Abstract
Carbohydrate structures on the cell surface encode complex information that through specific recognition by carbohydrate-binding proteins (lectins) modulates interactions between cells, cells and the extracellular matrix, or mediates recognition of potential microbial pathogens. Galectins are a family of ß-galactoside-binding lectins, which are evolutionary conserved and have been identified in most organisms, from fungi to invertebrates and vertebrates, including mammals. Since their discovery in the 1970s, their biological roles, initially understood as limited to recognition of endogenous carbohydrate ligands in embryogenesis and development, have expanded in recent years by the discovery of their roles in tissue repair and regulation of immune homeostasis. More recently, evidence has accumulated to support the notion that galectins can also bind glycans on the surface of potentially pathogenic microbes, and function as recognition and effector factors in innate immunity, thus establishing a new paradigm. Furthermore, some parasites ‘subvert’ the recognition roles of the vector/host galectins for successful attachment or invasion. These recent findings have revealed a striking functional diversification in this structurally conserved lectin family.
Keywords: pattern recognition receptors; galectins, ß-galactoside; carbohydrate recognition domain: glycans; structure; function; proto-type; chimera; tandem repeat
In this minireview, we discuss the structural basis of carbohydrate recognition by galectins, an evolutionary conserved family of ß-galactoside-binding lectins, and their binding and effector properties for endogenous (‘self’) and exogenous (‘non-self’) glycosylated ligands.
INTRODUCTION
The immediate recognition of distinct (‘non-self’) structures on the surface of potential pathogens and parasites is one of the key steps of innate immune responses. Among these microbial surface moieties, complex carbohydrates such as viral envelope glycoproteins, bacterial lipopolysaccharides and exopolysacharides, and various surface glycans from eukaryotic parasites encode rich information that is ‘decoded’ by the hosts’ carbohydrate-binding proteins (lectins) (Vasta and Ahmed 2008). The lectin-glycan interaction can trigger a series of downstream events that include activation of signaling pathways that promote phagocytosis and intracellular killing, and activation of prophenoloxidase and complement pathways that can promote phagocytosis and killing of the potential pathogen (Vasta et al. 2007). Thus, lectins are critical components of innate immune mechanisms in both invertebrates and vertebrates. However, unlike immunoglobulins and T-cell receptors which generate diversity in recognition by genetic recombination, lectins are ‘hard-wired’ in the germline, which is a feature shared by most innate immune receptors. Therefore, one of the outstanding questions is how the innate immune system is able to cope with the great diversity of potential microbial infectious challenges. Although the concept of pattern recognition proposes that only a handful of microbial conserved surface molecules need to be recognized for successful innate immune defense, the highly diversified microbial communities to which all organisms are exposed to, and the dynamic changes in surface expression components suggests that a substantial diversity in non-self-recognition mechanisms may be required for immune protection. The detailed analysis of the structural basis of lectin–ligand binding and the diversity and complexity of the lectin repertoires in taxa that lack adaptive immunity, such as invertebrates, strongly suggests that this is the case (Vasta et al. 2007, 2012). In this regard, the ongoing genome, transcriptome and proteome projects in non-mammalian organisms have provided a more realistic assessment of the diversity and complexity of the lectin repertoire in each species. This information, coupled to forward and reverse genetic approaches implemented in model organisms amenable to genetic manipulation, has contributed further insight into our understanding of their diversity in immune recognition and detailed functional aspects (Vasta et al. 2007, 2012).
Most soluble or membrane-associated lectins are oligomers of covalently or non-covalently bound peptide subunits, each characterized by the presence of one or more carbohydrate recognition domains (CRDs) (Taylor and Drickamer 2003). Based on the presence of conserved amino acid sequence motifs and structural fold of the CRD, and requirement of divalent cations or a reducing environment for ligand binding, lectins have been classified in several major families, such as C-, P-, X- and I-types, galectins (formerly S-type), heparin binding and others (Vasta and Ahmed 2008). The F-type lectin family (Bianchet et al. 2002; Odom and Vasta 2006; Vasta et al. 2008, 2012) is the most recently identified lectin family (Drickamer 2017, http://www.imperial.ac.uk/research/animallectins/), whereas the acute phase reactants C-reactive protein and serum amyloid P are now considered members of the pentraxin lectin family (Bottazzi et al. 2006).
Lectins can recognize endogenous ligands such as glycocoproteins, polysaccharides and glycolipids from the cell surface or the extracellular matrix (ECM) and promote cell–cell and cell–ECM interactions (Taylor and Drickamer 2003; Vasta and Ahmed 2008). Through these interactions, lectins can modulate various developmental processes, survival, proliferation and metastasis of cancer cells, and regulate immune homeostasis (Vasta and Ahmed 2008). Lectins can also decode ‘modified’ endogenous glycans, such as those displayed by tumor cells, and recognize foreign ‘non-self’ glycans on the surface of microbial pathogens or parasites (Vasta et al. 2007; Vasta and Ahmed 2008). Thus, the efficient, specific, discrimination between ‘self’, ‘modified-self’ and ‘non-self’ that is achieved by recognizing structural information encoded by cell surface sugar moieties constitutes a critical component of innate immune mechanisms (Ley and Kansas 2004; Barrionuevo et al. 2007). Among the several lectin families established so far, the critical roles of galectins in both adaptive and innate immunity responses, as well as their co-option by microbial pathogens for host entry, have recently generated great interest (Vasta 2009, 2012). This review article will discuss the multiple functions of galectins based in self and non-self-recognition, with emphasis on current research studies in our laboratory.
GALECTINS: BIOCHEMICAL, STRUCTURAL AND EVOLUTIONARY ASPECTS
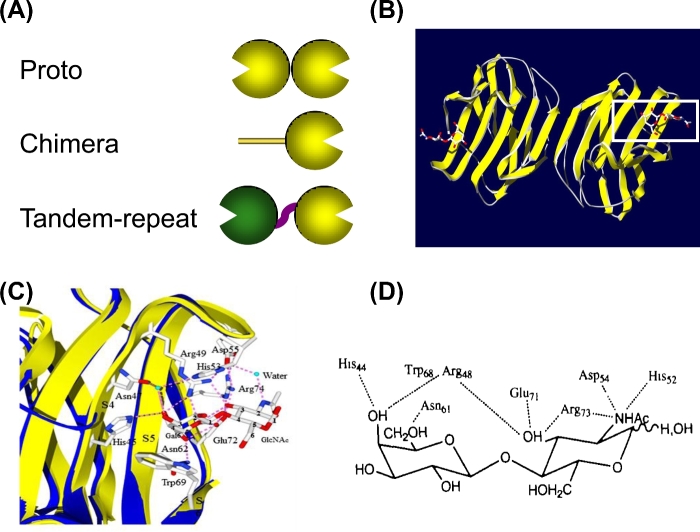
Galectins constitute a family of ß-galactoside-binding proteins characterized by a unique CRD sequence motif, that are ubiquitous and structurally conserved in eukaryotic taxa, including fungi, sponges and both invertebrates and vertebrates (Cooper 2002) (Fig. 1). Most galectins are non-glycosylated soluble proteins, although a few recently discovered exceptions have transmembrane domains (Lipkowitz et al. 2004). Galectins are synthesized in the cytosol, and some can be translocated into the nucleus (Fig. 2). Furthermore, although galectins lack a typical secretion signal peptide, they can be secreted into the extracellular space by direct translocation across the plasma membrane (Cleves et al. 1996). Based on structural features, mammalian galectins have been classified into three types: ‘proto’, ‘chimera’ and ‘tandem repeat’ (TR) (Hirabayashi and Kasai 1993) (Fig. 1). Proto-type galectins are non-covalently linked homodimers of peptide subunits that contain a single CRD. The chimera galectins have an N-terminal domain rich in proline and glycine with the CRD on the opposite C-terminal region. TR galectins display two CRDs that are bridged by a functional linker peptide. Galectins from invertebrates may have unique structural features, such as galectins with four CRDs per polypeptide described in clams, oysters and snails (Tasumi and Vasta 2007; Feng et al. 2013, 2015a; Kurz et al. 2013; Vasta et al. 2015), or the galectin MjGal from the shrimp Marsopenneus japonicus that resembles a chimera-type galectin but with the CRD located at the N-terminal end (Shi et al. 2014). Mammalian galectins comprise up to 15 distinct galectin subtypes, numbered following the order of their discovery: galectins-1, -2, -5, -7, -10, -11, -13, -14 and -15 are proto-type; galectin-3 is the only chimera-type, and galectins-4, -6, -8, -9 and -12 are TR type (Hirabayashi and Kasai 1993; Vasta and Ahmed 2008).
Figure 1.
Structural aspects of mammalian galectins. (A) Schematic display of three types of mammalian galectins. (B) Structure of the galectin-1 in complex with LacNAc (indicated by the white box) at 1.9 Å resolution. (C) Carbohydrate-binding site of galectin-1. Three continuous concave strands (S4–S6) contain all residues involved in direct interactions with LacNAc, plus additional interactions with His52, Asp54 and Arg73 bridged by a water molecule. (D) Schematic representation of interactions between amino acid residues of galectin-1 and LacNAc. (Adapted from Liao et al.1994 and Bianchet et al.2000).
Figure 2.
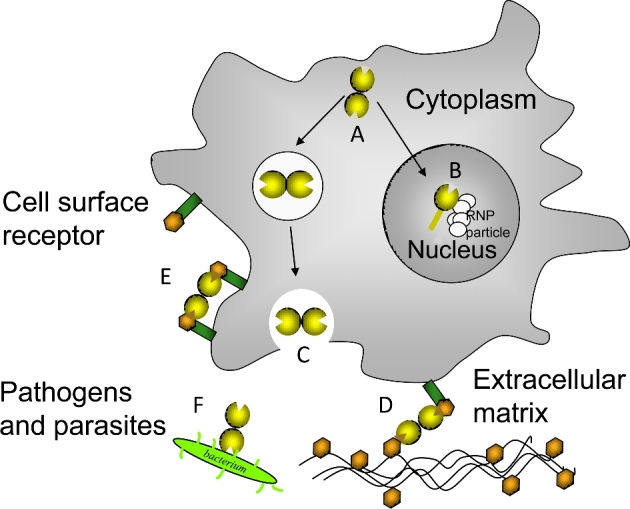
Multiple functions of galectins. Galectins are synthesized in the cytosol (A), and some can be translocated into the nucleus and interact with RNP particles (B). Most galectins are transported and secreted to the extracellular space (C), where they interact with glycans in the ECM (D), bridge the cell surface ligands (E) or function as PRRs by recognizing microbial pathogens (F).
Resolution of the structure of the galectin-1 in complex with N-acetyllactosamine (LacNAc) at 1.9 Å resolution revealed the galectin structural fold as a jellyroll topology typical of legume lectins, and enabled the identification of the amino acid residues that participate in interactions with the ligand, as well as the position and orientation of the sugar hydroxyls that interact with those amino acids (Liao et al. 1994; Bianchet et al. 2000) (Fig. 1). The galectin-1 subunit consists of an 11-strand antiparallel ß-sandwich, in which the single carbohydrate-binding site is formed by three continuous concave strands (ß4–ß6) that contain all residues involved in direct interactions with LacNac. Additional interactions involving a water molecule that bridges the nitrogen of the NAc group with His52, Asp54 and Arg73 explains the higher affinity of LacNAc over Lac (Liao et al. 1994; Bianchet et al. 2000). Thermodynamic approaches have enabled not only the rigorous assessment of the galectins’ carbohydrate-binding affinity but also the oligomeric organization of the protein. Based on microcalorimetry, the dissociation constants for the interactions of bovine galectin-1 with the preferred ligands (Lac, LacNAc, thiodigalactoside) were in the range of 10−5 M, with two binding sites per molecule (Schwarz et al. 1998). The dimerization of proto-type galectins such as galectin-1 is critical for their function in mediating cell–cell or cell–ECM interactions (Gabius 1997), and similar interactions via the N-terminus domain have been proposed for the chimera galectins such as galectin-3 (Colnot et al. 1997, 2001). However, unlike galectin-1, galectin-3 has an extended carbohydrate-binding site formed by a cleft open at both ends, thus enhancing affinity for glycans with multiple lactosamine units (polylactosamines), and with their substitution of the non-reducing terminal galactose moiety with ABH blood group oligosaccharides [Fucα1, 2; GalNAcα1,3(Fucα1,2); and Galα1,3(Fucα1,2)] (Seetharaman et al. 1998). For galectins from invertebrates, such as the Caenorhabditis elegans 16-kDa galectin and the oyster (Crassostrea virginica) galectins CvGal1 and CvGal2, the shorter length of the loop 4 determines its broader binding specificity for blood group oligosaccharides (Ahmed et al. 2002; Feng et al. 2013; Vasta et al. 2015) (Fig. 3).
Figure 3.
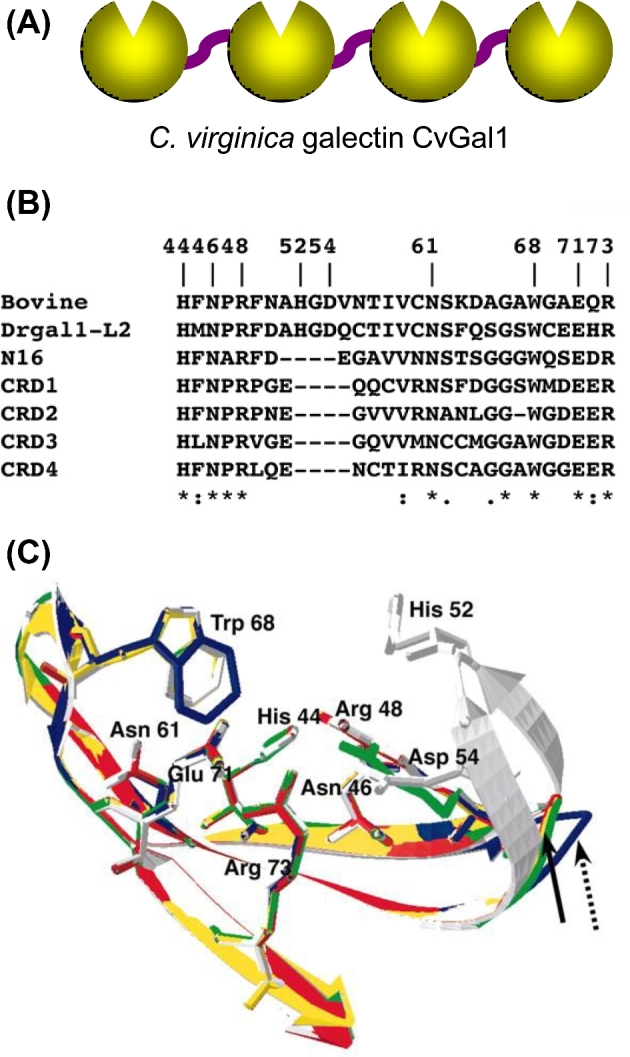
Structural aspects of galectins from invertebrates. (A) Schematic representation of the CRD organization of the galectin of CvGal1 from eastern oyster (C. virginica). (B) Sequence alignment of the carbohydrate-binding regions from bovine galectin-1, zebrafish Drgal1-L2, C. elegans N16 and the CRDs-1 to -4 of CvGal1. (C) Homology modeling of the CRDs from CvGal1: bovine galectin-1(white); CvGal1: CRD-1, -2, -3 and -4 are shown in blue, yellow, red and green, respectively. Numbering of amino acid residues is based on bovine galectin-1. The solid arrow shows loop 4 of CvGal1 CRD-2, 3 and 4, whereas a dashed arrow shows loop 4 of CRD-1. (Adapted from Tasumi and Vasta 2007 and Feng et al. 2013).
The discovery of galectin-like proteins in the fungus Coprinopsis cinerea and in the sponge Geodia cydonium revealed the early emergence and structural conservation of galectins in eukaryotic evolution along the lineages leading to the mammals (Saouros et al. 2005; Walser et al. 2005; Stalz et al. 2006). It is important to keep in mind that although galectins are considered a conserved lectin family, most species are endowed of a complex galectin repertoire, with members exhibiting multiple isoforms and more or less subtle variations in carbohydrate specificity, which together with a certain degree of plasticity in sugar binding of each CRD, suggests a substantial diversity in recognition properties (Sato and Hughes 1992; Vasta and Ahmed 2008; Vasta et al. 2012). In contrast, galectin-like proteins such as the mammalian lens galectin-related inter-fiber protein (GRIFIN) and the HSPC159 (hematopoietic stem cell precursor) lack carbohydrate-binding activity, and are considered products of evolutionary co-option (Ogden et al. 1998; Ahmed and Vasta 2008).
MAMMALIAN AND NON-MAMMALIAN ANIMAL MODELS FOR FUNCTIONAL STUDIES ON GALECTINS
It has been widely recognized in recent years that the use of murine models for the investigation of the biological roles of galectins has produced invaluable information. However, the use of non-mammalian animal models such as invertebrates [Drosophila (Pace et al. 2002); Caenorhabditis elegans (Dodd and Drickamer 2001; Ahmed et al. 2002)], protochordates [the sea squirt Ciona intestinalis (Azumi et al. 2003)] and fish [zebrafish (Danio rerio) (Vasta et al. 2004; Kirchmaier et al. 2015); medaka (Oryzias latipes) (Kirchmaier et al. 2015)] as alternative model organisms amenable to genetic manipulation has provided further insight into the biological function(s) of galectins (Ahmed et al. 2008). In addition to enabling the implementation of comparative approaches towards our understanding of the gene organization, structural aspects and biochemical properties of galectins, the aforementioned species offer multiple advantages over the murine models. Among these, their short generation time, the transparency of their embryos or larval stages and the possibility to carry out loss-of-function experiments in whole body systems have been key to the popularity of their use (Ahmed et al. 2008). Furthermore, comprehensive genetic toolboxes and large collections of mutants and transgenic lines have been developed for these alternative models, greatly facilitating functional research studies. The explosive increase in recent years in the availability of genome and transcriptomic public databases for invertebrates and ectothermic vertebrates of commercial or environmental relevance has also enabled the use of species such as oysters and shrimp as models for unraveling the roles of galectins in infectious disease (Tasumi and Vasta 2007; Feng et al. 2013, 2015a; Kurz et al. 2013; Shi et al. 2014; Vasta et al. 2015).
FUNCTIONS OF GALECTINS BASED ON THE RECOGNITION OF ENDOGENOUS (‘SELF’) GLYCANS
Glycans that contain N-acetyllactosamine chains [(Galβ1,4GlcNAc)n], such as laminin, fibronectin, lysosome-associated membrane proteins and mucins, are the preferred endogenous ligands for galectins on the cell surface and organelles and in ECM (Gabius 1997; Seetharaman et al. 1998; Elola et al. 2007) (Fig. 4A). The biological function of a particular galectin, however, may vary from site to site, depending on the availability of suitable ligands, and the redox properties of any particular intra- or extracellular microenvironment (Lobsanov et al. 1993; Liao et al. 1994; Bianchet et al. 2000). In solution, galectins can form multivalent species in a concentration-dependent equilibrium (Morris et al. 2004). Proto-type galectins associate as non-covalently bound dimers via a hydrophobic interphase, whereas galectin-3 associates via its N-terminal domain to form oligomers (trimers and pentamers) that in the presence of multivalent oligosaccharides in solution or at the cell surface display binding cooperativity (Brewer, Miceli and Baum 2002; Dam and Brewer 2008). The bivalent TR-type galectins can recognize different saccharide ligands with a single polypeptide, although they can also form higher order aggregates that enhances their avidity. Furthermore, the binding of galectins to cell surface ß-galactoside-containing glycolipids and glycoproteins can lead to the formation of lattices that cluster these ligands into lipid raft microdomains required for activation and optimal transmission of signals relevant to cell function (Brewer, Miceli and Baum 2002; Rabinovich et al. 2007; Dam and Brewer 2008). Galectin-mediated lipid raft assembly at the cell surface may function as an ‘on/off switch’ that regulates turnover of endocytic receptors, signal transduction pathways leading to T-cell activation and cytokine secretion, or apoptosis, B-cell maturation activation and tolerance and neutrophil activation leading to phagocytosis, oxidative burst and cytokine release (Brewer, Miceli and Baum 2002; Rabinovich et al. 2007; Dam and Brewer 2008).
Figure 4.
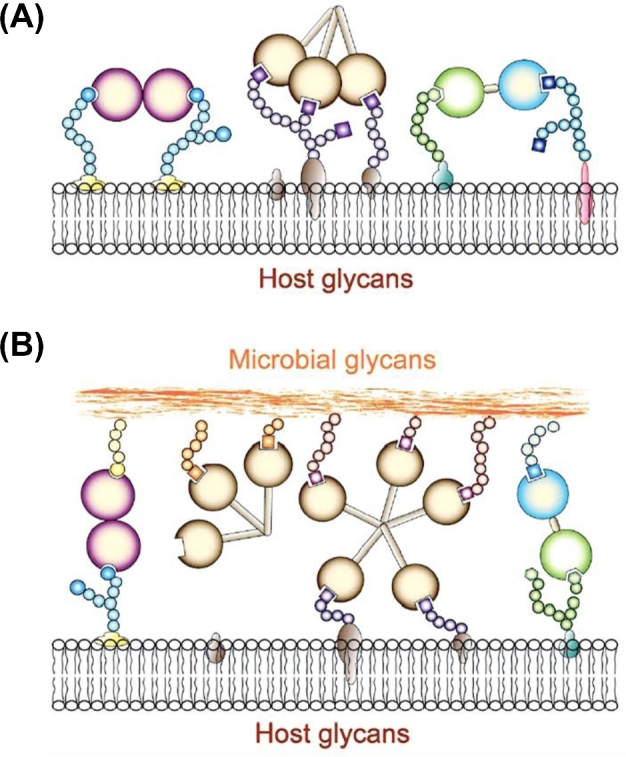
Recognition of ‘self’ and ‘non-self’ glycans by galectins. (A) In the extracellular space, galectins form multivalent oligomers that cross-link cell surface glycoproteins and glycolipids, form microdomains and activate signaling pathways. (B) Proto, chimera and tandem-repeat galectins can function as PRRs and establish transinteractions with the host cell surface and microbial glycans.
Early development and tissue regeneration
The initial description of galectins in chicken muscle as developmentally regulated proteins led to the proposal that their functions were related to embryogenesis and early development. Furthermore, the binding of chicken galectins to ‘self’ ligands such as polylactosamines abundant at the myoblast surface and the ECM suggested that they participate in myoblast fusion (Colnot et al. 1997, 2001; Gabius 1997). Later studies on murine galectin-1 and galectin-3 suggested roles in notochord development, somitogenesis, and development of muscle tissue and central nervous system (Cooper, Massa and Barondes 1991). Despite the increasing availability of genetically modified mice, however, some strains carrying null mutations for selected galectins only display subtle developmental phenotypes and the assignment of well-defined biological roles has not been straightforward (Colnot et al. 1997, 2001), and this was interpreted as functional redundancy or compensatory effects that would take place among the members of the galectin repertoire. More recently, however, as their binding properties and natural ligands are rigorously characterized, it has become clear that this is not the case, and their unique biological roles are being elucidated in increasing detail. For example, recent studies have unraveled the critical role of galectin-1 in angiogenesis, as the basis for resistance of certain tumors to otherwise effective anti-VEGF (vascular endothelial growth factors)-targeted therapy (Croci et al. 2014). Nevertheless, other genetically tractable model organisms endowed with a less diversified galectin repertoire such as Drosophila and zebrafish have become attractive alternatives for these selected galectins, with promising results (Pace et al. 2002; Ahmed et al. 2004; Vasta et al. 2004; Feng et al. 2015b).
Our current studies on the developmental roles of galectins using the zebrafish model were initiated by the characterization of the galectin repertoire in this species (Ahmed et al. 2004). We first identified and characterized the ontogenic expression and tissue distribution of galectin-1-like proteins: Drgal1-L1, Drgal1-L2, Drgal1-L3 and one splice variant of Drgal1-L2. Drgal1-L1 is maternal; Drgal1-L2 is zygotic and strongly expressed in the notochord; and Drgal1-L3 is both maternal and zygotic (Ahmed et al. 2004). Knockdown experiments in zebrafish embryos using a morpholino-modified antisense oligonucleotides targeted to the 5΄-UTR sequence of Drgal1-L2 resulted in a phenotype with a bent tail and disorganized muscle fibers. This phenotype was dose dependent and absent in Drgal1-L1 knockdown embryos, indicating that the observed effects are sequence-specific. Furthermore, ectopic expression of native Drgal1-L2 specifically rescued the phenotype (Vasta et al. 2004; Feng et al. 2015b). In vertebrates, the notochord is the primary source of signaling molecules required for patterning of the adjacent tissues, such as neural tube, heart and circulatory system, and the somites. Thus, these observations suggest that Drgal1-L2 expressed by the notochord cells plays a key role in differentiation and development of the myotome (Vasta et al. 2004; Feng et al. 2015b).
We also identified a homolog of the GRIFIN in zebrafish (designated DrGRIFIN), which like the mammalian equivalent is expressed in the lens, particularly in the fiber cells of 2 dpf (days post fertilization) embryos (Ahmed and Vasta 2008). In striking contrast with the mammalian counterparts, however, in adult zebrafish DrGRIFIN is also expressed in oocytes, brain and intestine. Most importantly, DrGRIFIN actively binds carbohydrates (preferentially blood group B type II oligosaccharides) and thus, behaves as a typical galectin. As silencing DrGRIFIN expression in embryos resulted in a significantly smaller lens, we hypothesize that in teleost fish GRIFIN modulates the differentiation of progenitor cells in the early eye lens structures by binding to cell surface glycans and activating the signaling pathways involved in lens development (Ahmed and Vasta 2008). We also proposed that through evolution along lineages leading to mammals, GRIFIN's carbohydrate- and protein-binding properties, as well as its spatio-temporal expression, became more restricted based on structural and functional constraints (Ahmed and Vasta 2008).
Studies on the zebrafish also revealed that galectins not only participate in early development but also in regeneration of injured adult tissues. Experimentally light-induced retinal injury in adult zebrafish was used in combination with an antisense knockdown approach to demonstrate that Drgal1-L2 is the first secreted factor in the retina shown to regulate aspects of regenerative neurogenesis. DrGal1-L2 is induced by photoreceptor cell death and secreted by stem cells and neuronal progenitors in the Müller glia, and selectively regulates the regeneration of rod photoreceptors but not the cones (Craig et al. 2010).
Cancer
The expression of galectins can be significantly altered upon neoplastic transformation. Some tumors such as melanoma, prostate and ovarian cancer show overexpression of selected galectins, particularly galectin-1, -3, -7, -8 and -9, and these profiles may correlate with malignancy stage, aggressiveness or metastatic potential (Hill et al. 2010; Blidner et al. 2015). For example, melanomas can display enhanced expression and secretion of galectin-1, which through its immunosuppressive and apoptotic effects for T cells can contribute to establish an immuneprivileged environment that enables tumor survival (Blidner et al. 2015). Furthermore, recent studies using galectin-1 knockout cell lines and mice have shown that expression of galectin-1 in tumor cell endothelium is essential for tumor angiogenesis. The mechanisms elucidated are based on the binding of galectin-1 to complex N-glycans on VEGF receptor 2 (VEGFR2), and galectin-1-mediated activation of VEGF-like signaling (Croci et al. 2014). Thus, galectins can play important roles in tumor progression and metastasis through indirect effects in regulating tumor immune responses and direct effects in tumor angiogenesis (Croci et al. 2014).
In other tumors, galectin expression can be downregulated or completely silenced by promoter methylation. For example, in the malignant prostate epithelial cell line LNCaP, expression of galectin-3 is silenced by high cytidine methylation of its promoter, as demonstrated by pre-treatment with azacytidine. This methylation is not observed in normal prostate cells, or cells from other tissues. Furthermore, the galectin-3 promoter is highly methylated in biopsies from early stages of prostate adenocarcinoma but not in those from benign prostate hyperplasia or normal prostate tissue. Based on this observation, a very reliable, highly sensitive and specific diagnostic assay for prostate cancer was developed and validated. Furthermore, that assay was adapted for use in plasma and urine samples, constituting a powerful tool for non-invasive diagnosis of prostate cancer at those early stages in which may be clinically undetectable (Ahmed, Banerjee and Vasta 2007; Ahmed et al. 2009; Ahmed 2010).
Galectin-3 also has a key role in metastasis of prostate adenocarcinoma. The Thomsen-Friedenreich disaccharide (TFD, Galβ1,3GalNAc), a preferred ligand of galectin-3, is present on the surface of most cancer cells and promotes angiogenesis, tumor-endothelial cell adhesion and metastasis of prostate cancer cells, as well as evading immune surveillance through killing of activated T cells. A glycopeptide from cod antifreeze protein (designated TFD100) that binds galectin-3 with picomolar affinity can also block galectin-3-mediated angiogenesis, tumor-endothelial cell interactions and metastasis of prostate cancer cells at nanomolar levels in a murine model. Thus, this high-affinity TFD100 should be a promising antimetastatic therapeutic treatment of prostate adenocarcinoma (Guha et al. 2013).
Adipogenesis and type 2 diabetes
Recently, the key roles of galectin-3 and galectin-12 in adipogenesis and their association with obesity, inflammation and type 2 diabetes have been characterized (Yang et al. 2011b; Pang et al. 2013; Pejnovic et al. 2013). Galectin-3 knockout mice display age-dependent increased adiposity, dysregulated glucose metabolism and systemic inflammation, including accelerated diabetes-associated kidney damage and diet-induced atherogenesis. In addition, these mice display proinflammatory changes in visceral adipose tissue and pancreas, the latter including infiltration of macrophages expressing NLRP3 inflammasome and IL-1β in pancreatic islets, accumulation of AGE, upregulation of RAGE expression and increased TNFα, IL-1β, IL-18 and IL-6. In contrast, galectin-12 is expressed in adipocytes and associated with lipid droplets, and functions as a negative regulator of lipolysis and insulin sensitivity. Galectin-12 knockout mice show increased lipolysis and reduced adiposity, suggesting that galectin-12 controls cAMP levels at the lipid droplet, increases protein kinase A signaling and modulates the recruitment or function of a phosphodiesterase (Yang et al. 2011b; Pang et al. 2013; Pejnovic et al. 2013).
Immune regulation
During the past decade, evidence has accumulated to support the critical roles of galectins in the regulation of both innate and adaptive immune homeostasis, as well as responses to infectious and allergic challenge, and cancer (Liu, Yang and Hsu 2012; Rabinovich, van Kooyk and Cobb 2012). Galectins are ubiquitously expressed and distributed in mammalian tissues, including most cells of the innate (dendritic cells, macrophages, mast cells, natural killer cells, gamma/delta T cells and B-1 cells) and adaptive (activated B and T cells) immune system, and as in other cell types (Stowell et al. 2008; Rabinovich, van Kooyk and Cobb 2012). Although the expression of galectins in animal tissues is tightly regulated, their expression can be modulated by a variety of stimuli, including infectious challenge, and this may be especially important in innate immune responses. For example, both galectin-1 and galectin-3 are upregulated in gastric epithelial cells that are infected by Helicobacter pylori. Galectin expression can also be modulated during infection by HIV, paramixovirus, herpesvirus 1, Newcastle and Epstein–Barr virus (reviewed by Vasta 2009). A recent report indicates that dengue infection of cells in vitro downregulates expression of galectin-1 in several different human cell lines (Toledo et al. 2014). Whether their expression is enhanced or attenuated upon infectious challenge, galectins can modulate neutrophil, macrophage, and B and T-cell function, thereby regulating host innate and adaptive immunity.
Galectins released by stromal and immune cells can oligomerize and form lattices at the cell surface through binding and clustering of polylactosamine-rich cell surface glycoconjugates into segregated membrane microdomains, leading to activation of transmembrane signaling pathways that modulate differentiation of immune cell precursors and diverse cell functions such as cell adhesion and migration, T-cell apoptosis, Th1/Th2 cytokine balance and mast cell degranulation (Liu, Yang and Hsu 2012; Rabinovich, van Kooyk and Cobb 2012). Galectins can have opposite roles as pro- or anti-inflammatory factors in innate immune responses. For example, galectin-1 has pro-apoptotic activity and functions as an anti-inflammatory agent by blocking or attenuating signaling events that lead to leukocyte infiltration, migration and recruitment (Stowell et al. 2008). In contrast, galectin-3 is normally expressed in various epithelia and inflammatory cells, such as activated macrophages, dendritic cells and Kupffer cells, and is upregulated during inflammation, cell proliferation and cell differentiation. Galectin-3 also exhibits anti-apoptotic activity for macrophages and enhances their interactions with basal lamina glycans, such as laminin and fibronectin, positively modulating their recruitment and antimicrobial activity (Hsu and Liu 2008; Liu, Yang and Hsu 2012). Recent studies in galectin-3 knockout mice show a link between galectin-3 and cardiomyopathy and cardiac fibrosis, conditions that are associated with heart failure (Filipe et al. 2015). Galectin-9 is highly expressed in various tissues of the immune system, such as bone marrow, spleen, thymus and lymph nodes, and functions as a selective chemoattractant for eosinophils, inducing their activation, oxidative activity and degranulation (Hirashima et al. 2004).
The roles of galectins as regulators of adaptive immune responses have been the focus of intense research in the past few years (Liu, Yang and Hsu 2012; Rabinovich, van Kooyk and Cobb 2012). Interactions between stromal cells from the bone marrow and thymic compartments and lymphocyte precursors are critical to their development, selection and further progression to the periphery (Rossi et al. 2006). Galectin-1 can regulate T-cell proliferation and apoptosis depending on their developmental stage and activation status, and the microenvironment in which the exposure takes place. The effects of galectin-3 in T-cell survival, however, are dependent on whether protein is produced endogenously (anti-apoptotic) or by exogenous exposure (pro-apoptotic) (Hsu and Liu 2008). Furthermore, the effects of galectins on T-cell cytokine synthesis and secretion ultimately determine the Th1/Th2 polarization of the immune response. For example, by reducing IFN-γ and IL-2 and enhancing IL-5, IL-10 and TGF-β production, galectin-1 skews the balance from a Th1- toward a Th2-polarized response, whereas by reducing IL-5 levels, galectin-3 has the opposite effect (Hsu and Liu 2008; Liu, Yang and Hsu 2012; Rabinovich, van Kooyk and Cobb 2012). Galectin-1-knockout mice show a ‘hyper-Th1 and Th17 response’ when challenged by antigens in vivo. They also exhibit heightened sensitivity to experimental autoimmune encephalomyelitis associated with elevated levels of Th1 and Th17 lymphocytes (Chen et al. 2014). Galectin-1 can also induce tolerogenic phenotypes in dendritic cells via their production of IL-27 and expansion of IL-10-secreting regulatory T cells that can suppress autoimmune neuroinflammation or promote fetomaternal tolerance (Blois et al. 2007). Therefore, the regulatory roles of galectins on immune homeostasis can lead to beneficial or detrimental effects on pathological conditions that have a basis on exacerbated or depressed immune function, such as inflammatory, allergic and autoimmune disorders, and cancer.
RECOGNITION OF EXOGENOUS (‘NON-SELF’) GLYCANS BY GALECTINS: A NEW PARADIGM
Most recently, the realization that galectins can bind exogenous (‘non-self’) glycans on the surface of viruses, bacteria, protista and fungi has led to a new paradigm about their potential roles as pattern recognition receptors (PRRs) (Vasta 2009) (Fig. 4B). This has been supported by our greater appreciation of the considerable diversity of the galectin repertoire in each organism and subtle variations in the specificity of each galectin towards the target glycans, which suggest an extensive diversity and plasticity in the capacity of galectins for non-self-recognition. Furthermore, because galectins can form oligomers, their multivalent binding properties, including increased avidity and cross-linking capacity, clearly enable galectins to participate in direct recognition of pathogens and parasites, and as effector factors in downstream processes that lead to modulation of innate and adaptive immune responses. However, whether galectin-mediated pathogen recognition has a clear benefit for the host as an effective defense mechanism is not entirely clear, except for a few examples (Vasta 2009).
Galectins as PRRs and effector factors in innnate immunity
A recent report that clearly illustrates galectin functions as a recognition and effector factor beneficial for host defense is the galectin MjGal from the kuruma shrimp Marsopenneus japonicus. This galectin is upregulated in hemocytes and hepatopancreas upon bacterial infection, and can bind to both Gram-positive and Gram-negative bacteria through the recognition of lipoteichoic acid and lipopolysaccharide, respectively. By also binding to the shrimp hemocyte surface, MjGal functions as an opsonin, promoting the phagocytosis of potentially pathogenic bacteria. Furthermore, as shown in vivo by RNA interference, MjGal participates in clearance of bacteria from circulation, and thereby contributes to the shrimp's immune defense against infectious challenge (Shi et al. 2014).
During infection by Nipah virus (NiV), a paramyxovirus that infect both humans and animals, the viral envelope glycoproteins attach and fuse to the host endothelial cells through their ephrinB2 or ephrinB3 receptors, also promoting cell–cell fusion and syncytia formation, causing endothelial disruption and hemorrhage (Levroney et al. 2005). Galectin-1 specifically cross-links the N glycans displayed in the NiV envelope glycoproteins and reduces cell–cell fusion, thereby attenuating the pathophysiological effects of NiV infection (Levroney et al. 2005). However, the beneficial effects of galectin-1 in NiV infection are conditioned by the timing of the virus–galectin interaction (Garner et al. 2015).
Galectin-1 can also bind to the envelope glycoprotein of influenza A virus (IAV) and reduce infection severity, although the mechanisms involved have not been fully elucidated yet (Yang et al. 2011a). Similarly, an in vitro study reported that galectin-1 can directly interact with dengue virus (DENV), a mosquito-transmitted enveloped RNA virus that can cause hemorrhagic fever. Galectin-1 directly binds to DENV and inhibits in vitro viral adhesion and internalization into host cells (Toledo et al. 2014). The role of galectin-1 was also examined in vivo using galectin-1 knockout mice, and demonstrated that the expression of endogenous galectin-1 contributes to resistance against DENV infection (Toledo et al. 2014). Recently, we have used the zebrafish model to examine the roles of galectins in viral adhesion and entry by the infectious hematopoietic necrosis virus (IHNV), which is responsible for significant losses in both farmed and wild salmon and trout populations (Nita-Lazar et al. 2016). Although IHNV enters the host through the skin at the base of the fins, the viral adhesion and entry mechanisms are not fully understood (Harmache et al. 2006). Results of the study showed that the zebrafish galectins Drgal1-L2 and Drgal3-L1 interact directly with the glycosylated envelope of IHNV and with the glycans on the fish epithelial cell surface, significantly reducing viral adhesion (Nita-Lazar et al. 2016).
In mammals, the TR galectins-4 and -8, which are expressed in the intestinal tract, can specifically recognize and kill Escherichia coli strains that display B-blood group oligosaccharides (BGB+ E. coli), while other E. coli strains or bacterial species are not affected (Stowell et al. 2010). The killing activity of both galectins is mediated by their C-terminal domains, and appears to be caused by disruption of integrity of the bacterial cell surface (Stowell et al. 2010). Mutation of key residues in either CRD revealed that the C-CRD mediates recognition the BGB+ E. coli but does not affect its viability, while the N-CRD might be endowed with killing activity (Stowell et al. 2010). Taken together, the results of these studies indicate that galectins can function not only in immune recognition but also as effector factors in innate immune responses against pathogens.
Subversion of the roles of galectins in innate immunity by pathogens and parasites
Although as indicated above, galectins can function as effective recognition and effector factors against viral and bacterial infection, some pathogens and parasites can ‘subvert’ the galectins’ defense roles, and use them for adhesion to or to gain entry into the host cells (Tasumi and Vasta 2007; Vasta 2009). For example, galectin-1 promotes infection by HIV-1 by facilitating viral attachment to CD4 receptor, and increasing infection efficiency by shortening the time required to establish an infection. Furthermore, galectin-1 would also function as a soluble scavenger receptor and enhance the uptake of the virus by macrophages (Sato et al. 2012). Although expression of galectin-3 is upregulated by the HIV Tat protein in several human cell lines, this galectin has no effect on HIV-1 adsorption, entry or infection (Fogel et al. 1999). This observation highlights the relevance of the subtle differences in galectin specificity and affinity that may determine the very different pathogen recognition outcomes. As recently reported, galectin-9 can also enhance HIV entry, albeit by an indirect mechanism (Bi et al. 2011; Merani, Chen and Elahi 2015). On Th2 cells, galectin-9 binds cell surface protein disulfide isomerase (PDI), increasing retention of PDI on the cell surface and altering the redox status at the plasma membrane. It has been established that cell surface PDI regulates integrin function on platelets and also enhances susceptibility of T cells to infection with HIV. Thus, the binding of galectin-9 to PDI on Th2 cells enhances cell migration via β3 integrins, and potentiates infection with HIV (Bi et al. 2011). It is noteworthy that HIV also uses recognition by DC-SIGN, a C-type lectin, to enter dendritic cells, thereby underscoring the multiple adaptations of the viral glycome for host infection via protein–carbohydrate interactions (Sato et al. 2012).
In the zebrafish–IHNV infection model described above, different members of the galectin repertoire can display opposite functions. While the proto-type galectin Drgal1-L2 and the chimera-type galectin DrGal3-L1 can inhibit viral adhesion to epithelial cells (Nita-Lazar et al. 2016), the TR galectin DrGal9-L1 can significantly promote viral adhesion and infection (Mancini et al, unpublished). For the NiV infection of human endothelial cells, however, the same galectin can have opposite effects depending on the timing of the virus–galectin interaction and determine the outcome of the viral infection. Although galectin-1 can inhibit syncytium formation and reduce production of progeny virus (Levroney et al. 2005), a recent study revealed that if galectin-1 is present during the initial phase of virus attachment, it can also enhance NiV adhesion to and infection of the endothelial cell surface by bridging glycans on the viral envelope to host cell glycoproteins (Garner et al. 2015).
Infection by eukaryotic parasites can also be modulated by the galectins of hosts and vectors, and this can be illustrated by Leishmania species, which spend part of their life cycle in the phlebotomine sandfly vectors for transmission to the vertebrate hosts. Leishmania amastigotes attach to the insect midgut epithelium via the sandfly galectin PpGalec that binds to the Gal(ß1-3) side chains on the Leishmania LPG, to prevent their excretion along with the digested bloodmeal and differentiate into free-swimming infective metacyclics (Kamhawi et al. 2004). Another interesting study identified galectin-1 as the receptor for the protozoan parasite Trichomonas vaginalis, the causative agent of the most prevalent non-viral sexually transmitted human infection in both women and men (Okumura, Baum and Johnson 2008). Trichomonas displays a surface LPG rich in galactose and N-acetyl glucosamine, which is recognized in a carbohydrate-dependent manner by galectin-1 expressed by the epithelial cells in the cervical linings, as well as placenta, prostate, endometrial and decidual tissue, also colonized by the parasite (Okumura, Baum and Johnson 2008). Similarly, the protozoan parasite Perkinsus marinus, a facultative intracellular parasite of the eastern oyster Crassostrea virginica, is recognized via the oyster's 4-CRD galectins CvGal1 and CvGal2 that recognize and promote phagocytosis of the parasite. The selective recognition of potentially pathogenic bacterial species such as Aeromonas spp., Carnobacterium spp., Streptococcus spp., Bacilus spp. and Vibrio spp. that may be internalized during filter feeding of phytoplankton suggests that the oyster CvGals may opsonize, phagocytose and kill the bacteria by the hemocyte respiratory burst, and digest them in the phagosome compartment by the lysosomal enzymes as part of the oyster's defense mechanisms. CvGal1 also selectively and specifically recognizes microalgal species such as Tetraselmis spp., an abundant phytoplankton component in the oyster's diet internalized by filter feeding, suggesting that CvGal1 can not only function as cell surface receptors or as opsonins for phagocytosis of bacterial pathogens but also for microalgal food. Therefore, the Perkinsus parasites may have co-evolved their glycocalyx to subvert the defense and feeding roles of the oyster galectins by being competitively recognized and phagocytosed by the oyster hemocytes where they inhibit respiratory burst and proliferate, eventually causing systemic infection and death of the oyster host (Tasumi and Vasta 2007; Feng et al. 2013, 2015a; Kurz et al. 2013; Vasta et al. 2015).
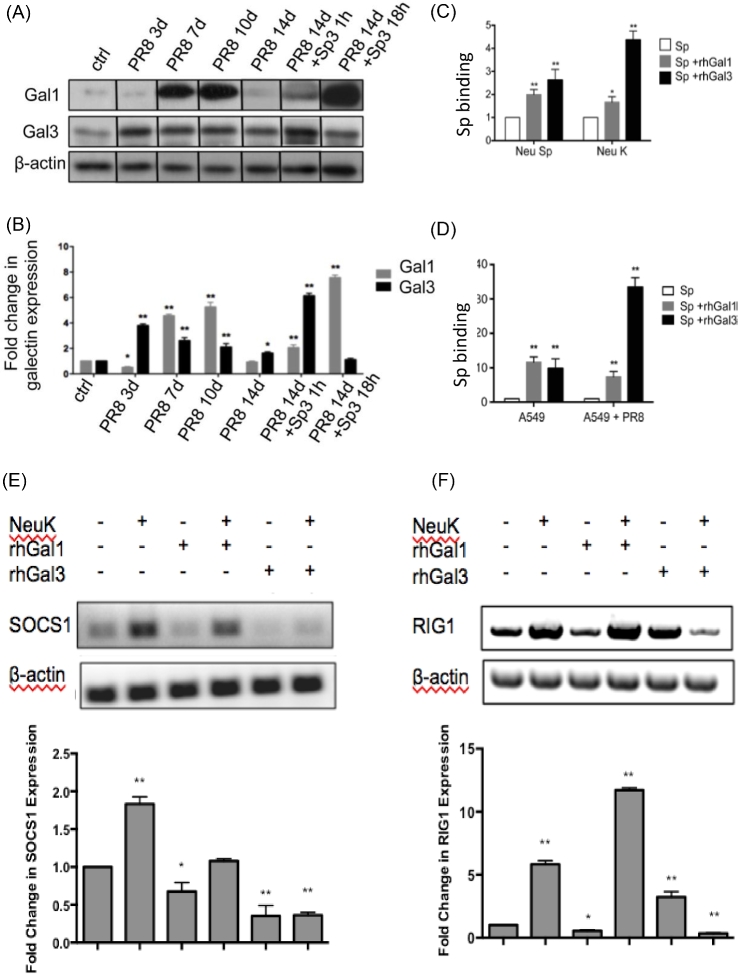
Recently, a murine model for influenza A infection and pneumococcal pneumonia revealed that the neuraminidases from IAV and Streptococcus pneumoniae significantly desialylate the airway epithelial surface and modulate expression and release of galectin-1 and galectin-3 to the bronchoalveolar space (Nita-Lazar et al.2015a) (Fig. 5). In vitro studies on the human airway epithelial cell line A549 were consistent with the observations made in the mouse model, and further revealed that galectin-1 and galectin-3 bind strongly to IAV and S. pneumoniae, and that exposure of the cells to viral neuraminidase or influenza infection increased galectin-mediated S. pneumoniae adhesion to the cell surface (Nita-Lazar et al. 2015a). Thus, these observations suggest that upon influenza infection, pneumococcal adhesion to the airway epithelial surface is enhanced by an interplay among the host galectins and viral and pneumococcal neuraminidases, and may be a contributing factor to the hypersusceptibility to pneumonia observed in influenza patients (Nita-Lazar et al. 2015a). Furthermore, the study revealed that the binding of secreted galectin-1 and galectin-3 to the epithelial cell surface modulates the expression of SOCS1 and RIG1, and activation of ERK, AKT or JAK/STAT1 signaling pathways, leading to a disregulated expression and release of proinflammatory cytokines (Nita-Lazar et al. 2015b). These results suggest that desialylation of the airway epithelial surface may act as a ‘danger signal’ that leads to rapid upregulation of SOCS1 expression to prevent an uncontrolled inflammatory response. While the binding of extracellular galectin-1 to the galactosyl moieties unmasked on the surface of airway epithelial cells can ‘fine-tune’ this process, the binding of galectin-3 can severely disregulate the cytokine response and lead to sepsis (Nita-Lazar et al.2015b).
Figure 5.
Galectins and microbial neuraminidases modulate bacterial adhesion and expression of SOCS1 and RIG1. (A and B) Mice were challenged with a sublethal dose of influenza PR8 strain (PR8) followed by a S. pneumoniae (Sp3) challenge 14 days afterwards. (A) Transcript levels of galectin-1 (Gal1) and galectin-3 (Gal3) were assessed by RT-PCR in broncho-alveolar lavage collected from control (unchallenged) or challenged groups of mice at indicated time points. (B) Bar graphs represent fold change of galectin transcription compared to control mice after normalized to β-actin. (C) A549 cells were treated with neuraminidases from Arthrobacter ureafaciens and Clostridium perfringens (Neu K) or S. pneumoniae neuraminidase (Neu Sp), and subsequently incubated with S. pneumoniae type 3 (Sp3, MOI 10) in medium (Sp) or with either 15 μg/ml of exogenous rhGal1 (Sp + rhGal1) or rhGal3 (Sp + rhGal3). The bound bacteria were released in water and quantified after 24 h incubation on 5% sheep's blood agar plates by counting colony-forming units (CFU). The fold changes of CFU from galectin-mediated Sp adhesion compared to that without exogenous galectin are shown. (D) A549 cells were exposed to PR8 (MOI 5) for 72 h (A549+PR8) or medium only (A549), and then incubated with Sp3 (MOI 10) in medium (Sp) or with either 15 μg/ml of exogenous rhGal1 (Sp + rhGal1) or rhGal3 (Sp + rhGal3) for bacterial adhesion, which was determined and shown as described above. (E and F) Total RNA was extracted from A549 control (Ctrl) or neuraminidase (A. ureafaciens and C. perfringens) treated cells (NeuK) incubated in presence or absence of 15 μg/ml exogenous rhGal1 or rhGal3 for 1 h. (E) SOCS1 transcript level was analyzed by RT-PCR. (F) RIG1 transcript levels were analyzed by RT-PCR. Bar graphs show the fold change in mRNA expression levels in neuraminidase-treated cells as well as galectin-treated cells in comparison with control cell without neuraminidase and galectin treatment (Ctrl) after normalized to β-actin. In all studies, representative data from at least three independent experiments are shown. *P < 0.05; **P < 0.001, non-paired Student's t test. (Adapted from Nita-Lazar et al. 2015a,b).
CONCLUSIONS
The recognition capacity of galectins from invertebrates and vertebrates for oligosaccharides displayed on the surface of virus, bacteria, protistan pathogens and parasites is now well established. Thus, like C-type lectins, galectins are currently considered bona fide PRRs. Based on the Medzhitov and Janeway model (Medzhitov and Janeway 2002), however, PRRs recognize pathogens via highly conserved microbial surface molecules of wide distribution such as lipopolysaccharide or peptidoglycan (pathogen- or microbe-associated molecular patterns), which are absent in the host. Therefore, galectins do not rigorously fit the definition of PRRs, as they also recognize ligands that are displayed on the host cell surface. This apparent paradox reveals our limited knowledge about the actual diversity in recognition of the host galectin repertoire and the structural and biophysical aspects of ligand-binding preference (Dam and Brewer 2010). This lack of detailed information particularly concerns the diverse architectural display of the galectin ligands within the complex carbohydrate moieties of the host cell and the microbial surface, and how these features impact the affinity and avidity of the oligomeric galectins in the extracellular space. Given that as described above, host galectins play key roles in early development, tissue repair and regulation of immune homeostasis via recognition of ‘self’ lactosamine moieties, the substantial conservation of this lectin family in evolution supports the notion that strong functional constraints would prevent any dramatic evolutionary changes in galectin carbohydrate specificity that would be detrimental to the host. Together with the well-recognized evolutionary plasticity for the colonization of new host tissues, it seems plausible that pathogens and parasites would have rather evolved their glycocomes to mimic their hosts’ to efficiently subvert the roles of galectins for attachment and entry into the host cells in a ‘Trojan horse’ model (Vasta 2009).
FUNDING
The authors' research reviewed herein was supported by Grant R01GM070589 from the National Institutes of Health and Grants IOS 1050518, IOB-0618409, and IOS-0822257 from the National Science Foundation (GRV). Post-doctoral fellowships for JM and NGM were awarded grant 5T32AI095190-03 from the National Institutes of Health.
Conflict of interest. None declared.
REFERENCES
- Ahmed H. Promoter methylation in prostate cancer and its application for the early detection of prostate cancer using serum and urine samples. Biomark Cancer 2010;2:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H, Banerjee PP, Vasta GR. Differential expression of galectins in normal, benign and malignant prostate epithelial cells: silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem Bioph Res Co 2007;358:241–6. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Bianchet MA, Amzel LM et al. Novel carbohydrate specificity of the 16-kDa galectin from Caenorhabditis elegans: binding to blood group precursor oligosaccharides (type 1, type 2, Talpha, and Tbeta) and gangliosides. Glycobiology 2002;12:451–61. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Cappello F, Rodolico V et al. Evidence of heavy methylation in the galectin 3 promoter in early stages of prostate adenocarcinoma: development and validation of a methylated marker for early diagnosis of prostate cancer. Transl Oncol 2009;2:146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H, Du S-J, O’Leary N et al. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology 2004;14:219–32. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Rabinovich GA, Jackson SS et al. Animal models for assessing the biological roles of lectins. In: Vasta GR, Ahmed H (eds) Animals Lectins: A Functional View. Boca Raton, FL: CRC Press, 2008. [Google Scholar]
- Ahmed H, Vasta GR. Unlike mammalian GRIFIN, the zebrafish homologue (DrGRIFIN) represents a functional carbohydrate-binding galectin. Biochem Bioph Res Co 2008;371:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumi K, De Santis R, De Tomaso A et al. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: “waiting for Godot”. Immunogenetics 2003;55:570–81. [DOI] [PubMed] [Google Scholar]
- Barrionuevo P, Beigier-Bompadre M, Ilarregui JM et al. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol 2007;178:436–45. [DOI] [PubMed] [Google Scholar]
- Bi S, Hong PW, Lee B et al. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. P Natl Acad Sci USA 2011;108:10650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchet MA, Ahmed H, Vasta GR et al. Soluble beta-galactosyl-binding lectin (galectin) from toad ovary: crystallographic studies of two protein-sugar complexes. Proteins 2000;40:378–88. [DOI] [PubMed] [Google Scholar]
- Bianchet MA, Odom EW, Vasta GR et al. A novel fucose recognition fold involved in innate immunity. Nat Struct Biol 2002;9:628–34. [DOI] [PubMed] [Google Scholar]
- Blidner AG, Mendez-Huergo SP, Cagnoni AJ et al. Re-wiring regulatory cell networks in immunity by galectin-glycan interactions. FEBS Lett 2015;589:3407–18. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 2007;13:1450–7. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Garlanda C, Salvatori G et al. Pentraxins as a key component of innate immunity. Curr Opin Immunol 2006;18:10–15. [DOI] [PubMed] [Google Scholar]
- Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol 2002;12:616–23. [DOI] [PubMed] [Google Scholar]
- Chen HL, Liao F, Lin TN et al. Galectins and neuroinflammation. Adv Neurobiol 2014;9:517–42. [DOI] [PubMed] [Google Scholar]
- Cleves AE, Cooper DN, Barondes SH et al. A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol 1996;133:1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Ripoche M, Fowlis D et al. The role of galectins in mouse development. Trends Glycosci Glyc 1997;9:31–40. [Google Scholar]
- Colnot C, Sidhu SS, Balmain N et al. Uncoupling of chondrocyte death and vascular invasion in mouse galectin 3 null mutant bones. Dev Biol 2001;229:203–14. [DOI] [PubMed] [Google Scholar]
- Cooper DN, Massa SM, Barondes SH. Endogenous muscle lectin inhibits myoblast adhesion to laminin. J Cell Biol 1991;115:1437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DNW. Galectinomics: finding themes in complexity. Biochim Biophys Acta 2002;1572:209–31. [DOI] [PubMed] [Google Scholar]
- Craig SEL, Thummel R, Ahmed H et al. The zebrafish galectin Drgal1-l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophth Vis Sci 2010;51:3244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci DO, Cerliani JP, Dalotto-Moreno T et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 2014;156:744–58. [DOI] [PubMed] [Google Scholar]
- Dam TK, Brewer CF. Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry 2008;47:8470–6. [DOI] [PubMed] [Google Scholar]
- Dam TK, Brewer CF. Maintenance of cell surface glycan density by lectin-glycan interactions: a homeostatic and innate immune regulatory mechanism. Glycobiology 2010;20:1061–4. [DOI] [PubMed] [Google Scholar]
- Dodd RB, Drickamer K. Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology 2001;11:71R–9R. [DOI] [PubMed] [Google Scholar]
- Drickamer K. A Genomics Resource for Animal Lectins. 2017. http://www.imperial.ac.uk/research/animallectins/ (date last accessed, 4 May 2017). [Google Scholar]
- Elola MT, Wolfenstein-Todel C, Troncoso MF et al. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci 2007;64:1679–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Ghosh A, Amin MN et al. The galectin CvGal1 from the eastern oyster (Crassostrea virginica) binds to blood group A oligosaccharides on the hemocyte surface. J Biol Chem 2013;288:24394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Ghosh A, Amin MN et al. Galectin CvGal2 from the Eastern Oyster (Crassostrea virginica) displays unique specificity for ABH blood group oligosaccharides and differentially recognizes sympatric Perkinsus species. Biochemistry 2015a;54:4711–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Nita-Lazar M, Gonzalez-Montalban N et al. Manipulating galectin expression in zebrafish (Danio rerio). Methods Mol Biol 2015b;1207:327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe MD, Meijers WC, Rogier van der Velde A et al. Galectin-3 and heart failure: prognosis, prediction & clinical utility. Clin Chim Acta 2015;443:48–56. [DOI] [PubMed] [Google Scholar]
- Fogel S, Guittaut M, Legrand A et al. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology 1999;9:383–7. [DOI] [PubMed] [Google Scholar]
- Gabius HJ. Animal lectins. Eur J Biochem 1997;243:543–76. [DOI] [PubMed] [Google Scholar]
- Garner OB, Yun T, Pernet O et al. Timing of galectin-1 exposure differentially modulates Nipah virus entry and syncytium formation in endothelial cells. J Virol 2015;89:2520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P, Kaptan E, Bandyopadhyaya G et al. Cod glycopeptide with picomolar affinity to galectin-3 suppresses T-cell apoptosis and prostate cancer metastasis. P Natl Acad Sci USA 2013;110:5052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmache A, LeBerre M, Droineau S et al. Bioluminescence imaging of live infected salmonids reveals that the fin bases are the major portal of entry for Novirhabdovirus. J Virol 2006;80:3655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M, Mazal D, Biron VA et al. A novel clinically relevant animal model for studying galectin-3 and its ligands during colon carcinogenesis. J Histochem Cytochem 2010;58:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi J, Kasai K. The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 1993;3:297–304. [DOI] [PubMed] [Google Scholar]
- Hirashima M, Kashio Y, Nishi N et al. Galectin-9 in physiological and pathological conditions. Glycoconjugate J 2004;19:593–600. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Liu F-T. Regulation of immune responses by galectin-3. In: Vasta GR, Ahmed H (eds) Animals Lectins: A Functional View. Boca Raton, FL: CRC Press, 2008. [Google Scholar]
- Kamhawi S, Ramalho-Ortigao M, Pham VM et al. A role for insect galectins in parasite survival. Cell 2004;119:329–41. [DOI] [PubMed] [Google Scholar]
- Kirchmaier S, Naruse K, Wittbrodt J et al. The genomic and genetic toolbox of the teleost medaka (Oryzias latipes). Genetics 2015;199:905–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz S, Jin C, Hykollari A et al. Hemocytes and plasma of the eastern oyster (Crassostrea virginica) display a diverse repertoire of sulfated and blood group A-modified N-glycans. J Biol Chem 2013;288:24410–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levroney EL, Aguilar HC, Fulcher JA et al. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J Immunol 2005;175:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol 2004;4:325–35. [DOI] [PubMed] [Google Scholar]
- Liao DI, Kapadia G, Ahmed H et al. Structure of S-lectin, a developmentally regulated vertebrate beta-galactoside-binding protein. P Natl Acad Sci USA 1994;91:1428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz MS, Leal-Pinto E, Cohen BE et al. Galectin 9 is the sugar-regulated urate transporter/channel UAT. Glycoconjugate J 2004;19:491–8. [DOI] [PubMed] [Google Scholar]
- Liu FT, Yang RY, Hsu DK. Galectins in acute and chronic inflammation. Ann N Y Acad Sci 2012;1253:80–91. [DOI] [PubMed] [Google Scholar]
- Lobsanov YD, Gitt MA, Leffler H et al. X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-A resolution. J Biol Chem 1993;268:27034–8. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002;296:298–300. [DOI] [PubMed] [Google Scholar]
- Merani S, Chen W, Elahi S. The bitter side of sweet: the role of Galectin-9 in immunopathogenesis of viral infections. Rev Med Virol 2015;25:175–86. [DOI] [PubMed] [Google Scholar]
- Morris S, Ahmad N, Andre S et al. Quaternary solution structures of galectins-1, -3, and -7. Glycobiology 2004;14:293–300. [DOI] [PubMed] [Google Scholar]
- Nita-Lazar M, Banerjee A, Feng C et al. Desialylation of airway epithelial cells during influenza virus infection enhances pneumococcal adhesion via galectin binding. Mol Immunol 2015a;65:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita-Lazar M, Banerjee A, Feng C et al. Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol Immunol 2015b;68:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita-Lazar M, Mancini J, Feng C et al. The zebrafish galectins Drgal1-L2 and Drgal3-L1 bind in vitro to the infectious hematopoietic necrosis virus (IHNV) glycoprotein and reduce viral adhesion to fish epithelial cells. Dev Comp Immunol 2016;55:241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom EW, Vasta GR. Characterization of a binary tandem domain F-type lectin from striped bass (Morone saxatilis). J Biol Chem 2006;281:1698–713. [DOI] [PubMed] [Google Scholar]
- Ogden AT, Nunes I, Ko K et al. GRIFIN, a novel lens-specific protein related to the galectin family. J Biol Chem 1998;273:28889–96. [DOI] [PubMed] [Google Scholar]
- Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol 2008;10:2078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace KE, Lebestky T, Hummel T et al. Characterization of a novel Drosophila melanogaster galectin. Expression in developing immune, neural, and muscle tissues. J Biol Chem 2002;277:13091–8. [DOI] [PubMed] [Google Scholar]
- Pang J, Rhodes DH, Pini M et al. Increased adiposity, dysregulated glucose metabolism and systemic inflammation in Galectin-3 KO mice. PLoS One 2013;8:e57915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejnovic NN, Pantic JM, Jovanovic IP et al. Galectin-3 is a regulator of metaflammation in adipose tissue and pancreatic islets. Adipocyte 2013;2:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Toscano MA, Jackson SS et al. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol 2007;17:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, van Kooyk Y, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci 2012;1253:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi B, Espeli M, Schiff C et al. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J Immunol 2006;177:796–803. [DOI] [PubMed] [Google Scholar]
- Saouros S, Edwards-Jones B, Reiss M et al. A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J Biol Chem 2005;280:38583–91. [DOI] [PubMed] [Google Scholar]
- Sato S, Hughes, RC Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem 1992;267:6983–90. [PubMed] [Google Scholar]
- Sato S, Ouellet M, St-Pierre C et al. Glycans, galectins, and HIV-1 infection. Ann N Y Acad Sci 2012;1253:133–48. [DOI] [PubMed] [Google Scholar]
- Schwarz FP, Ahmed H, Bianchet MA et al. Thermodynamics of bovine spleen galectin-1 binding to disaccharides: correlation with structure and its effect on oligomerization at the denaturation temperature. Biochemistry 1998;37:5867–77. [DOI] [PubMed] [Google Scholar]
- Seetharaman J, Kanigsberg A, Slaaby R et al. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J Biol Chem 1998;273:13047–52. [DOI] [PubMed] [Google Scholar]
- Shi X-Z, Wang L, Xu S et al. A galectin from the kuruma shrimp (Marsupenaeus japonicus) functions as an opsonin and promotes bacterial clearance from hemolymph. PLoS One 2014;9:e91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalz H, Roth U, Schleuder D et al. The Geodia cydonium galectin exhibits prototype and chimera-type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology 2006;16:402–14. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Dias-Baruffi M et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med 2010;16:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Qian Y, Karmakar S et al. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol 2008;180:3091–102. [DOI] [PubMed] [Google Scholar]
- Tasumi S, Vasta GR. A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J Immunol 2007;179:3086–98. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Drickamer K. Binding of oligosaccharide ligands to the selectins requires additional interactions with the carbohydrate-recognition domains. In: Taylor ME, Drickamer K (eds) Introduction of Glycobiology. Oxford, NY: Oxford University Press, 2003, 207. [Google Scholar]
- Toledo KA, Fermino ML, Andrade Cdel C et al. Galectin-1 exerts inhibitory effects during DENV-1 infection. PLoS One 2014;9:e112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR. Roles of galectins in infection. Nat Rev Microbiol 2009;7:424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR. Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol 2012;946:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR, Ahmed H. Animals lectins: a functional view. In: Vasta GR, Ahmed H (eds) Animals Lectins: A Functional View. Boca Raton, FL: CRC Press, 2008. [Google Scholar]
- Vasta GR, Ahmed H, Bianchet MA et al. Diversity in recognition of glycans by F-type lectins and galectins: molecular, structural, and biophysical aspects. Ann N Y Acad Sci 2012;1253:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR, Ahmed H, Du S et al. Galectins in teleost fish: Zebrafish (Danio rerio) as a model species to address their biological roles in development and innate immunity. Glycoconjugate J 2004;21:503–21. [DOI] [PubMed] [Google Scholar]
- Vasta GR, Ahmed H, Tasumi S et al. Biological roles of lectins in innate immunity: molecular and structural basis for diversity in self/non-self recognition. Adv Exp Med Biol 2007;598:389–406. [DOI] [PubMed] [Google Scholar]
- Vasta GR, Feng C, Bianchet MA et al. Structural, functional, and evolutionary aspects of galectins in aquatic mollusks: From a sweet tooth to the Trojan horse. Fish Shellfish Immunol 2015;46:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR, Odom EW, Bianchet MA et al. F-type lectins: a new family of recognition factors. In: Vasta GR, Ahmed H (eds), Animals Lectins: A Functional View. Boca Raton, FL: CRC Press, 2008. [Google Scholar]
- Walser PJ, Kues U, Aebi M et al. Ligand interactions of the Coprinopsis cinerea galectins. Fungal Genet Biol 2005;42:293–305. [DOI] [PubMed] [Google Scholar]
- Yang ML, Chen YH, Wang SW et al. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. J Virol 2011a;85:10010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RY, Yu L, Graham JL et al. Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. P Natl Acad Sci USA 2011b;108:18696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]