Abstract
Objective. A proportion of RA and SLE patients treated with standard doses of rituximab (RTX) display inefficient B cell deletion and poor clinical responses that can be augmented by delivering higher doses, indicating that standard-dose RTX is a sub-optimal therapy in these patients. This study aimed to investigate whether better responses could be achieved with mechanistically different anti-CD20 mAbs.
Methods. We compared RTX with obinutuzumab (OBZ), a new-generation, glycoengineered type II anti-CD20 mAb, in a series of in vitro assays measuring B cell cytotoxicity in RA and SLE patient samples.
Results. We found that OBZ was at least 2-fold more efficient than RTX at inducing B-cell cytotoxicity in in vitro whole blood assays. Dissecting this difference, we found that RTX elicited more potent complement-dependent cellular cytotoxicity than OBZ. In contrast, OBZ was more effective at evoking Fc gamma receptor-mediated effector mechanisms, including activation of NK cells and neutrophils, probably due to stronger interaction with Fc gamma receptors and the ability of OBZ to remain at the cell surface following CD20 engagement, whereas RTX became internalized. OBZ was also more efficient at inducing direct cell death. This was true for all CD19+ B cells as a whole and in naïve (IgD+CD27−) and switched (IgD−CD27+) memory B cells specifically, a higher frequency of which is associated with poor clinical response after RTX.
Conclusion. Taken together, these data provide a mechanistic basis for resistance to rituximab-induced B-cell depletion, and for considering obinutuzumab as an alternative B-cell depleting agent in RA and SLE.
Keywords: rheumatoid arthritis, systemic lupus erythematosus, B cells, rituximab, obinutuzumab
Rheumatology key messages
Obinutuzumab induces superior B cell cytotoxicity to rituximab in RA and SLE patient samples.
B cells from RA and SLE patients internalize rituximab more rapidly than obinutuzumab.
Obinutuzumab is superior to rituximab at evoking Fc gamma receptor-dependent and -independent effector mechanisms in RA and SLE.
Introduction
Incomplete B cell depletion following treatment with the anti-CD20 mAb rituximab (RTX) is associated with poor clinical response in both RA [1] and SLE [2], whereas enhanced B cell depletion achieved using additional doses of RTX in RA [3] and prolonged duration of depletion are associated with a better clinical response in SLE [4]. Therefore, these data indicate that achieving complete, durable B cell depletion will improve clinical response in both RA and SLE.
B cell subpopulations may be defined as naïve (IgD+CD27−), unswitched memory cells (IgD+CD27+), switched memory cells (IgD−CD27+) and double negative (DN) cells (IgD−CD27−). Poor clinical response to RTX in both RA and SLE is associated with a higher number and/or frequency of CD27+ memory cells [1, 2, 5] and also with DN B cells in RA [5], suggesting that resistance to depletion of different B cell subpopulations is clinically relevant [6]. Further, a greater frequency of IgD−CD27+ switched memory cells and DN cells, but not IgD+CD27− naïve or IgD+CD27+ unswitched memory cells, was detectable in peripheral blood of patients 4 weeks after a single low dose of RTX (500 mg), prior to organ transplantation. In contrast, B cell composition in lymph nodes and spleen [7] revealed the presence of IgD+CD27− naïve and IgD+CD27+ unswitched memory cells [8, 9], despite opsonization with RTX [8, 9], which suggests that in lymph nodes depletion by RTX was compromised. Collectively, these findings suggest that RTX depletes naïve cells and IgD+CD27+ unswitched memory cells more efficiently than IgD−CD27+ switched memory cells and DN cells, particularly in lymphoid tissues [10].
Anti-CD20 mAbs evoke distinct cytotoxic mechanisms: complement-dependent cellular cytotoxicity (CDC), Fc gamma receptor (FcγR)-mediated depletion through cellular effectors including antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis, and direct cell death (DCD). Good clinical response to rituximab in both RA [11] and SLE [12] is associated with the higher affinity 158 V polymorphism in CD16a (FcγRIIIa) suggesting that FcγR-mediated mechanisms are important for B cell depletion. Moreover, SLE-associated defects in complement [13], NK cells [14, 15] and neutrophils [16] and acquired defects in phagocytosis [16–18] may impact the efficiency of anti-CD20 mAbs [19].
Anti-CD20 mAbs can be categorized as types I and II. Type I anti-CD20 mAbs, such as RTX, redistribute CD20 into lipid rafts, a property that facilitates clustering and complement activation, but also mAb internalization, which is partly driven by cis-mediated engagement of FcγRIIb [20, 21], reducing surface accessible mAb for engagement with FcγR on effector cells [22] such as NK cells, neutrophils and macrophages [23]. Type II mAbs such as obinutuzumab (OBZ, GA101) do not undergo efficient redistribution, clustering or internalization. Accordingly, in follicular and mantle cell lymphoma high target cell expression of FcγRIIb was shown to be associated with poor clinical response to RTX [20, 24].
Other type I mAbs, ofatumumab and ocrelizumab, have been used in clinical trial settings in RA and/or SLE, respectively [25, 26]. To date, no type II mAb has been used in these diseases. However, OBZ has been used in chronic lymphocytic leukaemia and shown to be more effective than RTX [27]. OBZ has also been glycoengineered with an afucosylated Fc facilitating enhanced affinity for CD16a [28], which is the basis of its superior potency in NK-mediated ADCC [29] and antibody-dependent cellular phagocytosis [30]. Therefore, data on the pre-clinical activity of RTX and OBZ in RA and SLE would be of clear clinical importance to understanding whether OBZ may at least partly overcome autoimmune disease-related resistance mechanisms.
Our previous work showed that internalization of RTX compromised its ability to delete B cells in vitro and that glycosylated OBZ was superior to RTX in whole blood B cell depletion assays in both RA and SLE [31]. Here, we compared the ability of RTX and OBZ to evoke different effector mechanisms and delete target B cells from patients with RA and SLE. We show that OBZ is at least 2-fold more efficient than RTX at inducing cytotoxicity of these B cells, that it internalizes less rapidly than RTX from the autoimmune B cells, and that it is less efficient than RTX at recruiting complement, but significantly more potent at evoking FcγR-mediated activation of NK cells and neutrophils as well as FcγR-independent direct cell death. We also show that IgD−CD27+ switched memory cells and DN cells express significantly lower levels of CD20 than IgD+CD27+ unswitched memory cells, potentially contributing to their apparent resistance to RTX-induced depletion.
Methods
All participants of this study provided consent according to the Declaration of Helsinki and this study was approved by the National Research Ethics Service committee, London-Bentham. All patients with RA satisfied the ACR/EULAR classification criteria [32] and all patients with SLE met the ACR classification criteria [33]. The patient demographics are shown in the supplementary Tables S1 and S2, available at Rheumatology Online.
Antibodies and reagents
Anti-CD20 mAbs used in the studies include RTX, OBZ and non-glycoengineered, wild-type glycosylated OBZ (OBZGly) and in some experiments OBZ with a mutated Fc portion (P329G LALA) that does not engage any Fc-mediated effector functions [34] (OBZ-PG LALA). Roche Innovation Center Zürich, Switzerland generated all anti-CD20 mAbs except RTX, which was a kind gift from the pharmacy of University College Hospital, UK, and AT10 (FcγRII antagonist) [35] was produced in-house.
Flow cytometry and B cell isolation
Fluorochrome-conjugated mAbs anti-CD3 (phycoerythrin (PE)-Cy7), anti-CD15 (FITC): anti-CD16 (allophycoyanin), anti-CD19 (Alexa Fluor 700), anti-CD45 (PE), anti-CD56 (PE), anti-CD107a (Brilliant Violet 421), anti-CD11b (PE) and anti-CD62L (allophycoyanin), and propidium iodide (PI) and annexin V (Av) (FITC) were obtained from BD Biosciences (Oxford, UK) and Biolegend, London, UK. In addition to forward- and side-scatter characteristics, we identified B cells as CD19+, T cells as CD3+, NK cells as CD3−56+ and neutrophils as CD15+ by flow cytometry using a Becton Dickinson LSR Fortessa cell analyzer. Peripheral blood mononuclear cells were separated from whole blood by Ficoll-Hypaque density gradient and B cells were isolated using EasySep Human B Cell Enrichment Kit (Stemcell Technologies, Cambridge, UK).
Whole blood B cell depletion assays
Briefly, 300 μl of freshly drawn whole blood anti-coagulated with heparin was incubated with or without mAbs at 1 μg/ml for 24 h at 37 °C and 5% CO2 before analysing with a flow cytometer, as described previously [31]. The percentage B cell depletion was calculated from the proportion of B cells to T cells remaining after treatment and defined as the cytotoxicity index (CTI) as described previously [28, 31].
Surface fluorescence-quenching assays
Surface fluorescence-quenching assays were performed as described previously [23, 31] to assess internalization of mAbs by B cells. Isolated B cells were incubated for 6 h with Alexa-488 conjugated mAbs at a concentration of 5 μg/ml before analysing by flow cytometry.
CDC cytotoxicity assays
CDC assays were performed as previously described [36]. Isolated B cells were incubated with mAbs at a concentration of 10 μg/ml for 30 min at 37 °C and 5% CO2 stained with anti-CD19, Av and PI and the frequency of CD19+Av+PI+ cells assessed by flow cytometry. We used freshly collected normal healthy human serum as a source of complement and part of the serum was heat inactivated at 56 °C for 30 min to produce heat inactivated serum (HIS). The ability of mAbs to induce CDC was assessed by the relative frequency of CD19+Av+PI+ cells in samples incubated either with normal healthy serum or with HIS.
Direct cell death
Isolated B cells were incubated in RPMI supplemented with 10% heat inactivated fetal calf serum with or without mAbs at a concentration of 10 μg/ml for 6 h at 37 °C and 5% CO2 and stained and analysed as for CDC. The frequency of CD19+Av+ cells in samples incubated with mAbs compared with samples incubated without mAbs represented the ability of mAbs to induce DCD.
NK cell degranulation assays
NK cell degranulation was assessed using samples from the whole blood B cell depletion assay by measuring the expression of CD107a or lysosome associated membrane protein 1, which is up-regulated upon activation of NK cells and correlates with NK cell mediated ADCC [37, 38]. The extent of CD16a loss was also used as an indirect measure of NK cell activation [39, 40].
Neutrophil activation assays
We assessed neutrophil activation in the whole blood assay by measuring increases in the mean fluorescent intensity (MFI) of CD11b or decreases in MFI of CD62L on CD15+ neutrophils by flow cytometry [41, 42] in samples incubated with mAbs compared with samples incubated without mAbs.
Statistical analysis
Data were analysed using Prism version 5.0 (GraphPad Software, La Jolla, CA, USA). The Mann–Whitney test or Wilcoxon’s matched-pairs signed rank test was used to compare groups as appropriate. Spearman’s correlation coefficient was used to analyse for correlation.
Results
Type II mAbs are more efficient than type I at inducing B cell cytotoxicity
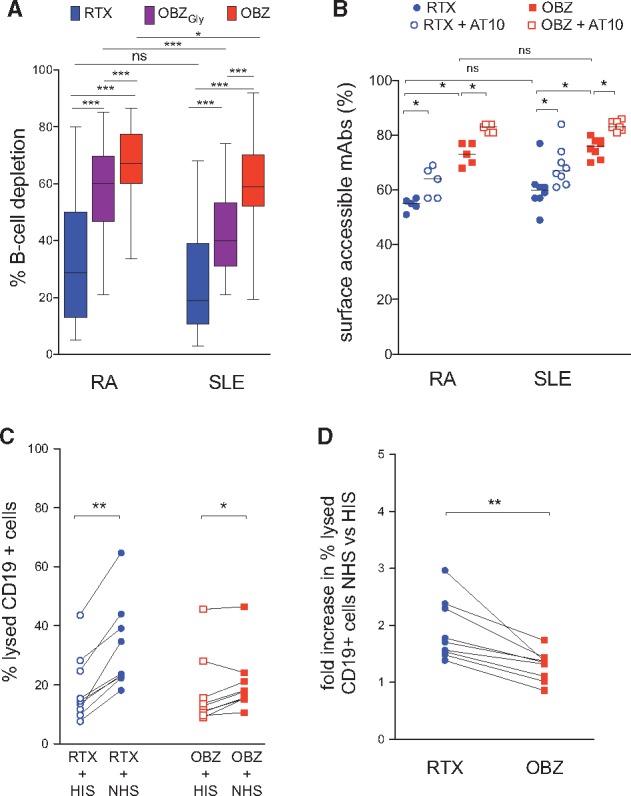
To assess the effect of types I and II mAbs on B cell cytotoxicity in RA and SLE samples, whole blood B cell depletion assays were performed as described previously [31] (supplementary Fig. S1, available at Rheumatology Online). OBZ was >2-fold more efficient than RTX at deleting B cells from patients with RA (n = 31) and SLE (n = 34) and both non-glycomodified OBZGly and OBZ were more efficient than RTX, in all samples tested (Fig. 1A and supplementary Table S3, available at Rheumatology Online). In both RA and SLE, the median CTI of OBZ was significantly greater than the CTI of OBZGly and RTX. The CTI of OBZGly was significantly higher than the CTI of RTX in both RA and SLE. In RA, the median (interquartile range) CTI of RTX, OBZGly and OBZ was 29 (13–50), 60 (47–70) and 67 (60–77), respectively, and in SLE was 19 (11–39), 40 (31–53) and 59 (52–70), respectively. Both type II anti-CD20 mAbs, OBZGly and OBZ, demonstrated superior efficiency of B cell cytotoxicity to the type I anti-CD20 mAb, RTX, in all individual samples from patients with RA and SLE (data not shown).
Fig. 1.
Whole blood B cell depletion, internalization and CDC elicited by anti-CD20 mAbs in RA and SLE patient samples
(A) Whole blood B-cell depletion in samples from patients with RA (n = 31) and SLE (n = 34). The horizontal line in the box represents the median, the box represents the interquartile range and the whiskers represent the range. (B) Surface fluorescence-quenching assay in RA (n = 5) and SLE (n = 8) samples with or without prior incubation with anti-FcγRII blocking mAb, AT10. (C) The frequency of lysed CD19+Av+PI+ B cells in SLE (n = 9) samples. (D) The fold increase in samples incubated with normal healthy serum (NHS) vs heat inactivated serum (HIS). *P < 0.05; **P < 0.005; ***P < 0.0001; and NS, not significant. OBZGly, obinutuzumab with glycosylated Fc; OBZ, obinutuzumab.
There were no significant correlations between CTI of RTX or CTI of OBZ and patient’s age, serum complement and/or IgA levels in samples from patients with SLE (data not shown).
Thus, in both RA and SLE, there was a hierarchy of mAb-induced B cell depletion: RTX < OBZGly < OBZ. The superior efficiency of OBZGly (having a non-glycomodified Fc similar to RTX) suggests that its type II nature accounts for the difference between the two types of mAbs in the efficiency of B cell depletion in the whole blood assay, whereas the increased efficiency of OBZ compared with OBZGly is attributable to afucosylation of the Fc portion.
B-cells internalize RTX more rapidly than OBZ
RTX was internalized more extensively than OBZ after 6 h of incubation with a median (range) percentage of surface accessible RTX vs OBZ of 55 (51–57) vs 83 (81–84), respectively, in RA (n = 5) and 60 (49–77) vs 76 (70–80), respectively, in SLE (n = 8) (Fig. 1B). Internalization of RTX and, to a smaller extent, OBZ, was partially inhibited in the presence of the FcγRII-blocking mAb AT10 (Fig. 1B), similar to our previous observations using a non-glycomodified type II antibody variant [31].
RTX is more efficient than OBZ at inducing CDC cytotoxicity
The frequency of lysed B cells (CD19+Av+PI+) was significantly greater in samples incubated with RTX in the presence of normal healthy serum compared with HIS (supplementary Fig. S2, available at Rheumatology Online) with a median (range) difference of 10.9% (8.1–21%) whereas the difference for OBZ was 4.8% (0.9–6.5%) (Fig. 1C). The mean (s.d.) fold increase in lysed cells in samples incubated with normal healthy serum vs HIS was 1.9 (0.5) and 1.2 (0.2) for RTX and OBZ, respectively (Fig. 1D). Thus, RTX was superior to OBZ at evoking CDC.
OBZ is more efficient than RTX at activating NK cells
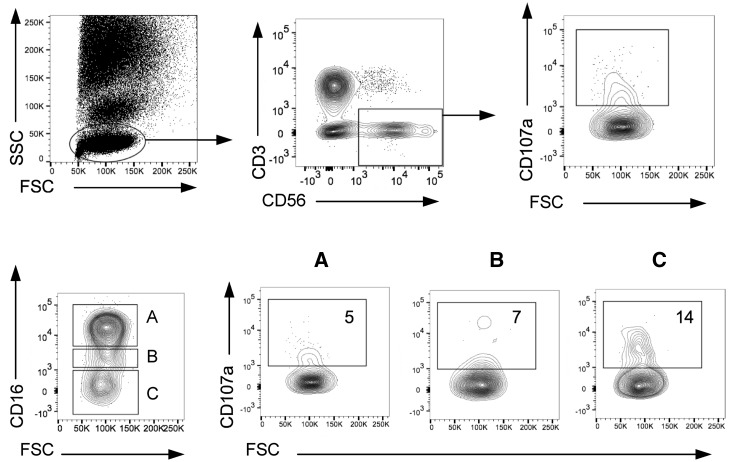
The ability of the mAbs to induce NK cell activation in the whole blood B cell depletion assay, shown in Fig. 2, allowed assessment of NK cell degranulation (CD107a increase) relative to expression of CD16a. The highest proportion of CD107a+ NK (CD3−CD56+) cells was seen in the CD56+CD16− fraction (Fig. 2) suggesting that degranulating NK cells had down-regulated CD16, as previously reported [39].
Fig. 2.
NK cell degranulation assay: relationship between NK cell expression of CD107a and CD16
Flow cytometry-gating strategy to assess NK cell degranulation. Whole blood samples were incubated with or without mAbs for 24 h before analysing by flow cytometry. NK cells were identified based on forward- and side-scatter characteristics and CD56+CD3−. The frequency of CD3−CD56+CD107a+ cells represented activated/degranulated NK cells. The relative frequency of activated CD107a+ NK cells based on CD16 expression in three subpopulations of CD3−CD56+ NK cells was identified based on the relative expression of CD16 (boxed as A, B and C above). FSC: forward-scatter; SSC: side-scatter.
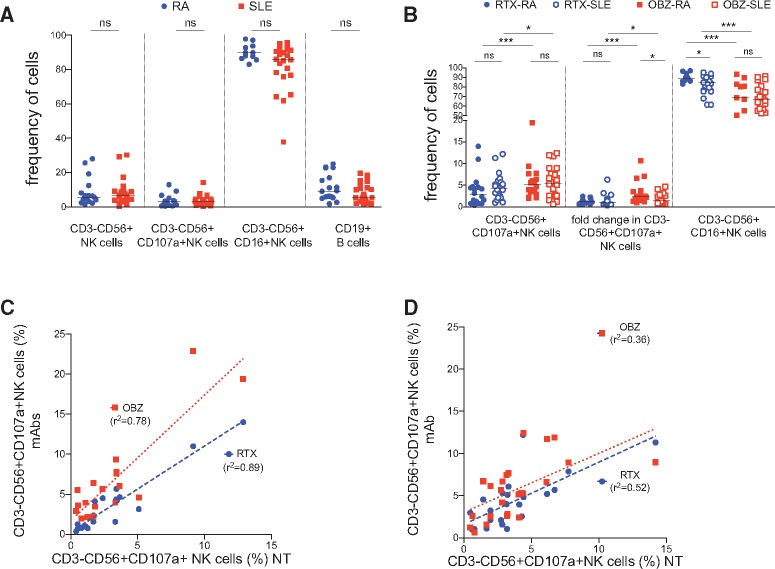
In equivalent assays comparing RTX and OBZ, after 24 h of incubation in the absence of mAbs, there was no significant difference in the frequency of NK cells, CD107a+ NK cells, CD16+ NK cells or B cells between patients with RA (n = 18) and SLE (n = 23) (Fig. 3A). However, in both RA and SLE, the median (range) frequency of CD3−CD56+CD107a+ activated NK cells was significantly higher in samples incubated with OBZ compared with RTX, 5.1% (1.9–22%) vs 2.8% (0.3–14%) and 5.5% (0.6–12%) vs 4.3% (1.2–8.9%), respectively, and the median (range) frequency of CD16+ NK cells was significantly lower, 69% (36–94%) vs 89% (83–97%) and 66% (42–91%) vs 84% (61–95%), respectively (Fig. 3B). Also, there was a significantly higher fold-increase in the frequency of CD3−CD56+CD107a+ NK cells in samples incubated with OBZ compared with RTX in SLE (Fig. 3B).
Fig. 3.
Obinutuzumab is more efficient than rituximab at activating NK cells in RA and SLE patient samples
NK cell activation was assessed in whole blood assay using samples from patients with RA (n = 18) and SLE (n = 23) by the frequency of CD3−CD56+ NK cells, CD3−CD56+CD107a+ NK cells, CD3−CD56−CD16+ NK cells as a percentage of total NK cells and CD19+ cells after incubation in the absence (A) or presence (B) of RTX and OBZ and their relationship in RA (C) and SLE (D). Horizontal lines represent the median. *P < 0.05; **P < 0.005; ***P < 0.0001; ns: not significant; r2: Spearman’s correlation coefficient. NT: not treated; OBZGly: obinutuzumab with glycosylated Fc; OBZ: obinutuzumab.
NK cell activation, as assessed by either gain of CD107a or loss of CD16, or the fold increase in the frequency of CD3−CD56+CD107a+ NK cells was greater in RA compared with SLE (Fig. 3B). NK cell activation, as assessed by the frequency of CD3−CD56+CD107a+ NK cells, by RTX and OBZ correlated significantly with that in samples incubated without mAbs, with r2 = 0.89, P < 0.05 and r2 = 0.78, P < 0.05, respectively, in RA (Fig. 3C) and r2 = 0.52, P < 0.05 and r2 = 0.36, P < 0.05, respectively, in SLE (Fig. 3D). However, correlations were stronger in RA compared with SLE.
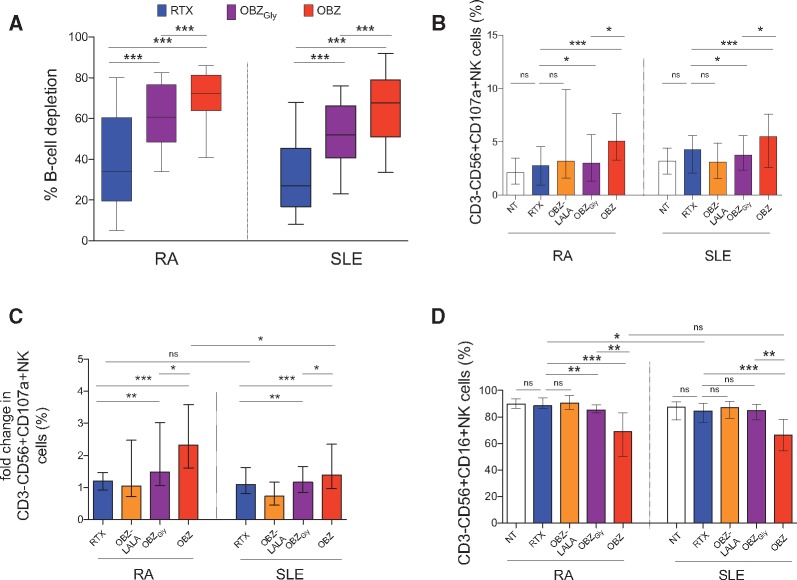
We next investigated the effect of Fc engineering on activation of NK cells using OBZGly and OBZ-PG LALA, which completely lacks FcγR engagement [43]. OBZ was more efficient than OBZGly and RTX in depleting B cells in the whole blood assay in both RA (n = 18) and SLE (n = 23) (Fig. 4A) with an increasing hierarchy in the frequency of, and fold-increase in, CD3−CD56+CD107a+ NK cells as follows: no mAbs = OBZ-PG LALA > RTX > OBZGly > OBZ (Fig. 4B–D). The frequency of CD3−CD56+CD16+ NK cells was significantly lower in samples incubated with OBZ compared with other samples (Fig. 4D). The frequency of CD3−CD56+CD16+ NK cells was also lower in samples incubated with OBZGly compared with RTX in RA, but not SLE (Fig. 4D).
Fig. 4.
Obinutuzumab induces superior NK cell-mediated cellular cytotoxicity to rituximab in RA and SLE patient samples
Whole blood B-cell depletion assays showing (A) the percentage B-cell depletion, (B) the frequency of CD3−CD56+CD107a+ NK cells, (C) the relative increase in % CD3−CD56+CD107a+ NK cells, and (D) the frequency of CD3−CD56+CD16+ NK cells in samples from a subgroup of patients with RA (n = 18) and SLE (n = 23) after 24-h incubation with RTX, OBZ-LALA, OBZGly and OBZ. For the bar graphs, the error bars represent the median and interquartile ranges. *P < 0.05; **P < 0.005; ***P < 0.0001; ns: not significant. NT: not treated with mAbs; OBZ-LALA: obinutuzumab-PG LALA; OBZGly: obinutuzumab with glycosylated Fc similar to RTX; OBZ: obinutuzumab.
Thus, the ability of mAbs to up-regulate the expression of CD107a on CD3−CD56+ NK cells was greater in RA compared with SLE, such that the mean fold difference in samples incubated with RTX, OBZ-PG LALA, OBZGly and OBZ compared with samples incubated without mAbs was 1.2, 1.5, 1.9 and 3.1, respectively, in RA and 1.5, 0.8, 1.4 and 1.8, respectively, in SLE (Fig. 4C).
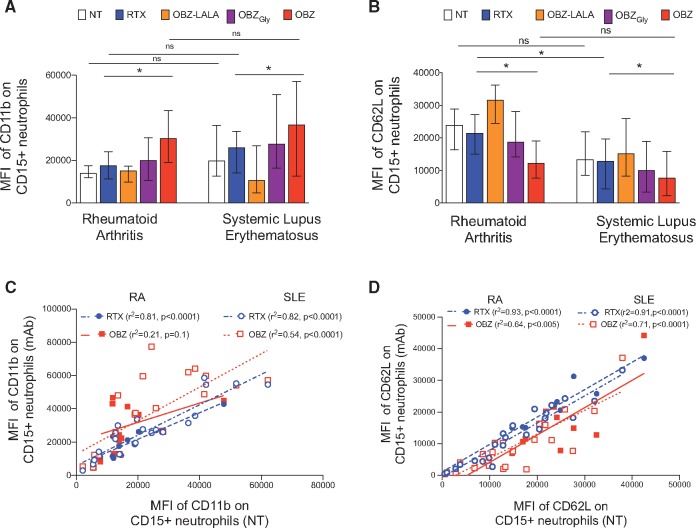
OBZ is more efficient than RTX at activating neutrophils
Neutrophils have been proposed as mAb effector cells [41]. We assessed the ability of mAbs to induce neutrophil activation by measuring the expression of CD11b and CD62L, as described previously [42] and shown in supplementary Fig. S3, available at Rheumatology Online. CD11b forms part of the β-integrin (Mac-1) complex and genetic variants of this complex have been associated with lupus-related phagocytic defects [44]. Upon neutrophil activation the surface expression of CD11b is up-regulated whereas the expression of the adhesion molecule CD62L is down-regulated [41, 42]. The MFI of CD11b in samples incubated with mAbs was significantly higher in both RA (n = 10) and SLE (n = 22) (Fig. 5A) compared with samples incubated without mAbs. In both RA and SLE, we noted significant correlations between the MFI of CD11b in samples incubated without mAbs and that in samples incubated with RTX (r2 = 0.81, 0.82, respectively) whereas significant correlation for OBZ was noted in SLE (r2 = 0.81), but not RA (Fig. 5B). We noted a hierarchy in the ability of mAbs to up-regulate CD11b such that the MFI of CD11b was lower in samples incubated with RTX < OBZGly < OBZ, as in the case of NK cell activation. The MFI of CD62L was also greater in samples incubated with RTX > OBZGly > OBZ (Fig. 5C). In both RA and SLE, we noted significant correlations between the MFI of CD62L in samples incubated without mAbs and that in samples incubated with RTX (r2 = 0.93, 0.91, respectively) and OBZ (r2 = 0.64, 0.71, respectively) (Fig. 5D). Thus, the hierarchy of mAbs in their ability to activate neutrophils was OBZ > OBZGly > RTX. Thus, these data indicated that type II mAbs are superior to RTX in activating neutrophils in the whole blood assay in both RA and SLE samples. OBZ-PG LALA did not elicit significant changes for either marker in both RA (n = 7) and SLE (n = 12) compared with samples incubated without mAbs.
Fig. 5.
Obinutuzumab is more efficient than rituximab at activating neutrophils in RA and SLE patient samples
Whole blood B-cell depletion assays showing (A) the mean fluorescence intensity (MFI) of CD11b, (B) the MFI of CD62L on CD15+ neutrophils, and the relationship between (C) the MFI of CD11b and (D) the MFI of CD62L on CD15+ neutrophils, in samples incubated with or without mAbs in RA (n = 10) and SLE (n = 22) samples. The median and interquartile ranges are represented by the error bars. r2: Spearman’s correlation coefficient; *P < 0.05; **P <0.005; ***P < 0.0001; ns: not significant. NT: not treated; OBZ-LALA: obinutuzumab-PG LALA; OBZGly: obinutuzumab with glycosylated Fc similar to RTX; OBZ: obinutuzumab.
OBZ is more efficient than RTX at inducing direct cell death
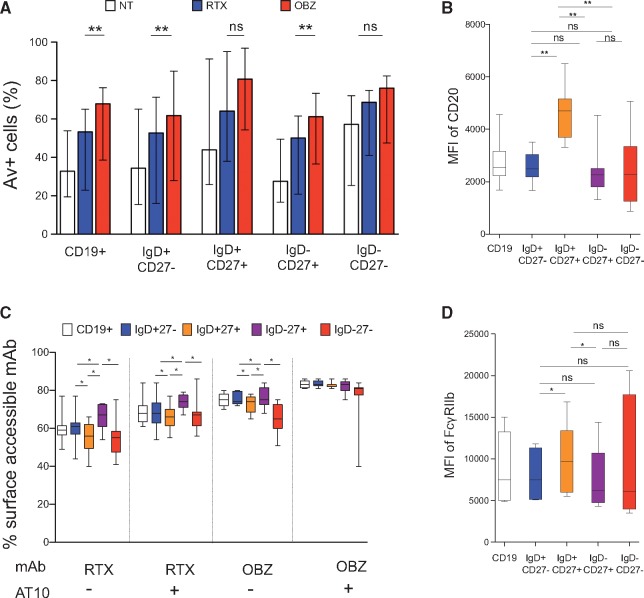
We assessed DCD, using the Av assay as shown in supplementary Fig. S4, available at Rheumatology Online. The ability of OBZ to induce DCD was greater than that of RTX for both CD19+ cells as a whole and the B cell subpopulations IgD+CD27− naïve cells and IgD−CD27+ switched memory cells (Fig. 6A; RA, n = 5 and SLE, n = 4). The proportion of Av+ cells was highest for DN cells > IgD+CD27+ unswitched memory cells > IgD−CD27+ switched memory cells > IgD+CD27− naïve cells. Nonetheless, OBZ was superior to RTX at inducing DCD.
Fig. 6.
Direct cell death, internalization and expression of CD20 and FcγRIIb in B cell subpopulations
In CD19+, IgD+CD27−, IgD+CD27+, IgD−CD27+ and IgD−CD27− cells from patients with RA (n = 5) and SLE (n = 4), (A) the frequency of annexin V+ cells in samples, (B) the mean fluorescence intensity (MFI) of CD20, (C) the frequency of surface accessible mAbs, and (D) the MFI of FcγRIIb. The error bars represent the median and interquartile ranges and box and whiskers the interquartile range, and the horizontal line in the box, the median. *P < 0.05; **P < 0.005; ns: not significant. AT10: anti-FcγRII mAb; OBZ: obinutuzumab.
Sensitivity of B cell subpopulations to deletion/DCD: relationship with expression of CD20, FcγRIIb and internalization
B cell subpopulations displayed varying ability to internalize mAbs such that IgD−CD27+ switched memory cells internalized mAbs less than other B cell subpopulations; and IgD+CD27+ unswitched memory cells internalized mAbs to a greater extent than other B cell subpopulations (Fig. 6C). Antagonizing the effects of FcγRIIb with AT10 significantly reduced internalization in both cases. When compared with naïve and IgD−CD27+ switched memory cells, IgD+CD27+ unswitched memory cells had significantly greater expression of CD20 (Fig. 6B) and FcγRIIb (Fig. 6D) and displayed significantly greater ability to internalize mAbs, whereas naïve and IgD−CD27+ switched memory cells had significantly lower expression of CD20 and FcγRIIb and displayed significantly lower levels of internalization. DN cells had variable levels of expression of CD20 and FcγRIIb, but internalized RTX to a significantly greater extent than IgD−CD27+ switched memory cells. B cells from both RA and SLE samples consistently displayed low levels of OBZ internalization. Thus, there was no clear relationship between the susceptibility of B cell subpopulations to mAb-induced DCD and the ability to internalize mAbs or to express CD20 or FcγRIIb.
Discussion
Our data show that OBZ, a type II anti-CD20 mAb with a glycomodified Fc, demonstrated at least 2-fold greater potency at deleting B cells from whole blood samples from patients with RA and SLE compared with RTX. This increased activity of OBZ was effected predominantly through FcγR-mediated effector mechanisms and DCD. In contrast, RTX recruited complement more efficiently for CDC, but was rapidly internalized and significantly less efficient at evoking ADCC and DCD. Our subsequent analysis revealed that the expression of the CD20 target molecule was less on IgD−CD27+ switched memory and DN cells, perhaps accounting for their relative resistance to removal by RTX.
Target B cells can be deleted with anti-CD20 mAb through multiple mechanisms, with type I mAb engaging complement more effectively than type II mAb. Our findings of superior efficiency of OBZ over RTX at inducing B cell death, despite its inferior ability to recruit complement, are consistent with previous data derived from in vitro studies on malignant B cells and/or cell lines [22, 28, 45, 46]. The superior efficiency of OBZ in the whole blood assay was noted in all individual samples. Complement defects are characteristic of certain autoimmune conditions, such as SLE [13], where, we speculate, OBZ may provide a mechanistic advantage over RTX.
The superior efficiency of OBZ in the whole blood assay despite inferior ability to evoke CDC suggests that the predominant mode of action of OBZ is through FcγR-mediated effector mechanisms and/or DCD. While there was no difference between patients with RA and SLE in the frequency of activated NK cells that lacked CD16 expression and/or expressed CD107a, NK cells from patients with both RA and SLE responded less well to stimulation with RTX compared with OBZ. We found that activation of NK cells by anti-CD20 mAbs is also associated with down-regulation of CD16 revealing remarkable differences in activation of NK-cell subpopulations based on the relative expression of CD16 and up-regulation of the degranulation marker, CD107a. Whereas RTX was less efficient at activating NK cells in both RA and SLE, OBZ induced a greater fold-increase in activating NK cells in samples from patients with RA compared with SLE, suggesting SLE-associated NK cell defects may also contribute to poor depletion with RTX [14, 15, 47].
The relative inefficiency of RTX at evoking ADCC in vitro may, at least partly, be due to internalization of mAbs leading to reduced engagement with FcγR-bearing effector cells, as shown previously [22, 36]. Afucosylation of Fc increases the affinity of IgG1 mAbs for CD16a with little effect on complement binding [48], which may explain the superior efficiency of OBZ at activating NK cells in the whole blood assay even in the presence of complement [49]. Therefore, the superior efficiency of OBZ at activating NK cells may be attributable to a greater surface accessibility owing to its type II nature and a greater affinity for CD16 conferred by afucosylation of Fc.
Our findings of superior neutrophil activation by OBZ compared with RTX in RA and SLE samples are consistent with studies in malignant B cells [41]. Polymorphisms of CD16b may at least partially account for the variability in mAb-induced activation, most notable for RTX whereas afucosylation may have reduced this variability, as described previously [41]. A number of polymorphisms of CD11b associated with SLE may have contributed to the variability between patients in neutrophil activation [44], but regardless, glycoengineered OBZ was more efficient than wild type OBZ and RTX at inducing neutrophil activation.
Following RTX treatment, a small number of IgD−CD27+ switched memory cells and DN cells are detectable in the peripheral blood of patients with RA and SLE [5, 6, 50] suggesting relative resistance to depletion by RTX, perhaps due to lower levels of CD20 on IgD−CD27+ switched memory cells and DN cells compared with IgD+CD27+ unswitched memory cells. Surface expression of IgD and the activation state of B cells may also influence internalization of mAbs, compromising their cytotoxicity [31]. Regardless, OBZ induced greater DCD in vitro in CD19+ cells and IgD−CD27+ switched memory cells from patients with RA and SLE, compared with RTX. These findings are similar to that in malignant B cells [28].
The main limitations of this study are that all experiments were performed in vitro. Therefore, these results showing superior efficiency of OBZ to RTX may not translate into more efficient B cell depletion in vivo and/or in different tissues such as the lymph node, kidney and joint. Further, concomitant therapies may influence the pharmacokinetics of OBZ and have an impact on its overall efficiency to deplete B cells in patients with RA and SLE.
Disease- and host-associated immune deficiencies may contribute to incomplete depletion with RTX in some patients with RA and SLE leading to worse clinical responses. Phase II clinical studies are on-going to evaluate the efficacy of OBZ in patients with lupus nephritis (NCT02550652) and hypersensitized patients with end-stage renal disease awaiting transplantation (NCT02586051). Our results showing superior efficiency of OBZ over RTX, noted in the whole blood assay, are likely due to FcγR-mediated effector mechanisms and DCD.
This study provides compelling mechanistic reasons for expecting better outcomes with OBZ as an alternative B cell depleting agent for patients with RA, and SLE in particular.
Supplementary Material
Acknowledgements
V.R. was funded by an Arthritis Research UK Fellowship (20488) to and supported by Doris Hillier Grant (2013). D.A.I., V.R. and M.J.L. acknowledge the support of the BRC award to University College London and University College Hospital. The authors would like to thank Eve McLoughlin, Pauline Buck, Nichola Whitbread and Samantha Moore for their help with recruiting and obtaining samples from patients for this study. Obinutuzumab was provided by Hoffmann La Roche.
Funding: This study was supported by Arthritis Research UK and Hoffmann La Roche.
Disclosure Statement: C.K. is an employee of and shareholder of Roche. M.S.C. acts as a consultant for Bioinvent and has received research grant support from Roche, GlaxoSmithKline and Gilead. V.R. has received a research grant from Roche. M.J.L. acted as a consultant for Roche and Genentech and received funds less than $10 000 and support to attend educational conferences.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Vital EM, Rawstron AC, Dass S. et al. Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B cell depletion. Arthritis Rheum 2011;63:603–8. [DOI] [PubMed] [Google Scholar]
- 2. Vital EM, Dass S, Buch MH. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011;63:3038–47. [DOI] [PubMed] [Google Scholar]
- 3. Vital EM, Dass S, Buch MH, Rawstron AC, Emery P.. An extra dose of rituximab improves clinical response in rheumatoid arthritis patients with initial incomplete B cell depletion: a randomised controlled trial. Ann Rheum Dis 2015;74:1195–201. [DOI] [PubMed] [Google Scholar]
- 4. Dias SS, Rodriguez-Garcia V, Nguyen H, Pericleous C, Isenberg D.. Longer duration of B cell depletion is associated with better outcome. Rheumatology 2015;54:1876–81. [DOI] [PubMed] [Google Scholar]
- 5. Adlowitz DG, Barnard J, Biear JN. et al. Expansion of activated peripheral blood memory B cells in rheumatoid arthritis, impact of B cell depletion therapy, and biomarkers of response. PLoS One 2015;10:e0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther 2013;15 (Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iso Y, Sawada T, Kita J. et al. Discrepancy of B cell frequency between periphery and spleen after rituximab treatment in ABO-incompatible liver transplantation. Hepatogastroenterology 2013;60:1624–6. [PubMed] [Google Scholar]
- 8. Kamburova EG, Koenen HJ, Borgman KJ. et al. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant 2013;13:1503–11. [DOI] [PubMed] [Google Scholar]
- 9. Wallin EF, Jolly EC, Suchanek O. et al. Human T-follicular helper and T-follicular regulatory cell maintenance is independent of germinal centers. Blood 2014;124:2666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong Q, Ou Q, Ye S. et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 2005;174:817–26. [DOI] [PubMed] [Google Scholar]
- 11. Ruyssen-Witrand A, Rouanet S, Combe B. et al. Fcgamma receptor type IIIA polymorphism influences treatment outcomes in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 2012;71:875–7. [DOI] [PubMed] [Google Scholar]
- 12. Anolik JH, Campbell D, Felgar RE. et al. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 2003;48:455–9. [DOI] [PubMed] [Google Scholar]
- 13. Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res 2002;4 (Suppl 3):S279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz P, Zaytoun AM, Lee JH Jr, Panush RS, Longley S.. Abnormal natural killer cell activity in systemic lupus erythematosus: an intrinsic defect in the lytic event. J Immunol 1982;129:1966–71. [PubMed] [Google Scholar]
- 15. Neighbour PA, Grayzel AI, Miller AE.. Endogenous and interferon-augmented natural killer cell activity of human peripheral blood mononuclear cells in vitro. Studies of patients with multiple sclerosis, systemic lupus erythematosus or rheumatoid arthritis. Clin Exp Immunol 1982;49:11–21. [PMC free article] [PubMed] [Google Scholar]
- 16. Fossati-Jimack L, Ling GS, Cortini A. et al. Phagocytosis is the main CR3-mediated function affected by the lupus-associated variant of CD11b in human myeloid cells. PLoS One 2013;8:e57082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaipl US, Voll RE, Sheriff A. et al. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev 2005;4:189–94. [DOI] [PubMed] [Google Scholar]
- 18. Frank MM, Hamburger MI, Lawley TJ, Kimberly RP, Plotz PH.. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med 1979;300:518–23. [DOI] [PubMed] [Google Scholar]
- 19. Reddy V, Dahal LN, Cragg MS, Leandro M.. Optimising B-cell depletion in autoimmune disease: is obinutuzumab the answer? Drug Discov Today 2016;21:1330–8. [DOI] [PubMed] [Google Scholar]
- 20. Lim SH, Vaughan AT, Ashton-Key M. et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011;118:2530–40. [DOI] [PubMed] [Google Scholar]
- 21. Vaughan AT, Iriyama C, Beers SA. et al. Inhibitory FcgammaRIIb (CD32b) becomes activated by therapeutic mAb in both cis and trans and drives internalization according to antibody specificity. Blood 2014;123:669–77. [DOI] [PubMed] [Google Scholar]
- 22. Tipton TR, Roghanian A, Oldham RJ. et al. Antigenic modulation limits the effector cell mechanisms employed by type I anti-CD20 monoclonal antibodies. Blood 2015;125:1901–9. [DOI] [PubMed] [Google Scholar]
- 23. Beers SA, Chan CH, James S. et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood 2008;112:4170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee CS, Ashton-Key M, Cogliatti S. et al. Expression of the inhibitory Fc gamma receptor IIB (FCGR2B, CD32B) on follicular lymphoma cells lowers the response rate to rituximab monotherapy (SAKK 35/98). Br J Haematol 2015;168:145–8. [DOI] [PubMed] [Google Scholar]
- 25. Taylor PC, Quattrocchi E, Mallett S. et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis 2011;70:2119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rigby W, Tony HP, Oelke K. et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 2012;64:350–9. [DOI] [PubMed] [Google Scholar]
- 27. Goede V, Fischer K, Busch R. et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101–10. [DOI] [PubMed] [Google Scholar]
- 28. Mossner E, Brunker P, Moser S. et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010;115:4393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE.. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 1999;17:176–80. [DOI] [PubMed] [Google Scholar]
- 30. Herter S, Birk MC, Klein C. et al. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol 2014;192:2252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddy V, Cambridge G, Isenberg DA. et al. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 33. Tan EM, Cohen AS, Fries JF. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 34. Herter S, Herting F, Muth G. et al. Abstract 2460: dissecting the in vitro and in vivo mechanism of action of obinutuzumab (GA101) in preclinical models using an immune effector-dead version of obinutuzumab. Cancer Res 2015;75:2460. [Google Scholar]
- 35. Greenman J, Tutt AL, George AJ. et al. Characterization of a new monoclonal anti-Fc gamma RII antibody, AT10, and its incorporation into a bispecific F(ab')2 derivative for recruitment of cytotoxic effectors. Mol Immunol 1991;28:1243–54. [DOI] [PubMed] [Google Scholar]
- 36. Cragg MS, Glennie MJ.. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 2004;103:2738–43. [DOI] [PubMed] [Google Scholar]
- 37. Alter G, Malenfant JM, Altfeld M.. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004;294:15–22. [DOI] [PubMed] [Google Scholar]
- 38. Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G.. Relationship between CD107a expression and cytotoxic activity. Cell Immunol 2009;254:149–54. [DOI] [PubMed] [Google Scholar]
- 39. Grzywacz B, Kataria N, Verneris MR.. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia 2007;21:356–9; author reply 9. [DOI] [PubMed] [Google Scholar]
- 40. Bowles JA, Wang SY, Link BK. et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood 2006;108:2648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Golay J, Da Roit F, Bologna L. et al. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 2013;122:3482–91. [DOI] [PubMed] [Google Scholar]
- 42. Wittmann S, Rothe G, Schmitz G, Frohlich D.. Cytokine upregulation of surface antigens correlates to the priming of the neutrophil oxidative burst response. Cytometry A 2004;57:53–62. [DOI] [PubMed] [Google Scholar]
- 43. Hessell AJ, Hangartner L, Hunter M. et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007;449:101–4. [DOI] [PubMed] [Google Scholar]
- 44. Zhou Y, Wu J, Kucik DF. et al. Multiple lupus-associated ITGAM variants alter Mac-1 functions on neutrophils. Arthritis Rheum 2013;65:2907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bologna L, Gotti E, Manganini M. et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol 2011;186:3762–9. [DOI] [PubMed] [Google Scholar]
- 46. Ysebaert L, Laprevotte E, Klein C, Quillet-Mary A.. Obinutuzumab (GA101) is highly effective against chronic lymphocytic leukemia cells in ex vivo B-cell depletion irrespective of high-risk prognostic markers. Blood Cancer J 2015;5:e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Henriques A, Teixeira L, Ines L. et al. NK cells dysfunction in systemic lupus erythematosus: relation to disease activity. Clin Rheumatol 2013;32:805–13. [DOI] [PubMed] [Google Scholar]
- 48. Shields RL, Lai J, Keck R. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002;277:26733–40. [DOI] [PubMed] [Google Scholar]
- 49. Kern DJ, James BR, Blackwell S. et al. GA101 induces NK-cell activation and antibody-dependent cellular cytotoxicity more effectively than rituximab when complement is present. Leuk Lymphoma 2013;54:2500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC.. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:613–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.