Abstract
Percutaneous coronary intervention for chronic total occlusions (CTOs) is still today a challenge even for experienced operators. In the hands of the most experienced the success rate increased from about 60 to 90 % in the past 10 years; paralleled by a long-term patency with drug-eluting stents exceeding 90 %. These results are comparable or even superior to surgical revascularisation. Thanks to Japanese and European CTO club online registries and live courses we are able to rapidly understand and adopt new strategies, techniques and materials to master morphology deemed untreatable 10 years ago. Several of the persistent challenges and solutions unique to CTO interventions are discussed.
Keywords: Chronic total coronary occlusion, revascularisation, technique, results, complications
Definition and Prevalence
Chronic coronary occlusions (CTOs) are defined as lesions with Thrombolysis in Myocardial Infarction (TIMI) 0 flow older than three months (either angiographically proven or with high clinical likelihood).[1] According to a recent Canadian registry, CTOs are detected in about 30 % of patients with symptomatic coronary artery diseases (CAD).[2] Likewise a very large German monitor controlled registry found that 27.5 % of 45,722 consecutive patients with CAD had a non-acute total occlusion.[3]
Indication of Chronic Coronary Occlusion Percutaneous Coronary Intervention
The overriding principle in medicine is to improve symptoms and/or prognosis. Thus revascularisation of CTO is indicated only in the presence of angina or ischaemia related to the respective territory.[4] It has been shown that upon successful reopening angina will improve, functional tests will be normalised, left ventricular (LV) function will improve and coronary artery bypass graft (CABG) will be avoided.[5–10,11] Improved LV dysfunction correlates with the presence of myocardial viability in the respective LV segments,[12] and it has been shown to attain better prognosis. In about 60 % of CTO patients it appears sufficient to simply prove that no Q-waves are present in the territory of the occluded vessel in order to achieve recovery of the LV function upon recanalisation.[8] The EuroCTO registry 2008–2010 of 4,820 patients clearly elucidates that more than 80 % of the CTO patients had no prior ST-elevation myocardial infarction (STEMI) in the region of the occluded vessel, and we might deduct a prognostic impact of successful CTO percutaneous coronary intervention (PCI) in these patients. Frankly, we do not yet know if we are improving life-expectancy. In the Occluded Artery Trial (OAT) patients with a recent myocardial infarction (MI) of 3–28 days there was no advantage of the interventional approach in terms of survival and there were more recurrent MIs than with the conservative approach.[13] However, this trial is dealing with a different subset of patients that had infarctions and only poor proof of viability or residual ischaemia. A meta-analysis of 7,288 patients observed over a weighted average follow-up of six years confirms that successful attempts appear to be associated with an improvement in mortality and with a reduction for the need for CABG as compared with failed recanalisation.[14] In summary, patients who are symptomatic or ischaemic despite optimal medical therapy as well as those with relevant viable territory (>5 % of myocardium) should deserve revascularisation.
Current Challenges
Operator Experience
Ten to 20 years ago most of the reports of CTO results included ‘well selected cases’ and used a more liberal definition including recent occlusions so that the success rates of >60 % are overestimated.[10,15,16] Even experienced non-CTO operators, as the SYNTAX participants, still today achieve success rates of about 50 % only, a figure not far from CABG either.[17] The classical predictors of failure until the late 1990s included no stump, occlusion at side branch, orthograde collaterals (caput medusae) and occlusion age >3 months. Severe calcifications, marked tortuosity or long occlusions were considered undoable. Material and strategies, namely retrograde approach, were recently refined predominantly by a few Japanese pioneers. Unfortunately, most centres still do not restrict the recanalisations of CTO to a few selected operators, which results in insufficient experience (<30/year) and less favourable outcomes. Only high-volume CTO operators will be able to deal with difficult morphology and achieve success rates >80 % even in unselected cases. Since 2006 the EuroCTO club has been collecting clinical and procedural data of all consecutive CTO procedures – and in an online registry (www.ercto.org) since 2008. During these seven years data of more than 14,000 CTO procedures by more than 35 ERCTO operators were collected. The overall success rate increased from 75 to 85 %, in 2012 reaching 91 % in the hands of the most experienced who enrolled >100 procedures annually (see Figure 1). The result of the EuroCTO club online registry depicts a clear relation of caseload and success even in highly experienced CTO operators (see Figure 1). Furthermore in this survey a retrograde approach was chosen in 12 % of cases with a success rate of 65 %, underscoring that this is a good strategy after antegrade failure.
Figure 1: Success Rate of Experienced European Operators 2008–2012 According to Their Level of Experience.
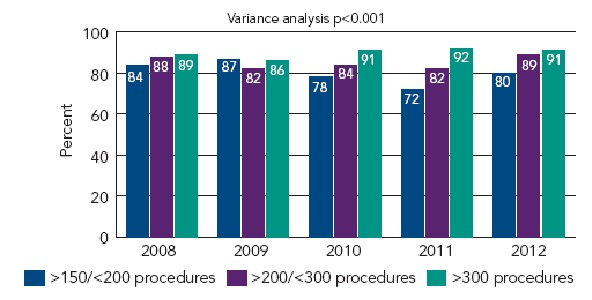
The mean success rate in 2012 was 85 %, in those with an annual caseload >100 it was >90 %.
Operators With and Without Retrograde Experience
This European registry more recently depicted that the success rate of the less experienced operators who do not apply the retrograde approach is about 80 % as opposed to 90 % of those who do.[18]
Similarly in 2009 a US retrospective analysis of 636 consecutive CTO procedures (overall success 69 %) confirmed that less experienced operators who did not adopt the novel retrograde strategy had a lower success rate than those who did (59 versus 75 %).[19]
Morphology
An analysis of the registry data 2008–2010 comprising 1,914 consecutive patients of then 16 centres revealed as independent predictors of antegrade failure: blunt stump, occlusion length >20 millimetres (mm), severe calcification and previously failed attempt (76 versus 83 %).[20] Noteworthy is that the success rate of a second attempt was 85 % if a different operator had failed before (n=636) and 69 % if it was the same operator who failed before (n=260).[21]
In the Japanese J-CTO registry from 12 enrolling centres 498 patients with 528 CTOs the success rate was 88.6 % for first attempt and 65.8 % for retry cases, which confirms the European experience.[22] Other independent predictors of lower success rates in this registry were calcification, bending, blunt stump and occlusion length >20 mm. The retrograde approach was chosen in 26 % of cases, and was successful without trying antegrade first in 79 %, and after futile antegrade attempt in 68–74 %, similar to European results.
In summary, complex morphology, that until recently was deemed impossible to negotiate is barely influencing success rates nowadays.
According to contemporary experience the most important factor inversely influencing success independent from the approach is severe calcification. No doubt other factors like tortuosity, absence of stump, length of occlusion and failed recanalisation will cumber the procedure, but are by no means a reason to deny an attempt by experienced operators.[20,22] There is only one exception to this rule, chronically occluded vessels with no visible distal target should not be addressed at all because the ‘blind penetration’ bears an unpredictable risk of perforation and life-threatening tamponade.
Crucial Steps to Success
The most important prerequisite is operator and team experience in CTO procedures. The operator should be skilful, trained specifically in CTO intervention, be patient, persistent and yet cautious. Every operator should select patients according to their level of expertise or seek for expert backup or refer those beyond their reach to CTO masters.
Patient Preparation
It is mandatory to carefully review the film for best views, occlusion entry and exit, and possible mistakes of previous unsuccessful attempts.
The patient’s glomerular filtration rate (GFR) (millilitre/minute [ml/min]) should be considered as a limit to dye consumption that should not exceed 4–8 times the nominal number of GFR in ml.[23] An activated clotting time (ACT) measurement has to be accomplished every 30–40 minutes (min) to keep the level above 250 seconds (sec) and all lines need to be flushed every 10–20 min to avoid clotting and embolisation that is life-threatening if it occurs at the contralateral access. Since wire exits are not uncommon during the process of recanalisation IIb/IIIa inhibitors should be avoided.
Access Route and Guiding Catheters
Although radial approach is feasible, 90 % of experienced operators prefer femoral access mainly because they are used to it and because this access allows for use of larger guiding catheters. For chronic occlusions a good passive support with coaxial alignment and sufficient lumen to host several wires, an anchor balloon and a microcatheter or Corsair® or even sometimes intravascular ultrasound (IVUS) guidance is crucial. This can only be achieved with larger guiding catheters (7 and 8 French [Fr]). For the left coronary system extra backup-type catheters (Voda left, extra backup, geometric left, left support) are preferable. For the right coronary artery we prefer left Amplatz 0.75–2.00 shapes, hockey stick upon gentle superior origin of the right coronary artery (RCA), Judkins shape for slightly inferior origin and internal mammary artery (IMA) or Shepherd’s Crook Replacement (SCR) type guiding for upward origin of RCA. For the RCA I strongly recommend guides with side-holes to prevent aorto-ostial disssections or progressive spiral dissections caused by forceful dye injection into a subintimal space.
It is absolutely mandatory to visualise the vessel distal to the occlusion to avoid blind poking or immensely dangerous dilatation of a false wire exit. When the distal vessel is not filled by orthograde collaterals or these collaterals disappear during manipulation, contralateral injection is a must. The contralateral approach can also be achieved easily by puncturing the same groin with a 4–6 Fr catheter, which may overcome an operators inhibition to insert a second sheath in the other groin.
Over the wire kink resistant microcatheters ease wire manipulation and allow atraumatic rapid exchange or reshaping of the wires and its use is therefore strongly recommended.
Wires and Handling
The most popular current strategy is to start with the atraumatic tapered and highly lubricious Fielder XT®, which according to the EuroCTO registry will be successful in 39 % of cases. The second most popular selection – especially in old and calcified occlusions that are not very tortuous is to select stiffer tapered wires, like the Confianza 9 Pro® early in the process to minimise the risk of large dissection as well as shorten and simplify the procedure. Another recent line of action is to start with a soft tapered polymer wire (e.g. Fielder XT), stepping up quite early (Confianza 9 Pro or Miracle 6g®) and then step down to a softer wire again.
The three fundamental elements of wire handling are rotating, pushing and pulling the wire. It is important to feel the resistance at the wire tip, when pulling the wire, since a wrong channel often exerts much higher resistance than the correct lumen. Any wire has the tendency to follow the outer part of the vessel curve, which can often cause the tip to exit the lumen. It is therefore preferable to direct the wire-curve towards the inner part of the vessel bend.
Parallel Wire Technique
First described in 1995 this is still the best technique to correct a false wire position.[24] If the first wire has entered a false lumen, it is left in place to mark the dissection channel and a second wire (typically the same stiffness or stiffer and often tapered) with a slightly different tip curve to allow creating a slightly different course supported by an over-the-wire (OTW) (balloon) catheter, is passed along the same path parallel to the first wire, with care taken to avoid wire twisting. This technique allows for penetration towards the distal cap more centrally, avoiding the false channel that is marked by the wire left in situ. Occasionally, three or more wires are used in parallel.
How to Overcome Specific Challenges
Tortuous Access
The most common approach for CTO-PCI is via the groin. However, in some elderly patients catheter manipulation via femoral approach is barred by tremendous friction due to severe kinking of the iliac artery, most probably because of atherosclerotic vessel remodelling.[25]
A common solution is to use a larger rigid kink resistant long sheath with a stiff guidewire. Nevertheless in rare cases the kinking cannot be overcome and the friction remains high that the investigator has to puncture the contralateral side or switch to a transradial approach, which might be extremely difficult as well because the atherosclerotic disease is often generalised, and last but not least in more than 70 % of the cases we do need two arterial catheters for contralateral injections. Finally up to 1 % of the CTO procedures may fail because of insurmountable access problems.
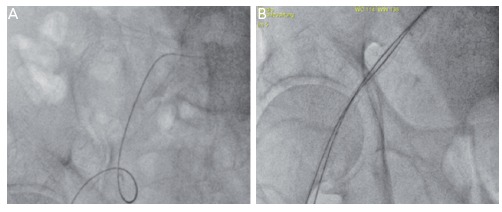
Inserting two parallel sheaths and extra stiff guidewires into the same common femoral artery is a simple novel technique not only to ease contralateral access but also to overcome serious iliac tortuosity that otherwise prevents diagnostic or therapeutic percutaneous interventions. With this technique the external and common iliac will be straightened impressively and the friction reduced tremendously thus enabling easy guiding catheter manipulations and intervention (see Figure 2A and B).[26]
Figure 2: (A) Extreme Iliac Tortuosity that Could Not be Overcome by a Single Kink Resistant 7 Fr Sheath and a Regular Guidewire (0.035). No Guiding Catheter Could be Inserted into the Coronary Ostium Because of Serious Friction (B) Only After Inserting a Second 5 Fr Long Sheath and Placing Three Extra Stiff 0.035 Amplatz Guidewires, the Iliac was Well-straightened and the Intervention Continued Successfully.
No Stump, Ostial Occlusion or Occlusion at Take-off
Without a doubt recanalisation is much easier if a stump can be identified, and tapered stumps are more favourable than blunt stumps, which are more likely to be experienced in very old occlusions. If no stump can be identified, some operators primarily prefer a retrograde approach via collaterals although antegrade wiring may still be successful in >50 % of cases. This is why I do start antegrade in almost all ‘no stump-cases’. Several techniques were developed to help identify the correct antegrade entry. If a side branch is available at the suspected take-off, IVUS may be used to guide the wire entry. Less expensive strategies are the Sesame-open technique that requires a wire left in the side branch at take-off. The wire not only may serve as a marker for the take-off but also sometimes lever the side branch and enlarge the bifurcation angle and thus demask a tiny stump. A more aggressive approach is the side branch technique, an attempt to break the proximal cap with a balloon inflated in the main vessel and side branch across the take-off. This technique may successfully open the entry in 30–40 % of cases with a risk of subintimal dissection requiring re-entry with a tapered stiff wire.
If I do not succeed antegradely within 5–10 minutes of fluoroscopy time or a dissection occurs with the risk of extending distally I do stop the antegrade approach and search my cine runs for optimal collaterals to continue retrograde.
Heavy Calcification
About 60 % of chronic occlusions involve calcification that can be detected during angiography. The EuroCTO registry ERCTO grades calcification as:
absent;
mild (spots of calcium);
moderate (<50 % of vessel circumference involved); and
severe (>50 % of vessel circumference calcified) – severe calcification was an independent predictor of lower success rate.
Although some operators prefer to start antegrade with a soft tapered polymer wire (e.g. Fielder XT) to probe invisible microchannels, in my experience this is a waste of time in 90 % of cases since in most cases it is only possible to break up the rocky proximal cap with a tapered stiff wire like Confianza 9 or 12. I do recognise that in 10 % of cases even severe calcification may mainly be located outside the former vessel lumen and what initially appeared like a rock-hard barrier is quite easy to cross with a moderately stiff wire. After having entered the CTO I continue with the same wire if the course is straight and switch to a softer steerable wire (e.g. Ultimate 3g or Pilot 150) if the course is tortuous. The distal cap is always softer than the proximal but often still requires to be punctured preferably at its centre. If the distal vessel cannot be re-entered correctly one should avoid extensive manipulations that can easily shear off collaterals and prohibit visualisation of the distal vessel. Instead of losing distal patency and time the operator should leave the antegrade wire in place and switch to retrograde wire crossing or reversed controlled antegrade and retrograde subintimal tracking (CART). In general it is easier to enter a calcified CTO from retrograde than antegrade because the distal cap is always softer.
Balloon Failure
After successful antegrade wire crossing it may be impossible to follow with a small balloon even after having increased the guiding support with an anchoring wire or balloon in a proximal side branch.
Rotablator may be successful but it is not wise to pull the wire and a change for a rotawire unless all other options failed. The options that will be successful in >70 % are Tornus®, twisted in counter-clockwise, or Corsair, twisted in clockwise and counter-clockwise, although the latter is more fragile and operators should avoid extensive rotations on the same spot indicating that the device might be captured and break.
In about 10 % of the balloon failures Tornus and Corsair will fail as well. In these cases Laser is the best option; however, it is available only in a few institutions. Then it is worth trying to increase the lumen of the channel by paralleling a second stiff wire, but staying strictly parallel along the whole occlusion is not easy either. Before giving up we try to advance a micocatheter as close as possible and then exchange the CTO wire for a rotablator wire and then rotablator with a 1.25 burr.
Tortuous Coronary Artery
Tortuosity in the ERCTO registry is defined as none (straight: no bend >70 °, slight: one bend >70 °, moderate: two bends >70 ° or one bend >90 °, severe: two bends >90 °or one bend >120 °).
The best strategy to overcome tortuosity is to use a Corsair, which thanks to its flexibility, kink resistance and lubricious surface render optimal steerability even to non-polymer wires. Polymer wires should be selected in very tortuous anatomy as depicted in Figure 3 (A–C). Sometimes progress can only be achieved by keeping more than one wire in place to straighten the vessel.
Figure 3: (A) Short CTO Proximal to Anastomosis of LIMA to LAD and Short Stenosis More Distal. Extreme Tortuosity Due to Curved LIMA and Three Angles of 180 Degrees (B) Corsair, Straightening First and Second Bend and a Lubricious Wire (Fielder XT®) Enabled Retrograde Crossing of the Occlusion (C) Final Result After PCI of Both LAD Lesions with DES.
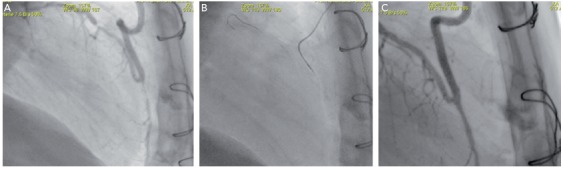
CTO = chronic total occlusion; DES = drug-eluting stents; LAD = left anterior descending; LIMA = left internal mammary artery; PCI = percutaneous coronary intervention.
Dissection
Almost all recanalisations to some extent involve a wire exit and re-entry, and thus limited dissection with subintimal stenting. This limited mistreatment of the coronary appears not to unfavourably influence short and long-term results. However, intentional extended subintimal tracking, what I view as Ramboplasty and stenting as practised with STAR-like techniques, not only may amputate side branches but also bear the risk of unacceptable reocclusion rates of more than 50 %.[27] Therefore if a subintimal wire cannot be steered back to the lumen after 10–15 mm, one should consider either switching to retrograde or to a more sophisticated re-entry technique like IVUS-guided re-entry or the BridgePoint Stingray® system.[28]
Complementary Imaging as Solution to Challenges
Unlike in the US and Japan complementary imaging is still unpopular in Europe firstly because the predominant philosophy is to keep procedures simple and secondly because of restricted reimbursement.
No wonder that up until 2010 IVUS and Multislice Computed Tomography (MSCT) were used in 1.5 % (IVUS) and 4.0 % (MSCT) of cases only by the members of the EuroCTO club. With a redoubling of the retrograde approach in 2012 the use of IVUS increased to a still modest 12.5 %. No doubt IVUS appears helpful to identify the take-off of the occluded vessel when no stump is discernible, to facilitate re-entry after having created a large false channel and to identify the position of the retrograde wire in the dissection channel as well as to measure the vessel diameter and safely select the proper balloon size. MSCT is used to depict long and tortuous occlusions and to locate calcifications not visible angiographically[29,30] – definitely helpful information if the first attempt to recanalise failed. Since only a few studies with small numbers are reported so far, the importance of MSCT with its adverse additional X-ray exposure and additional costs remains unsolved.
Retrograde Approach
If a vigorous antegrade attempt to open the vessel failed a second attempt then mostly a retrogradely approach is recommendable in most patients. Until recently, it was strongly suggested to schedule the retrograde attempt a few weeks after the antegrade failure.[31] Many operators, including me, nowadays favour to switch ad hoc after 10–15 min of unsuccessful antegrade wiring with different wires and wiring techniques well before the distal vessel gets affected by enlarged false channels.
The first report of retrograde recanalisation of a CTO was published by a French group in 1996.[32] In 2005, Katoh opened an important new window, pioneering the modern era of retrograde CTO recanalisation with the CART technique.[33] The novelties introduced in this procedure were the targeted septal collateral crossing and the connection of an antegrade and retrograde subintimal channel.
To facilitate this approach several tools were newly developed like the channel dilator Corsair, a long wire for externalisation (ASAHI RG3®), Short (90–95 centimetres [cm]) or shortened guiding catheters (GCs) are sometimes necessary especially in long epicardial connections. Monitoring of anticoagulation during the procedure is very important. An ACT should be measured every 30 min and should be maintained over 300 sec until the end of the procedure is foreseeable, to avoid any thrombotic complications in the donor artery, which are potentially lethal.
Septal collaterals are most often used (about 75 % of cases) followed by epicardial collaterals that are in general larger but more tortuous and more difficult to traverse. Stronger and straight collaterals are easier to be crossed than invisible and tortuous connections, but it is not rare to discover that the collateral that appeared less favourable was quite easy to cross.
There are three main techniques to cross and dilate the occlusion:
Retrograde marker wire at the distal end of occlusion as a target for the antegrade stiff and tapered wire.
Retrograde penetration of occlusion with wire and Corsair and externalisation of a long wire like Rotablator Wire or ASAHI RG3 via guiding catheter exiting the ‘antegrade sheath’ followed by antegrade stenting.
Connecting both false wire channels either via retrograde subintimal dilation (CART technique) or antegrade subintimal dilation (reverse-CART technique) followed by antegrade stenting.
Since the retrograde approach is generally more difficult and time consuming all CTO procedures should be started in an antegrade way with very few exceptions. The more complex nature is documented by the EuroCTO club registry 2008–2010 with a procedural time of antegrade approach 87 min, dye consumption 268 millilitres (ml) in 4,299 patients versus 154 min and 383 ml dye consumption in 501 patients with retrograde approach. As patients selected for retrograde approach are generally more awkward and the procedure is more tricky, the current success rate is roughly 15 % lower than antegrade. To make matters worse the retrograde approach is handicapped by a 2–3 fold higher rate incidence of severe in-hospital complications.
Retrograde Challenges
The most difficult part is the successful transition of collaterals, which might only be possible in 60–70 % of CTO patients. The most favourable collaterals are the well visible straight interseptals and the worst are the tiny cork screw like epicardials. Although ‘surfing’ collaterals may be successful even if the vessel course is not clearly depicted angiographically, it is recommendable to take advantage of superselective dye injection via the retrograde Corsair. This is best done by a 3 ml syringe with Luer-lock and forceful injection. To prove proper position in the vessel and avoid detrimental forceful intramural injection one should be able to aspirate blood before injecting dye.
Connecting an antegrade and retrograde dissection again should aim at limiting the extension of a subintimal track to avoid long subintimal stenting. Antegrade IVUS is very helpful to identify the optimal balloon size for retrograde CART and the optimal position for re-entry (where both wires are closest).
Complications
In-hospital complications are rare, but not negligible – quite similar to PCI of non-occluded vessels in the EuroCTO online registry 2008– 2010 (N=4,820 patients) death occurred in 0.30 %, any MI in 2.70 %, emergency CABG in 0.21 % and cardiac tamponade in 0.45 % of cases.
As mentioned earlier the complication rate with retrograde approach exceeds that of antegrade by at least 2–3 times (major adverse cardiac events [MACE] 4.5 %)[34] and according to G. Werner infarctions defined as CK rise to >3 times upper limit were 3.1 % following antegrade and 7.1– 20.0 % following retrograde approach via septal or epicardial collaterals.[35] Some of the complications of retrograde approach are quite unusual like collateral perforation, septum haematoma, aortic dissection, dissection and thrombotic occlusion of the collateral donating vessel or severe wire entrapment. In general I assume that complications after retrograde approach do occur more often than reported in registries and I strongly recommend adopting this technique only after extensive proctoring.
In summary CTO PCI is probably the most challenging percutaneous intervention, but almost every vehement assignment in CTO may nowadays be mastered with high success rates, low complication rates and good long-term results, provided that indication, operator experience and strategy are fit and proper.
References
- 1.Di Mario C, Werner GS, Sianos G et al. European Perspective in Recanalisation of Chronic Total Occlusions: Consensus Document from the EuroCTO-club. Eurointervention. 2007;3(1):30–43. [PubMed] [Google Scholar]
- 2.Werner GS, Gitt AK, Zeymer U et al. Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin Res Cardiol. 2009;98(7):435–41. doi: 10.1007/s00392-009-0013-5. [DOI] [PubMed] [Google Scholar]
- 3.Reifart NSG, Levenson B. Effect of chronic total coronary occlusion on treatment strategy in 2008: analysis of a monitor controlled registry (QUIK) Submitted for publication. 2011.
- 4.Wijns W, Kolh P, Danchin N et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31(20):2501–55. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 5.Pfisterer ME, Buser P, Osswald S et al. Time dependence of left ventricular recovery after delayed recanalization of an occluded infarct-related coronary artery: findings of a pilot study. J Am Coll Cardiol. 1998;32(1):97–102. doi: 10.1016/s0735-1097(98)00188-0. [DOI] [PubMed] [Google Scholar]
- 6.Sirnes PA, Myreng Y, Mølstad P et al. Improvement in left ventricular ejection fraction and wall motion after successful recanalization of chronic coronary occlusions. Eur Heart J. 1998;19(2):273–81. doi: 10.1053/euhj.1997.0617. [DOI] [PubMed] [Google Scholar]
- 7.Melchior JP, Doriot PA, Chatelain P et al. Improvement of left ventricular contraction and relaxation synchronism after recanalization of chronic total coronary occlusion by angioplasty. J Am Coll Cardiol. 1987;9(4):763–8. doi: 10.1016/s0735-1097(87)80230-9. [DOI] [PubMed] [Google Scholar]
- 8.Surber R, Schwarz G, Figulla HR, Werner GS. Resting 12-lead electrocardiogram as a reliable predictor of functional recovery after recanalization of chronic total coronary occlusions. Clin Cardiol. 2005;28(6):293–7. doi: 10.1002/clc.4960280608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren RJ, Black AJ, Valentine PA et al. Coronary angioplasty for chronic total occlusion reduces the need for subsequent coronary bypass surgery. Am Heart J. 1990;120(2):270–4. doi: 10.1016/0002-8703(90)90069-a. [DOI] [PubMed] [Google Scholar]
- 10.Bell MR, Berger PB, Bresnahan JF et al. Initial and long-term outcome of 354 patients after coronary balloon angioplasty of total coronary artery occlusions. Circulation. 1992;85(3):1003–11. doi: 10.1161/01.cir.85.3.1003. [DOI] [PubMed] [Google Scholar]
- 11.Kirschbaum SW, Baks T, van den Ent M et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am J Cardiol. 2008;101(2):179–85. doi: 10.1016/j.amjcard.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Senior R, Kaul S, Lahiri A. Myocardial viability on echocardiography predicts long-term survival after revascularization in patients with ischemic congestive heart failure. J Am Coll Cardiol. 1999;33:1848–54. doi: 10.1016/s0735-1097(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 13.Hochman JS, Lamas GA, Buller CE et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355(23):2395–407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am Heart J. 2010;160(1):179–87. doi: 10.1016/j.ahj.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Prasad A, Rihal CS, Lennon RJ et al. Trends in outcomes after percutaneous coronary intervention for chronic total occlusions: a 25-year experience from the Mayo Clinic. J Am Coll Cardiol. 2007;49(15):1611–8. doi: 10.1016/j.jacc.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Suero JA, Marso SP, Jones PG et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. 2001;38(2):409–14. doi: 10.1016/s0735-1097(01)01349-3. [DOI] [PubMed] [Google Scholar]
- 17.Serruys PW. Cardiovascular Research Technologies. Washington DC, US: Mar 4-6, 2009. SYNTAX trial: Chronic total occlusion subsets. Presented at: [Google Scholar]
- 18.Galassi AR. CTO Summit 2013. New York, US: Feb, 2013. Updates from the EuroCTO Club. Presented at: [Google Scholar]
- 19.Thompson CA, Jayne JE, Robb JF et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv. 2009;2(9):834–42. doi: 10.1016/j.jcin.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Galassi AR, Tomasello SD, Reifart N et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. Eurointervention. 2011;7(4):472–9. doi: 10.4244/EIJV7I4A77. [DOI] [PubMed] [Google Scholar]
- 21.Reifart N. Percutaneous Revascularisation of Coronary Chronic Total Occlusion – Outcomes and development of strategies 2006–2010. Interventional Cardiology Review. 2011;6(2):128–33. [Google Scholar]
- 22.Morino Y, Abe M, Morimoto T et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4(2):213–21. doi: 10.1016/j.jcin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Laskey WK, Jenkins C, Selzer F et al. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(7):584–90. doi: 10.1016/j.jacc.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 24.Reifart N. The parallel wire technique for chronic total occlusions. Interventional Course Frankfurt. 1995. p. personal communication.
- 25.Nasser TK, Mohler ER 3rd, Wilensky RL, Hathaway DR. Peripheral vascular complications following coronary interventional procedures. Clin Cardiol. 1995;18(11):609–14. doi: 10.1002/clc.4960181105. [DOI] [PubMed] [Google Scholar]
- 26.Reifart N. The Parallel Sheath Technique in Severe Iliac Tortuosity. Eurointerv. 2013. p. in print. [DOI] [PubMed]
- 27.Valenti R, Vergara R, Migliorini A et al. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol. 2013;61(5):545–50. doi: 10.1016/j.jacc.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Werner GS. The BridgePoint devices to facilitate recanalization of chronic total coronary occlusions through controlled subintimal reentry. Expert Rev Med Devices. 2011;8(1):23–9. doi: 10.1586/erd.10.76. [DOI] [PubMed] [Google Scholar]
- 29.Mollet NR, Hoye A, Lemos PA et al. Value of preprocedure multislice computed tomographic coronary angiography to predict the outcome of percutaneous recanalization of chronic total occlusions. Am J Cardiol. 2005;95(2):240–3. doi: 10.1016/j.amjcard.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Kaneda H, Saito S, Shiono T et al. Sixty-four-slice computed tomography-facilitated percutaneous coronary intervention for chronic total occlusion. Int J Cardiol. 2007;115(1):130–2. doi: 10.1016/j.ijcard.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 31.Di Mario C, Werner GS, Sianos G et al. European perspective in the recanalisation of Chronic Total Occlusions (CTO): consensus document from the EuroCTO Club. Eurointervention. 2007;3(1):30–43. [PubMed] [Google Scholar]
- 32.Silvestri M, Parikh P, Roquebert PO et al. Retrograde left main stenting. Cathet Cardiovasc Diagn. 1996;39:396–9. doi: 10.1002/(SICI)1097-0304(199612)39:4<396::AID-CCD15>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Surmely JF, Tsuchikane E, Katoh O et al. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique. J Invasive Cardiol. 2006;18(7):334–8. [PubMed] [Google Scholar]
- 34.Rathore S, Katoh O, Matsuo H et al. Retrograde Percutaneous Recanalization of Chronic Total Occlusion of the Coronary Arteries. Circulation: Cardiovascular Interventions. 2009;2(2):124–32. doi: 10.1161/CIRCINTERVENTIONS.108.838862. [DOI] [PubMed] [Google Scholar]
- 35.Werner G. Transcatheter Cardiovascular Therapeutics. Washington, US: Sep 21–25, 2010. The retrograde aproach increases complications and adds little to CTO angioplasty. Presented at: [Google Scholar]


