Abstract
Background
Thymidylate synthase (TS) has a predictive role in pemetrexed treatment of mesothelioma; however, additional chemoresistance mechanisms are poorly understood. Here, we explored the role of the reduced-folate carrier (RFC/SLC19A1) and proton-coupled folate transporter (PCFT/SLC46A1) in antifolate resistance in mesothelioma.
Patients and methods
PCFT, RFC and TS RNA and PCFT protein levels were determined by quantitative RT-PCR of frozen tissues and immunohistochemistry of tissue-microarrays, respectively, in two cohorts of pemetrexed-treated patients. Data were analyzed by t-test, Fisher’s/log-rank test and Cox proportional models. The contribution of PCFT expression and PCFT-promoter methylation to pemetrexed activity were evaluated in mesothelioma cells and spheroids, through 5-aza-2′-deoxycytidine-mediated demethylation and siRNA-knockdown.
Results
Pemetrexed-treated patients with low PCFT had significantly lower rates of disease control, and shorter overall survival (OS), in both the test (N = 73, 11.3 versus 20.1 months, P = 0.01) and validation (N = 51, 12.6 versus 30.3 months, P = 0.02) cohorts. Multivariate analysis confirmed PCFT-independent prognostic role. Low-PCFT protein levels were also associated with shorter OS. Patients with both low-PCFT and high-TS levels had the worst prognosis (OS, 5.5 months), whereas associations were neither found for RFC nor in pemetrexed-untreated patients. PCFT silencing reduced pemetrexed sensitivity, whereas 5-aza-2′-deoxycytidine overcame resistance.
Conclusions
These findings identify for the first time PCFT as a novel mesothelioma prognostic biomarker, prompting prospective trials for its validation. Moreover, preclinical data suggest that targeting PCFT-promoter methylation might eradicate pemetrexed-resistant cells characterized by low-PCFT expression.
Keywords: malignant pleural mesothelioma, pemetrexed, outcome, PCFT, expression, methylation
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor of the pleura with increasing incidence [1]. Most MPM patients are not amenable to radical surgery, and the combination of pemetrexed and cisplatin (or carboplatin) is the standard first-line treatment [2]. A few studies highlighted the predictive role of thymidylate synthase (TS), the primary target of pemetrexed [3, 4]. However, this remains controversial [5, 6], prompting research on additional biomarkers.
Three transport systems are currently known to mediate cellular uptake of antifolates. These include the family of folate receptors (encoded by three different genes, FRα/β/γ), the reduced-folate carrier (RFC/SLC19A1) and the proton-coupled folate transporter (PCFT/SLC46A1) [7]. Despite high expression of FRα in 72% of MPM specimens, no difference was observed in overall survival (OS) compared with patients with negative immunohistochemical staining of FRα [8]. Conversely, impaired RFC function was associated with resistance to pemetrexed and methotrexate in MPM and leukemia, respectively [6, 9]. These data are consistent with the cross-resistance to several antifolates in cells which have lost RFC expression [10].
However, in HCT-15 colorectal cancer cells, loss of RFC expression led to a reduction in pemetrexed transport, but this was associated with an increased sensitivity to pemetrexed, attributed to a substantial level of pemetrexed transport via an RFC-independent route [11]. The collateral sensitivity to pemetrexed reflected enhanced inhibition of TS and de novo purine biosynthesis (a secondary target of pemetrexed), attributable to decreased pools of reduced folates, which compete with pemetrexed for intracellular targets.
More recently, the RFC-independent transport of pemetrexed has been attributed to PCFT [12]. The key role of PCFT in folate/antifolate transport has been demonstrated in various models, including MPM cells [13], but has not yet been characterized in human MPM specimens. In contrast to RFC, which displays optimal transport activity at physiological pH, the optimal pH for PCFT-dependent transport is 5.5, approximating the acidic microenvironment of solid tumors [14]. Notably, PCFT exhibits a high-transport affinity for pemetrexed, and PCFT transfection increased pemetrexed cytotoxicity [15]. These findings support the unique role that PCFT plays in the pharmacologic activity of pemetrexed.
The PCFT promoter can be silenced via dense DNA methylation [14], eventually reducing its transcriptional activity. Indeed, promoter silencing through methylation accounted for the loss of PCFT activity in antifolate-resistant HeLa-R1-11 cells [15].
The current study was aimed at evaluating the possible association of RFC and PCFT expression, and PCFT promoter methylation with pemetrexed outcome in MPM. To this end, we used a large repository of well-characterized MPM specimens, including two independent cohorts of patients uniformly treated with pemetrexed-based therapies. Moreover, using appropriate in vitro models, we provide new mechanistic insights that may contribute to the development of novel therapeutic interventions for this devastating disease.
Materials and methods
Patients
A test and a validation cohort of 73 and 51 patients were consecutively enrolled at Humanitas-Cancer-Center (Milan, Italy) between 2003 and 2008, and 2008–2013, respectively. Protein levels were evaluated in tissue microarrays (TMA) from 35 patients from the validation cohort. An additional cohort included 40 pemetrexed-untreated patients. This study was approved by the appropriate ethical review board (ClinicalTrials.gov NCT00867711).
Quantitative RT-PCR
RFC and PCFT expression were assessed using RNA isolated from paraffin-embedded samples (5–516 ng), as described previously [4].
Immunohistochemistry
PCFT analysis was carried out using an affinity purified human PCFT-specific polyclonal antibody, as described in supplementary material and supplementary Figure S1, available at Annals of Oncology online.
Statistics
RECIST criteria were used to classify tumor response. Patients with disease control, including partial response and stable disease, were compared with patients with progressive disease. Progression-free survival (PFS) and OS were calculated from the start of chemotherapy until progression and death (or last contact), respectively. Kaplan–Meier and log-rank methods were used to compare the curves, using SPSS-Version.20 (IBM-SPSS, Chicago, IL). Significant prognostic variables (P < 0.05) identified by univariate analysis were included in the multivariate analysis, using Cox’s proportional hazards model.
In vitro studies
Preclinical studies were carried out in four human MPM cell lines. Pemetrexed was a gift from Eli Lilly (Indianapolis, IN), while 5-aza-2′-deoxycytidine was purchased from Sigma-Aldrich (St. Louis, MO).
PCFT promoter methylation was evaluated by bisulfite DNA sequencing, while PCFT mRNA and protein levels were quantified by quantitative RT-PCR and immunocytochemistry, respectively, as detailed in the supplementary material, available at Annals of Oncology online. The effects of PCFT modulation were assessed with ID#141241-siRNA (Ambion® Silencer). Additional experiments evaluated PCFT expression, cell proliferation and pemetrexed metabolites after DNA demethylation, using 1 μM 5-aza-2′-deoxycytidine (supplementary material, available at Annals of Oncology online).
Results
Clinical characteristics
Baseline characteristics of the two cohorts were comparable, except EORTC-prognostic score (Table 1), and patients older than 65 years or of male gender had significantly shorter OS (supplementary Tables S1 and S2, available at Annals of Oncology online), while none of the other characteristics were associated with outcome.
Table 1.
Baseline characteristics of MPM patients enrolled in the present study
| Clinical characteristics |
n of patients (%) |
|
|---|---|---|
| Test cohort | Validation cohort | |
| Age, years [median, range] | 63 [40–85] | 73 [51–85] |
| Gender | ||
| Male | 56/73 (76) | 32/51 (63) |
| Female | 17/73 (24) | 19/51 (37) |
| EORTC prognostic score | ||
| Good | 29/73 (40) | 39/51 (76) |
| Poor | 44/73 (60) | 12/51 (24) |
| Histologic subtype | ||
| Epithelial | 68/73 (93) | 44/51 (86) |
| Non-epithelial | 5/73 (7) | 7/51 (14) |
| IMIG stage | ||
| Stage III | 30/73 (41) | 23/51 (45) |
| Stage IV | 43/73 (59) | 28/51 (55) |
| Surgical therapies EPP | 4/73 (5) | 1/51 (2) |
| CT response | ||
| SD | 38 (52) | 30 (59) |
| PD | 14 (19) | 4 (8) |
| PR | 21 (29) | 17 (33) |
| Median PFS (months) | 6.8 | 7.5 |
| Median OS (months) | 17.5 | 19.3 |
Survival data were available for all patients. Among the 15 patients still alive the minimum follow-up at the time of analysis was 22.4 months.
CT, computerized tomography; EPP, extrapleural pneumonectomy (after chemotherapy); IMIG, International Mesothelioma Interest Group, OS, overall survival; PD, progressive disease, PFS, progression-free survival; PR, partial response; SD, stable disease.
Correlation of PCFT mRNA expression with outcome
Quantitative RT-PCR reactions were successfully carried out in 69 out of the 73 specimens (macrodissected according to pathologists’ indications, i.e. with tumor content >70%) from the test cohort and in all the specimens from the validation cohort. Gene expression data were evaluated continuously, but the cut-off values were not identified. Therefore, the two cohorts of patients were categorized according to their mRNA median values. Significant correlations were neither detected between gene expression and baseline characteristics, nor between PCFT or RFC levels and clinical response. However, low PCFT expression levels significantly correlated with higher risk of developing a progression compared with disease-control, considering both the test and the validation cohorts (P = 0.01 and P = 0.04, respectively). Notably, disease-control correlated with OS (supplementary Tables S1 and S2, available at Annals of Oncology online).
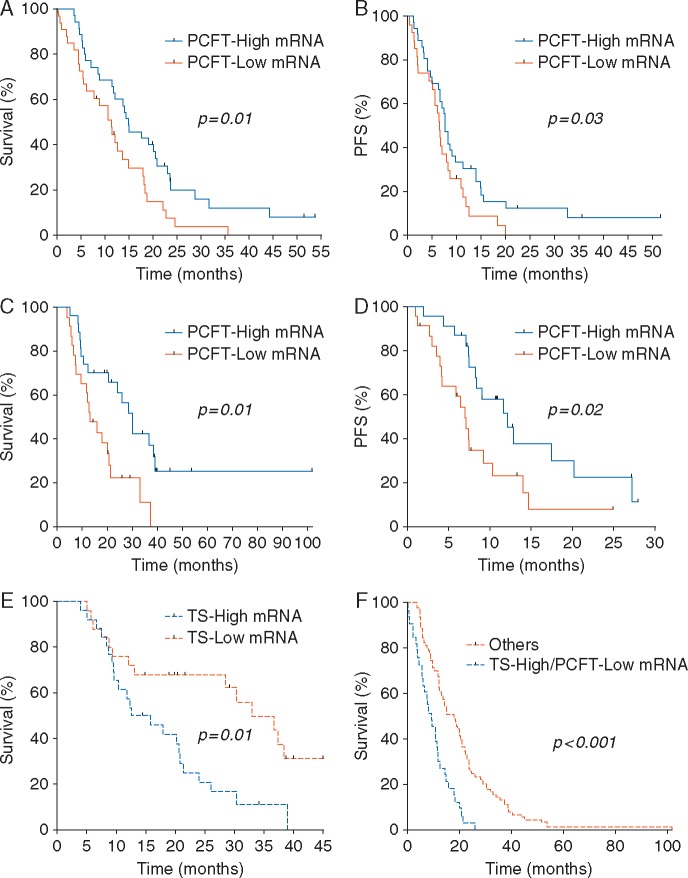
No correlation was found for RFC expression with either OS or PFS in both cohorts (supplementary Figure S2A and S2B, available at Annals of Oncology online). Conversely, PCFT expression was significantly associated with OS. In particular, significantly longer survival was observed in patients with high PCFT expression (median OS, 20.1 months, 95% CI: 10.1–30.0), in comparison to patients displaying low PCFT expression (11.3 months, 95% CI: 10.1–12.5, P = 0.01, Figure 1A). A significant association was also observed for PFS (Figure 1B). Similarly, in the validation cohort, patients with low PCFT expression had the worst prognosis (median OS, 12.6 months, 95% CI: 6.3–17.1), compared with the patients with high PCFT expression (30.3 months, 95% CI: 15.3–36.7, P = 0.01, Figure 1C). A significant difference was also observed for PFS (Figure 1D). Multivariate analysis confirmed the prognostic relevance of PCFT (Table 2).
Figure 1.
Correlation of PCFT mRNA expression with clinical outcome. (A and B) Overall survival (OS) and progression-free survival (PFS) curves segregated according to PCFT mRNA expression in the MPM patients from the test cohort. High/low PCFT levels are relative to the median values acquired by RT-PCR data available from 69 patients; (C and D) OS and PFS curves according to RT-PCR data of PCFT mRNA expression in the MPM patients from the validation cohort (mRNA expression data were available from 51 patients); (E) OS curves subgrouped according TS mRNA expression levels in the MPM patients from the validation cohort. High/low levels are relative to the median values acquired by RT-PCR data available from 51 patients; (F) OS curves according to RT-PCR data of both TS and PCFT mRNA levels in the MPM patients from both the test and the validation cohorts (mRNA expression data were available for 120 patients). The curves were compared using the log-rank test.
Table 2.
Factors associated with overall survival (OS) and progression-free survival (PFS) in the multivariate analysis
| HR (95% CI) | Wald P | |
|---|---|---|
| Covariates for PFS | ||
| Test cohort (n=73) | ||
| Sex: male versus female | 1.52 (0.98–2.11) | 0.06 |
| Age: below versus >65 years | 1.25 (0.71–1.82) | 0.23 |
| PCFT mRNA: low versus high PCFT | 2.27 (1.12–3.74) | 0.02 |
| TS mRNA: low versus high TS | 0.59 (0.37–0.93) | 0.02 |
| Validation cohort (n=51) | ||
| Sex: male versus female | 1.58 (1.00–2.11) | 0.05 |
| Age: below versus >65 years | 1.23 (0.72–1.86) | 0.23 |
| PCFT mRNA: low versus high PCFT | 1.92 (1.32–3.05) | 0.03 |
| TS mRNA: low versus high TS | 0.60 (0.37–0.98) | 0.04 |
| Covariates for OS | ||
| Test cohort (n=73) | ||
| Sex: male versus female | 1.48 (0.61–2.25) | 0.47 |
| Age: below versus above median | 0.92 (0.51–3.10) | 0.39 |
| PCFT mRNA: low versus high PCFT | 2.12 (1.35–3.14) | 0.03 |
| TS mRNA: low versus high TS | 0.72 (0.44–0.95) | 0.03 |
| Validation cohort (n=51) | ||
| Sex: male versus female | 1.56 (0.72–3.49) | 0.65 |
| Age: below versus above median | 0.86 (0.37–2.82) | 0.35 |
| PCFT mRNA: low versus high PCFT | 2.43 (1.44–3.58) | 0.02 |
| TS mRNA: low versus high TS | 0.82 (0.65–0.97) | 0.04 |
HR, hazard ratio, OS, overall survival; PFS, progression-free survival.
The potential predictive role versus prognostic role of PCFT mRNA expression was further investigated in a series of 40 patients who were not treated with pemetrexed. The median OS of this population was 11.9 months (95% CI, 3.4–20.4), and a correlation was neither found between PCFT mRNA levels and OS nor with PFS (supplementary Figure S3A and S3B, available at Annals of Oncology online).
Correlation of combined expression of TS and PCFT with outcome
We previously reported a correlation between the expression of the main target of pemetrexed, TS, and both PFS and OS, in patients belonging to the first cohort [4]. Therefore, we repeated this analysis in our second/validation cohort of patients, categorized on the basis of TS median value, showing again a highly significant correlation (P = 0.01) with OS (Figure 1E). Then, we carried out a subgroup analysis for the patients with both low-PCFT mRNA and high-TS mRNA levels (16/69 in the first and 15/51 patients in the second cohort). In this subgroup, we observed a statistically significantly shorter OS, compared with all the other patients in both the test and validation cohorts, as well as when the two cohorts were combined (P < 0.001, Figure 1F). PFS data showed a similar association, as reported in supplementary Figure S4A, available at Annals of Oncology online. Similarly, the subgroup analysis for patients with both high-PCFT mRNA and low-TS showed significantly longer OS and PFS (supplementary Figure S4B and S4C, available at Annals of Oncology online).
Correlation of PCFT protein expression with outcome
PCFT protein levels were analyzed by immunohistochemistry on TMAs (Figure 2A). This analysis showed a variable PCFT protein expression, related to mRNA expression. Tissues characterized by high-PCFT mRNA levels presented a strong and diffuse staining, while tissues with low-PCFT levels had only a few scattered positive cells with a weak membrane staining. Patients were categorized according to their high- versus low-PCFT levels, using the scoring system reported in supplementary Figure S1, available at Annals of Oncology online, and the univariate analysis revealed a significant correlation between high PCFT and longer OS (P = 0.02, Figure 2B), as well as a trend towards a significant association with PFS (P = 0.08, Figure 2C),
Figure 2.
Correlation of PCFT protein expression with clinical outcome. (A) Representative pictures of the tissue-microrrays (TMAs) including cores obtained from paraffin-embedded tumor material (from four different tumor areas from each of the 35 MPM patients of the validation cohort) incubated with an anti-PCFT specific antibody, as described in the methods; (upper panels) TMA slide (original magnification 1× magnification) and single spots of negative and positive controls (original magnification 20×); (Lower panels) Example of weak, intermediate and strong PCFT staining (original magnification 20×). Staining results were evaluated using a four-tier system (supplementary Figure S1A, available at Annals of Oncology online). (B and C) Overall survival (OS) and progression-free survival (PFS) curves segregated according to PCFT protein expression levels in the MPM patients from the validation cohort.
PCFT promoter methylation and expression affect pemetrexed cytotoxicity
The DNA methylation status of the PCFT promoter was evaluated in four MPM cell lines, and compared with Caco-2 and CCRF-CEM cells, as described previously [14]. Promoter methylation (Figure 3A and supplementary Figure S5, available at Annals of Oncology online), showed a strong inverse correlation with PCFT gene expression and protein levels (Figure 3B and supplementary Figure S6, available at Annals of Oncology online). CCRF-CEM and H28 cells, which exhibited low but detectable levels of PCFT, displayed PCFT promoter methylation, with ∼85% of the CpG-islands methylated in H28 cells. Further, Caco-2 cells exhibited the highest PCFT levels and showed almost no promoter methylation, whereas the MSTO-211, H2452 and H2052 cells had ∼40%, 60% and 65%, respectively, of their CpG-sites methylated.
Figure 3.
PCFT promoter methylation and PCFT expression affect pemetrexed cytotoxicity. (A) DNA methylation of the PCFT promoter involves a 414-bp-long fragment (termed fragment-1) spanning nucleotide positions −309/+104. Fragment-1 was subdivided into fragment-1A and fragment-1B for bisulfite-based DNA sequencing in H28 and MSTO-211H MPM cells. Each column represents a different clone, whereas each row represents a different CpG site. (B) Quantitative RT-PCR analysis of PCFT mRNA expression in MPM cell lines. Results are presented relative to the expression levels of PCFT in CCRF-CEM cells, assigned a value of 1. Columns, mean values obtained from three independent experiments; bars, SEM. (C) PCFT silencing successfully reduced PCFT mRNA levels in the three MPM cell lines. Conversely, exposure to the demethylating agent 5-aza-2′-deoxycytidine (5-aza-CdR, 1 μM) increased the mRNA expression of PCFT. *Significantly different (P < 0.05) from untreated cells (cells treated with the siRNA negative control had similar results, data not shown). (D) Representative growth inhibition curves of MPM cells treated with pemetrexed (PMX, 0.001–50 μM, 72-h exposure), with and without transfection with a specific anti-PCFT siRNA; Points, mean values obtained from three independent experiments; bars, SEM. (E) (Upper panels) Representative images of trypan blue-stained H2452 cells following treatment with PMX after exposure to 5-aza-CdR; (Lower panel). Cell growth of cells treated with PMX (at the cell line specific IC50) after exposure to 5-aza-CdR (1 μM). Cell growth of treated cells was compared to growth of untreated control cells set at 100%. *Significantly different (P < 0.05) from cells treated with PMX alone.
A concentration-dependent inhibition of cell growth was seen in all MPM cells treated with pemetrexed (Figure 3D). MSTO-211 H cells, harboring the highest PCFT levels, were the most sensitive cells (IC50, 0.021 ± 0.003 μM), whereas H28 cells, displaying the lowest PCFT mRNA/protein levels, were the most inherently pemetrexed resistant (IC50, 10.96 ± 2.46 μM). Notably, we previously found that H28 cells also express low levels of RFC [16]. This suggests that PCFT is the dominant entry route for pemetrexed in these tumor cells.
The role of PCFT expression on the anti-proliferative activity of pemetrexed was further evaluated using siRNA-silencing, which repressed PCFT mRNA levels by 80% (Figure 3C), and resulted in four- and threefold increased IC50 values in MSTO-211 H and H2452 cells, respectively (Figure 3D). A slight change in pemetrexed sensitivity accompanying PCFT modulation was also detected in the H28 cells, showing an IC50 of 15.65 ± 2.39 μM.
We then treated the cells with 1 μM 5-aza-2′-deoxycytidine, which reduced the methylation of the PCFT promoter by 70% in H28, and by 45% in H2452 and MSTO-211 H cells, with consequently increased PCFT mRNA levels (Figure 3C). Consequently, this demethylating agent significantly increased the entry of pemetrexed and the concentration of its di-glutamate metabolite, as well as its growth inhibitory activity in both monolayers and spheroids (Figure 3E and supplementary Figures S7–S9, available at Annals of Oncology online).
Discussion
This study explored the differential expression of key folate transporters in MPM specimens and, to the best of our knowledge, it is the first study showing a correlation between PCFT mRNA and protein levels and OS in two large cohorts of MPM patients who received a pemetrexed/carboplatin regimen. Moreover, our in vitro studies demonstrated a correlation between PCFT promoter methylation and silencing and pemetrexed resistance. These findings suggest novel therapeutic interventions to overcome pemetrexed resistance mediated by loss of PCFT expression in MPM.
The prognosis of MPM patients varies among different tumors and patient characteristics, and novel biomarkers and therapies are urgently needed [1]. A comprehensive genomic analysis identified four distinct MPM molecular subtypes correlated with survival [17], and recent trials evaluated new agents such as FAK inhibitors [18].
However, only a few studies identified potential predictors of responsiveness to chemotherapy. The two largest multicenter studies on pemetrexed-based regimens reported that both TS mRNA and protein expression are inversely correlated with OS and response [3, 4]. Conversely, a similar study with a smaller cohort did not find this correlation, but rather suggested a prognostic role for RFC and folylpolyglutamate synthetase (FPGS), responsible for polyglutamylation and cellular retention of antifolates [6]. Yet another study showed that neither TS nor FPGS were useful markers of response to pemetrexed [5]. These controversial results might be explained by different quantification procedures, since a consensus has not yet been reached regarding TS immunohistochemistry scoring. However, recent studies, including a meta-analysis on 526 patients, and a prospective randomized trial clearly supported the role of TS in predicting the outcome of non-small-cell lung cancer (NSCLC) patients treated with pemetrexed regimens [19, 20]. Accordingly, TS had a strong prognostic value in our validation cohort. Furthermore, we showed for the first time that PCFT expression is another important prognostic factor. As might be predicted, patients with both low-PCFT and high-TS levels had the worst prognosis, suggesting that multiple mechanisms affecting pemetrexed pharmacokinetics/-dynamics play key roles in tumor sensitivity.
PCFT has recently emerged as the main transporter mediating pemetrexed influx, with remarkable transport Km values of 0.2–0.8 μM, and its expression has been linked to pemetrexed cytotoxicity [15]. Consistently with this notion, we observed that PCFT silencing increased pemetrexed IC50 values.
Previous studies demonstrated that the PCFT promoter is highly methylated, thereby silencing PCFT expression, accounting for the loss of PCFT activity in antifolate-resistant cells [14, 15]. In the current study MPM cells with the lowest levels of PCFT mRNA/protein exhibited PCFT promoter hypermethylation and were more resistant to pemetrexed. Thus, one plausible modality to overcome pemetrexed resistance could involve the simultaneous treatment with a DNA-demethylating agent. Therefore, we tested a combination of pemetrexed and 5-aza-2′-deoxycytidine, a well-tolerated compound used in myelodysplastic syndrome and under investigation for other malignancies [21]. Our results suggest that tumor cells with low PCFT levels, comparable with low-PCFT expression in MPM tissues, can be successfully targeted by this combination. Moreover, a recent study supported the immune-related antitumor activity of 5-aza-2′-deoxycytidine in MPM models [22], while a phase I/II trial of combined epigenetic therapy with inhibitors of DNA methylation, in metastatic NSCLC, showed some clinical responses, with the best change in defined target lesions after pemetrexed treatment [23].
In conclusion, PCFT expression emerged as a novel biomarker for predicting potential therapeutic outcome after pemetrexed-based chemotherapy in a homogeneous setting of patients with MPM, hence prompting prospective trials. Moreover, preclinical data suggest that modulation of promoter methylation by 5-aza-2′-deoxycytidine may eradicate antifolate-resistant tumor cells displaying low PCFT levels.
Supplementary Material
Acknowledgements
We thank Dr Elena Lorenzi and Dr Letizia Gianoncelli (Department of Oncology; Humanitas Clinical and Research Hospital, Rozzano (Milan), Italy; University of Milan) for the isolation of RNA from the initial MPM samples, and Ietje Kathmann (Lab Medical Oncology, VUmc, Amsterdam) for performing the MTX uptake experiments. We extend our thanks to Dr Ilaria Carnevale and Dr Leticia G. Leon (Cancer Pharmacology Lab, AIRC Start-Up Unit, University of Pisa, Italy) for the help with the cell cultures and list of references.
Funding
This work was partially supported by ‘the Law Offices of Peter G. Angelos Grant’ from the Mesothelioma Applied Research Foundation (MARF), United States (EG, PAZ, and GJP), Fondazione Humanitas, Milano, Italy (EG, PAZ, MG, and MP), an Eli Lilly educational grant, The Netherlands (GJP), a Netherlands Organization for Scientific Research, NWO-Veni grant, The Netherlands (EG), Italian Association for Cancer Research, AIRC/Start-Up, Italy (EG), CCA Foundation grants 2013 and 2015, The Netherlands (EG and GJP), Tuscany Region grant FAS-DIAMANTE, Italy (NF and EG), R01 CA53535 from the National Cancer Institute, National Institutes of Health (LHM and ZH), the Eunice and Milton Ring Endowed Chair for Cancer Research (LHM) and the Fred Wyszkowski Cancer Research Fund (YGA).
Disclosure
The authors have declared no conflicts of interest to disclose. GJP received an educational grant from Eli Lilly, The Netherlands.
References
- 1. Carbone M, Kanodia S, Chao A. et al. Consensus report of the 2015 Weinman international conference on mesothelioma. J Thorac Oncol 2016; 11: 1246–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ceresoli GL, Zucali PA, Favaretto AG. et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 2006; 24: 1443–1448. [DOI] [PubMed] [Google Scholar]
- 3. Righi L, Papotti MG, Ceppi P. et al. Thymidylate Synthase but not excision repair cross-complementation group-1 tumor expression predicts outcome in patients with mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol 2010; 28: 1534–1539. [DOI] [PubMed] [Google Scholar]
- 4. Zucali PA, Giovannetti E, Destro A. et al. Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatin. Clin Cancer Res 2011; 17: 2581–2590. [DOI] [PubMed] [Google Scholar]
- 5. Lustgarten DE, Deshpande C, Aggarwal C. et al. Thymidylate synthase and folyl-polyglutamate synthase are not clinically useful markers of response to pemetrexed in patients with mesothelioma. J Thorac Oncol 2013; 8: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mairinger F, Vollbrecht C, Halbwedl I. et al. Reduced folate carrier and folylpolyglutamate synthetase, but not thymidylate synthase predict survival in pemetrexed-treated patients suffering from mesothelioma. J Thorac Oncol 2013; 8: 644–653. [DOI] [PubMed] [Google Scholar]
- 7. Gonen N, Assaraf YG.. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Update 2012; 15: 183–210. [DOI] [PubMed] [Google Scholar]
- 8. Nutt JE, Razak AR, O’Toole K. et al. The role of folate receptor alpha in the response of mesothelioma to pemetrexed-containing chemotherapy. Br J Cancer 2010; 102: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wojtuszkiewicz A, Peters GJ, van Woerden NL. et al. Methotrexate resistance in relation to treatment outcome in childhood acute lymphoblastic leukemia. J Hematol Oncol 2015; 8: 61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaufman Y, Ifergan I, Rothem L. et al. Coexistence of multiple mechanisms of PT523 resistance in human leukemia cells harboring 3 reduced folate carrier alleles: transcriptional silencing, inactivating mutations, and allele loss. Blood 2006; 107: 3288–3294. [DOI] [PubMed] [Google Scholar]
- 11. Chattopadhyay S, Zhao R, Krupenko S. et al. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther 2006; 5: 438–449. [DOI] [PubMed] [Google Scholar]
- 12. Qiu A, Jansen M, Sakaris A. et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006; 127: 917–928. [DOI] [PubMed] [Google Scholar]
- 13. Cherian C, Kugel-Desmoulin S, Wang L. et al. Therapeutic targeting malignant mesothelioma with a novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate via its selective uptake by the proton-coupled folate transporter. Cancer Chemother Pharmacol 2013; 71: 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonen N, Bram EE, Assaraf YG.. PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun 2008; 376: 787–792. [DOI] [PubMed] [Google Scholar]
- 15. Diop-Bove NK, Wu J, Zhao R. et al. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther 2009; 8: 2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giovannetti E, Zucali PA, Assaraf YG. et al. Preclinical emergence of vandetanib as a potent antitumour agent in mesothelioma: molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin. Br J Cancer 2011; 105: 1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bueno R, Stawiski EW, Goldstein LD. et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016; 48: 407–416. [DOI] [PubMed] [Google Scholar]
- 18. Soria JC, Gan HK, Blagden SP. et al. A phase-I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann Oncol 2016; 27: 2268–2274. [DOI] [PubMed] [Google Scholar]
- 19. Wang L, Wang R, Pan Y. et al. The pemetrexed-containing treatments in non-small-cell lung cancer: is −/low thymidylate synthase expression better than +/high thymidylate synthase expression: a meta-analysis. BMC Cancer 2014; 14: 205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun JM, Ahn JS, Jung SH. et al. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non-small-cell lung cancer: a biomarker-stratified randomized phase-II trial. J Clin Oncol 2015; 33: 2450–2456. [DOI] [PubMed] [Google Scholar]
- 21. Nervi C, De Marinis E, Codacci-Pisanelli G.. Epigenetic treatment of solid tumours: a review of clinical trials. Clin Epigenet 2015; 7: 127.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Covre A, Coral S, Nicolay H. et al. Antitumor activity of epigenetic immunomodulation combined with CTLA-4 blockade in syngeneic mouse models. Oncoimmunology 2015; 4: e1019978.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juergens RA, Wrangle J, Vendetti FP. et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small-cell lung cancer. Cancer Discov 2011; 7: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.