i editing of coding and non-coding rnas by adars. Nature reviews. Molecular cell biology. 2016;17:83–96. doi: 10.1038/nrm.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of adar1 rna editing deaminase gene. The Journal of biological chemistry. 2004;279:4952–4961. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an ampa receptor gene rescues lethality in mice deficient in the rna-editing enzyme adar2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 5.Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the rna-specific adenosine deaminase gene family, adar3, contains both single- and double-stranded rna binding domains. RNA (New York, NY) 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slotkin W, Nishikura K. Adenosine-to-inosine rna editing and human disease. Genome medicine. 2013;5:105. doi: 10.1186/gm508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stellos K, Gatsiou A, Stamatelopoulos K, Perisic Matic L, John D, Lunella FF, Jae N, Rossbach O, Amrhein C, Sigala F, Boon RA, Furtig B, Manavski Y, You X, Uchida S, Keller T, Boeckel JN, Franco-Cereceda A, Maegdefessel L, Chen W, Schwalbe H, Bindereif A, Eriksson P, Hedin U, Zeiher AM, Dimmeler S. Adenosine-to-inosine rna editing controls cathepsin s expression in atherosclerosis by enabling hur-mediated post-transcriptional regulation. Nature medicine. 2016;22:1140–1150. doi: 10.1038/nm.4172. [DOI] [PubMed] [Google Scholar]
- 8.Ramaswami G, Li JB. Radar: A rigorously annotated database of a-to-i rna editing. Nucleic acids research. 2014;42:D109–113. doi: 10.1093/nar/gkt996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Z, Cheng Y, Tan BC, Kang L, Tian Z, Zhu Y, Zhang W, Liang Y, Hu X, Tan X, Guo J, Dong Z, Liang Y, Bao L, Wang J. Comprehensive analysis of rna-seq data reveals extensive rna editing in a human transcriptome. Nature biotechnology. 2012;30:253–260. doi: 10.1038/nbt.2122. [DOI] [PubMed] [Google Scholar]
- 10.Borik S, Simon AJ, Nevo-Caspi Y, Mishali D, Amariglio N, Rechavi G, Paret G. Increased rna editing in children with cyanotic congenital heart disease. Intensive care medicine. 2011;37:1664–1671. doi: 10.1007/s00134-011-2296-z. [DOI] [PubMed] [Google Scholar]
- 11.Witman NM, Behm M, Ohman M, Morrison JI. Adar-related activation of adenosine-to-inosine rna editing during regeneration. Stem cells and development. 2013;22:2254–2267. doi: 10.1089/scd.2013.0104. [DOI] [PubMed] [Google Scholar]
- 12.Diroma MA, Ciaccia L, Pesole G, Picardi E. Elucidating the editome: Bioinformatics approaches for rna editing detection. Briefings in bioinformatics. 2017 doi: 10.1093/bib/bbx129. [DOI] [PubMed] [Google Scholar]
- 13.John D, Weirick T, Dimmeler S, Uchida S. Rnaeditor: Easy detection of rna editing events and the introduction of editing islands. Briefings in bioinformatics. 2016 doi: 10.1093/bib/bbw087. [DOI] [PubMed] [Google Scholar]
- 14.Tan MH, Li Q, Shanmugam R, Piskol R, Kohler J, Young AN, Liu KI, Zhang R, Ramaswami G, Ariyoshi K, Gupte A, Keegan LP, George CX, Ramu A, Huang N, Pollina EA, Leeman DS, Rustighi A, Goh YPS, Chawla A, Del Sal G, Peltz G, Brunet A, Conrad DF, Samuel CE, O’Connell MA, Walkley CR, Nishikura K, Li JB. Dynamic landscape and regulation of rna editing in mammals. Nature. 2017;550:249–254. doi: 10.1038/nature24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang IX, So E, Devlin JL, Zhao Y, Wu M, Cheung VG. Adar regulates rna editing, transcript stability, and gene expression. Cell reports. 2013;5:849–860. doi: 10.1016/j.celrep.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, Zhang F. Rna editing with crispr-cas13. Science (New York, NY) 2017;358:1019–1027. doi: 10.1126/science.aaq0180. [DOI] [PMC free article] [PubMed] [Google Scholar]