Abstract
Chlamydia trachomatis (hereafter CT) is Gram-negative, obligate intracellular pathogen. It causes the world's most common non-viral sexually transmitted disease. India is home to the world's greatest burden of infectious diseases, yet information on prevalence rates of CT is scarce. This article systematically reviews the literature for the prevalence rates and testing methods in India. A total of 27 studies were included. Four main patients groups (symptomatic women, infertile women, pregnant women and asymptomatic population groups) could be identified with varying rates of CT (0.1%–32% using PCR, 2.4%–75% using ELISA serology). Most of the studies originated from urban settings, 11 of them from New Delhi. In-house PCR was the most common diagnostic technique used generating the following ranges in prevalence for the four group studies: symptomatic women 10%–50%, pregnant women 0.1%–2.5% and asymptomatic populations 0.9%–24.5%. The rates among infertile women were 9%–68% based on serology results. The prevalence rates featured in this paper are in line with other locations across the Indian subcontinent. This review highlights the extreme heterogeneity in the limited studies available in India on CT and the need for standardized guidelines for diagnosis and management of CT in India. The availability of resources should be considered in the formulation of recommendations.
Keywords: Chlamydia trachomatis, India, C. trachomatis, Sexually Transmitted Diseases, Infertility
The first systematic literature review on Chlamydia trachomatis in India.
INTRODUCTION
Chlamydia trachomatis is a Gram-negative, obligate intracellular pathogen that can lead to a broad spectrum of clinical diseases in human populations. It is known to cause a significant burden of preventable blindness in third world countries. Chlamydia trachomatis is also known as the most common bacterial sexually transmitted infection worldwide (Newman et al.2015). With a total of over 130 million new cases per year worldwide in 2015, C. trachomatis infections represent a major problem worldwide. Latest trends furthermore seem to indicate that this number is likely to increase (WHO 2008). Urogenital infections with C. trachomatis have been associated with a wide range of genitourinary conditions including cervicitis and salpingitis in women as well as epididymitis and urethritis in men. Infection with the pathogen is however often asymptomatic and hence frequently remains undiagnosed, leading to an array of severe long-term consequences (Haggerty et al.2010). Studies indicate that chlamydial infections can lead to severe impairments such as pelvic inflammatory disease, tubal damage and ultimately tubal factor infertility in women if they are not treated in a timely and adequate fashion (den Hartog, Morré and Land 2006; Haggerty et al.2010). Additionally, infections with C. trachomatis can severely impact the reproductive health of women, causing severe conditions such as ectopic pregnancies, repeated and spontaneous abortions and stillbirths(Tiller 2002; Baud and Greub 2011). These features render timely identification and reliable diagnosis of C. trachomatis an issue of public health importance, as the disease can effectively be treated using antibiotics. This is particularly true in developing countries, where infectious disease already significantly burdens the populations and the healthcare systems (Gangolli, Duggal and Shukla 2005; John et al.2011). The asymptomatic nature of the disease also requires evidence-based guidelines for the implementation of population-wide screening programs. In fact, studies have claimed that, due to the low prevalence of Chlamydia at the population level, screening in the general population may not be cost-effective (Low et al.2007). The identification of high-risk groups or chlamydial infections is however a public health issue (Althaus et al.2010). This aspect is particularly significant for low and middle-income countries, where healthcare resources and budgets are limited (CDC and World Bank 2008).
With a total population of approximately 1.2 billion inhabitants, India is after China the second most populous country in the world (Perianayagam and Goli 2012). It is similarly known to be home to one of the greatest burden of Infectious diseases in the world (John et al.2011). There is however a paucity of data and a lack of overview concerning the burden of C. trachomatis infections in India. Insights regarding the strategies for diagnosis and management of C. trachomatis in healthcare settings are also limited (Malhotra et al.2013). As a result of the varying levels of specificity and sensitivity of the diagnostic tools utilized in the clinical settings, the choice of method is of primordial importance (Chernesky 2005).
The aim of this paper is to systematically review the available scientific literature to investigate the urogenital C. trachomatis burden in India, across different regions and patient groups. The initial scope of this review is to provide an overview of the prevalence of the disease among the different patient groups. Additionally, the techniques used to identify C. trachomatis in patients need to be assessed, to lead the way for formulation of future best practices and potential evidence-based guidelines for diagnosis and management of C. trachomatis across the Indian subcontinent. Finally, in light of the significant impact of C. trachomatis on the female reproductive tract, and the importance of maternal and child health in India, the disease's contributions to the national burden of reproductive health needs to be clarified (Inhorn and Bharadwaj 2007; Ganguly and Unisa 2010; Mahesh 2013) in order to aid policy decisions.
METHODS
Peer-reviewed articles included in this review were obtained from the major databases namely PubMed and Embase using the search headlines ‘Chlamydia trachomatis’ and ‘India’. Additionally, Google Scholar was screened with the same search criteria to include non-indexed articles and ‘gray’ literature (Mahood, Van Eerd and Irvin 2014). Lastly, all the references and work cited by the articles included in this review were screened to identify further articles to be included. The above databases were screened for available data from August 2015 until December 2015.
In light of the previously mentioned lack of consistent data originating from the Indian settings, the scope of the literature search was designed to include all the patient groups that are at a higher risk of infection or subsequent sequelae when considering C. trachomatis. Taking into account the exploratory nature of the review, the authors screened all articles based on the type of patient population, the diagnostic method utilized to test for a urogenital C. trachomatis and the geographical location of the study. All of the studies that suited the scope of the review were included for further analysis based on the abstract and reviewed by at least two of the authors and checked for duplicates. The included articles were then scrutinized individually in their full-text versions. The final inclusion of discordant studies was then discussed with the authors.
If the data screened did not feature population testing, described laboratory techniques in relation with C. trachomatis detection or Chlamydia typing of established positive samples, they were excluded from this review. Additionally, all duplicates were excluded.
RESULTS
The combined literature search yielded a total of 27 unique articles; the details of the inclusion are summarized in Fig. 1.
Figure 1.
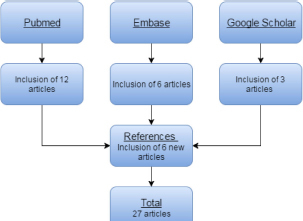
Sources of included articles.
The literature search succeeded in unveiling scientific studies originating from a wide range of geographical locations. However, the city of New Delhi was over-represented, as over a third of all the included studies (11/27 = 40%) could be traced back to this location. Similarly, three studies originated from the city of Mumbai and three others from the city of Chennai, respectively. Additionally, it should be noted that most of the studies originated from urban settings, as only two studies were conducted in rural settings (2/27 = 7%). The locations of the different studies included are summarized in Fig. 2.
Figure 2.
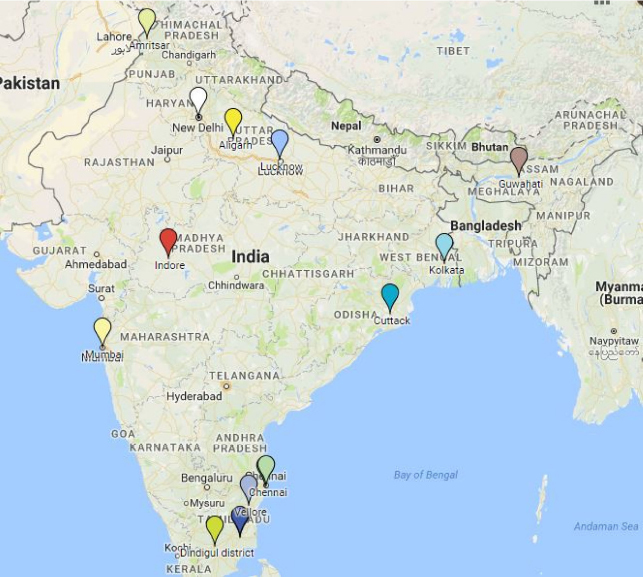
Geographical locations of studies (obtained with Google mymaps).
The differences in the testing methods used to diagnose Chlamydia trachomatis and the differences in patient populations enclosed in this review render a meta-analysis of the data difficult. Some distinct patient groups could however be consistently identified and grouped together for analysis. A total of four patient groups could be identified as listed below (see Table 1 for details):
Symptomatic patients presenting in healthcare settings: 13 identified studies.
Infertile and subfertile women: seven identified studies.
Pregnant women: two identified studies and four control groups.
Asymptomatic population groups: three studies and one control group included
Table 1.
The articles are color-coded based on the patient groups they feature: Group 1 (green): symptomatic women; Group 2 (blue): infertile and subfertile women; Group 3 (red): pregnant women; Group 4 (white): asymptomatic population groups.
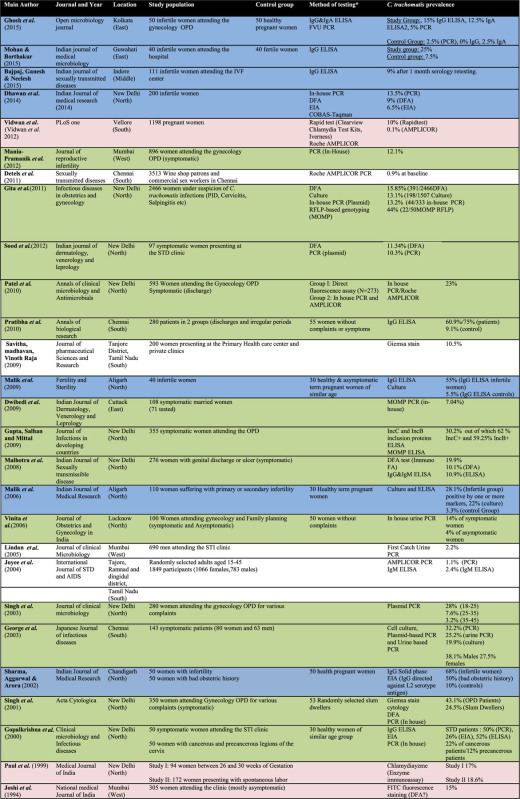
|
Symptomatic patients presenting at the gynecology outpatient department (OPD)
The first patient population was the most represented in the review with a total of 13 studies identified. The details of the studies are featured in Table 2. The prevalence of C. trachomatis among the studies ranged from 10% in the study by Sood et al. (2012) to 50% in the study by Gopalkrishna et al. (2000) using polymerase chain reaction (PCR), both in New Delhi (Gopalkrishna et al.2000; Sood et al.2012). However, another study from Chennai by Pushpa Innocent (2010) using ELISA reported a prevalence of 75%. The predominance of studies from New Delhi could also be observed in this group. Eight of the studies conducted on symptomatic patients were in fact conducted in New Delhi.
Table 2.
Chlamydia trachomatis prevalence among symptomatic patients (results given for PCR or most sensitive technique).
| Authors | Location | Testing | Cohort size | Prevalence |
|---|---|---|---|---|
| Mania-Pramanik et al. (2012) | Mumbai | PCR | 896 | 12% |
| Sood et al. (2012) | New Delhi | DFA, PCR | 97 | 10% |
| Gita et al. (2011) | New Delhi | DFA, PCR Culture, | 2466 | 15% (DFA)/13.2% (PCR) |
| Patel et al. (2010) | New Delhi | PCR | 593 | 23% |
| Pushpa Innocent (2010) | Chennai | IgG ELISA | 280 | 60%/75% (two cohorts) |
| Dwibedi et al. (2009) | Cuttack | PCR | 108 | 7.0% |
| Gupta, Salhan and Mittal (2009) | New Delhi | IgG ELISA | 355 | 30% |
| Malhotra et al. (2008) | New Delhi | DFA, IgG IgM ELISA | 276 | 19% |
| Vinita et al. (2006) | Lucknow | PCR | 100 | 14% |
| Singh et al. (2003) | New Delhi | PCR | 280 | 28% |
| George et al. (2003) | Chennai | Culture and PCR | 143 | 32% |
| Singh et al. (2002) | New Delhi | DFA, PCR | 350 | 43% |
| Gopalkrishna et al. (2000) | New Delhi | IgG ELISA, PCR | 50 | 50% |
A similar study testing on men attending the STD clinic in Mumbai was also identified. The authors however did not specify patient details. The study reported 2.2% C. trachomatis prevalence using first-void urine (FVU) PCR (Lindan et al.2005).
Infertile and subfertile women
Female patients (repeatedly) infected with C. trachomatis are known to be at an elevated risk for late complications,i.e. tubal factor infertility (den Hartog, Morré and Land 2006). Seven studies conducted among infertile and subfertile women were included. The results are summarized in Table 3. The prevalence ranged from 9% in Indore in central region of India to 68% in a study performed in Amritsar, making use of enzyme immunoassay (Sharma, Aggarwal and Arora 2002; Bajpai, Ganesh and Neelesh 2015). A further study from Kolkata in the eastern region of India reported an even lower prevalence using PCR (2.5%) (Ghosh et al.2015). The same study nonetheless reported a seroprevalence of the pathogen of 15% while testing with ELISA (Ghosh et al.2015).
Table 3.
Prevalence among infertile and subfertile women (results given for PCR or most sensitive technique).
| Authors | Location | Testing | Cohort size | Prevalence |
|---|---|---|---|---|
| Ghosh et al. (2015) | Kolkata | IgG ELISA, PCR | 50 | 2.5%PCR 15% ELISA |
| Mohan and Borthakur (2015) | Guwahati | IgG ELISA | 40 | 25% |
| Bajpai, Ganesh and Neelesh (2015) | Indore | IgG ELISA | 111 | 9% |
| Dhawan et al. (2014) | New Delhi | PCR,DFA, EIA | 200 | 13.5% |
| Malik et al. (2009) | Aligarh | IgG ELISA | 20 | 55% |
| Malik et al. (2006) | Aligarh | Culture, IgG ELISA | 110 | 28.1% |
| Sharma, Aggarwal and Arora (2002) | Amritsar | IgG EIA | 50 | 68% |
Pregnant women
The third group of interest is pregnant women. Two studies were identified where testing was conducted in pregnant women. Pregnant women were also used as a control group in four previously mentioned studies (Sharma, Aggarwal and Arora 2002; Malik et al.2006, 2009; Ghosh et al.2015). All results are summarized in Table 4. The reported prevalence of C. trachomatis in this group for studies reporting the use of PCR ranged from 0.1% in Vellore, South India, to 2.5% in the study by Ghosh et al. in Kolkata, East India (Vidwan et al.2012; Ghosh et al.2015). The prevalence range is even greater in the studies utilizing ELISA tests. These results range from 3.3% in Aligarh reported by Malik et al. (2006) to 18.6% in the study by Paul et al. (1999). The results featured in this group also display higher prevalence in the studies utilizing serology-based testing.
Table 4.
Chlamydia trachomatis prevalence among pregnant women (results given for PCR or most sensitive technique).
| Authors | Location | Testing | Cohort size | Prevalence |
|---|---|---|---|---|
| Ghosh et al. (2015) | Kolkata | ELISA, PCR | 50 | 2.5% |
| Vidwan et al. (2012) | Vellore | PCR | 1198 | 0.1% |
| Malik et al. (2009) | Aligarh | IgG ELISA, culture | 30 | 5.5% |
| Malik et al. (2006) | Aligarh | IgG ELISA, culture | 30 | 3.3% |
| Sharma, Aggarwal and Arora (2002) | Amritsar | IgG EIA | 50 | 10% |
| Paul et al. (1999) | New Delhi | Enzyme immunoassay | 94 and 172 | 17%/18.6% |
Asymptomatic population groups
Data from studies performing testing at the population level could only be found in three studies and one control group. The population screening group is hence the least represented group, the results and prevalence of the study are summarized in Table 5. The populations screened in this patient group present with major differences in both sample size, origin and prevalence of C. trachomatis. The lowest prevalence (0.9%) was observed by Detels et al. among shop owners and commercial sex workers in Chennai, while the highest prevalence (24.5%) was reported for residents of slum areas in New Delhi by Singh et al. (2002). Both the aforementioned results were provided using PCR testing.
Table 5.
Prevalence of Chlamydia trachomatis in population screening (results given for PCR or most sensible technique).
| Authors | Location | Testing | Cohort size | Prevalence |
|---|---|---|---|---|
| Detels et al. (2011) | Chennai | PCR | 3513 (males and females | 0.9% |
| Savitha, Madhavan and Vinoth Raja (2009) | Tanjore district, Tamil Nadu | Giemsa Stain | 200 (females) | 10.5% |
| Joyee et al. (2004) | Tanjore, Ramnad and Dingidul districts, Tamil Nadu | PCR | 1849 (males and females) | 1.1% |
| Singh et al. (2002) | New Delhi | PCR | 53 (males and females) | 24.5% |
Testing and diagnostic methods
The methods used to diagnose the disease and identify the pathogen in clinical settings are of pivotal importance. In fact, C. trachomatis can be identified through different tests, all of which have different characteristics. Although PCR is currently the gold standard for the identification of the bacteria in human subjects, it however can also be identified through culture (Centers for Disease Control and Prevention 2014; Lanjouw et al.2016). On the other hand, serology-based tests such as ELISA are only suitable for screening in subfertile women. Serology tests help to ascertain the presence of an immune response against the pathogen through the detection of specific antibodies. These antibodies highlight a previous infection which is particularly relevant in the case of C. trachomatis infections (Keltz, Gera and Moustakis 2006). Previous infections may in fact be the cause of tubal pathologies which can be traced back as the cause of infertility. Table 6 summarizes the testing methods featured in the studies included in this review. It should be noted that many of the studies made use of more than one diagnostic test.
Table 6.
Diagnostic and tests in the studies included in the review.
| Testing method | Number of studies |
|---|---|
| In-house PCR | 14 |
| ELISA | 13 |
| Commercial PCR tests | 5 |
| DFA | 4 |
| Culture/Giemsa stain | 3 |
The most commonly reported test in the review was in-house PCR testing, which was performed in 14 studies. ELISA immunological testing was the second most common test, present in 13 studies.
Material for PCR testing
Material for the conduction of (or sample for conducting the) PCR testing can be obtained from different parts of the body as well as different bodily fluids. Each of the products requires a specific sampling procedure which might be more or less invasive. The origin of the sampled material for PCR among the different studies is summarized below. It should be specified that some of the studies featured more than one PCR assays requiring different materials each.
It can be seen that the most commonly used biological material for the conduction of PCR assays were endocervical swabs. It can be seen that the most commonly used biological material for the conduction of PCR assays were endocervical swabs, As featured in Table 7. This method of sampling was used in 11 studies. Other methods included FVU and urine sampling, which were conducted in three studies each. Vaginal samples were used in only two studies.
Table 7.
Origin of the material for PCR testing.
| Material for PCR testing | Number of studies |
|---|---|
| Endocervical swabs | 11 |
| Urine | 3 |
| FVU | 3 |
| Vaginal swabs | 2 |
DISCUSSION
This review provides the first overview on the Chlamydia trachomatis prevalence in the Indian subcontinent. Articles and studies on the subject could be traced back to various geographical regions of India, and the results could be classified into symptomatic women, infertile and subfertile women and asymptomatic population groups. All of them display varying values of C. trachomatis prevalence, which can be attributed to the different settings where testing was conducted, the different population characteristics, but most importantly the tools used to diagnose the pathogen. The findings of this constitute a first attempt at identifying existing discrepancies attributable to differences in population testing techniques and locations regarding the current situation surrounding C. trachomatis in India. In fact, in all patient groups investigated in this review, the prevalence results stemming from studies making use of serology-based testing such as ELISA were as expected higher when compared with the results of studies using PCR.
It was observed that, in patient groups 1 and 2, the prevalence reported using ELISA serology is higher than results obtained through PCR testing. This highlights that the seroprevalence is higher among symptomatic and infertile patients when compared to other groups. The presence of an immune response to the pathogen as supported by positive results of serological tests in fact suggests a past or chronic exposure to the pathogen (Horner et al.2013). The high seroprevalences as compared to PCR prevalences suggest that previous Chlamydia trachomatis infection is related to long term consequences. In fact, PCR detects bacterial DNA inside of the patient's genital tract, which suggests a current infection rather than a past one. This finding underscores the role played by C. trachomatis in chronic infections leading to adverse health outcomes, and particularly, infertility.
Lack of studies featuring male participants could also be observed in group 1. In fact, only one study could be identified, where testing was conducted among men. This study could furthermore not be included in the tables, as the patients tested were not listed as symptomatic, but as attendees of the STD clinic, who may be present only for information. The only testing group where accounts for both men and women could be obtained was the group regarding population screening. Huge variations in terms of sample sizes in this group however restrict the scope for extrapolation. In fact, great differences in sample sizes were reported from this group. The sample size of the featured testing group ranges from 53 participants featured in the control group by Singh et al. (2002) in New Delhi to 3513 in the study by Detels et al. (2011) performed in the south Indian city of Chennai. This represents a testing group almost 70 times bigger.
The prevalence of C. trachomatis across the Indian Territory appears to vary across geographical regions and patient groups. The results suggest that the prevalence rates of C. trachomatis may be influenced by a variety of factors. Aspects influencing the prevalence of C. trachomatis may be attributable to differences in sensibility and specificity of testing, varied cultural backgrounds as well as healthcare practices and health-related beliefs. Additionally, the performance of local, state-run healthcare delivery systems varies greatly between the different states (Goli et al.2014). Moreover, research in different settings has highlighted the role of host genetic markers in the development of symptoms and consequences related to pathogens such as C. trachomatis. Genetic and genomic differences present within the different ethnicities that inhabit the Indian subcontinent could lead to varying prevalence of C. trachomatis. Furthermore, it should be stated that, despite the various represented patient groups included in this review, only three individual studies featured male patient groups. It should be furthermore stated that the only patient group where only men were represented was part of the asymptomatic population groups category. This limited data representing the male populations not only questions the completeness of the picture regarding the prevalence of C. trachomatis in the population but also raises the question of public health relevance associated with an undetected reservoir of infections, which could be held accountable for propagation of the disease. In addition, there are no mentions of contact tracing and partner testing in the different studies. Nonetheless, the data regarding prevalence featured in this article, with the exception of some very high prevalence numbers, match to some extent the prevalence data from European countries, when considering similar patient groups (ECDC 2014; Redmond et al.2015). Additionally, and in a similar fashion compared with the Indian scenario, data from neighboring countries in similar situations and geographical locations are scarce. Some studies from Sri Lanka and Bangladesh nonetheless seem to suggest similar results across comparable patient groups. A study from the Colombo district in Sri Lanka in fact highlighted a C. trachomatis prevalence of 8.3% using PCR amid women attending the STI clinics (Kamani Mangalika et al.2014). In another study from Bangladesh, a prevalence rate of 23% from sexually active women was found using in-house PCR (Hoque et al.2013). The results from these studies support the range of prevalence, which was observed in similar groups in India. These results support the idea that C. trachomatis is associated with a significant burden of disease on the Indian subcontinent, particularly among high-risk groups such as sex workers and patients at STI clinics.
An important factor that may render the Indian settings unique regarding testing and identification of C. trachomatis and other sexually transmittable diseases in the community is the cultural stigma. Stigma was briefly mentioned in the study by Dwibedi et al. (2009) as a significant factor hindering women for seeking assistance and medical care for STI-related symptoms. Although no work could be identified highlighting the stigma against C. trachomatis, there exists a body of evidence surrounding the stigma surrounding HIV/AIDS in India. Many articles in fact support the idea that stigma is a major factor, present at multiple levels which hinders proper and timely management of HIV/AIDS, and that impacts the daily life of people affected by the diseases (Bharat 2011; Ekstrand et al.2012, 2013). It is hence plausible that many people in the community shy away from getting diagnosed and treated with a sexually transmitted infection, even in spite of the fact that treatments and cures may exist, like in the case of C. trachomatis. This is further supported by experience of co-authors (Indian researchers/medical doctors) in this paper. This highlights the need for additional research into psychosocial factors influencing the health-seeking behavior of Indian patients. Stigma might in fact cause many patients to renounce seeking care and hence expose themselves to long-term health impairments. The absence of acknowledgement of the role of stigma related to STIs may suggest that the issue is widely ignored.
Furthermore, there seems to be discrepancies between the internationally recognized gold standards for C. trachomatis detection in industrialized countries and the practices currently implemented in India. As a matter of fact, only slightly short of half of all the studies included in this review featured testing using PCR. Most the PCR tests were performed in-house, while only five studies featured commercially available and internationally acclaimed tests such as the Roche AMPLICOR assay. It should be stressed that, as per the co-authors’ experience, due to the elevated prices of some of the commercial PCR assays, in-house PCR tests might represent a valuable alternative for identification of C. trachomatis in healthcare settings. More research is nonetheless necessitated to better understand the properties of such tests. The values of specificity and sensitivity of in-house PCR tests ought to be judged against internationally acclaimed tests to ensure appropriate diagnosis. Additionally, there would be a need for evidence-based guidelines regarding the conduction of in-house PCR for the detection of C. trachomatis. Research conducted in Indian settings has highlighted that in-house PCR testing displays highly satisfactory results when compared to the AMPLICOR assay (Sachdeva et al.2009). Research from Trinidad and Tobago has further highlighted the use of in-house PCR as a valuable option for settings where the availability of commercial testing kits is limited (Rampersad et al.2007). Further research from South America has highlighted that a set of well-defined in-house PCR primers can be used for the detection of C. trachomatis, showcasing high levels of sensitivity and specificity when compared to gold standard kits (Aguilera-Arreola et al.2014).
Furthermore, many of the articles referred to cell culture as the gold standard for the detection and diagnosis of C. trachomatis in India. These aspects might be associated with the resource intensity of PCR when compared to more affordable testing methods such as culture, direct fluorescence assay (DFA) or ELISA. PCR testing in fact necessitates a vast array of equipment and expertise and can hence only be performed in some facilities and by trained staff. Identification through culture or serology can conversely be performed in traditional laboratory settings which are present in clinics and primary health centers. This further supports the need for cost-effective diagnostic tools for detection in resource-limited settings such as in India. It should hence be stated that research is needed for the implementation of novel testing and diagnosis methods for C. trachomatis and other pathogens in resource-limited settings.
Another important point brought up by the review is the discrepancies in research methodology among the included studies. The control group included in some of the studies did not match with the study populations. This holds in the article by Singh et al. (2002) for instance. The study in fact compares female patients in healthcare settings to randomly selected inhabitants of the slums. This also holds for the lack of information regarding the inclusion of these populations. Additionally, in the two studies by Malik et al. (2006, 2009), both testing groups composed of infertile women were compared to control groups that were smaller in size. The population tested for C. trachomatis ought to be thorough and representative of their populations in order to provide sound information to policy makers. This is especially true in studies aiming at screening populations, as they may guide future screening schemes for sexually transmitted diseases. Overall, there should be a drive towards high-quality research designs, in order to facilitate policy implementations aimed at addressing the burden of C. trachomatis among the Indian population. In fact, there is a need for high-quality research to enable the development of evidence-based guidelines for the management of C. trachomatis in Indian healthcare settings. The current standard operating procedure regarding the diagnosis and management of sexually transmissible diseases are in fact hard to identify and appear to be incomplete. The current guidelines issued by the government of India indeed miss C. trachomatis as a potential cause of vaginal discharge, although it is one of the most common manifestation of C. trachomatis infection once it presents with symptoms (Government of India 2014). There is thus a need for clarity at the policy level for the identification, screening, prevention and treatment of pathogens like C. trachomatis. In addition of the need for cost-effective and readily implementable testing solutions, there is a need for more research in the policy strata. There is in fact a need for an encompassing policy framework taking all the relevant variables into account in order to foster safe, effective and evidence-based management of C. trachomatis infections as well as the long-term consequences they may cause.
This paper touches upon the topic of female infertility in India. This topic remains vastly unexplored, although some accounts suggest that the burden might underreported in India (Jejeebhoy 1998; Malhotra et al.2013). There has been an increase in the number of couples that make use of in vitro fertilization and other methods in order to conceive (Widge and Cleland 2009). Experts have also argued that the issue is not properly addressed and is not sufficiently investigated in Indian settings (Pande 2013). Results from national surveys suggest a rise in the number of infertile couples (Mahesh 2013). These factors ought to draw more attention on the burden of infertility, of which Chlamydia trachomatis is a major cause alongside other diseases such as N. Gonorrhea. In fact, not only is the detection of the pathogen straightforward, but a timely management and treatment of the infection may lead to a full recovery and replenished reproductive health.
CONCLUSION
This review is the first endeavor to systematically review the prevalence of Chlamydia trachomatis across India. The studies included for analysis differed greatly based on research methodology, patient populations and testing methods used to diagnose C. trachomatis highlighting a need for standardization and guidelines for the identification and diagnosis of C. trachomatis in India. The prevalence of the disease across states and patient groups has been shown to vary quite significantly, suggesting the influential role of a wide range of factors including diagnostic tools and perhaps the genetic makeup of the different populations on the Indian subcontinent. More research is needed to develop novel diagnosis tests which are cost-effective and which can be implemented in resource-limited settings such as India. This paper highlights in-house PCR as a technique yielding high levels of sensitivity and specificity for pathogen identification. Tools and techniques however ought to be streamlined. More research is also necessary to ascertain the role of C. trachomatis in primary and secondary infertility in India as well as in adverse pregnancy outcomes. Further research will also need to be conducted in order to identify population subgroups that are at a high risk for sexually transmitted diseases and, particularly, for C. trachomatis. Policies and guidelines defining the identification and management of C. trachomatis in India also ought to be updated to maximize patient outcomes.
FUNDING
This study was supported by the NDBE Foundation, The Netherlands
Conflict of interest. None declared.
REFERENCES
- Aguilera-Arreola M, González-Cardel A, Tenorio A et al. Highly specific and efficient primers for in-house multiplex PCR detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma hominis and Ureaplasma urealyticum. BMC Res Notes 2014;7:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus CL, Heijne JCM, Roellin A et al. Transmission dynamics of Chlamydia trachomatis affect the impact of screening programmes. Epidemics 2010;2:123–31. [DOI] [PubMed] [Google Scholar]
- Bajpai T, Ganesh BS, Neelesh G. Prevalence of Chlamydia trachomatis immunoglobulin G antibodies in infertile women attending an in vitro fertility center. Indian J Sex Transm Dis 2015;36:215–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud D, Greub G. Intracellular bacteria and adverse pregnancy outcomes. Clin Microbiol Infect 2011;17:1312–22. [DOI] [PubMed] [Google Scholar]
- Bharat S. A systematic review of HIV/AIDS-related stigma and discrimination in India: Current understanding and future needs. SAHARA J-J Soc Asp H 2011;8:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Sexually Transmitted Infections in Developing Countries. Current Concepts and Strategies on Improving STI Prevention, Treatment, and Control. 2008. [Google Scholar]
- Centers for Disease Control and Prevention Papp JR, Schachter J et al. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR-RecommRep 2014;63:1–19. [PMC free article] [PubMed] [Google Scholar]
- Chernesky MA. The laboratory diagnosis of Chlamydia trachomatis infections. Can J Infect Dis Med 2005;16:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog JE, Morré SA, Land JA. Chlamydia trachomatis-associated tubal factor subfertility: Immunogenetic aspects and serological screening. Hum Reprod Update 2006;12:719–30. [DOI] [PubMed] [Google Scholar]
- Detels R, Green AM, Klausner JD et al. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis 2011;38:503–9. [PMC free article] [PubMed] [Google Scholar]
- Dhawan B, Rawre J, Ghosh A et al. Diagnostic efficacy of a real time-PCR assay for Chlamydia trachomatis infection in infertile women in north India. Indian J Med Res 2014;140:252–61. [PMC free article] [PubMed] [Google Scholar]
- Dwibedi B, Pramanik JM, Sahu P et al. Prevalence of genital Chlamydia infection in females attending an Obstetrics and Gynecology out patient department in Orissa. Indian J Dermatol Ve 2009;75:614–6. [DOI] [PubMed] [Google Scholar]
- ECDC. Chlamydia Control in Europe: Literature Review. Stockholm, 2014. [Google Scholar]
- Ekstrand ML, Bharat S, Ramakrishna J et al. Blame, symbolic stigma and HIV misconceptions are associated with support for coercive measures in urban India. AIDS Behav 2012;16:700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand ML, Ramakrishna J, Bharat S et al. Prevalence and drivers of HIV stigma among health providers in urban India: implications for interventions. J Int AIDS Soc 2013;16, DOI: 10.7448/IAS.16.3.18717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangolli LV, Duggal R, Shukla A. Review of Healthcare in India, Mumbai: Centre for enquiry into health and allied themes; 2005. [Google Scholar]
- Ganguly S, Unisa S. Trends of infertility and childlessness in India: findings from NFHS data. Facts Views Vis ObGyn 2010;2:131–8. [PMC free article] [PubMed] [Google Scholar]
- George JA, Panchatcharam TS, Paramasivam R et al. Evaluation of diagnostic efficacy of PCR methods for Chlamydia trachomatis infection in genital and urine specimens of symptomatic men and women in India. Jpn J Infect Dis 2003;56:88–92. [PubMed] [Google Scholar]
- Ghosh M, Choudhuri S, Ray RG et al. Association of genital Chlamydia trachomatis infection with female infertility, study in a tertiary care hospital in Eastern India. Open Microbiol J 2015;9:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gita S, Suneeta M, Anjana S et al. C. trachomatis in female reproductive tract infections and RFLP-based genotyping: A 16-year study from a tertiary care hospital. Infect Dis Obstet Gynecol 2011, DOI: 10.1155/2011/548219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goli S, Arokiasamy P, Barro RJ et al. Trends in health and health inequalities among major states of India: assessing progress through convergence models. Health Econ Policy L 2014;9:143–68. [DOI] [PubMed] [Google Scholar]
- Gopalkrishna V, Aggarwal N, Malhotra VL et al. Chlamydia trachomatis and human papillomavirus infection in Indian women with sexually transmitted diseases and cervical precancerous and cancerous lesions. Clin Microbiol Infect 2000;6:88–93. [DOI] [PubMed] [Google Scholar]
- Government of India. Prevention, Management and Control of Reproductive Tract Infections and Sexually Transmitted Infections. National AIDS control Policy & Guidelines. New Delhi: Ministry of Health and Family Welfare; 2014. [Google Scholar]
- Gupta R, Salhan S, Mittal A. Seroprevalence of antibodies against Chlamydia trachomatis inclusion membrane proteins B and C in infected symptomatic women. J Infect Dev Countr 2009;3:191–8. [DOI] [PubMed] [Google Scholar]
- Haggerty CL, Gottlieb SL, Taylor BD et al. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010;201Suppl:S134–55. [DOI] [PubMed] [Google Scholar]
- Hoque SM, Hossain MA, Paul SK et al. Detection of Chlamydia trachomatis by immunological and genetic methods in female sex workers and the local female population of reproductive age in Mymensingh, Bangladesh. Jpn J Infect Dis 2013;66:256–9. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Wills GS, Reynolds R et al. Effect of time since exposure to Chlamydia trachomatis on chlamydia antibody detection in women: a cross-sectional study. Sex Transm Infect 2013;89:398–403. [DOI] [PubMed] [Google Scholar]
- Inhorn MC, Bharadwaj A. Reproductively disabled lives: Infertility, stigma, and suffering in Egypt and India, in Ingstad B, Reynolds Whyte S, (eds). Disability in Local and Global Worlds. Berkeley: University of california press; 2007, 78–106. [Google Scholar]
- Jejeebhoy DSJ. Infertility in India - levels, patterns and consequences: Priorities for social science research. J Fam Welf 1998;44:15–24. [Google Scholar]
- John TJ, Dandona L, Sharma VP et al. Continuing challenge of infectious diseases in India. Lancet 2011;377:252–69. [DOI] [PubMed] [Google Scholar]
- Joshi J V, Palayekar S, Hazari KT et al. The prevalence of Chlamydia trachomatis in young women. Natl Med J India 1994;7:57–9. [PubMed] [Google Scholar]
- Joyee AG, Thyagarajan SP, Rajendran P et al. Chlamydia trachomatis genital infection in apparently healthy adult population of Tamil Nadu, India: a population-based study. Int J STD AIDS 2004;15:51–5. [DOI] [PubMed] [Google Scholar]
- Kamani Mangalika GHA, Cankanamge SK, Priyadarshana D et al. Prevalence of Chlamydia trachomatis in women attending sexually transmitted disease clinics in the Colombo district, Sri Lanka. Indian J Pathol Micr 2014;57:55–60. [DOI] [PubMed] [Google Scholar]
- Keltz MD, Gera PS, Moustakis M. Chlamydia serology screening in infertility patients. Fertil Steril 2006;85:752–4. [DOI] [PubMed] [Google Scholar]
- Lanjouw E, Ouburg S, De Vries H et al. 2015 European guideline on the management of Chlamydia trachomatis infections. 2016, DOI: 10.1177/0956462415618837. [DOI] [PubMed]
- Lindan C, Mathur M, Kumta S et al. Utility of pooled urine specimens for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in men attending public sexually transmitted infection clinics in Mumbai, India, by PCR. J Clin Microbiol 2005;43:1674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low N, McCarthy A, Macleod J et al. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection. Health Technol Assess 2007;11:iii–iv, ix–xii, 1–165. [DOI] [PubMed] [Google Scholar]
- Mahesh R. Infertility rate among Indian couples on the Rise, Says Survey. International Business Times. 2013, http://www.ibtimes.co.in/infertility-rate-among-indian-couples-on-the-rise-says-survey-507672. (7 April 2016, date last accessed). [Google Scholar]
- Mahood Q, Van Eerd D, Irvin E. Searching for grey literature for systematic reviews: Challenges and benefits. Res Synth Methods 2014;5:221–34. [DOI] [PubMed] [Google Scholar]
- Malhotra M, Bala M, Muralidhar S et al. Prevalence of Chlamydia trachomatis and its association with other sexually transmitted infections in a tertiary care center in North India. Indian J Sex Transm Dis & AIDS 2008;29:82–5. [Google Scholar]
- Malhotra M, Sood S, Mukherjee A et al. Genital Chlamydia trachomatis: an update. Indian J Med Res 2013;138:303–16. [PMC free article] [PubMed] [Google Scholar]
- Malik A, Jain S, Hakim S et al. Chlamydia trachomatis infection & female infertility. Indian J Med Res 2006;123:770–5. [PubMed] [Google Scholar]
- Malik A, Jain S, Rizvi M et al. Chlamydia trachomatis infection in women with secondary infertility. Fertil Steril 2009;91:91–5. [DOI] [PubMed] [Google Scholar]
- Mania-Pramanik J, Kerkar S, Sonawane S et al. Current Chlamydia trachomatis infection, a major cause of infertility. J Reprod Infertil 2012;13:204–10. [PMC free article] [PubMed] [Google Scholar]
- Mohan DG, Borthakur AK. Seroprevalence of Chlamydia trachomatis in infertile women in a tertiary care hospital: a pilot study. Indian J Med Microbiol 2015;33:331–2. [DOI] [PubMed] [Google Scholar]
- Newman L, Rowley J, vander Hoorn S et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015;10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A. Commercial surrogacy in India: manufacturing a perfect mother‐worker. Signs 2013;35:969–92. [Google Scholar]
- Patel AL, Sachdev D, Nagpal P et al. Prevalence of Chlamydia infection among women visiting a gynaecology outpatient department: evaluation of an in-house PCR assay for detection of Chlamydia trachomatis. Ann Clin Microb Anti 2010;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul VK, Singh M, Gupta U et al. Chlamydia trachomatis infection among pregnant women: prevalence and prenatal importance. Natl Med J India 1999;12:11–4. [PubMed] [Google Scholar]
- Perianayagam A, Goli S. Provisional results of the 2011 Census of India. Int J Soc Econ 2012;39:785–801. [Google Scholar]
- Pushpa Innocent JD. Prevalence of Chlamydia trachomatis infection in women in Chennai, India. Ann Biol Res 2010;1:76–81. [Google Scholar]
- Rampersad J, Wang X, Gayadeen H et al. In-house polymerase chain reaction for affordable and sustainable Chlamydia trachomatis detection in Trinidad and Tobago. Rev Panam Salud Públ 2007;22:317–22. [DOI] [PubMed] [Google Scholar]
- Redmond SM, Alexander-Kisslig K, Woodhall SC et al. Genital chlamydia prevalence in Europe and non-European high income countries: systematic review and meta-analysis. PLoS One 2015;10:e0115753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva P, Patel AL, Sachdev D et al. Comparison of an in-house PCR assay, direct fluorescence assay and the Roche AMPLICOR Chlamydia trachomatis kit for detection of C. trachomatis. J Med Microbiol 2009;58:867–73. [DOI] [PubMed] [Google Scholar]
- Savitha S, Madhavan S, Vinoth Raja R. Incidence of chlamydial infection in women. J Pharm Sci Res 2009;1:26–33. [Google Scholar]
- Sharma K, Aggarwal A, Arora U. Seroprevalence of Chlamydia trachomatis in women with bad obstetric history and infertility. Indian J Med Sci 2002;56:216–7. [PubMed] [Google Scholar]
- Singh V, Rastogi S, Garg S et al. Polymerase chain reaction for detection of endocervical Chlamydia trachomatis infection in women attending a gynecology outpatient department in India. Acta Cytol 2002;46:540–4. [DOI] [PubMed] [Google Scholar]
- Singh V, Salhan S, Das BC et al. Predominance of Chlamydia trachomatis serovars associated with urogenital infections in females in New Delhi, India. J Clin Microbiol 2003;41:2700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Mukherjee A, Bala M et al. A pilot study for diagnosis of genital Chlamydia trachomatis infections by polymerase chain reaction among symptomatic Indian women. Indian J Dermatol Venereol Leprol 2012;78:443–7. [DOI] [PubMed] [Google Scholar]
- Tiller CM. Chlamydia during pregnancy: implications and impact on perinatal and neonatal outcomes. J Obstet Gynecol Neonatal Nurs 2002;31:93–8. [DOI] [PubMed] [Google Scholar]
- Vidwan NK, Regi A, Steinhoff M et al. Low prevalence of Chlamydia trachomatis infection in non-urban pregnant women in Vellore, S. India. PLoS One 2012;7, DOI: 10.1371/journal.pone.0034794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinita D, Meenal A, Jyotsna A et al. Prevalence of genital Chlamydia trachomatis by first void urine polymerase chain reaction test in women attending out patient clinic. Obstet Gynecol 2006;56:511–3. [Google Scholar]
- WHO. Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections. Geneva, 2008. [Google Scholar]
- Widge A, Cleland J. The public sector's role in infertility management in India. Health Policy Plan 2009;24:108–15. [DOI] [PubMed] [Google Scholar]


