Abstract
Genital infection by Chlamydia trachomatis is the most common bacterial sexually transmitted disease worldwide. It causes serious reproductive health complications, including pelvic inflammatory disease and infertility. Stress is implicated as a risk factor for various infections; however, its effect on chlamydia genital infection is unknown. We previously showed that repeated exposure of mice to cold water results in increased severity of chlamydia genital infection. In this study, cold water-induced stress resulted in (i) elevated levels of norepinephrine (NE) and epinephrine in the spleen and genital tract of stressed mice; (ii) elevated IL-1β, TNF-α, IL-6 and nitric oxide production in macrophage-rich peritoneal cells of mice; (iii) supplement of NE in vitro exerts an immunosuppressive effect on splenic T-cell production of cytokines; (iv) decreased C. muridarum shedding in the genital tract of β1Adr/β2Adr receptor KO mice; and (v) a higher rate of infertility in infected mice. These results suggest that cold water stress induces the production of catecholamines, which may play a critical role in the modulation of the immune system leading to increased intensity of C. muridarum genital infection.
Keywords: cold-induced stress, chlamydia, immune response
Cold water-induced stress increases the intensity of chlamydia infection, changes pathology and reduces fertility in mice.
INTRODUCTION
Chlamydia genital infection caused by Chlamydia trachomatis, the most common bacterial sexually transmitted disease worldwide, is a leading cause of pelvic inflammatory disease, fallopian tube scarring, ectopic pregnancy, infertility and neonatal conjunctivitis (Westrom et al. 1992; Cohen and Brunham 1999; Stamm 1999; Haggerty et al.2010). Epidemiologic data from the Centers for Disease Control and Prevention and World Health Organization (WHO) indicate that C. trachomatis genital infection is a serious public health problem, with more than 90 million new cases occurring annually worldwide, and 4 million in the USA alone (CDC 2008). In the USA, chlamydia genital infection disproportionately affects populations of low socioeconomic status, particularly African-Americans (Ellen et al. 1995; Owusu-Edusei et al.2013). The reasons are not well known, but stress and other unknown factors may have a role in the persistent high rate of Chlamydia genital infection associated with low socioeconomic conditions (Beatty, Morrison and Byne 1994; Pampel, Krueger and Denney 2010).
It is well recognized that genital infection with C. trachomatis leads both to the recruitment of protective immune responses to the site of infection and infiltration of inflammatory products that may also contribute to pathological damage in the host. Previous studies demonstrated that immune response to chlamydia genital infection may contribute to pathology diseases following infection (Westrom et al. 1992; Cohen and Brunham 1999; Stamm 1999). This has been demonstrated in patients with repeated chlamydia genital infection having a much greater risk of tubal obstruction than those with less exposure to C. trachomatis.
Various animal model studies have been undertaken to identify and characterize factors that may be involved in the development of immunopathogenesis after chlamydia genital infection (Patton et al. 1987; Rank 1994; Kelly et al. 2000; Rank, Bowlin and Kelly 2000). Those murine model studies have exhibited vital aspects of human genital chlamydial infection including similar course of active infection, pathological consequences or sequelae of an infection, tubal inflammation, hydrosalpinx formation and infertility (Rank 1999).
More studies in animal models have demonstrated that anti-chlamydial T-cell response in the local genital mucosa plays a role in the clearance of C. trachomatis from the genital tract (Igietseme and Rank 1991; Cain and Rank 1995; Morrison, Feilzer and Tumas 1995; Cotter et al. 1997; Johansson et al. 1997; Rank 1999; Igietseme, Portis and Perry 2001). It was demonstrated that immunity to C. trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and independent pathways (Perry, Feilzer and Caldwell 1997).
Chlamydia trachomatis infection of the genital tract has gained much attention because of its impact on female infertility (Tuffrey, Alexander and Taylor-Robinson 1990; Pal et al.1998) and no reliable vaccine against the infection has been developed. Experimental vaccines showed that vaccine-induced immunity has the ability to prevent infection compared to immunity generated by previous infection (Igietseme, Eko and Black 2011). In that study, vaccinated mice with dendritic cells pulsed with Fc-major outer membrane protein were protected from infertility, whereas C. muridarum perfected mice were not, suggesting that infection-induced immunity does not prevent the pathological process leading to infertility.
It is documented that psychological or physical stress, resulting from competition, pressure and hardship of life in society, has major impacts on public health (Solomon 1987; Ziemessen et al.2007). Stress may regulate the host immune response through neuroendocrine–immune system interactions (Sheridan et al.1994; Madden, Sanders and Felten 1995; Borghetti et al. 2009). Glucocorticoids and catecholamines are hormones that serve as the major mediators of stress responses by modulating signaling events, which result in either immunosuppression or immunostimulation in the host (Solomon 1987; Ziemessen et al.2007).
Norepinephrine (NE) is one of the catecholamines of the sympathetic nervous system released during stressful conditions including cold-water application. NE binds and stimulates the beta2 adrenergic receptor (β2Adr), which is predominantly expressed on CD4+ and B cells (Sanders et al.2003; Sanders and Virginia 2012). Studies document the existence of at least eight different alpha or beta subtypes of NE receptors (Bylund et al.1994). Studies have further shown that the direct binding of NE to β2Adr modulates immune responses, including altering cytokine production, lymphocyte proliferation and antibody secretion (Elenkov et al.1996; Ramer-Quinn, Baker and Sanders 1997; Kohm et al.2000; Goyarts et al.2008; Nakai et al.2014; Lorton and Bellinger 2015).
Cold water-induced stress in mice has proven useful in defining the relationships between the central nervous and immune defense systems (Kubera et al. 1998; Aviles et al.2004). Application of cold water as a stressor in animal models, including mice, has resulted in changes in immune responses that correlate with the activity of the neuroendocrine system of corticosteroids and catecholamines (Aviles and Monroy 2001; Rober et al.2006)
The role of stress in the immunopathogenesis of chlamydia genital infection remains unknown. The objective of this study was to investigate the role of cold water-induced stress on the immune response, histopathology and fertility in a mouse model during chlamydia genital infection. The hypothesis was that exposure of mice to cold water-induced chronic stress increases the intensity of chlamydia genital infection and results in a more progressive infection and severe pathology. A better understanding of how stress contributes to the immunopathogenesis of chlamydia genital infection in a mouse model may allow us to develop more effective prevention and treatment options in humans.
MATERIALS AND METHODS
Chlamydia stock culture and McCoy cell line
McCoy mouse fibroblast cell line and Chlamydia muridarum (previously known as Chlamydia trachomatis) agent of mouse pneumonitis (MoPn) were kindly provided by Dr Joseph Igietseme, Morehouse School of Medicine, Atlanta, GA. Stock of C. muridarum was prepared by propagating elementary bodies in McCoy cells, as previously described (Belay et al.2002).
Animals
Six- to seven-week-old female BALB/c strain mice and proven fertile males for mating were purchased from Harlem-Sprague Daley (Indianapolis, IN, USA), or Hilltop Lab Animals, Inc. (Scottsdale, PA, USA). A β1Adr/β2Adr receptor knockout (KO) mouse and three wild-type mice, representing the C57BL/6J, DBA/2J and FVB/NJ background strains from which the β1Adr/β2Adr receptor KO strain was derived purchased from Jackson laboratories (Bar Harbor, ME, USA), were used in the study. Mice were housed in a vivarium at Bluefield State College (BSC) located in the Basic Science Building of the School of Arts and Sciences. Mice were given food and water ad libitum in an environmentally controlled room with equal daylight and night hours. Before starting experiments, all animal experimental protocols were approved by the BSC Institutional Animal Care and Use Committee.
Cold water stress protocol
Stress was induced by placing five mice in a packet filled with 2 cm of cold water (1°C–4°C) for 5 min daily for 24 days, as previously described (Aviles and Monroy 2001; Belay and Woart 2013). The water level was deep enough to cover the backs of the mice while they were swimming in the cold water. At the end of each stressing period, mice were dried with towels to avoid hypothermia. Non-stressed mice were kept at room temperature without the treatment of cold water-induced stress. In preparation for secondary infection, mice were stressed as above on day 60 of the primary infection for 10 days and re-infected on day 70 of primary infection.
Restraint stress protocol
As an alternative to cold stress, a restraint stressing method was applied to and randomly selected group of mice as previously described (Bonneau 1996). Briefly, each mouse was placed in an adequately ventilated 60 mL conical syringe tube with holes for 5 min every day for 24 days. Mice could rotate along the horizontal axis, but could not turn head to tail.
Peritoneal fluid harvesting protocol
After stressing mice for 19 days, at least five mice of each experimental group were injected with 2 mL thioglycollate intraperitoneally. At the conclusion of the 24-day stressing period, these mice were euthanized by CO2 inhalation followed by cervical dislocation. Peritoneal cells were harvested from mice as previously described (Aviles et al.2004). Briefly, 7 mL of RPMI 1640 without phenol red was gently inoculated into the lower abdominal part of each mouse using a 20-gauge needle and syringe. Cells were then harvested by low speed centrifugation and seeded at 5 × 105 cells per well in 100 μL of RPMI-1640 complete medium and stimulated with lipopolysaccharide (LPS) (5 μg mL−1) and incubated at 37°C supplemented with 5% CO2. Culture supernatant was collected at different time points for immediate nitric oxide and enzyme-linked immunosorbent assay (ELISA) protocols, respectively.
Nitric oxide assay
Nitrite concentrations were determined using the Greiss reagent (1% sulfanilamide and 0.1% naphthyl ethylene diamine hydrochloride in 5% phosphoric acid) with reference to a standard curve for sodium nitrate as previously described (Aviles et al.2004).
T-cell isolation from mouse spleen cells
Spleens from mice in each experimental group were removed aseptically. Tissues were kept in 2 mL of complete RPMI 1640 medium supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO, USA), 1% penicillin/streptomycin (Sigma) and 0.1% gentamicin (Sigma). The spleen tissues then were minced and teased with forceps and cells were pressed through a 70 μm size cell strainer (Becton Dickinson, Franklin Lakes, NJ, USA) and placed in an additional 5 mL of complete RPMI 1640 medium. Untouched mouse T cells from stressed and non-stressed mice were isolated using a Dynabead Magnetic Separation kit from Life Technologies (Carlsbad, CA, USA) following the manufacturer's instructions. Cell counts were determined using a TC20 Automatic Cell Counter from Bio Rad (Hercules, CA, USA) and the counts were at least 1 × 107 cells mL−1.
Antigen-presenting cell preparation
Antigen-presenting cells (APCs) from splenocytes of non-stressed mice were prepared by treatment with mitomycin C following the manufacturer's instructions. Briefly, splenocyte cells were washed by centrifugation at 300 × g for 10 min and freshly prepared mitomycin C at the concentration of 25 μg mL−1 was added to 107 splenocyte cells mL−1 and incubated at 37°C for 30 min. Cells were washed four times as above and a final cell count of 50 × 105 mitomycin C-treated cells was prepared for proliferation assay.
T-cell proliferation assay
T-cell proliferation assay was performed as described previously (Aviles et al.2004) with some modifications. Briefly, cells were seeded in triplicate at a density of 5 × 105 cells mixed with 5 × 105 mitomycin C-treated cells per well in a 96-well culture plate (Becton Dickinson). The cells were stimulated with 2.5 μg mL−1 of concanavalin A (Con A) and 5 μg mL−1 of LPS and NE at 10−6 M or in combination and cultured for 72 h in a water-jacketed incubator at 37°C and 5% CO2 (NuAire, Plymouth, MN, USA). Culture supernatants were collected after 72 h of proliferation then stored at −80°C to measure cytokine production by ELISA.
Testing the action of synthetic NE on T-cell proliferation
To investigate the influence of synthetic NE bitartrate salt (Sigma) on cytokine production, we investigated whether NE inhibits immune cell activities as measured by the amount of important cytokines produced. Spleens were harvested as above from stressed/infected (SI), non-stressed/infected (NSI) and non-stressed/non-infected (NSNI) mice. T cells were isolated using Dynabeads® Magnetic Separation following the manufacturer's instructions. Purified T cells were seeded at a density of 5 × 105 per well in 96-well plates along with the same number of APCs. Cells were proliferated for 72 h at 37°C in the presence/absence of Con A (5 μg mL−1), (0.3 μg mL−1) irradiated chlamydia antigen or 100 μL NE at the concentration of 10−4M. Culture supernatants were collected after 72 h of proliferation and immediately stored at –20°C to be tested for the production of selected cytokines as determined by ELISA.
Cytokine measurement using ELISA
The amount of cytokines in supernatants collected from peritoneal fluid cells of stressed and non-stressed mice was measured by using an ELISA kit (Invitrogen, Camarillo, CA) following the manufacturer's instructions. Briefly, standard samples and test samples were diluted and added to the wells in duplicate and incubated as directed. Supernatant fluids from parallel cell cultures of spleen cell proliferation and from the peritoneal functional assays were used to determine cytokine production (Aviles et al.2004). Optical densities were measured at 450 nm with an ELISA plate reader from Biotech (Winooski, VT, USA). The concentration of the cytokine in each sample was obtained by extrapolation from the standard calibration curve generated in each assay.
Evaluation of the course of Chlamydia muridarum genital infection in mice
On day 17 of the 24-day stressing period, all mice were injected subcutaneously with 2.5 mg per mouse of progesterone in 100 μL of phosphate buffered saline (PBS) to regulate the mice's estrous cycles. On the final day of the stressing period, stressed and non-stressed mice were infected intravaginally with 103 IFU of C. muridarum in a volume of 30 μL of PBS while under Ketamine-Xylazine-induced anesthesia. Infection was monitored by cervico-vaginal swabbing at 3-day intervals during primary infection as previously described (Belay and Woart 2013). To isolate C. muridarum shedding from genital swabs, individual wells of McCoy cell monolayer cultured in complete Dulbecco's modified Eagle's medium (DMEM) in 96-well plates were inoculated with 200 μL of vortexed solution of the genital swab from the –80°C freezer, followed by a centrifugation at 300 rpm for 1 h at room temperature. Culture plates were incubated at 37°C for 32 h, and then fixed with methanol after washing twice with PBS. Approximately 50 μL of Fluorescein isothiocyanate-labeled, genus-specific anti-chlamydial antibody from Remel (Lenexa, KS, USA) was added to each well and incubated for 2 h at room temperature. After washing and drying wells, fluorescence was observed with a fluorescence microscope to illuminate inclusions. The number of inclusions was determined by counting inclusion bodies within 10 fields (×40), as previously described (Belay and Woart 2013).
Detection of Chlamydia muridarum shedding in regions of the genital tract
Alternatively, approximate quantification of inclusion bodies was achieved by culturing homogenized tissues of the regions of the genital tract. Briefly, female mice were stressed and infected as above. The genital tracts were aseptically removed and separated into three regions, cervix, uterus, oviduct, and placed in 1 mL complete DMEM and homogenized using a QIAGEN Tissue Raptor (Valencia, CA). A volume of 200 μl of each homogenized tissue supernatant was added to monolayers of McCoy cell lines seeded in 96-well plates and incubated at 37°C supplemented with 5% CO2. Isolation of C. muridarum was performed as described above.
Determination of catecholamines levels in spleen and genital tract of stressed mice
The level of epinephrine (EP) or NE in the spleens and genital tracts of mice was determined as previously described (Belay and Woart 2013). Briefly, mice were euthanized and tissues were harvested and homogenized as above. The level of EP or NE was determined using a 3-cat EIA kit and following the manufacturer's instructions (Labor Diagnostika Nord GmbH and Co. KG, Germany). Absorbance was read using a microreader plate set to 450 nm. Concentrations of EP or NE were determined by extrapolation from the standard values.
Harvesting tissues for gross examination and pathology analysis in mice
To reveal histological-grade differences of the genital tracts between stressed and non-stressed mice during primary infection at several time points (2, 14, 42 days) and secondary infection (75 days), mice were euthanized during primary or at 15 day of secondary of chlamydia genital infection. The existence of hydrosalpinx fluid and any other related abnormalities of the entire genital tract harvested at 15 day of secondary infection was carried out by gross examination with naked eyes. Each genital tract was sectioned into regions containing the cervix, uterus and oviduct for pathological analysis. After photographing, excised tissues were transferred to 10% neutral formalin and processed for histology following procedures, processed and stained with H&E (Rank, Sanders and Patton 1995; Kelly et al.2000). The slides were imaged and examined under a microscope digital camera for the presence of neutrophils (acute inflammation), lymphocytes and plasma cells of fibrosis (chronic inflammation) by a pathologist at St. Mary's Hospital, Huntington, WV.
Fertility studies
After primary and secondary C. muridarum genital infection and resolution, the stressed, non-stressed and non-stressed/non-infected mice were mated with proven fertile males and monitored for pregnancy by daily weight-gain assessment as previously described (Pal et al.1998). The mating was monitored for 19–21 days, a duration covering murine gestational and litter production. Pregnancy was determined by measuring weight gained and the mice that were considered pregnant euthanized. After the first mating, female mice that were not pregnant were mated again with a male mouse that had successfully mated with others. The percentage of pregnant mice and the number of embryos or pups in each group was compared during the 19–21 days of experimentation. The experiment was repeated three times for comparison purposes.
Statistical analysis
Statistical significance between any two groups was tested using Student's t-test and ANOVA was used to test a statistical difference between more than two groups. Level of statistical significance was at the level of P < 0.05.
RESULTS
Cold water-induced stress increases NE and EP levels in the spleen and genital tract of mice
We previously reported that cold-induced stress increases plasma levels of catecholamines (NE and EP) in mice (Belay and Woart 2013). To further assess the presence of these stress hormones in lymphoid organs and the genital tract during Chlamydia muridarum genital infection, we measured the levels of NE and EP in the spleens and genital tracts of cold-induced stressed and non-stressed mice using ELISA. As shown in Fig. 1a and b, mice exposed to cold stress had a statistically significant increased production of NE in the spleen (p < 0.008) and genital tract (P < 0.002), compared to that of non-stressed mice. Similarly, the EP level in the spleen (P < 0.001) and genital tract (P < 0.002) of stressed mice was higher than that of non-stressed mice. The secretion level of stress hormones by cold water-treated and restraint-stressed mice were almost identical, suggesting application of cold water as a physical stressor and restraint stress as a psychological stressor in mice resulted in the same changes in the neuroendocrine system of corticosteroids and catecholamines. This elevated level of catecholamines in the spleen and the genital tract as a result of cold stress treatment may suggest that these stress hormones contribute to impairment of the immune system during chlamydia genital infection.
Figure 1.
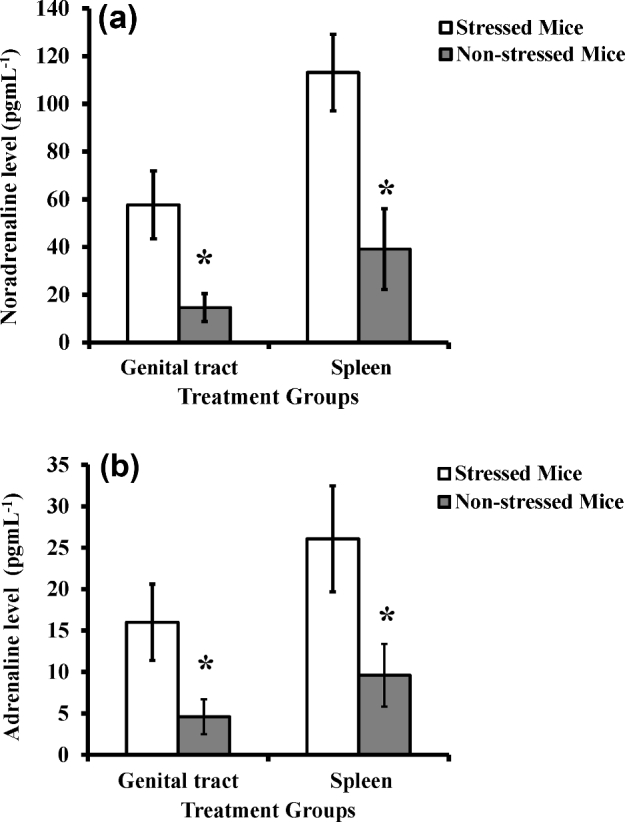
Effect of cold-induced stress on production of catecholamines in genital tract and spleen: noradrenaline (a) and adrenaline (b) measured by ELISA. Each point represents the means ± standard deviation of two experiments (n = 5 to 6 mice for each experiment). Statistical differences between treatments were evaluated by Student's t test. Asterisk denotes significant statistical differences between the experimental groups at the level of (P ≤ 0.05).
Cold water-induced stress increases production of proinflammatory cytokines and nitric oxide in peritoneal macrophages
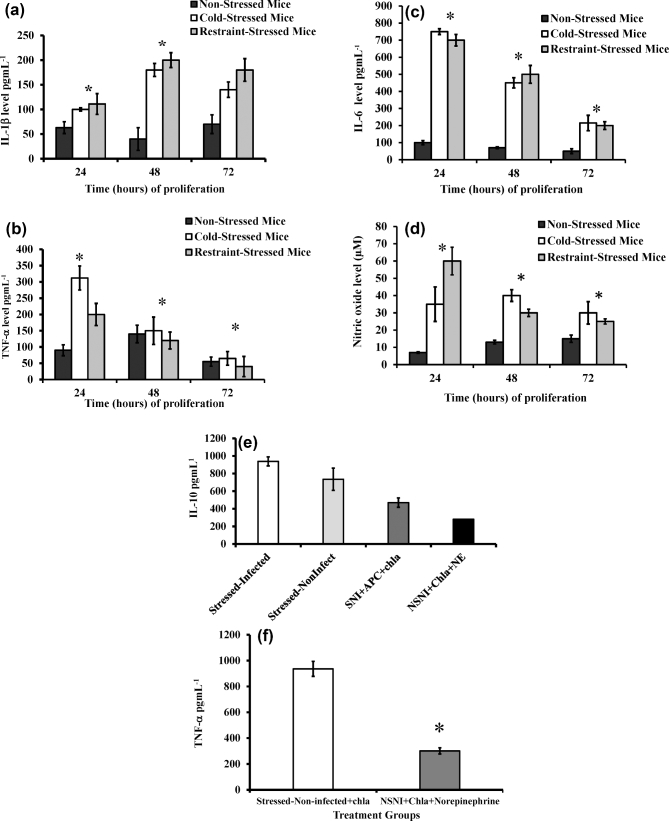
This study was to examine the effect of stress on the secretion of proinflammatory cytokines and production of reactive NO by peritoneal cells. We tested the hypothesis that stress would alter the pattern of secretion of proinflammatory cytokines and NO. As shown in Fig. 2a–c, cold water-induced stress or restraint stress markedly increased production of proinflammatory cytokines of interleukins (IL) 1β and 6, and tumor necrosis factor-alpha (TNF-α) in culture supernatant of LPS-treated peritoneal fluid cells compared to that of non-stressed mice. Similarly, high level of NO was produced in culture supernatant of stressed mice compared to that of non-stressed mice (Fig. 2d).
Figure 2.
Relative production of proinflammatory cytokines in peritoneal cells of mice stressed with cold water. The levels of cytokines IL-1β (a), TNF-α (b) and IL-6 (c) were measured by using ELISA. Nitric oxide concentrations determined using the Greiss reagent (d), IL-10 (e), TNF-α (f), IL-2 (g) and INF-γ (h). SI, stressed/infected; NSI, non-stressed/infected; NSNI, non-stressed/non-infected mice; APC, antigen-presenting cell; CHL, Chlamydia trachomatis antigen (irradiated); NE, norepinephrine. Each point value represents the means ± standard deviation of 2 or 3 wells (n = 5 or 6 mice for each group of experiments). Statistical differences between treatments were evaluated by Student's t test. Asterisk denotes significant statistical differences between the experimental groups at the level of (P ≤ 0.05).
Exposure of naive splenic T cells to NE leads to a reduced production of cytokines
The purpose of this experiment was to examine whether synthetic NE contributes to decreased in vitro cytokine production by T cells. Similar to our previous studies, exposure of mice to cold water-induced stress resulted in an increase in the secretion of IL-10 and TNF-α (Belay and Woart 2013), while addition of synthetic NE to naïve T cells resulted in a reduced production of IL-10 (Fig. 2e), TNF-α (Fig. 2f) and IL-2 (Fig. 2g). T cells isolated from C. muridarum-infected stressed mice showed increased production of IL-2 (Fig. 2g) and INF-γ (Fig. 2h) compared to controls.
Cold water-induced stress increases the intensity of Chlamydia muridarum genital infection during primary infection
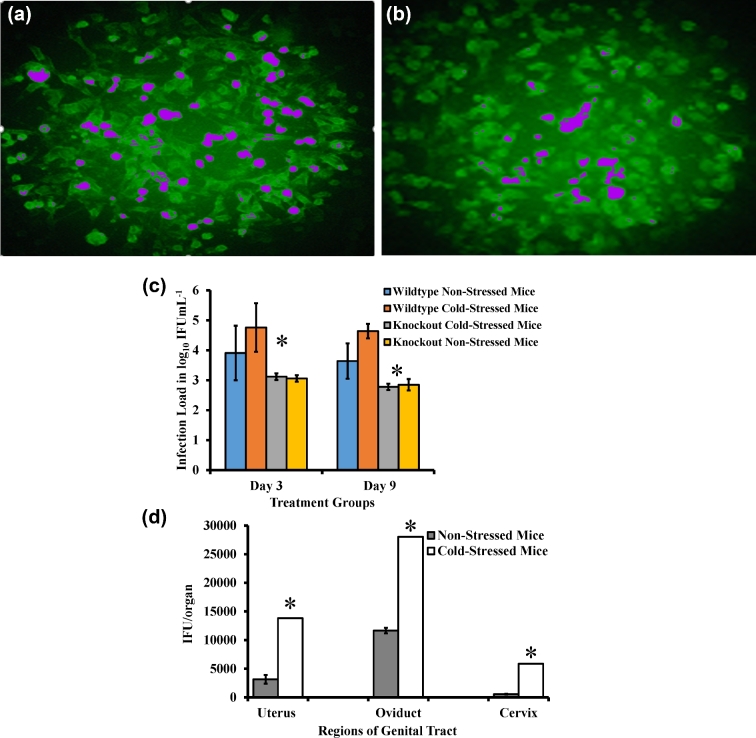
To determine the level of infection in stressed and non-stressed mice, we measured the number of viable C. muridarum present on vaginal swabs collected every 3 days post infection. As shown in Fig. 3a, there are greater numbers of chlamydia inclusion counts in the swabs of stressed mice compared to non-stressed mice (Fig. 3b), indicating that cold-induced stress increases the intensity of C. muridarum genital infection in mice.
Figure 3.
Determination of C. muridarum shedding in the genital tract of wild type and knockout stressed mice. Stressed (a) and non-stressed (b) mice were infected with C. muridarum. Cervicovaginal swabs were collected at 3-day intervals (c). Alternately, cervix, uterus and oviduct sections were harvested after 48 h of infection (d). McCoy mouse fibroblasts were seeded into 96-well plates and incubated at 37°C with 5% carbon dioxide supply until monolayers were 90%–95% confluent. After 32 h of infection, C. muridarum isolation was performed by staining with fluorescence-tagged anti-chlamydia antibodies and inclusion bodies were counted. Each data point is mean ± standard deviation of log10 inclusion forming unit/milliliter representing combined results of two separate experiments (n = 5 to 6 mice per group). False color has been added to (a) and (b) to aid visualization of the inclusion bodies.
Increased Chlamydia muridarum shedding in regions of the genital tract of stressed mice
Since cervical-vaginal swabs do not show the amount of chlamydiæ burden in each region of the genital tract, we also measured number of C. muridarum in tissue homogenates of different genital regions: cervix, uterine horn and oviduct. Similar to Fig. 3a, there are greater numbers of IFUs in the homogenates of stressed mice compared to homogenates of non-stressed mice (Fig. 3c). Although the deposition of C. muridarum was at the cervix, high IFU counts were detected in the oviduct of stressed mice compared to non-stressed mice (P < 0.05). This significant difference in chlamydia shedding shows a rapid spread of C. muridarum to the upper region of the genital tract, suggesting the existence of differential distribution of C. muridarum and disease severity in mouse genital tract.
Deficiency in β1Adr/β2Adr receptors results in reduced shedding of Chlamydia muridarum in the genital tract of stressed mice
To assess the relative role of NE receptors in stress-induced responsiveness to chlamydia genital infection, we examined the susceptibility of a β1Adr/β2Adr receptor KO mouse exposed to cold water-induced stress. We observed that β1Adr/β2Adr receptor-deficient mice shed less C. muridarum IFUs in the genital tract compared to the control wild-type mice (representing the C57BL/6J, DBA/2J and FVB/NJ background strains from which the β1Adr/β2Adr receptor KO strain was derived). The decrease in C. muridarum burden in the genital tract of β1Adr/β2Adr receptor KO mice exposed to cold water-induced stress, as compared to cold water-induced stressed wild type mice, provides an initial indication that an absence of NE receptors reduces the effect of stress on chlamydia genital infection. After euthanizing the mice, necropsy was performed as previously described (Shah et al.2005). As shown in Fig. 3d, IFU counts were significantly higher in the genital tract regions of stressed mice compared to that of non-stressed mice. With respect to the regions, the oviduct showed the highest IFU count, then cervix and uterus. Even though chlamydia deposition was through the cervix, it had a significantly lower IFU count than did the uterus.
Cold water induced-stress enhances leukocyte infiltration into regions of the genital tract of mice during Chlamydia muridarum genital infection
We hypothesized that prolonged cold-induced stress enhances production of immunopathogenic cytokines that cause histopathological changes in the genital tract of mice during chlamydia genital infection. To test the relationship between stress-induced inflammation and chlamydial infection, the cervix, uterine and oviduct tissues were assessed independently for histologically alterations at two, 14 and 42 days during primary infection and at day 15 of secondary infection. As represented in Fig. 4, cold water-induced stress treatment resulted in a marked increase of leukocyte infiltration, especially in the cervix of stressed mice compared to non-stressed mice at day 2 of primary infection. At days 14 and 42 of primary infection, insignificant counts of leukocytes were observed (data not shown). At day 15 of secondary infection, leukocyte infiltration in the cervix was comparable to the primary infection, but the leukocyte counts in the uterine and oviduct was lowered to half the primary infection (Table 1).
Figure 4.
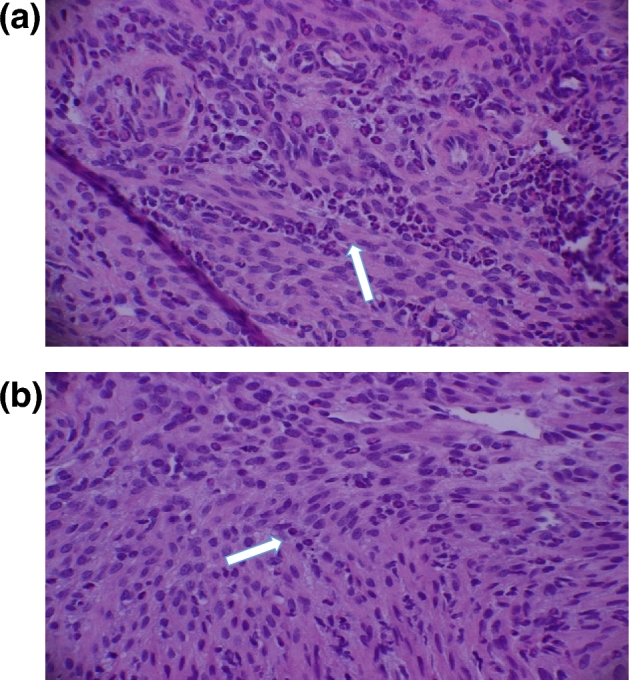
Infiltration of leukocytes to the regions of genital tract of mice during C. muridarum genital infection. Arrows show neutrophils the cervix of stressed mice (a) and non-stressed mice (b) during C. muridarum infection. Mice were stressed, infected as above. After 48 h of infection, mice were euthanized and the tract was aseptically harvested and excised into cervix, uterus and oviduct sections and placed in buffered formalin. The sections were stained with hematoxylin and eosin (H&E) at Marshall University pathology laboratory services.
Table 1.
Effect of cold-induced stress on infiltration of leukocytes to the regions of genital tract of mice during C. muridarum genital infection.
| Treatment groups | ||||
|---|---|---|---|---|
| Infection | Genital | Non-stressed | Non-stressed | Stressed |
| Phasea | Tract | Non-infected | Infected | Infected |
| region | (n = 5) | (n = 10) | (n = 10) | |
| Primaryb | Cervix | 130 ± 42 | 198.5 ± 20 | 154 ± 32 |
| Uterus | 88.5 ± 57 | 65 ± 22 | 133.5 ± 7 | |
| Oviduct | 1 ± 0 | 1 ± 0 | 124 ± 17 | |
| Secondaryc | Cervix | 20 ± 0 | 130 ± 92 | 149.5 ± 21 |
| Uterus | 2 ± 0 | 53 ± 17 | 65 ± 42 | |
| Oviduct | 2 ± 0 | 14 ± 11 | 71.3± 67 | |
Genital tract samples were harvested 48 h after primary infection.
Genital tract samples were harvested 48 h after secondary infection.
Results are the average of two separate experiments.
Representatives of the complete genital tract of stressed and non-stressed mice after 15 days of C. muridarum secondary infection are shown in Fig. 5. Evaluation with naked eyes showed three to four hydrosalpinx in the oviducts and uterine horns of stressed mice compared to a few hydrosalpinx in non-stressed mice. Fluid in the uterine horns and abscesses on the oviducts and inflammatory response (redness) of genital tract were higher in stressed mice than that of non-stressed mice. This study indicates that stress can induce acute inflammation in the cervical region of mice, suggesting that this inflammation might predispose mice to increased susceptibility to chlamydial genital infection and histopathological changes. Although the shedding of C. muridarum and hydrosalpinx development was significant in the oviduct of stressed mice, leukocyte infiltration was modest in uterine and oviducts.
Figure 5.
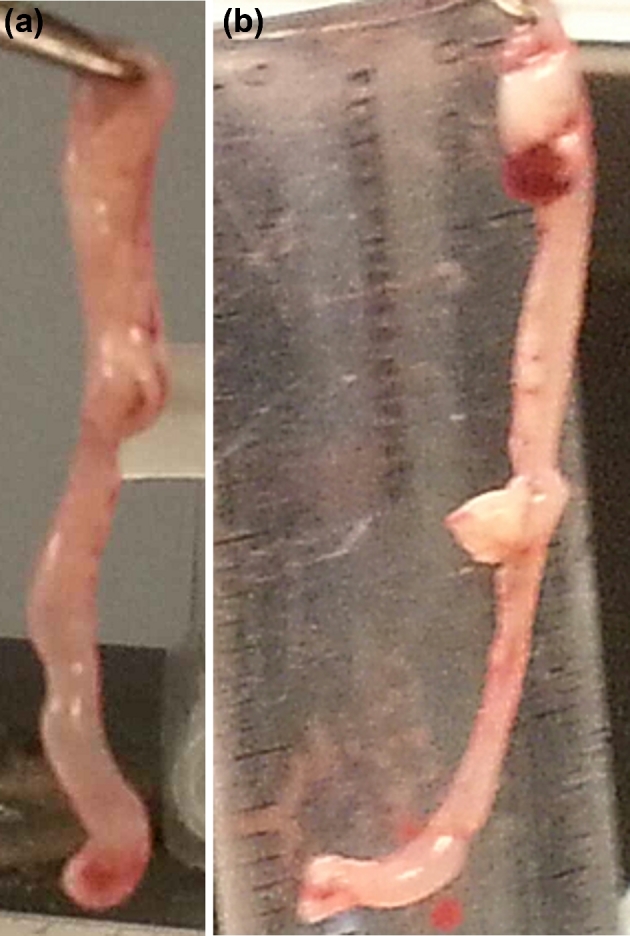
Gross genital tract pathology in stressed and non-stressed C. muridarum infected mice. At day 15 after secondary infection, tissues were removed from mouse for evidence of the number of oviduct hydrosalpinx scored by observing clearly visible with naked eyes. Uterine horn filled with clear fluid and any other related abnormalities of the entire genital tract carried out by gross examination.
Cold water-induced stress enhances Chlamydia muridarum genital infection-induced infertility in mice
This study was undertaken to examine the effect of stress on induction of infertility in a mouse model. After recovery from secondary infection, mice were mated with proven breeder male mice and monitored for fertility for 19–21 days. As shown in Table 2, non-stressed and non-infected mice had 100% fertility rates. Non-stressed but infected mice had a lower fertility rate (84%) but had significantly higher fertility rates than stressed and infected mice (56%) (P < 0.05). All stressed mice had two or three times fewer embryos than control animals, and non-stressed, non-infected mice had significantly more embryos than stressed and infected mice (P < 0.05). This significant impact of cold water-induced stress in combination with chlamydia genital infection on fertility indicates that stress increases infertility complications associated with C. trachomatis genital infection. This study is in agreement with the finding that stressed and C. muridarum infected mice had the least fertility outcome compared to other experimental groups. Overall, mice mated 40 days following secondary infection had a significant decrease in fertility rate compared with the control groups, as shown by a reduction in pregnancy and a decrease in the number of embryos.
Table 2.
Effect of cold water-induced stress on the rate of pregnancies and litter sizes in BALB/c mouse strain after C. muridarum genital infectiona.
| Animal group | Fertility at day 19 | Number of embryos/mouse | Gross appearance of genital tract |
|---|---|---|---|
| Non-stress | 19/19 (100%) | 7 (11b), 8(2 b), 9(6 b) | Normal appearance |
| Non-infected | Average no. of embryos c = 7.74 ± 0.9 | ||
| Non-stressed | 21/25 (84%) | 6 (16 b) to 7 (6 b) | Inflammation and necrosis |
| Infected | Average no. of embryos c = 6.6 ± 0.6 | ||
| Stressed | 14/25 (56%) | 3 (11 b), 3 (6 b) | Inflammation and necrosis |
| Infected | Average no. of embryos c = 3.6 ± 0.8 |
Pregnancy testing was performed after the resolution secondary C. muridarum genital infection. Mating occurred 120 days after primary infection or 40 days after secondary infection
The number in parenthesis denotes the number of mice that had the specified number of embryos.
The average number of embryos of each animal group was obtaining by dividing the total number of embryos over the total number of the mice of the group.
In summary, we have demonstrated that prolonged exposure of mice to cold water-induced stress results in (i) elevated levels of NE and EP in the spleen and genital tract of stressed mice; (ii) elevated IL-1β, TNF-α, IL-6 and NO production in macrophage-rich peritoneal cells of mice; (iii) supplement of NE in vitro exerts an immunosuppressive effect on splenic T-cell production of cytokines; (iv) increased intensity of C. muridarum genital infection; decreased C. muridarum shedding in the genital tract of β1Adr/β2Adr receptor KO mice; and (v) a higher rate of infertility in infected mice.
DISCUSSION
We have investigated the possible role of cold water-induced stress in the outcomes of chlamydia genital infection and subsequent complications in a mouse model to help understand the differential incidence and disease severity of chlamydia genital infection in humans. The significance of this study intends to expand a mounting evidence that stress increases susceptibility to infections by modulating the immune system in animal models, which may be applicable in humans.
Evidence in the literature shows that, depending on the duration and timing, cold stress has either immunosuppressive effects leading to decreased resistance to infection or enhancement in immune response (Yin et al.1996). In establishing our study, preliminary experiments indicated that application of cold water-induced stress at least for 8 days increased productions of interferon gamma by splenic T cells, suggesting enhancement of the immune response. These observations led us to adopt extending the stressing frequency of the mouse model for 5 min every day for 24 days in order to establish chronic stress. This was a crucial step because many published studies reported that long-term stress suppresses the ability of the immune system to fight viral, bacterial, fungal and parasitic infections (Yin et al. 1996; Kubera et al.1998). All data presented in this study were from mice subjected to chronic stress compared to non-stressed mice.
Similarly, preliminary experiments included two stressing methods: cold and restraint. Initial results were that the restraint method of stressing produces comparable results to cold stressing. In an effort to simplify the experiment and reduce the number of experimental groups, and thus the number of mice, required for testing, we needed to select one stressing method. We chose the cold-stress method because it was observationally clear that even after repeated exposure, the mice were experiencing discomfort, whereas with the restraint stress it seemed that repeated stressing induced habituation, as the mice would no longer struggle in the restraint syringe. Unless indicated to be restraint stress, data presented in this study were from mice subjected to cold stress.
Previous studies in a mouse model showed a correlation between susceptibility to chlamydia genital infection and increased stress hormone production in response to cold water-induced stress application (Belay and Woart 2013). Our previous results also revealed decreased production of stimulatory cytokines, such as IFN-γ, and chemokines after cold-stress treatment, which are consistent with previous reports that exposure of mice to cold water leads to a decrease in the secretion of cytokines (Aviles and Monroy 2001). These findings prompted us to investigate further the impact of cold water-induced stress on immune response, pathological changes and fertility following chlamydia genital infection in the mouse model we developed.
Our current data indicate that cold water-induced stress modulates production of proinflammatory cytokines and nitric oxide by peritoneal macrophages. These results are consistent with previous reports that exposure of mice to cold water leads to an increase in the secretion of proinflammatory cytokines, suggesting that cold-water treatment of mice stimulates the release of peptide substance that may alter the function of peritoneal macrophages (Zhu et al. 1996). Whether the increased production of proinflammatory cytokines by peritoneal cells favors protection or disease remains to be determined. The increased production of proinflammatory cytokines by peritoneal macrophages and failure to clear Chlamydia muridarum suggests that these cytokines probably are involved in the development of immunopathology, rather than protection. There is a possibility that catecholamines may actually enhance IL-1β, TNF-α and primarily IL-6 productions that are detrimental effects of proinflammatory cytokines. Previous studies showed that altered macrophages and dysregulated macrophage function may well contribute to the generalized suppression of the immune response in the host as described (Cheng et al. 1990). Although produced by CD8+ T cells, it has been demonstrated that TNF-α contributes significantly to upper genital tract pathology in mice (Murthy et al.2011).
The present data also show that exposure of mice to cold water-induced stress leads to elevated shedding of chlamydia, indicating suppression of the immune system and subsequently leading to infection that is more progressive. Our results are consistent with other reports that stress suppresses the ability of the immune system to fight viral, bacterial, fungal and parasitic infections (Bonneau 1996; Borghetti et al. 2009;; Zieziulewicz et al.2013). However, the mechanism(s) involved in the increased intensity of the infection process are not well defined.
We hypothesized that NE through binding to androgenic receptors expressed on immune cells leads to decreased resistance to chlamydia genital infection. Our results revealed that the catecholamines, EP and NE, were significantly more expressed in the lymphoid and genital tract tissues of stressed, compared to non-stressed mice. Because increased intensity of chlamydia genital infection was associated with high levels of stress in stressed mice (Belay and Woart 2013), we questioned whether mice deficient in adrenergic receptors would develop reduced infection. We infected stressed β1Adr/β2Adr receptor KO mice with C. muridarum and found that the NE receptor-deficient mice developed low burden of C. muridarum compared to the stressed wild type, indicating that the receptors are associated with suppressing the immune response against C. muridarum infection. In addition, stress-mediated suppression of host immune response against Chlamydia and the increased susceptibility of the animals may be due to a lack of recruitment and activation of inflammatory cells by chemotactic factors (Mormède et al. 2000; Simhan et al.2003). Our results appear to be substantiated by documented studies that sympathetic nerves in the lymphoid organs release the stress hormone, NE, which stimulates the β2Adr receptor expressed on CD4+ and B cells, leading to modulation of the immune system (Yin et al.1996; Swanson, Lee and Sanders 2001; Shimizu et al.2005). Interestingly, our results also showed that NE in vitro significantly alters the production of different cytokines possibly exerting an immunosuppressive effect on proliferation of T cells. We believe that the increased release of cytokines from T cells of stressed mice compared to naïve T cells is a result of combined influences such as other stress hormones compared to action of NE.
Because T cells express the β2Adr, which is the receptor of NE, we decided to examine the influence of synthetic NE on cytokine production. To determine whether synthetic NE has an inhibitory effect on T cells, we evaluated the proliferation of T cells supplemented with synthetic NE in vitro. Different cytokine production profiles in vitro and in vivo are obtained in this study. Although the findings in vivo and in vivo are difficult to compare, it is important to note that the effects observed in vivo could be a result of a combined influence from other hormones such as corticosteroids (Ziemessen et al. 2007). Compared to the in vitro action of synthetic NE, a number of cellular and molecular events and mechanisms, such as coordination with different signaling pathways and/or transcription factors that occur in vivo, may influence the level of cytokine production. As previously stated, the actual effects of NE on lymphocytes can be blocked by other types of immune cells (Slota et al.2015). NE appears to play an important role in modulating T-cell function, and thus further studies are underway utilizing T lymphocyte subsets to have a better understand the impact of NE on these cells.
Inoculation of C. muridarum intravaginally showed persistent infiltration of polymorphonuclear leukocytes into the cervix and a rapid spread to the upper regions of the genital tract as shown in IFU counts. Our results demonstrate that stress worsens the pathological outcomes, as well as increased infertility outcomes following chlamydia genital infection.
These results confirm that the rate and extent of the acute inflammatory infiltrate corresponds with severe pathological outcomes (Patton and Kuo 1989; Shah et al.2005; Danville 2010). Increased infiltration of neutrophils shows active inflammation, whereas challenged mice displayed significant decreases in tissue infiltration of inflammatory leukocytes, with marked reduction in the frequency of neutrophils. Moreover, previous studies have reported that large infiltration of neutrophils in the genital tract is directly related to increased burden of C. muridarum in the genital tract (Maxion et al.2004). Greater infiltration of polymorphonuclear cells in the cervical tissue, a large number of viable chlamydia IFU, more hydrosalpinx in the oviduct and dilatation of the uterus with clear fluid were observed in stressed mice compared to controls. This shows that the immune response is insufficient to clear C. muridarum in the genital tract of stressed mice.
One objective of the study was to test the hypothesis that cold-induced stress enhances infertility in infected mice. We showed that chlamydia genital-infected mice were more infertile with the cold-induced stress treatment, whereas unstressed controls were fertile. This is in agreement with previous studies (Tuffrey, Alexander and Taylor-Robinson 1990). Our results are also consistent with previous results in decreased fertility and premature delivery in mice (de la Maza et al.1994). Previous studies have shown that the fertility of BALB/c strains following chlamydia genital infection was not different from that of controls (Tuffrey et al. 1992). Our results, however, showed that stress enhances chlamydia-induced infertility, which is consistent with previous observations that cold water-induced stress modulates cytokine production that subsequently leads to inhibition of host defense against C. muridarum.
Although several studies have demonstrated that C. muridarum infection results in pathological outcomes and infertility, the current study is the first report of its kind that stress likely worsens pathological disease and increases chlamydia-induced infertility in mice. The results of this study showed a greater number of hydrosalpnix linked with low rate of fertility in stressed group compared to non-stressed C. muridarum-infected mice. The poor fertility outcome exhibited in C. muridarum-infected stressed mice could be linked to the negative impact of hydrosalpnix in the host. Our results are in agreement with previous reports that hydrosalpnix fluid inhibits in vitro fertilization and embryonic development in a mouse model (Beyler et al. 1997). We speculate that stress is a problem and may play a major role in enhancing acute inflammation during chlamydia genital infection that leads to worsened pathology and infertility in our mouse model.
Despite the fact that C. trachomatis is the causative agent of humans, we used the mouse agent, C. muridarum, in this study for the following reasons. Studies show that inoculation of C. trachomatis in mice does not cause productive infection, nor does it cause reproductive pathologies as observed following C. muridarum infections in mice (Darville et al.1997; Lyons et al.2005). Furthermore, C. trachomatis in mice may not directly mimic C. trachomatis infections in human because C. trachomatis and C. muridarum have evolved host-specific mechanisms for IFN-gamma evasion (tryptophan synthetize and GTPase, respectively (Nelson et al.2005). It is true that human and mouse immunology differs with respect to downstream IFN-gamma signaling that controls chlamydial replication. Such features are only produced by the naturally adapted pathogen in mice, C. muridarum. Moreover, studies have shown that primary genital infection of mice with C. trachomatis induces immunity against challenge with E and L human biovars (Ramsey et al. 1999; Igietseme et al. 2009). Thus, the mouse strain, C. muridarum has been used as a model organism for the study of human C. trachomatis urogenital and respiratory tract infections (Shao et al.2012; De Clercq, Kalmar and Vanrompay 2013). Several studies of the mouse immune system have led to significant advances in our knowledge of the human immune system. While we agree that no model is perfect, extensive literature presents little evidence to suggest that immunological findings made using C. muridarum infection in mice is vastly different from those that occur with C. trachomatis infections in human. The closeness of the mouse and human serovars has been described, and thus the results of our studies in mice might extend to what happens in humans that are stressed and exposed to C. trachomatis, which could explain the increased incidence of chlamydial genital infection in some populations.
In conclusion, the initial observations in this study could serve as the basis for designing studies to assess in detail how stressful conditions may promote susceptibility to chlamydia genital infection and disease onset, the associated immunological alterations and immunomodulatory mechanisms. We feel that this study is important because psychological or physical stressors may lead to increased stress hormone production and have profound effects on health, including prevalence of chlamydia genital infection. Evidence shows that these stressors generally are greater in populations of lower socioeconomic status, in which there are increased health concerns. However, this study is preliminary and does not fully define the underlying similarities between our animal model and humans. The present finding, taken together within previous observations (Belay and Woart 2013) indicates that cold-water induced stress increases the intensity of chlamydia genital infection in mice. Furthermore, the present results show that stress increases disease progression, contributing to pathology and increased chlamydia-induced infertility. The long term of our study will employ the cold water-induced stress in a β2Adr receptor KO mouse model, and the hypothesis to be tested is that cold water-induced stress leads to NE modulation of the immune response against C. muridarum genital infection by enhancing the production of immunopathogenic cytokines that result in disease sequelae. We believe that this study may offer important direction for further investigation of the mechanisms of stress on chlamydia genital infection and immunity in humans.
Acknowledgments
The authors are grateful to Dr Joseph Igiesteme; at the Centers for Disease Control and Prevention, for his expertise and consultation in the Chlamydia project and for providing us Chlamydia stock cultures throughout the study. The authors are also thankful to Dr Guy Sims's Title III Office at Bluefield State College for the financial assistance to prepare the manuscript. The authors are also very thankful to Dr Julie Kalk, Associate Professor of Physics and MS. Beth Hash at Bluefield State College, for editing our complete manuscript.
FUNDING
This work was supported by National Institution of Health (NIH) grant P20GM103434 to the West Virginia IDeA Network Biomedical Research Excellence (WV-INBRE), awarded to Bluefield State College, and National Institute of Allergy and Infectious Diseases at NIH grant R15AI124156 awarded to Bluefield State College.
Conflict of interest. None declared.
REFERENCES
- Aviles H, Belay T, Vance M et al. . Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions. J Appl Physiol 2004;97:1437–44. [DOI] [PubMed] [Google Scholar]
- Aviles H, Johnson MT, Monroy FP. Effects of cold stress on spleen cell proliferation and cytokine production during chronic Toxoplasma gondii infection. Neuroimmunomodulation 2004;11:93–102. [DOI] [PubMed] [Google Scholar]
- Aviles H, Monroy FP. Immunomodulatory effects of cold stress on mice infected intraperitoneally with a 50% lethal dose of Toxoplasma gondii. Neuroimmunomodulation 2001;9:6–12. [DOI] [PubMed] [Google Scholar]
- Beatty WL, Morrison RP, Byne GI. Persistent Chlamydia: form cell culture to a paradigm for Chlamydial pathogenesis. Microbiol Rev 1994;58:686–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay T, Eko FO, Ananaba GA et al. . Chemokine and chemokine receptor dynamics during genital chlamydia infection. Infect Immun 2002;70:844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay T, Woart A. Cold-induced stress increases the intensity of Chlamydia genital infection in mice. J Microbiol Immunol 201345:330–7. [DOI] [PubMed] [Google Scholar]
- Beyler SA, James KP, Fritz MA et al. . Hydrosalpingeal fluid inhibits in-vitro embryonic development in a murine model. Hum Reprod 1997;12:2724–28. [DOI] [PubMed] [Google Scholar]
- Bonneau RH. Stress-induced effects on integral immune components involved in herpes simplex virus (HSV)-nonspecific memory cytotoxic T lymphocyte activation. Brain Behav Immun 1996;10:139–63. [DOI] [PubMed] [Google Scholar]
- Borghetti P, Saleri R, Mocchegiani E et al. . P. Infection, immunity and the neuroendocrine response. Vet Immunol Immunop 2009;130:141–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP et al. . International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev 1994;46:121–36. [PubMed] [Google Scholar]
- Cain TK, Rank RG. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun 1995;63:1784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center of Disease Control: National surveillance Data for Chlamydia, Gonorrhea, and Syphills. Published in CDC's report, Sexually Transmitted Disease Surveillance, 2008, www.cdc.gov/std/stats(18 April 2017, date last accessed). [Google Scholar]
- Cheng GJ, Morrow-Tesch JL, Beller ID et al. . Immunosuppression in mice induced by cold water stress. Brain Behav Immun 1990;4:278–91. [DOI] [PubMed] [Google Scholar]
- Cohen CR, Brunham RC. Pathogenesis of Chlamydia induced pelvic inflammatory disease. Sex Transm Infect 1999;75:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter TW, Ramsey KH, Miranpuri GS et al. . Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun 1997;65:2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danville T. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 2010;Suppl 2:S114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville T, Andrews CW, Laffoon K et al. . Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun 1997;65:3065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun 2013;81:3060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza LM, Pal S, Khamesipour A et al. . Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 1994;62:2094–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen JM, Kohn RP, Bolan GA et al. . Socioeconomic differences in sexually transmitted disease rates among black and white adolescents, San Francisco, 1990 to 1992. Am J Public Health 1995;85:1546–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Papanicolaou DA, Wilder RL et al. . Modulatory effects of glucocoricoids and catecholamines on human interlukin-12 and interleukin-10 production: clinical implication. P Assoc Am Physician 1996;108:474–81. [PubMed] [Google Scholar]
- Goyarts E, Matsui M, Mammone T et al. . Norepinephrine modulates human dendritic cell activation by altering cytokine release. Exp Dermatol 2008;17:188–96. [DOI] [PubMed] [Google Scholar]
- Haggerty C, Gottlieb S, Taylor B et al. . Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010;201(Suppl 2):S134–5. [DOI] [PubMed] [Google Scholar]
- Igietseme JU, Eko FO, Black CM. Chlamydia vaccines: recent developments and the role of adjuvants in future formulations. Expert Rev Vaccines 2011;10:1585–96. [DOI] [PubMed] [Google Scholar]
- Igietseme JU, He Q, Joseph K et al. . Role of T lymphocytes in the pathogenesis of Chlamydia Disease. J Infect Dis 2009;200:926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igietseme JU, Portis JL, Perry LL. Inflammation and Clearance of Chlamydia trachomatis in enteric and non-enteric mucosae. Infect Immun 2001;69:1832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen specific T cells in the genital tract. Infect Immun 1991;59:1346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Schon K, Ward M et al. . Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun 1997;65:1032–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AK, Walker JC, Jameel SH et al. . Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia trachomatis. Infection Infect Immun 2000;68:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm PA, Tang Y, Sanders VM et al. . Activation of antigen-specific CD4+Th2 cells and B cell in vivo increases norepinephrine release in the spleen and bone marrow. J Immunol 2000;165:725–33. [DOI] [PubMed] [Google Scholar]
- Kubera M, Basta-Kaim A, Holan V et al. . Effect of mild chronic stress, as a model of depression, on the immunoreactivity of C57BL/6 mice. Int J Immunopharmaco 1998;20:781–9. [DOI] [PubMed] [Google Scholar]
- Lorton D, Bellinger DL. Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci 2015;16:5635–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM, Ito JI, Peña AS et al. . Differences in growth characteristics and elementary body associated cytotoxicity between Chlamydia trachomatis oculogenital serovars D and H and Chlamydia muridarum. J Clin Pathol 2005;58:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neuralmodulation of immune responsiveness. Annu Rev Pharmacol 1995;35:417–48. [DOI] [PubMed] [Google Scholar]
- Maxion HK, Liu W, Chang MH et al. . The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection. Infect Immun 2004;72:6330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormède C, Castanon N, Médina C et al. . Chronic mild stress in mice decreases peripheral cytokine and increases central cytokine expression independently of IL-10 regulation of the cytokine network. Neuroimmunomodulation 2000;10:359–66. [DOI] [PubMed] [Google Scholar]
- Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 1995;63:4661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK, Li W, Chaganty BK et al. . Tumor necrosis factor alpha production form CD8+ T cell mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun 2011;79:2928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Hayano Y, Furuta F et al. . Control of lymphocyte egress form lymph nodes through β2-adrenergic receptors. J Exp Med 2014;211:2583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Virok DP, Wood H et al. . Chlamydial IFN-gamma immune evasion is linked to host infection tropism. P Natl Acad Sci USA 2005;102:10658–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Edusei K, Chesson WH, Leichliter JS et al. . The association between racial disparity in income and reported sexually transmitted infections. Am J Public Health 2013;103:910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Hui W, Peterson EM et al. . Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J Med Microbiol 1998;47:599–605. [DOI] [PubMed] [Google Scholar]
- Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol 2010;36:349–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Kuo CC. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil 1989;85:647–56. [DOI] [PubMed] [Google Scholar]
- Patton DL, Kuo CC, Wang SP et al. . Chlamydial infection of subcutaneous fimbrial transplants in cynomolgus and rhesus monkeys. J Infect Dis 1987;155:229–35. [DOI] [PubMed] [Google Scholar]
- Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper cells through INF-gamma-dependent and independent pathways. J Immunol 1997;158:3344–352. [PubMed] [Google Scholar]
- Ramer-Quinn DS, Baker RA, Sanders VM. Activated Th1 and Th2 cells differentially express the b2-adrenergic receptor: a mechanism for selective modulation of Th1 cell cytokine production. J Immunol 1997;15:4857–456. [PubMed] [Google Scholar]
- Ramsey KH, Cotter TW, Salyer RD et al. . Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect Immun 1999;67:3019–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG. Animal models of urogenital infections. Methods Enzymol 1994;235:83–93. [DOI] [PubMed] [Google Scholar]
- Rank RG. Models of immunity. In: Stephens RS. (ed). Chlamydia: Intracellular Biology, Pathogenesis And Immunity. Washington, DC: ASM Press, 1999, 239–95. [Google Scholar]
- Rank GR, Bowlin AK, Kelly KA. Characterization of lymphocyte response in the female genital tract during ascending chlamydial genital infection in the guinea pig model. Infect Immun 2000;68:5293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, Sanders MM, Patton DL. Increased incidence of oviduct pathology in the guinea pig after repeat vaginal inoculation with the chlamydial agent of guinea pig inclusion conjunctivitis. J Sex Transm Dis 1995;22:48–54. [DOI] [PubMed] [Google Scholar]
- Rober SO, Obermeier LM, Straub HR et al. . Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology 2006;147:4968–76. [DOI] [PubMed] [Google Scholar]
- Sanders M, Kasprowicz DJ, Swanson-Mungerson MA et al. . Adaptive immunity in mice lacking the beta (2)-adrenergic receptors. Brain Behav Immun 2003;17:55–67. [DOI] [PubMed] [Google Scholar]
- Sanders M, Virginia M. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun 2012;26:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Schripsema JH, Imtiaz MT et al. . Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 2005;32:49–56. [DOI] [PubMed] [Google Scholar]
- Shao R, Wang X, Wang W et al. . From mice to women and back again: causalities and clues for Chlamydia-induced tubal ectopic pregnancy. Fertil Steril 2012;98:1175–85. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Dobbs C, Brown D et al. . Psychoneuroimmunology: stress effects on pathogenesis and immunity during infection. Clin Microbiol Rev 1994;7:200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Kaizuka Y, Hori T et al. . Immobilization increases norepinephrine release and reduces NK cytotoxicity in spleen of conscious rat. Am J Physiol-Reg I 2005;271:R537–44. [DOI] [PubMed] [Google Scholar]
- Simhan N, Caritis N, Krohn A et al. . Decreased cervical proinflammatory cytokines permit subsequent upper genital tract infection during pregnancy. Am J Obstet Gynecol 2003;189:560–57. [DOI] [PubMed] [Google Scholar]
- Slota C, Shi A, Chen G et al. . Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun 2015;46:168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon GF. Psychoneurology: interaction between central nervous system and immune system. J Neurosci Res 1987;18:1–9. [DOI] [PubMed] [Google Scholar]
- Stamm WE. Chlamydia trachomatis infections of the adult. in: Holmes KK, et al. (eds). Sexually Transmitted Diseases. New York: McGraw Hill, 1999, 407–22. [Google Scholar]
- Swanson MA, Lee WT, Sanders VM. INF-gamma production by Th1 cells generated form naïve CD4+ T cells exposed to Norepinephrine. J Immunol 2001;166:232–40. [DOI] [PubMed] [Google Scholar]
- Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol 1990;71:403–10. [PMC free article] [PubMed] [Google Scholar]
- Tuffrey M, Alexander F, Woods C et al. . Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil 1992;95:31–8. [DOI] [PubMed] [Google Scholar]
- Westrom L, Joesoef R, Reynolds G et al. . Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparocacople results. Sex Trans Dis 1992;19:185–92. [PubMed] [Google Scholar]
- Yin D, Tuthill D, Mufson RA et al. . Chronic restraint stress promotes lymphocyte apoptosis by modulating Cd95 expression. J Exp Med 1996;191:1423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FG, Chancellor-Freeland C, Berman AS et al. . Endogenous substance P mediates cold water stress-induced increase in interleukin-6, peritoneal macrophages. J Neurosci 1996;16:3745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemessen T, Mondal TK, Gao D et al. . Psychoneuroimmunology-cross-talk between the immune and nervous system. J Neurol 2007;254(Suppl 2):II8–11. [DOI] [PubMed] [Google Scholar]
- Zieziulewicz T J, Mondal TK, Gao D et al. . Stress-induced effects, which inhibit host defenses, alter leukocyte trafficking. Cell Stress Chaperones 2013;18:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]