Abstract
Background
Multiple pilot studies, including one in colorectal cancer patients, suggest that creatine, an amino acid derivative, augments muscle, improves strength, and thereby could palliate the cancer anorexia/weight loss syndrome.
Patients and methods
In this randomized, double-blind, placebo-controlled trial, incurable patients with this syndrome were assigned creatine (20 g/day load×5 days followed by 2 g/day orally) versus identical placebo. Patients were weighed once a week for 1 month and then monthly. Patients were also assessed over 1 month for appetite and quality of life (validated questionnaires), fist grip strength, body composition (bioelectrical impedance), and adverse events. The primary endpoint was 10% or greater weight gain from baseline during the first month.
Results
Within this combined cohort of 263 evaluable patients (134 received creatine and 129 placebo), only 3 gained ≥10% of their baseline weight by 1 month: two creatine-treated and the other placebo-exposed (P = 1.00). Questionnaire data on appetite, quality of life, and activities of daily living showed no statistically significant differences between groups. Similarly, no statistically significant differences between groups were observed for fist-grip strength or body composition. Rates and severity of adverse events were comparable between groups. Finally, a median survival of 230 and 239 days were observed in the creatine and placebo groups, respectively (P = 0.70).
Conclusion
Creatine, as prescribed in this trial, had no effect on the cancer anorexia/weight loss syndrome.
Keywords: weight loss, cachexia, nutrition, creatine, supportive care
Key Message
This study suggests that creatine, a readily available supplement, is ineffective for the treatment of cancer-associated weight loss.
Introduction
Over 50 clinical trials have tested creatine supplements and, in total, suggest this amino acid derivative improves strength, augments muscle mass, and leads to weight gain [1]. Replete in dietary sources such as meat and fish, creatine is integrated into human skeletal and cardiac muscle, brain, and other organs, playing a pivotal role in energy production by transporting the extra phosphate that converts adenosine diphosphate to adenosine triphosphate. Because the latter fuels daily activities and bodily functions, creatine phosphate serves an important role by maintaining this key energy source. Creatine is thought to improve athletic performance, thereby enhancing muscle bulk and weight gain. Furthermore, emerging research suggests that creatine has direct muscle effects. A study in healthy men demonstrated large shifts in mRNA expression after creatine supplementation with patterns indicative of cytoskeletal muscle remodeling [2].
Such observations in healthy individuals spawned studies focused on patients with various disease states, such as congestive heart failure, Duchenne muscular dystrophy, McArdle’s disease, amyotrophic lateral sclerosis, sarcopenia of aging as well as a small study in colon cancer [3–8]. At times, these trials have suggested clinical benefit in accordance with that observed in healthy adults who take creatine supplements. However, such factors as limited sample sizes and heterogeneity of study populations prompted a meta-analysis to conclude ‘larger clinical studies are needed to confirm these observations’ and another review to conclude ‘further research is required’ [9, 10].
The trial reported here was designed to determine whether creatine improves clinical outcomes among incurable cancer patients who are suffering from the cancer anorexia/weight loss syndrome. The latter is a debilitating, life-limiting syndrome; no intervention, apart from successful cancer treatment, reverses all aspects [11]. At best, progesterone analogs and corticosteroids improve appetite but fail to address patients’ decline in functional status and compromised survival [11]. In view of the foregoing provocative clinical and mechanistic data, the mandate to pursue more definitive clinical trials with creatine, and a clear lack of therapeutic options in solid tumor patients who suffer from the cancer anorexia/weight loss syndrome, the current multi-institutional, double-blind, placebo-controlled trial was undertaken.
Patients and methods
Overview
The North Central Cancer Treatment Group (NCCTG), a cooperative group funded by the National Cancer Institute and now known as the Alliance, conducted this trial. All institutions had their institutional review boards approve the study protocol (NCT00081250). All patients had to provide written informed consent at the time of enrollment.
Eligibility criteria
To be eligible, patients had to meet all the following criteria: (i) age ≥18 years; (ii) incurable malignancy other than a primary brain tumor; (iii) life expectancy of ≥3 months, as per the judgment of the treating healthcare provider; (iv) an Eastern Cooperative Oncology Group performance score of 0-2; (v) patient-reported weight loss of at least 5 pounds (2.3 kg) over the preceding 2 months and/or a healthcare provider-estimated caloric intake of <20 calories/kg of body weight/day; (vi) alert and reliably able to take oral medication; and (vii) under the care of a healthcare provider who believed that weight gain would potentially benefit the patient, and the patient perceived weight loss as a problem. Concurrent chemotherapy and/or radiation therapy was permitted.
Patients were excluded with any one of the following: (i) ongoing tube feedings or parenteral nutrition; (ii) edema or ascites; (iii) recent (within the preceding month) or anticipated treatment with adrenal corticosteroids (except for short-term dexamethasone around the time of chemotherapy), androgens, progestational agents, or other appetite stimulants; (iv) obstruction of the alimentary canal, malabsorption, or intractable vomiting or ongoing radiation to the bowel or stomach; (v) serum creatinine above the institutional upper limit of normal; (vi) symptomatic or untreated brain metastases; (vii) insulin-requiring diabetes; (viii) pregnancy, nursing, or unwilling to use oral contraceptives; (ix) poorly controlled hypertension or congestive heart failure; or (x) creatine use at study entry.
At baseline, patients underwent a history, physical examination, and assignment of performance score. Serum creatinine, a hemogram, and, if appropriate, a pregnancy test were obtained at baseline. All initial testing and follow up was to be done in a nonhospital setting.
Stratification and randomization
In a double blind manner, patients were randomly assigned to creatine versus an identical-appearing placebo with the following stratification factors: (i) cancer type: lung cancer versus gastrointestinal cancer versus other; (ii) degree of weight loss in the previous 2 months: <10 pounds versus ≥10 pounds; (iii) planned or ongoing concurrent chemotherapy: yes versus no; (iv) sex: male versus female; (v) age: <50 years versus ≥50 years; and (vi) a previously utilized index that categorized patients’ prognosis as good versus bad versus unsure [12].
Description of intervention
Creatine monohydrate with 99% purity as assessed by high-performance liquid chromatography as per the manufacturer was used. Both the creatine and identical placebo were provided by Hi-Health in Scottsdale, AZ. The intervention was to start no later than 14 days after randomization.
Patients started with a loading dose of creatine/placebo powder at 20 g/day orally for days 1–5. A measuring scoop was provided, and patients were advised to add the powder to any beverage. On day 6, patients started 2 g/day orally and could continue with the intervention indefinitely as long as no deleterious effects were observed. This dose was based on previous pilot studies that suggested benefit [13].
Instructions for dose reductions or intervention cessation were outlined in the protocol. A serum creatinine was checked during the loading dose, and, if this laboratory parameter increased beyond 1.5 times the baseline value, the intervention was discontinued. If at any point, patients manifested weight gain beyond 120% of their ideal body weight, then dosing was to be cut by 50% at the discretion of the patient’s healthcare provider. If ‘unacceptable’ weight loss occurred after a dose reduction, the original dose could be resumed. Healthcare providers were allowed discretion in adjusting the dose based on other adverse events, as long as the rationale for doing so was outlined in the patient’s study record.
Baseline and follow-up assessments
At baseline, patients completed previously validated questionnaires to assess weight and appetite and were provided a weight diary. These items were to be completed weekly for 4 weeks, and then monthly as long as the patient remained on the creatine/placebo. The questionnaires consisted of an NCCTG appetite questionnaire, the Functional Assessment of Anorexia/Cachexia Treatment (FAACT) instrument (version 4); a series of linear analog self-assessment (LASA) scales on overall quality of life, mental well-being, physical well-being, emotional well-being, and degree of social activity; and a previously utilized Frailty Index to assess extent and spectrum of daily activities [14, 15].
Furthermore, at baseline, patients underwent assessment of fist-grip strength with a JAMAR® hydraulic hand dynamometer and bioelectrical impedance. The latter was measured with the HYDRA ECF-ICF device at 5 and 200 frequencies. Patients underwent this testing monthly while on study.
Adverse events were itemized regardless of perceived attribution with Common Terminology Criteria for Adverse Events (version 3). Events were assessed and recorded at least monthly but more frequently if a severe event occurred.
Statistical considerations
The primary endpoint was the percentage of patients who gained ≥10% of their baseline weight in each study arm by 1 month. Weight was assessed monthly over time. Secondary endpoints included the percentage of patients who manifested weight stability (within 5% of baseline at 1 month), appetite changes, quality of life assessment scores, fist-grip strength, bioelectrical impedance, and intervention-related adverse events. Secondary endpoints were assessed monthly. Overall survival was also assessed as a secondary endpoint.
Patients who developed edema or ascites were censored with respect to weight data, and patients who dropped out after initiating creatine/placebo but before the 1-month time point were considered treatment failures. A Fisher’s exact test was used to analyze differences between study groups for the primary endpoint and for other categorical endpoints. Two sample t-tests or Wilcoxon methodology was employed to explore differences between arms for continuous variables, such as quality of life ratings. All testing was carried out using a two-sided hypothesis test and a 5% type I error rate.
Sample size calculations were derived from an anticipated detection of a 15% difference in the true percentage of patients able to gain more than 10% of their baseline weight if that percentage was no more than 16% in the inferior group. In other words, the study team anticipated a 10% baseline weight gain in 16% of the placebo-exposed patients and in 31% of the creatine-treated patients. A sample size of 125 patients per arm allowed for >80% power for detecting the above.
Results
Baseline and general follow-up data
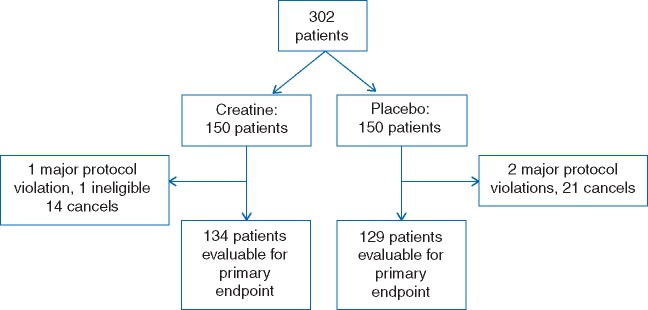
A total of 302 patients were recruited. Of these, 263 were evaluable for the primary study endpoint (Figure 1).
Figure 1.
A total of 134 creatine-treated and 129 placebo-exposed patients were assessed for the primary endpoint.
Baseline demographics that included age, gender, weight loss, and anticipated concurrent chemotherapy administration showed comparability between study arms (Table 1). The median time on the intervention was not statistically different between study arms: 54.5 days versus 64 days in patients assigned to the creatine and placebo arms, respectively (P = 0.14).
Table 1.
Baseline demographics of assessable patients
| Creatine n=134 | Placebo n=129 | P-valuea | |
|---|---|---|---|
| Age, mean in years (standard deviation) | 65 (11) | 65 (12) | 0.69 |
| Sex | |||
| Male | 83 (62) | 80 (62) | 0.99 |
| Female | 51 (38) | 49 (38) | |
| Concurrent chemotherapy | 105 (78) | 105 (81) | 0.54 |
| Primary malignancy | |||
| Lung | 48 (36) | 46 (36) | 0.96 |
| Gastrointestinal | 35 (26) | 32 (25) | |
| Other | 51 (38) | 51 (40) | |
| Prognostic index | |||
| Good | 12 (9) | 12 (9) | 0.89 |
| Bad | 26 (19) | 22 (17) | |
| Unsure | 96 (72) | 95 (74) | |
| Weight loss | |||
| <10 pounds | 67 (50) | 63 (49) | 0.85 |
| ≥10 pounds | 67 (50) | 66 (51) |
Numbers in parentheses refer to percentage of the cohort, unless otherwise specified.
A χ2 test was used for all analyses except for the age comparison for which a Kruskal–Wallis test was used.
Efficacy outcomes
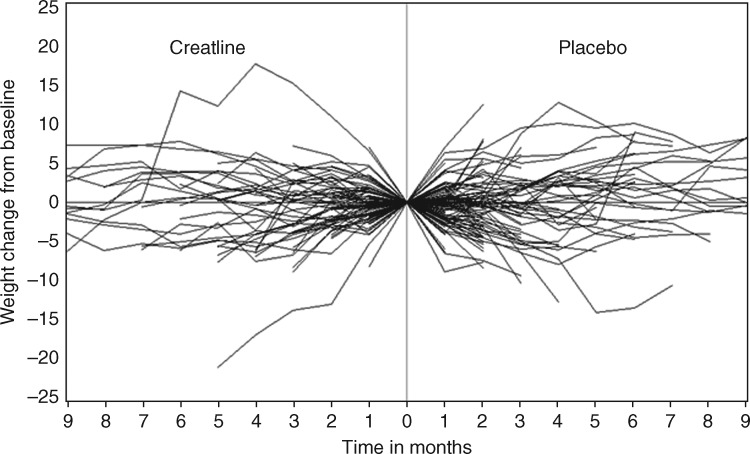
Within this entire cohort of 263 evaluable patients, only 3 gained ≥10% of their baseline weight over 1 month: two in the creatine arm and the other in the placebo arm (P = 1.00). Over time, 5 (4%) patients in the creatine arm and 14 (11%) in the placebo arm gained ≥10% of their baseline weight (P = 0.03). In terms of less than a 5% change in weight (gain or loss), 95 (71%) of creatine-treated patients and 88 (68%) of placebo-exposed patients manifested such weight stability at 1 month (P = 0.64) (Figure 2).
Figure 2.
Over 1 month, only three patients gained ≥10% of their baseline weight: two in the creatine arm and the other in the placebo arm (P = 1.00). Over time, no trends emerged to indicate that creatine was effective.
In response to the 1-month NCCTG appetite questionnaire response with items, such as (i) ‘How would you compare your appetite now to what it was before your present illness?’ (ii) ‘What is your current food intake in comparison to before your present illness?’ (iii) ‘How would you rate/describe your appetite?’ (iv) ‘How is your appetite now in comparison to before you started the study medications?’ and (v) ‘What effect, if any, do you feel the study medications have had on your food intake?’ no statistically significant differences in responses were observed in arms over time (Table 2). The FAACT questionnaire generated similar negative neutral results, as did LASA scores when assessed as change from baseline (data not shown). One-month Frailty Index responses based on intervention revealed no statistically significant differences between groups (Table 3).
Table 2.
Select NCCTG Anorexia Questionnaire 1-month responses
| Creatine n=114 | Placebo n=117 | P value | |
|---|---|---|---|
| How would you compare your appetite to what it was before your illness? | |||
| Same | 12 (17) | 20 (27) | 0.43 |
| Increased | 20 (29) | 23 (32) | |
| Slightly reduced | 16 (23) | 10 (14) | |
| Moderately reduced | 14 (20) | 15 (21) | |
| Markedly reduced | 7 (10) | 5 (7) | |
| What is your current food intake in comparison to before your illness? | |||
| Same | 10 (15) | 14 (20) | 0.86 |
| Increased | 19 (28) | 22 (31) | |
| Slightly reduced | 15 (22) | 12 (17) | |
| Moderately reduced | 17 (25) | 15 (21) | |
| Markedly reduced | 8 (12) | 8 (11) | |
| How would you rate your appetite? | |||
| Very good | 3 (4) | 8 (11) | 0.47 |
| Good | 16 (23) | 20 (27) | |
| Fair | 33 (48) | 26 (36) | |
| Poor | 12 (17) | 13 (18) | |
| very poor | 5 (7) | 6 (8) | |
| How is your appetite now in comparison to before you started the study medication? | |||
| Same | 19 (28) | 26 (36) | 0.49 |
| Increased | 8 (12) | 6 (8) | |
| Slightly reduced | 18 (26) | 21 (29) | |
| Moderately reduced | 19 (28) | 13 (18) | |
| Markedly reduced | 4 (6) | 7 (10) | |
| What effect, if any, do you feel the study medication has on your food intake? | |||
| Eat the same | 20 (29) | 26 (35) | 0.67 |
| Eat less | 10 (15) | 6 (8) | |
| Eat slightly more | 21 (31) | 26 (35) | |
| Eat moderately more | 12 (18) | 11 (15) | |
| eat considerably more | 4 (6) | 5 (7) | |
| Do the study medications make food taste better? | |||
| Yes | 9 (13) | 4 (6) | 0.22 |
| No | 30 (44) | 39 (55) | |
| Do not know | 29 (43) | 28 (39) | |
| Do the study medications allow you to eat more at one time by preventing you from getting ‘full’ soon after you start eating? | |||
| Yes | 13 (19) | 11 (16) | 0.8 |
| No | 33 (48) | 33 (47) | |
| Do not know | 23 (33) | 27 (38) | |
| Do you feel the study medications are helping or hindering you? | |||
| Helping | 29 (43) | 28 (41) | 0.8 |
| Hindering | 0 | 0 | |
| Neither | 39 (57) | 41 (59) |
Numbers in parentheses are percentages and do not always sum to 100% either because of missing data or rounding.
Table 3.
Select frailty index responses
| Baseline |
One month |
|||||
|---|---|---|---|---|---|---|
| Creatine | Placebo | P value | Creatine | Placebo | P value | |
| Do you do light housework? | ||||||
| Never | 31 (29) | 23 (22) | 0.5 | 22 (32) | 16 (24) | 0.33 |
| Sometimes | 36 (33) | 33 (31) | 28 (41) | 26 (38) | ||
| Mostly | 20 (19) | 22 (21) | 5 (7) | 11 (16) | ||
| Always | 21 (19) | 28 (26) | 13 (19) | 15 (22) | ||
| Do you do heavy housework? | ||||||
| Never | 66 (61) | 52 (50) | 0.3 | 41 (60) | 33 (49) | 0.49 |
| Sometimes | 24 (22) | 24 (23) | 17 (25) | 18 (27) | ||
| Mostly | 6 (6) | 9 (9) | 3 (4) | 6 (9) | ||
| Always | 13 (12) | 20 (19) | 7 (10) | 10 (15) | ||
| Do you prepare warm meals yourself, or do you assist in preparing them? | ||||||
| Never | 29 (27) | 23 (22) | 0.11 | 20 (29) | 15 (22) | 0.21 |
| Sometimes | 45 (41) | 32 (30) | 29 (43) | 22 (33) | ||
| Mostly | 24 (22) | 33 (31) | 12 (18) | 16 (24) | ||
| Always | 11 (10) | 18 (17) | 7 (10) | 14 (21) | ||
| How many flights of stairs do you walk up per day? | ||||||
| I never walk up stairs | 52 (48) | 36 (34) | 0.03 | 26 (39) | 25 (36) | 0.39 |
| 1–5 | 40 (37) | 59 (55) | 32 (48) | 27 (39) | ||
| 6–10 | 11 (10) | 5 (5) | 7 (10) | 12 (17) | ||
| More than 10 | 5 (5) | 7 (7) | 2 (3) | 5 (7) | ||
| If you go somewhere in your hometown, what kind of transportation do you use? | ||||||
| I never go out | 4 (4) | 0 | 0.16 | 1 (2) | 1 (2) | 0.72 |
| Car | 100 (93) | 101 (96) | 65 (96) | 64 (96) | ||
| Public transportation | 4 (4) | 3 (3) | 2 (3) | 1 (2) | ||
| Bicycle | 0 | 0 | 0 | 0 | ||
| walking | 0 | 1 (1) | 0 | 1 (2) | ||
| How often do you go shopping? | ||||||
| Never or less than once a week | 42 (39) | 35 (33) | 0.45 | 31 (46) | 15 (22) | 0.02 |
| Once per week | 40 (37) | 42 (39) | 18 (27) | 29 (42) | ||
| Twice to four times per week | 22 (21) | 29 (27) | 18 (27) | 25 (36) | ||
| Every day | 3 (3) | 1 (1) | 1 (2) | 0 | ||
| If you go shopping, what kind of transportation do you use? | ||||||
| I never go shopping | 15 (14) | 9 (9) | 0.12 | 10 (15) | 4 (6) | 0.2 |
| Car or truck | 93 (86) | 94 (89) | 57 (84) | 60 (88) | ||
| Public transportation | 0 | 3 (3) | 1 (2) | 3 (4) | ||
| Bicycle | 0 | 0 | 0 | 0 | ||
| walking | 0 | 0 | 0 | 1 (2) | ||
| Do you play a sport? | ||||||
| No | 102 (94) | 97 (91) | 0.43 | 62 (95) | 64 (94) | 0.74 |
| Yes | 7 (6) | 10 (9) | 3 (5) | 4 (6) | ||
| Do you work outside your home? | ||||||
| No | 85 (83) | 86 (83) | 0.97 | 51 (82) | 9 (15) | 0.65 |
| yes | 18 (18) | 18 (17) | 11 (18) | 52 (85) | ||
Numbers in parentheses are percentages and do not always sum to 100% either because of missing data or rounding.
Fist-grip strength and bioelectrical impedance were assessed in cohort subgroups. In terms of fist-grip strength, 89 creatine-treated and 92 placebo-exposed patients participated. The mean scores (standard deviation) among the patients in these respective arms at baseline were 25 kg (9.2) and 26 (9.9); P = 0.42. At 1 month, these scores were similar at 25 (10.5) and 25 (11.0); P = 0.76. Evaluating changes from baseline between arms at this 1-month interval also revealed no statistically significant differences between groups with a change of –0.2 (7.7) in the creatine arm and –0.8 (7.2) in the placebo arm; this comparison was also statistically insignificant (P = 0.94). Bioelectrical impedance was carried out in a subgroup that included 20 creatine-treated patients and 15 placebo-exposed patients. Again, readings at baseline and 1 month and assessing changes over time revealed no statistically significant differences between study arms (data not shown).
Grade 3 and 4 hematologic and nonhematologic adverse events were not statistically different between arms in their frequency (Table 4). The more frequent events included such symptoms as nausea, vomiting, abdominal pain, constipation, and shortness of breath. Four deaths occurred during active intervention on the creatine arm and seven on the placebo arm.
Table 4.
Adverse events
| Number of patients with at least one such adverse event (%) |
||
|---|---|---|
| Creatine | Placebo | |
| All grade 3 or worse | 45 (37) | 50 (42) |
| All grade 4 or worse | 14 (11) | 14 (12) |
| Grade 3 or worse hematologic | 5 (4) | 15 (12) |
| Grade 4 or worse hematologic | 1 (1) | 7 (6) |
| Grade 3 or worse nonhematologic | 39 (32) | 45 (38) |
| Grade 4 or worse nonhematologic | 13 (11) | 9 (8) |
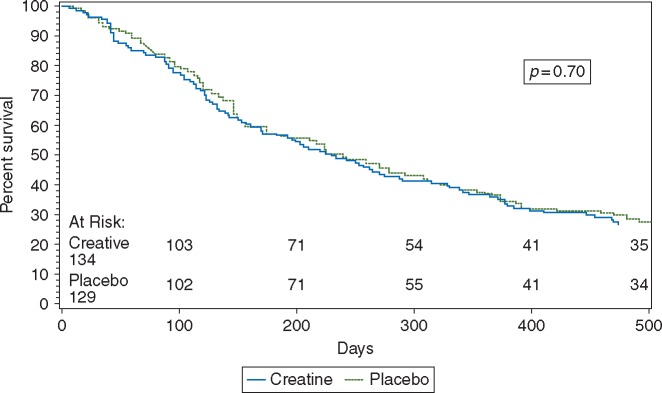
No statistically significant differences in survival were observed between the treatment arms: median survival (days): 230 versus 239 in the creatine and placebo arms, respectively (P = 0.70 by log-rank test) (Figure 3).
Figure 3.
Median survival was 230 days versus 239 days in the creatine and placebo patients, respectively (P = 0.70 by log-rank test).
Discussion
This trial showed that creatine, as prescribed here does not treat the cancer anorexia/weight loss syndrome. Evaluating multiple clinical endpoints—including weight, appetite, fist grip strength, body composition, and survival—we observed that creatine does not improve outcomes. This agent joins a long list of others, such as infliximab, etanercept, dronabinol, omega 3 fatty acids, fluoxymesterone, pentoxifylline, and hydrazine sulfate, which have yielded disappointment [16–21].
Importantly, the negative findings reported here do not negate earlier trials that portrayed creatine as a promising for a variety of other maladies, including sarcopenia of aging and other disease states. It is possible that the pathophysiology of the cancer anorexia/weight loss syndrome differs from that of these other disease entities. One might also argue that creatine supplementation might be more effective in a deficient state, and, at least a modicum of data suggests that cancer patients might not be deficient [22]. The current study should, however, motivate other investigators to pursue larger, more definitive trials, with clinically meaningful endpoints to determine assuredly whether creatine does have a role in treating these other muscle disorders.
This study has both strengths and weaknesses. With respect to weaknesses, one might argue that our enrollment of patients with marked weight loss selected for patients at late stages of the cancer anorexia/weight loss syndrome and that these patients had reached a point of nonreversal. Although we acknowledge that certain interventions might be more effective with earlier stage disease, the fact that this study showed absolutely no benefit with creatine indicates that further study of creatine for this specific syndrome might be difficult to justify. The strengths of this study are its robust sample size; randomized, double-blinded, placebo-controlled study design; and reliance on clinically important endpoints—all of which we believe counterbalance the above limitation. In this context, we view the results reported here as important and definitive.
Funding
This work was supported by the United States National Cancer Institute of the National Institutes of Health [Grant Award Numbers UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA037404, U10CA063848, U10CA035195, U10CA035101, U10CA035269, U10CA037417, U10CA035448, U10CA063844, U10CA035267, U10CA063849, U10CA035113, U10CA035103, U10CA035415, and U10CA035431]. This work was also supported R21CA098477. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Lanhers C, Pereira B, Naughton G. et al. Creatine supplementation and upper limb strength performance: a systemic review and meta-analysis. Sports Med 2017; 47: 163–173. [DOI] [PubMed] [Google Scholar]
- 2. Safdar A, Yardley NJ, Snow R. et al. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Gen 2008; 32: 219–228. [DOI] [PubMed] [Google Scholar]
- 3. Johannsmeyer S, Candow DG, Brahms CM. et al. Effect of creatine supplementation and drop set resistance training in untrained aging adults. Exp Gerontol 2016; 83: 112–119. [DOI] [PubMed] [Google Scholar]
- 4. Pinto CL, Botelho PB, Carneiro JA, Mota JF.. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J Cachexia Sarcopenia Muscle 2016; 7: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilkinson TJ, Lemmey AB, Jones JG. et al. Can creatine supplementation improve body composition and objective physical function in rheumatoid arthritis patients? A randomized controlled trial. Arthritis Care Res 2016; 68: 729–737. [DOI] [PubMed] [Google Scholar]
- 6. Fuld JP, Kilduff LP, Neder JA. et al. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2005; 60: 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tarnopolsky MA. Creatine as a therapeutic strategy for myopathies. Amino Acids 2011; 40: 1397–1407. [DOI] [PubMed] [Google Scholar]
- 8. Norman K, Stubler D, Baier P. et al. Effects of creatine supplementation on nutritional status, muscle function, and quality of life in patients with colorectal cancer – a double blind randomized controlled trial. Clin Nutr 2006; 25: 596–605. [DOI] [PubMed] [Google Scholar]
- 9. http://www.cochrane.org/CD005184/HTN_effectiveness-of-creatine-analogues-in-cardiac-disease (14 November 2016, date last accessed).
- 10. Cooper R, Naclerio F, Allgrove J, Jimenez A.. Creatine supplementation with specific view to exercise/sports performance: an update. J Int Soc Sports Nutr 2012; 9: 33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le-Rademacher JG, Jatoi A, Cachexia JA In Abeloff’s Clinical Oncology. Elsevier, Churchill Livingstone, 6th edition. [Google Scholar]
- 12. Sloan JA, Loprinzi CL, Laurine JA. et al. A simple stratification factor prognostic for survival in advanced cancer: the good/bad/uncertain index. J Clin Oncol 2001; 19: 3539–3546. [DOI] [PubMed] [Google Scholar]
- 13. Hultman E, Soderlund K, Timmons JA. et al. Muscle creatine loading in men. J Appl Physiol 1996; 81: 232–237. [DOI] [PubMed] [Google Scholar]
- 14. Ribaudo JM, Cella D, Hahn EA. et al. Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 2000; 9: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 15. Voorrips LE, Ravelli ACJ, Dongelmans PCA. et al. A physical activity questionnaire for the elderly. Med Sci Sports Exerc 1991; 23: 974–979. [PubMed] [Google Scholar]
- 16. Loprinzi CL, Kuross SA, O’Fallon JR. et al. Randomized placebo-controlled evaluation of hydrazine sulfate in patients with advanced colorectal cancer. J Clin Oncol 1993; 11: 762–767. [DOI] [PubMed] [Google Scholar]
- 17. Jatoi A, Dakhil SR, Nguyen PL. et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 form the North Central Cancer Treatment Group. Cancer 2007; 110: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 18. Jatoi A, Ritter HL, Dueck A. et al. A placebo-controlled trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 2010; 68: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jatoi A, Rowland K, Loprinzi CL. et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 2004; 22: 2469–2476. [DOI] [PubMed] [Google Scholar]
- 20. Jatoi A, Windschitl HE, Loprinzi CL. et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002; 20: 567–573. [DOI] [PubMed] [Google Scholar]
- 21. Goldberg RM, Loprinzi CL, Mailliard JA. et al. Pentoxyfulline for treatment of cancer anorexia and cachexia? A randomized, double-blind, placebo-controlled trial. J Clin Oncol 1995; 13: 2856–2859. [DOI] [PubMed] [Google Scholar]
- 22. Bergstrom J, Alvestrand A, Furst P.. Influence of severe potassium depletion and subsequent repletion with potassium on muscle electrolytes, metabolites, and amino acids in man. Clin Sci 1976; 51: 589–599. [DOI] [PubMed] [Google Scholar]